Abstract
Context:
Inconsistent associations between maternal vitamin D status and fetal size have been published in small studies.
Objective:
Our objective was to examine the association between maternal 25-hydroxyvitamin D [25(OH)D] levels and measures of newborn and placental weight.
Design and Setting:
We measured maternal 25(OH)D in mothers from the Collaborative Perinatal Project, an observational cohort conducted in 12 U.S. medical centers from 1959 to 1965.
Participants:
Women delivering singleton, term, live births with 25(OH)D measured at a gestation of 26 wk or less (n = 2146).
Main Outcome Measures:
Birth weight, ponderal index, placental weight, the placental to fetal weight ratio, and small for gestational age were measured. Hypotheses were formulated after data collection.
Results:
After confounder adjustment, mothers with 25(OH)D of 37.5 nmol/liter or greater gave birth to newborns with 46 g [95% confidence interval (CI), 9–82 g] higher birth weights and 0.13 cm (0.01–0.25 cm) larger head circumferences compared with mothers with less than 37.5 nmol/liter. Birth weight and head circumference rose with increasing 25(OH)D up to 37.5 nmol/liter and then leveled off (P < 0.05). No association was observed between 25(OH)D and ponderal index, placental weight, or the placental to fetal weight ratio. Maternal 25(OH)D of 37.5 nmol/liter or greater vs. less than 37.5 nmol/liter in the first trimester was associated with half the risk of small for gestational age (adjusted odds ratio 0.5; 95% CI 0.3–0.9), but no second-trimester association was observed.
Conclusions:
Maternal vitamin D status is independently associated with markers of physiological and pathological growth in term infants. Adequately powered randomized controlled trials are needed to test whether maternal vitamin D supplementation may improve fetal growth.
Maternal vitamin D deficiency is a significant problem in the United States (1) and across the globe (2, 3) due to lack of sunlight exposure and inadequate intake (4, 5). Placental and maternal decidual cells express the enzyme to synthesize the active 1,25-dihydroxyvitamin D3 and have vitamin D receptors (6), allowing for numerous potential roles of vitamin D in pregnancy including fetal growth restriction (7).
There is mixed evidence in observational studies (8–12) and randomized supplementation trials (13–17) worldwide connecting vitamin D to fetal growth. Methodologies and quality of studies widely differ, with late pregnancy vitamin D assessment and small sample size seeming to contribute to null findings. Most vitamin D studies have assessed birth weight, a nonspecific summary measure of physiological fetal growth, yet some have also investigated small for gestational age (SGA), a marker of pathological growth.
Growth restriction in utero increases both immediate risk of infant morbidities and mortality (18) and latent risk of adult chronic diseases (19). Head circumference, ponderal index, placental weight, and the placental to fetal weight ratio have also been used as markers of fetal growth adequacy (20) and are associated with poor perinatal outcomes in offspring (21, 22) yet are lacking from vitamin D and birth size investigations.
Periconceptional nutritional status may be important in setting the fetal growth trajectory, and, like other exposures, vitamin D status in early pregnancy may be influential for later fetal growth. Our objective was to examine the associations between maternal vitamin D status at gestations of 26 wk or less and measures of newborn and placental weight, as markers of physiological and pathologic growth, in a large U.S. cohort of singleton, term, live births.
Subjects and Methods
This analysis was part of an ongoing investigation of vitamin D status and adverse pregnancy outcomes and was granted exemption from ethical approval by the Institutional Review Board at the University of Pittsburgh because we used deidentified data. Data and blood samples came from the Collaborative Perinatal Project (CPP), which enrolled a cohort of pregnant women from 12 medical centers across the United States from 1959 to 1965 (n = 55,908 births) (23, 24). Nonfasting maternal venous blood was sampled at the first prenatal visit and every 8 wk thereafter. Extensive interviews on participant demographics and medical histories were conducted. A summary of the labor and delivery was recorded by the obstetrician responsible for each patient's care, and infant and placental weights were measured after birth.
