Abstract
Context:
Adrenocortical carcinoma (ACC) is a rare malignancy with high recurrence and mortality rates. The role of adjuvant radiation therapy (RT) to improve outcome remains unclear.
Objective:
The aim of this study was to evaluate the impact of adjuvant RT on overall survival and recurrence rates of ACC patients.
Design:
We conducted a retrospective cohort study of select ACC patients who were seen at The University of Texas MD Anderson Cancer Center (MDACC) between 1998 and 2011. All patients in this study underwent primary tumor resection and received adjuvant RT within 3 months of primary surgical resection prior to referral to the MDACC. We compared patients who had surgery and adjuvant RT with patients who had surgery alone.
Results:
Baseline characteristics and adjuvant mitotane use were not significantly different between the adjuvant RT group (n = 16) and the non-RT group (n = 32). Local recurrence occurred in seven patients (43.8%) who received RT and 10 patients (31.3%) in the control group. At 5 yr, the estimated local recurrence-free rate (95% confidence interval) was 53% (32–87%) in the RT group and 67% (52–86%) in the non-RT group (P = 0.53). The distributions of time to distant recurrence and recurrence-free survival were not significantly different between the two groups. Using a multivariate Cox proportional hazards model for overall survival, the hazard ratio for RT use was 1.593 (95% confidence interval, 0.707–3.589; P = 0.26) after adjusting for stage and adjuvant mitotane therapy.
Conclusions:
ACC has high rates of recurrence. In our study, RT did not improve clinical outcomes in patients who received their initial care in the community. We believe there is a need for a collaborative, multicenter, prospective randomized trial to evaluate the role of adjuvant treatments (both mitotane and RT) to assess their impact on recurrence patterns and survival.
Adrenocortical carcinoma (ACC) is a rare malignancy with an annual incidence of one to two cases per million population (1–3). In general, ACC has poor prognosis and high recurrence and mortality rates (4). Although many patients present with metastatic disease, surgery remains the primary treatment for those with local or locoregional disease (4–6), and local and distant recurrence rates remain high even after seemingly complete surgical resection (7–10). Retrospective evidence suggested that adjuvant mitotane therapy can reduce recurrence rates and improve survival; a prospective investigation is ongoing to validate these findings (11, 12). The role of radiation therapy (RT) is less clear. ACC typically has been considered radio-resistant, although recent studies have found improved local control associated with adjuvant RT use (13–16). Considering the very small number of reported ACC patients who have received RT, the evidence regarding use of adjuvant RT has become suitable for meaningful analysis. We hypothesized that RT can reduce local recurrence rates after surgery, especially in high-risk patients, thus improving overall survival (OS) in ACC. To test this hypothesis, we conducted a historical cohort study to evaluate the effect of adjuvant RT in ACC patients on recurrence rates and OS. By gaining more insight into the possible role of adjuvant RT for ACC, we believe clinicians and patients will be able to make more informed decisions about the treatment modalities available after primary surgery.
Patients and Methods
This retrospective cohort study was conducted at The University of Texas MD Anderson Cancer Center (MDACC) after institutional review board approval. We selected two groups of patients from the 330 ACC patients included in the MD Anderson ACC registry (1998- 2011): an RT group (n = 16) of those who had surgery followed by adjuvant RT within 3 months of surgical resection; and a non-RT control group (n = 32), those who had surgery without RT. All 16 patients in our RT group had their initial surgery and RT at an outside institution and were then followed up at MDACC. The non-RT control group initially consisted of 45 patients matched to the RT group patients on the basis of resection margin [no evidence of tumor in resection margins (R0), microscopic evidence of tumor in margins (R1), macroscopic residual disease (R2), or unknown status of margins (Rx)] and disease stage at diagnosis (17, 18). To reduce the effect of referral bias pattern in our final comparisons between groups, we only analyzed the 32 patients who also had their first surgery outside MDACC in the non-RT controls (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
All patients had a minimum follow-up period of 6 months. OS time was calculated from the date of first surgery to the date of death or last follow-up. Patients were censored at the last follow-up dates if death did not occur. Time to local recurrence was calculated from the date of first surgery to that of documentation of local recurrence, death, or last follow-up. Data for patients who died without local recurrence were censored at the death date. Data for patients were censored at the last follow-up date if local recurrence or death did not occur. Recurrence-free survival time was calculated from the date of first surgery to the date of first recurrence (whether local or distant) or death. Patient data were censored at the last follow-up date if recurrence or death did not occur. Case matching was done by a team independent from that handling data analysis and was done using baseline data at the time of initial diagnosis.