Pregnancies were eligible for the parent vitamin D study if they met the following criteria: singleton gestation; White, Black, or Puerto Rican maternal race/ethnicity; no preexisting diabetes or hypertension; entry to prenatal care at 26 wk or less; available stored serum sample at 26 wk or less; and gestational age at birth 20–42 wk (n = 28,429). Of these, 3074 were randomly selected for maternal 25-hydroxyvitamin D [25(OH)D] assessment to meet sample size requirements for studying preeclampsia and preterm birth, the main outcomes of the parent trial. If a woman had multiple blood samples available at 26 wk or less, a sample was selected at random for 25(OH)D measurement. For the current study, we excluded stillbirths (n = 32), preterm births (<37 wk, n = 636), and pregnancies for which serum was unsuitable for vitamin D measurement (n = 93). We then excluded pregnancies with missing covariates (n = 167) for a final analytical sample of 2146 singleton, live born, term infants born to 2096 mothers.
Exposure
The best biochemical marker of vitamin D status from all sources is circulating 25(OH)D (25). We measured total serum 25(OH)D [25(OH)D2 + 25(OH)D3] by liquid chromatography-tandem mass spectrometry. This method has a lower detection limit of 1 ng/ml and no upper limit. No total 25(OH)D concentrations fell below the detectable range. The intraassay coefficient of variation was 8.2 and 5.9% for 25(OH)D2 and 25(OH)D3, respectively. Samples had been stored more than 40 yr at −20 C; thus, we conducted a pilot study demonstrating that significant degradation of 25(OH)D was not likely (26). Additionally, others have shown 25(OH)D not likely to degrade over time, from UV exposure or from multiple freeze-thaw cycles (27).
Outcomes
Birth weight was measured just after birth, and infant head circumference and length as well as placental weight (trimmed of fetal membranes and umbilical cord, large clots removed) were measured within 24 h of birth. Ponderal index (28) and the placental to fetal weight ratio (29) were calculated as follows:
Ponderal index = birth weight (grams)/length (cubic centimeters) × 100.
Placental to fetal weight ratio = placental weight (grams)/birth weight (grams) × 100.
SGA was classified as birth weight less than the 10th percentile of a sex- and gestational age-specific national birth weight reference (30). Gestational age was based on the self-reported date of last menstrual period because ultrasound was not available when the CPP was conducted.
Covariates
Mothers were White, Black, or Puerto Rican, and we classified them as White or other because vitamin D associations were similar among Blacks and Puerto Ricans. Season of maternal blood draw was categorized by winter (December-February), spring (March-May), summer (June-August), and fall (September-November). We also assessed gestational age at maternal blood draw, prepregnancy body mass index [BMI; self-reported weight (kilograms) divided by measured height (squared meters)], height, parity (primiparous/multiparous), smoking at registration (yes/no), marital status (married/not married), socioeconomic status [continuous scale as described previously (24)], maternal age (continuous), study site (which also accounts for latitude), and infant sex.
Statistical analysis
Continuous outcomes were approximately normally distributed by visual observation using kernel density plots and were not transformed. We identified five low newborn lengths that were biologically implausible and set those to missing. Other statistical outliers were biologically plausible and retained. Because there is controversy around cut points to define vitamin D deficiency (31), we used different cut points to dichotomize 25(OH)D: 37.5, 50, and 80 nmol/liter. Results were not meaningfully different using these different cutoffs, but statistical significance was often lost when using 50 or 80 nmol/liter, so we maintained the 37.5 nmol/liter cut point. In addition, we examined 25(OH)D as a continuous variable. We tested the independent association between maternal serum 25(OH)D at 26 wk or less and the continuous outcomes of interest using multivariable linear regression, and the association with SGA using multivariable logistic regression. Nonlinear relations were tested using linear spline regression with a knot at the deficiency cut point.
Effect modification by trimester of maternal blood draw, maternal race, and infant sex was tested using the likelihood-ratio test (α = 0.10) in full models with all potential confounders (maternal race/ethnicity, season and gestational age at blood draw, prepregnancy BMI, height, parity, smoking, marital status, socioeconomic status, maternal age, study site, and infant sex). Potential confounders were then removed from full models if they did not change the main effect by greater than 10%. Maternal race/ethnicity, trimester of maternal blood draw, BMI, height, and smoking status met our definition of confounding in one or more models and were retained for all models for comparability.
To test the sensitivity of our results to missing data, we used multiple imputation by creating five data sets that imputed infant length, head circumference, placental weight, 25(OH)D, season of blood draw, prepregnancy weight, height, smoking status, socioeconomic status, and parity by including race/ethnicity, maternal age, marital status, gestational age at blood draw, CPP study site, infant sex, birth weight, and SGA in the imputation model (32). Results were not meaningfully different from results with the complete data set. We fitted generalized estimating equation regression models to account for the clustering of infants within women (n = 50 mothers with repeated pregnancies) and found no meaningful differences with our results, so we ignored the clustering. We conducted analysis with STATA 12.0 (StataCorp, College Station, TX).
Results
Most women were young, parous, married, and enrolled in the second trimester; almost half reported smoking (Table 1). Mean ± sd maternal 25(OH)D was 51.3 ± 28.0 nmol/liter, with 34.8% of women less than 37.5 nmol/liter and 55.9% less than 50 nmol/liter. Incidence of SGA was high (Table 1).
Table 1.
Characteristics of pregnant women and their newborns (n = 2146)
| n (%) or mean ± sd | Range (min, max) | |
|---|---|---|
| Pregnant women | ||
| Race | ||
| White | 1117 (52.1) | |
| Black | 893 (41.6) | |
| Puerto Rican | 136 (6.3) | |
| Maternal age (yr) | ||
| <20 | 470 (21.9) | |
| 20–29 | 1338 (62.4) | |
| ≥30 | 338 (15.8) | |
| Parity | ||
| 1 | 713 (33.2) | |
| ≥2 | 1430 (66.6) | |
| Married | 1757 (81.9) | |
| Smoking at study entry | 1020 (47.5) | |
| Prepregnancy BMI (kg/m2) | 22.3 ± 3.7 | 13.0, 42.7 |
| Trimester at registration | ||
| First (<14 wk) | 618 (28.8) | |
| Second (14–26 wk) | 1528 (71.2) | |
| Season of blood sampling | ||
| Winter (December–February) | 513 (23.9) | |
| Spring (March–May) | 555 (25.9) | |
| Summer (June–August) | 551 (25.7) | |
| Fall (September–November) | 527 (24.6) | |
| Median [IQR] gestational age at blood sampling (wk) | 20.6 [15.7, 23.4] | 4.1, 26.0 |
| Latitude of study site | ||
| ≥41° North | 1351 (63.0) | |
| 38–40° North | 619 (28.8) | |
| ≤35° North | 176 (8.2) | |
| Infantsa | ||
| Male | 1071 (49.9) | |
| Gestational age at delivery | 39.7 ± 1.3 | 37, 42 |
| Birth weight (g) | 3184 ± 408 | 1644, 4026 |
| Head circumference (cm) | 33.8 ± 1.3 | 29, 41 |
| Ponderal indexb | 2.54 ± 0.28 | 1.54, 3.59 |
| Placental weight (g) | 438 ± 90 | 150, 842 |
| Placental to fetal weight ratiob | 13.8 ± 2.4 | 7.7, 23.6 |
| SGAc | 395 (18.4) | |
IQR, Interquartile range.
Head circumference, 48 missing values; ponderal index, 65 missing values; placental weight and placental to fetal weight ratio, 194 missing values.
Ponderal index = birth weight (grams)/length (cubic centimeters) × 100; placental to fetal ratio = placental weight (grams)/birth weight (grams) × 100.
Defined by Alexander 1996 birth weight reference at less than the 10th percentile for males or females (30).