We calculated frequencies and percentages for categorical variables (such as gender and surgery type). Summary statistics were calculated for continuous data (such as age and primary tumor size).
The Fisher's exact test or χ2 test was used to evaluate the association between two categorical variables. The Wilcoxon rank sum test was used to evaluate the difference in distribution of a continuous variable between patient groups. The Kaplan-Meier method was used to analyze the time-to-event endpoints, including OS, time to local recurrence, time to distant recurrence, time to any recurrence, and recurrence-free survival. The log-rank test was used to evaluate differences on these endpoints between patient groups. Multivariate Cox proportional hazards models were fitted to include important demographic and clinical variables. All tests were two-sided. P values of less than 0.05 were considered statistically significant. Statistical software SAS version 9.1.3 (SAS Institute, Cary, NC) and S-Plus 8.0 (TIBCO Software Inc., Palo Alto, CA) were used for all the analyses.
Results
Patient characteristics
The median follow-up time for patients with censored observations was 1.84 yr (range, 0.89–4.81) in the RT group and 2.77 yr (range, 0.62–14.06) in the non-RT group. The median time from surgery to RT was 46 d (range, 19–69). The median radiation dose was 50.4 Gy (range, 36.0–59.4), and the median number of fractions was 25 (range, 20–30). In general, RT was well tolerated, with reports of mild to moderate nausea (n = 9), fatigue (n = 5), abdominal pain (n = 1), and anorexia (n = 1) that did not halt the administration of RT. Table 1 summarizes the clinical parameters for the two groups.
Table 1.
Patient characteristics by RT status
| Variable | RT (n = 16) | No RT (n = 32) | P value |
|---|---|---|---|
| Median age in years at diagnosis (range) | 48 (22–61) | 44 (24–72) | 0.77 |
| No. of females (%)/males (%) | 10 (62.5)/6 (37.5) | 21 (65.6)/11 (34.4) | 0.83 |
| Median primary tumor size in cm (range) | 12 (5.3–23) | 11 (6–25) | 0.69 |
| Hormonal overproduction, yes (%) | 8 (50) | 15 (46.9) | 0.73 |
| Site, right (%)/left (%) | 5 (31.2)/11 (68.8) | 15 (46.9)/17 (53.1) | 0.36 |
| Surgery, open resection (%)/laparoscopic resection (%) | 12 (75)/4 (25) | 28 (87.5)/4 (12.5) | 0.41 |
| Preoperative biopsy, yes (%) | 1 (6.3) | 2 (6.3) | 1.0 |
| Adjuvant mitotane, yes (%) | 4 (25) | 10 (31.3) | 0.75 |
| Margin status, n (%) | |||
| R0 | 7 (43.8) | 13 (40.6) | 1.0 |
| R1 | 6 (37.5) | 11 (34.4) | |
| R2 | 1 (6.3) | 2 (6.3) | |
| Rx | 2 (12.5) | 6 (18.8) | |
| Disease stage (%) | |||
| 2 | 9 (56.3) | 17 (53.1) | 0.84 |
| 3 | 7 (43.8) | 15 (46.9) |
Local recurrence
In the RT group, seven patients (43.8%) had a local recurrence during their follow-up period, compared with 10 (31.3%) in the non-RT group (Fig. 1A). Three years after primary surgery, the estimated local recurrence-free rate [95% confidence interval (CI)] was 62% (42–91%) in the RT group and 67% (52–86%) in the non-RT group. At 5 yr, the estimated local recurrence-free rate (95% CI) was 53% (32–87%) in the RT group and 67% (52–86%) in the non-RT group (P = 0.53). Multivariate Cox proportional hazard model was fitted for time to local recurrence and is presented as supplemental material only because of the small number of events (Supplemental Tables 2 and 3).
Fig. 1.
Time to local (A) and distant (B) recurrence for patients based on radiation use.
Distant recurrence
In the RT group, 14 patients (88%) had a distant recurrence during their follow-up period, compared with 24 patients (75%) in the non-RT group (Fig. 1B). The median distant recurrence-free time (95% CI) was 0.87 yr (0.52-not reached) in the RT group and 1.39 yr (0.54–4.37) in the non-RT group. At 3 yr, the estimated distant recurrence-free rate (95% CI) was 29% (13–64%) in the RT group and 33% (19–56%) in the non-RT group (P = 0.33).
Using the multivariate Cox proportional hazards model fitted for time to distant recurrence, the hazard ratio of distant recurrence during the follow-up period in patients who had received RT was 1.319 (95% CI, 0.618–2.816; P = 0.47) relative to that in non-RT group after adjusting for stage, surgery type, and adjuvant mitotane use (Table 2).
Table 2.
Multivariate Cox proportional hazard model fitted for time to distant recurrence analysis after adjusting for stage, surgery type, and adjuvant mitotane use
| Variable | P value | Hazard ratio | 95% CI for HR |
|---|---|---|---|
| Stage: III vs. IIa | 0.0048 | 2.680 | 1.351–5.319 |
| Surgery type: laparoscopic vs. opena | 0.2139 | 1.882 | 0.694–5.100 |
| RT: yes vs. noa | 0.4747 | 1.319 | 0.618–2.816 |
| Adjuvant mitotane: yes vs. noa | 0.2409 | 0.617 | 0.275–1.383 |
For each covariate, the second level is the reference group.
Subanalysis: time to any recurrence
We applied more stringent selection criteria, including only patients with known resection margins, and considered all recurrences together. We excluded 16 patients with Rx (n = 8) or R2 (n = 3) margins and those whose disease recurred within 3 months after the surgical resection (n = 5; two with distant recurrence and three with both local and distant recurrences) to ensure that these very early recurrences were not misdiagnosed at the time of surgery. Our final new subpopulation consisted of 32 patients: 13 who received RT, and 19 who did not. The median follow-up for patients in the RT group was 39.9 months (range, 8.4–83 months) and for patients in the non-RT group was 34.4 months (range, 7.5–240 months). Recurrence of disease (local and distant) was found in 27 of 32 patients (84%).
We analyzed the time to any recurrence in this selected subcohort (n = 32 patients) by margin status, stage, and RT (Fig. 2, A–C). Although RT use did not have a statistically significant effect on time to any recurrence, we found that staging and achieving negative resection margins were important determinants of any recurrence. In particular, achieving R0 resection had significant impact to reduce local recurrence when compared with R1 resection, as shown in Fig. 3.
Fig. 2.
Time to any recurrence based on margin status (A), stage (B), and RT (C) in a subgroup of 32 patients who had R0 or R1 resection.
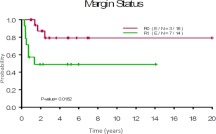
Fig. 3.
Time to local recurrence by margin status.
Overall survival
The median OS time for all 48 patients was 3.84 yr (range, 2.95–7.11). In univariate analysis, the median OS time was 4.81 yr in the RT group and 3.46 yr in control group (P = 0.49). Using the multivariate Cox proportional hazards model for OS, we calculated that the hazard ratio of death during the follow-up period for patients who had received RT was 1.593 (95% CI, 0.707–3.589; P = 0.26) compared with that of control patients after adjusting for stage and adjuvant mitotane therapy.
Given that the median follow-up time for patients in the non-RT group was longer than that in the RT group, we performed additional analyses to evaluate whether this difference in follow-up would affect the results. After truncating the follow-up time at 8 yr, we analyzed all time-to-event end points by RT status and obtained very similar results.
Discussion
To further the understanding of the effect of adjuvant RT in ACC patients, we conducted a retrospective cohort study to evaluate the recurrence patterns and survival rates of 16 ACC patients who had undergone RT and compared their results with 32 similar patients who had not. This group represents the largest number of reported cases studied to date, and our findings could not confirm prior reports that suggested reduced local recurrence rates with adjuvant RT use (15, 16, 19). The high rate of distant recurrence and poor OS we found are in line with prior reports and illustrate the tendency of patients with ACC to develop distant metastases despite best efforts to achieve local control (16). Thus, the findings of our study did not show a clear advantage to performing adjuvant RT routinely.
Traditionally, ACC has been viewed as radio-resistant malignancy (13, 14), and only 12% of patients with resectable ACCs received RT (20). Earlier small reports suggested improved outcomes with RT use (21), especially when it was combined with mitotane (22, 23), whereas others did not confirm this finding (24, 25).
In the largest series published before our study, Fassnacht et al. (16) compared the recurrence and survival outcomes of 14 ACC patients in whom adjuvant RT was used with those of 14 patients in whom it was not. The RT group had a significantly lower local recurrence rate than the controls (21 vs. 88%, respectively), whereas OS and distant recurrence were not improved with RT use (16). The matching criteria in that study included adjuvant mitotane use and tumor size. We selected stage and resection margin as the most important factors to affect outcome. Other factors were eventually balanced between the two groups, including tumor size and adjuvant mitotane use as shown in Table 1.