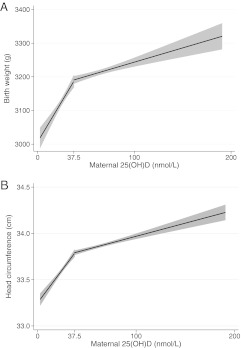
In a bivariate analysis, women with 25(OH)D of 37.5 or greater compared with less than 37.5 nmol/liter had infants with higher birth weights, head circumference, and ponderal index, but placental weight and placental to fetal weight ratio were not different (Table 2). After adjustment for trimester at maternal blood draw, maternal race/ethnicity, prepregnancy BMI, height, smoking, season, and study site, differences in birth weight and head circumference by vitamin D deficiency remained (Table 2). We found a nonlinear relation between 25(OH)D and birth weight as well as head circumference (P < 0.01). Birth weight and head circumference increased by 3.6 [95% confidence interval (CI), 1.1–6.1] g and 0.010 (0.002–0.018) cm, respectively, per 1 nmol/liter increase in maternal 25(OH)D up to 37.5 nmol/liter and then leveled off thereafter (Fig. 1). No relationship was observed between 25(OH)D and ponderal index, placental weight, or the placental to fetal weight ratio in adjusted analyses, regardless of how vitamin D was specified. None of these associations were modified by trimester of vitamin D assessment, maternal race/ethnicity, or infant sex.
Table 2.
Associations between maternal vitamin D deficiency at gestation of 26 wk or less and measures of infant and placental size
| Mean (sd) |
Unadjusted difference |
Adjusted differencea |
||||||
|---|---|---|---|---|---|---|---|---|
| 25(OH)D <37.5 nmol/liter | 25(OH)D ≥37.5 nmol/liter | β | (95% CI) | P value | β | (95% CI) | P value | |
| Birth weight (g), n = 2146 | 3127 (15) | 3215 (11) | 87.3 | (51.3–123.4) | <0.001 | 45.7 | (9.1–82.3) | 0.014 |
| Head circumference (cm), n = 2098 | 33.6 (0.05) | 33.9 (0.0.3) | 0.24 | (0.13–0.36) | <0.001 | 0.13 | (0.01–0.25) | 0.032 |
| Ponderal index,b n = 2081 | 2.52 (0.01) | 2.55 (0.01) | 0.027 | (0.002–0.052) | 0.033 | 0.021 | (−0.005 to 0.047) | 0.117 |
| Placental weight (g), n = 1952 | 435 (3.4) | 440 (2.5) | 4.67 | (−3.67 to 13.01) | 0.272 | 3.59 | (−5.16 to 12.33) | 0.421 |
| Placental to fetal weight ratio,c n = 1952 | 13.9 (0.09) | 13.7 (0.07) | −0.16 | (−0.38 to 0.07) | 0.166 | −0.03 | (−0.26 to 0.21) | 0.821 |
Maternal serum 25(OH)D is expressed as mean (sd).
Adjusted for trimester at maternal blood draw, maternal race/ethnicity (white/other), prepregnancy BMI, height, and smoking (yes/no), season, and study site. Adjustment for parity, marital status, socioeconomic status, age, and infant sex had no meaningful impact on the findings.
Ponderal index = birth weight (grams)/length (cubic centimeters) × 100.
Placental to fetal ratio = placental weight (grams)/birth weight (grams) × 100.
Fig. 1.
Nonlinear association between 25(OH)D and birth weight (A) and head circumference (B) based on linear spline regression models with knot at 37.5 nmol/liter and adjusted for trimester at maternal blood draw, maternal race (white/other), prepregnancy BMI, height, smoking, season, and study site. P < 0.01 for spline terms in each model.
The association between maternal 25(OH)D and risk of SGA was modified by the trimester of vitamin D assessment (P < 0.05). After adjustment for maternal race/ethnicity, prepregnancy BMI, height, smoking, season, and study site, maternal 25(OH)D of 37.5 or greater vs. less than 37.5 nmol/liter in the first trimester was associated with almost half the risk of SGA (Table 3). In addition, each 1 sd increase in 25(OH)D reduced the risk of SGA by 34% (95% CI 0.45, 0.98). There was no evidence of a nonlinear association. No relationship was observed between second-trimester 25(OH)D and SGA, regardless of how vitamin D was specified.