In the most recent report on a series of patients treated and not treated with adjuvant radiation, adjuvant RT was used in 10 ACC patients (nine received external-beam RT and one brachytherapy). Local recurrence occurred in 33% of the patients who underwent surgery alone, whereas it occurred in only 20% of those who received surgery and RT; RT use did not reduce the risk of distant metastases (15). That report may have been limited by the significant baseline differences between the groups, including disease stage, adjuvant mitotane use, and disease type (primary vs. recurrent). That study also included patients with recurrent and metastatic disease and did not provide any information about resection margin status, which limits the conclusions that can be drawn. We believe our findings differ from theirs because our groups were closely matched in terms of baseline characteristics related to prognosis, including our decision to exclude patients with metastatic disease at the time of initial diagnosis.
The reliability of our findings is further bolstered by the number of patients studied, the careful selection of patients in the intervention and control groups on the basis of disease stage and resection margin, and the adjustment for referral pattern. We also ensured that other factors in our analysis, including the tumor's functional status and size, patient age, and mitotane use, did not have confounding effects.
Our study was limited by our inability to account for other parameters known to affect recurrence rate and overall prognosis (because information was not available for all patients), including Weiss score and proportion of cells expressing proliferative markers (e.g. Ki-67) (26, 27). Furthermore, our study was retrospective and may have been susceptible to referral bias, i.e. patients in the RT group may have been selected for RT because they were perceived to have worse risk factors for recurrence. Our matching process and multivariate analysis may not have been able to account for all of these factors. Because most ACC patients receive their initial care at outside major referral centers (2), as our patients had, we believe that our findings are likely to be relevant to ACC cases initially treated in the community.
Considering the limitations of our study, we were unable to show that routine use of adjuvant RT improves clinical outcome in most ACC patients. However, RT may benefit selected patients.
Conclusions
ACC has high rates of distant recurrence despite attempts of local control. To our knowledge, this is the largest report about adjuvant RT use in ACC. In our study, RT did not improve clinical outcomes in ACC patients who received their initial care in the community. Considering the rarity of ACC, we believe there is a need for a collaborative multicenter prospective randomized trial to evaluate the role of adjuvant treatments (both mitotane and RT) to assess their impact on recurrence patterns and survival.
Acknowledgments
We thank Ms. Tamara Locke and Ms. Kathryn Carnes for their editorial support.
This paper is supported in part by the National Institutes of Health through The University of Texas MD Anderson Cancer Center Support Grant CA016672 and The Beverlin Fund for Adrenal Cancer Research.
Disclosure Summary: The authors made no disclosures.
Footnotes
- ACC
- Adrenocortical carcinoma
- CI
- confidence interval
- OS
- overall survival
- RT
- radiation therapy.
References
- 1. Paton BL, Novitsky YW, Zerey M, Harrell AG, Norton HJ, Asbun H, Kercher KW, Heniford BT. 2006. Outcomes of adrenal cortical carcinoma in the United States. Surgery 140:914–920 [DOI] [PubMed] [Google Scholar]
- 2. Bilimoria KY, Shen WT, Elaraj D, Bentrem DJ, Winchester DJ, Kebebew E, Sturgeon C. 2008. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer 113:3130–3136 [DOI] [PubMed] [Google Scholar]
- 3. Schteingart DE, Doherty GM, Gauger PG, Giordano TJ, Hammer GD, Korobkin M, Worden FP. 2005. Management of patients with adrenal cancer: recommendations of an international consensus conference. Endocr Relat Cancer 12:667–680 [DOI] [PubMed] [Google Scholar]