Table 3.
Associations between maternal serum 25(OH)D at 26 wk or less and SGA, by trimester at blood draw
| n (%) |
Unadjusted |
Adjustedb |
||||
|---|---|---|---|---|---|---|
| Non-SGA | SGAa | OR | (95% CI) | OR | (95% CI) | |
| Overall | ||||||
| 25(OH)D ≥37.5 nmol/liter | 1161 (82.9) | 239 (17.1) | 0.77 | (0.62–0.97) | 0.94 | (0.73–1.20) |
| 25(OH)D <37.5 nmol/liter | 590 (79.1) | 156 (20.9) | 1.0 | Reference | 1.0 | Reference |
| First trimesterc | ||||||
| 25(OH)D ≥37.5 nmol/liter | 202 (88.2) | 27 (11.8) | 0.43 | (0.24–0.76) | 0.50 | (0.27–0.91) |
| 25(OH)D <37.5 nmol/liter | 93 (76.2) | 29 (23.8) | 1.0 | Reference | 1.0 | Reference |
| Second trimesterc | ||||||
| 25(OH)D ≥37.5 nmol/liter | 959 (81.9) | 212 (18.1) | 0.87 | (0.68–1.11) | 1.05 | (0.81–1.37) |
| 25(OH)D <37.5 nmol/liter | 497 (79.7) | 127 (20.4) | 1.0 | Reference | 1.0 | Reference |
SGA defined by Alexander 1996 birth weight reference at less than the 10th percentile by gestational age for males or females (30).
Adjusted for trimester at maternal blood draw, maternal race/ethnicity (white/other), prepregnancy BMI, height, and smoking (yes/no), season, and study site. Adjustment for parity, marital status, socioeconomic status, age, and infant sex had no meaningful impact on the findings.
Effect modification by trimester at blood draw, P = 0.024; first trimester, less than 14 wk; second trimester, 14–26 wk.
Discussion
In this large U.S. pregnancy cohort of singleton infants born at term, maternal vitamin D status at a gestation of 26 wk or less was positively related to birth weight and head circumference, and, in the first trimester, was negatively associated with risk of SGA. No associations were observed between maternal vitamin D status and ponderal index, placental weight, or the placental to fetal weight ratio.
Studies of vitamin D and measures of birth weight are limited and heterogeneous. Results are mixed but the preponderance of evidence supports a positive association between vitamin D and birth weight. Two randomized trials in India testing third-trimester vitamin D supplementation (14, 15), and three observational studies of maternal first-trimester (8) or third-trimester 25(OH)D (9, 33) found a positive vitamin D and birth weight relationship. There are three randomized supplementation trials (13, 16, 17) and four observational studies (10, 11, 34, 35) that found no relation between maternal vitamin D and birth weight, yet Morley et al. (9) later found that vitamin D receptor genotype modified the vitamin D and birth weight association. Null findings may be explained by the way 25(OH)D was statistically analyzed (e.g. only continuously), small sample size, lack of vitamin D-deficient mothers, and studying different time points in gestation. None of the observational studies mentioned examining nonlinear relationships, and had we only looked at the continuous relationship, we would have reported null findings as well.
Few previous studies have examined measures of fetal growth other than birth weight. Our finding of a positive association between 25(OH)D and head circumference in term infants disagrees with two previous studies that found no association in preterm and term infants (10, 11), whereas our null association with term placental weight agrees with the one available study to also address this outcome (34). Ponderal index and the placental to fetal weight ratio are lacking from related published studies. Our results suggest that maternal vitamin D during pregnancy is associated with neonatal head circumference in a similar fashion as overall term birth weight (not lending support to a head sparing hypothesis) yet is not associated with infant thinness nor overall placental growth. Future studies should examine these additional measures as well as examine other markers of placental growth and function to elucidate the maternal vitamin D status and birth weight connection.
Randomized trials have demonstrated third-trimester vitamin D supplementation to reduce risk of SGA [15 vs. 28% (13)] or low birth weight [4 vs. 19% (15)]. In addition, two first-trimester observational studies found a vitamin D and SGA connection in term infants (8, 12). In our previous investigation, we found a U-shaped relationship between maternal 25(OH)D at approximately 10 wk and risk of SGA in U.S. white women (lowest risk at 60–80 nmol/liter) and no association among Black women (12). Leffelaar et al. (8) reported 2.4 times higher risk of SGA for mothers with 25(OH)D less than 29.9 nmol/liter vs. 50 nmol/liter or greater at 13 wk in a multiethnic cohort. We did not find the same U-shaped association again in this diverse U.S. population, yet our findings in the first trimester correspond to our earlier study in that vitamin D deficient mothers had 2 times the risk of delivering an SGA infant compared with nondeficient mothers.
There are several biological mechanisms plausibly connecting maternal vitamin D to fetal growth. By classical functions, maternal vitamin D deficiency may impede the typical increase in calcium absorption and affect bone metabolism to in turn reduce fetal bone accretion, but this hypothesis has not been adequately tested (36). The CYP27B1 enzyme, which hydroxylates 25(OH)D into 1,25(OH)2D, as well as vitamin D receptors that mediate the hormone's biological actions are expressed and functional in the human placenta (6, 37). Vitamin D receptors and 1,25(OH)2D regulate placental secretion of human placental lactogen and other hormones that affect maternal glucose and fatty acid metabolism to provide for fetal energy needs (36, 37). Thus, we speculate that vitamin D might influence neonatal mass through an effect on transplacental glucose and fatty acid transport and accretion of fat and nonskeletal lean mass.
We were concerned in our study that the effect modification by trimester at vitamin D measurement could be due to the fact that women who came into prenatal care earlier and had an available sample were different than those that entered care later. Nevertheless, after limiting our analysis to women who began prenatal care early, we found similar results. Our study is limited in the use of data in the 1960s, when obesity was low and smoking rates were high; thus, findings may not be generalizable to the United States today. In addition, those maternal characteristics (smoking and leanness) may explain the high rates of SGA we found. However, the CPP collected a tremendous amount of data on mothers and their offspring, affording us the unique opportunity to study vitamin D and multiple measures of newborn and placental size in a large study with a diverse population. Selection bias was unlikely given our findings were similar after multiple imputation. This study is among the few in U.S. populations (12) and has one of the largest sample sizes, and additionally, findings were robust to multiple sensitivity analyses. Another strength is the focus on term infants because it is highly problematic to accurately estimate SGA in preterm births (38) (even when there is essentially no iatrogenic prematurity, as is the case in CPP). Future studies should examine longitudinal maternal samples and ultrasound measures of fetal size to provide insight into the temporal effect of vitamin D on fetal growth.
As a recent review of vitamin D and pregnancy outcomes concluded, the quality of evidence in this area is low (39). Our study is an important contribution to the epidemiology evidence that maternal vitamin D status, especially in early pregnancy, may contribute to both pathological and physiological fetal growth, but not placental growth, in term infants. Randomized controlled trials that begin early in pregnancy are needed to provide causal evidence for clinical recommendations regarding vitamin D intake and potential screening in the care of pregnant women.
Acknowledgments
We thank Jill Diesel for her assistance with data preparation.
This work was supported by the National Institutes of Health Grant HD056999 (principal investigator, L.M.B.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- Body mass index
- CI
- confidence interval
- CPP
- Collaborative Perinatal Project
- 25(OH)D
- 25-hydroxyvitamin D
- SGA
- small for gestational age.
References
- 1. Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. 2011. Vitamin D status: United States, 2001–2006. NCHS Data Brief 1–8 [PubMed] [Google Scholar]
- 2. van der Meer IM, Karamali NS, Boeke AJ, Lips P, Middelkoop BJ, Verhoeven I, Wuister JD. 2006. High prevalence of vitamin D deficiency in pregnant non-Western women in The Hague, Netherlands. Am J Clin Nutr 84:350–353; quiz 468–469 [DOI] [PubMed] [Google Scholar]
- 3. Sachan A, Gupta R, Das V, Agarwal A, Awasthi PK, Bhatia V. 2005. High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. Am J Clin Nutr 81:1060–1064 [DOI] [PubMed] [Google Scholar]
- 4. Holick MF. 1987. Photosynthesis of vitamin D in the skin: effect of environmental and life-style variables. Fed Proc 46:1876–1882 [PubMed] [Google Scholar]
- 5. Moore CE, Murphy MM, Holick MF. 2005. Vitamin D intakes by children and adults in the United States differ among ethnic groups. J Nutr 135:2478–2485 [DOI] [PubMed] [Google Scholar]
- 6. Henry HL, Norman AW. 1984. Vitamin D: metabolism and biological actions. Annu Rev Nutr 4:493–520 [DOI] [PubMed] [Google Scholar]
- 7. Bodnar LM, Simhan HN. 2010. Vitamin D may be a link to black-white disparities in adverse birth outcomes. Obstet Gynecol Surv 65:273–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leffelaar ER, Vrijkotte TG, van Eijsden M. 2010. Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: results of the multi-ethnic Amsterdam Born Children and their Development cohort. Br J Nutr 104:108–117 [DOI] [PubMed] [Google Scholar]
- 9. Morley R, Carlin JB, Pasco JA, Wark JD, Ponsonby AL. 2009. Maternal 25-hydroxyvitamin D concentration and offspring birth size: effect modification by infant VDR genotype. Eur J Clin Nutr 63:802–804 [DOI] [PubMed] [Google Scholar]
- 10. Prentice A, Jarjou LM, Goldberg GR, Bennett J, Cole TJ, Schoenmakers I. 2009. Maternal plasma 25-hydroxyvitamin D concentration and birthweight, growth and bone mineral accretion of Gambian infants. Acta Paediatr 98:1360–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, Godfrey KM, Cooper C. 2008. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr 62:68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bodnar LM, Catov JM, Zmuda JM, Cooper ME, Parrott MS, Roberts JM, Marazita ML, Simhan HN. 2010. Maternal serum 25-hydroxyvitamin D concentrations are associated with small-for-gestational age births in white women. J Nutr 140:999–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brooke OG, Brown IR, Bone CD, Carter ND, Cleeve HJ, Maxwell JD, Robinson VP, Winder SM. 1980. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. Br Med J 280:751–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marya RK, Rathee S, Lata V, Mudgil S. 1981. Effects of vitamin D supplementation in pregnancy. Gynecol Obstet Invest 12:155–161 [DOI] [PubMed] [Google Scholar]
- 15. Marya RK, Rathee S, Dua V, Sangwan K. 1988. Effect of vitamin D supplementation during pregnancy on foetal growth. Indian J Med Res 88:488–492 [PubMed] [Google Scholar]
- 16. Mallet E, Gügi B, Brunelle P, Hénocq A, Basuyau JP, Lemeur H. 1986. Vitamin D supplementation in pregnancy: a controlled trial of two methods. Obstet Gynecol 68:300–304 [DOI] [PubMed] [Google Scholar]
- 17. Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. 2011. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res 26:2341–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McIntire DD, Bloom SL, Casey BM, Leveno KJ. 1999. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med 340:1234–1238 [DOI] [PubMed] [Google Scholar]
- 19. Barker DJ. 2006. Adult consequences of fetal growth restriction. Clin Obstet Gynecol 49:270–283 [DOI] [PubMed] [Google Scholar]
- 20. Dombrowski MP, Berry SM, Johnson MP, Saleh AA, Sokol RJ. 1994. Birth weight-length ratios, ponderal indexes, placental weights, and birth weight-placenta ratios in a large population. Arch Pediatr Adolesc Med 148:508–512 [DOI] [PubMed] [Google Scholar]
- 21. Shehata F, Levin I, Shrim A, Ata B, Weisz B, Gamzu R, Almog B. 2011. Placenta/birthweight ratio and perinatal outcome: a retrospective cohort analysis. BJOG 118:741–747 [DOI] [PubMed] [Google Scholar]
- 22. Patterson RM, Pouliot MR. 1987. Neonatal morphometrics and perinatal outcome: who is growth retarded? Am J Obstet Gynecol 157:691–693 [DOI] [PubMed] [Google Scholar]
- 23. Niswander K. 1972. The Collaborative Perinatal Study of the National Institute of Neurological Diseases and Stroke: The women and their pregnancies. Philadelphia: WB Saunders [Google Scholar]
- 24. Hardy JB. 2003. The Collaborative Perinatal Project: lessons and legacy. Ann Epidemiol 13:303–311 [DOI] [PubMed] [Google Scholar]
- 25. Seamans KM, Cashman KD. 2009. Existing and potentially novel functional markers of vitamin D status: a systematic review. Am J Clin Nutr 89:1997S–2008S [DOI] [PubMed] [Google Scholar]
- 26. Bodnar LM, Catov JM, Wisner KL, Klebanoff MA. 2009. Racial and seasonal differences in 25-hydroxyvitamin D detected in maternal sera frozen for over 40 years. Br J Nutr 101:278–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zerwekh JE. 2004. The measurement of vitamin D: analytical aspects. Ann Clin Biochem 41:272–281 [DOI] [PubMed] [Google Scholar]
- 28. Fay RA, Dey PL, Saadie CM, Buhl JA, Gebski VJ. 1991. Ponderal index: a better definition of the ‘at risk’ group with intrauterine growth problems than birth-weight for gestational age in term infants. Aust N Z J Obstet Gynaecol 31:17–19 [DOI] [PubMed] [Google Scholar]
- 29. Risnes KR, Romundstad PR, Nilsen TI, Eskild A, Vatten LJ. 2009. Placental weight relative to birth weight and long-term cardiovascular mortality: findings from a cohort of 31,307 men and women. Am J Epidemiol 170:622–631 [DOI] [PubMed] [Google Scholar]
- 30. Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. 1996. A United States national reference for fetal growth. Obstet Gynecol 87:163–168 [DOI] [PubMed] [Google Scholar]
- 31. Institute of Medicine 2011. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; [PubMed] [Google Scholar]
- 32. Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. 2009. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bowyer L, Catling-Paull C, Diamond T, Homer C, Davis G, Craig ME. 2009. Vitamin D, PTH and calcium levels in pregnant women and their neonates. Clin Endocrinol (Oxf) 70:372–377 [DOI] [PubMed] [Google Scholar]
- 34. Farrant HJ, Krishnaveni GV, Hill JC, Boucher BJ, Fisher DJ, Noonan K, Osmond C, Veena SR, Fall CH. 2009. Vitamin D insufficiency is common in Indian mothers but is not associated with gestational diabetes or variation in newborn size. Eur J Clin Nutr 63:646–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morley R, Carlin JB, Pasco JA, Wark JD. 2006. Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Metab 91:906–912 [DOI] [PubMed] [Google Scholar]
- 36. Lapillonne A. 2010. Vitamin D deficiency during pregnancy may impair maternal and fetal outcomes. Med Hypotheses 74:71–75 [DOI] [PubMed] [Google Scholar]
- 37. Shin JS, Choi MY, Longtine MS, Nelson DM. 2010. Vitamin D effects on pregnancy and the placenta. Placenta 31:1027–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hutcheon JA, Platt RW. 2008. The missing data problem in birth weight percentiles and thresholds for “small-for-gestational-age.” Am J Epidemiol 167:786–792 [DOI] [PubMed] [Google Scholar]
- 39. Thorne-Lyman A, Fawzi WW. 2012. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatr Perinat Epidemiol 26(Suppl 1):75–90 [DOI] [PMC free article] [PubMed] [Google Scholar]