- 4. Fassnacht M, Libé R, Kroiss M, Allolio B. 2011. Adrenocortical carcinoma: a clinician's update. Nat Rev Endocrinol 7:323–335 [DOI] [PubMed] [Google Scholar]
- 5. Icard P, Goudet P, Charpenay C, Andreassian B, Carnaille B, Chapuis Y, Cougard P, Henry JF, Proye C. 2001. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg 25:891–897 [DOI] [PubMed] [Google Scholar]
- 6. Dackiw AP, Lee JE, Gagel RF, Evans DB. 2001. Adrenal cortical carcinoma. World J Surg 25:914–926 [DOI] [PubMed] [Google Scholar]
- 7. Brix D, Allolio B, Fenske W, Agha A, Dralle H, Jurowich C, Langer P, Mussack T, Nies C, Riedmiller H, Spahn M, Weismann D, Hahner S, Fassnacht M. 2010. Laparoscopic versus open adrenalectomy for adrenocortical carcinoma: surgical and oncologic outcome in 152 patients. Eur Urol 58:609–615 [DOI] [PubMed] [Google Scholar]
- 8. Porpiglia F, Fiori C, Daffara F, Zaggia B, Bollito E, Volante M, Berruti A, Terzolo M. 2010. Retrospective evaluation of the outcome of open versus laparoscopic adrenalectomy for stage I and II adrenocortical cancer. Eur Urol 57:873–878 [DOI] [PubMed] [Google Scholar]
- 9. Gonzalez RJ, Shapiro S, Sarlis N, Vassilopoulou-Sellin R, Perrier ND, Evans DB, Lee JE. 2005. Laparoscopic resection of adrenal cortical carcinoma: a cautionary note. Surgery 138:1078–1085; discussion 1085–1076 [DOI] [PubMed] [Google Scholar]
- 10. Miller BS, Ammori JB, Gauger PG, Broome JT, Hammer GD, Doherty GM. 2010. Laparoscopic resection is inappropriate in patients with known or suspected adrenocortical carcinoma. World J Surg 34:1380–1385 [DOI] [PubMed] [Google Scholar]
- 11. Terzolo M, Angeli A, Fassnacht M, Daffara F, Tauchmanova L, Conton PA, Rossetto R, Buci L, Sperone P, Grossrubatscher E, Reimondo G, Bollito E, Papotti M, Saeger W, Hahner S, Koschker AC, Arvat E, Ambrosi B, Loli P, Lombardi G, Mannelli M, Bruzzi P, Mantero F, Allolio B, Dogliotti L, Berruti A. 2007. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med 356:2372–2380 [DOI] [PubMed] [Google Scholar]
- 12. Berruti A, Fassnacht M, Baudin E, Hammer G, Haak H, Leboulleux S, Skogseid B, Allolio B, Terzolo M. 2010. Adjuvant therapy in patients with adrenocortical carcinoma: a position of an international panel. J Clin Oncol 28:e401–e402; author reply e403 [DOI] [PubMed] [Google Scholar]
- 13. Hutter AM, Jr, Kayhoe DE. 1966. Adrenal cortical carcinoma. Clinical features of 138 patients. Am J Med 41:572–580 [DOI] [PubMed] [Google Scholar]
- 14. Luton JP, Cerdas S, Billaud L, Thomas G, Guilhaume B, Bertagna X, Laudat MH, Louvel A, Chapuis Y, Blondeau P, Bonnin A, Bricaire H. 1990. Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N Engl J Med 322:1195–1201 [DOI] [PubMed] [Google Scholar]
- 15. Sabolch A, Feng M, Griffith K, Hammer G, Doherty G, Ben-Josef E. 2011. Adjuvant and definitive radiotherapy for adrenocortical carcinoma. Int J Radiat Oncol Biol Phys 80:1477–1484 [DOI] [PubMed] [Google Scholar]
- 16. Fassnacht M, Hahner S, Polat B, Koschker AC, Kenn W, Flentje M, Allolio B. 2006. Efficacy of adjuvant radiotherapy of the tumor bed on local recurrence of adrenocortical carcinoma. J Clin Endocrinol Metab 91:4501–4504 [DOI] [PubMed] [Google Scholar]
- 17. Lee JE, Berger DH, el-Naggar AK, Hickey RC, Vassilopoulou-Sellin R, Gagel RF, Burgess MA, Evans DB. 1995. Surgical management, DNA content, and patient survival in adrenal cortical carcinoma. Surgery 118:1090–1098 [DOI] [PubMed] [Google Scholar]
- 18. Fassnacht M, Johanssen S, Quinkler M, Bucsky P, Willenberg HS, Beuschlein F, Terzolo M, Mueller HH, Hahner S, Allolio B. 2009. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a revised TNM classification. Cancer 115:243–250 [DOI] [PubMed] [Google Scholar]
- 19. Polat B, Fassnacht M, Pfreundner L, Guckenberger M, Bratengeier K, Johanssen S, Kenn W, Hahner S, Allolio B, Flentje M. 2009. Radiotherapy in adrenocortical carcinoma. Cancer 115:2816–2823 [DOI] [PubMed] [Google Scholar]
- 20. Kebebew E, Reiff E, Duh QY, Clark OH, McMillan A. 2006. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J Surg 30:872–878 [DOI] [PubMed] [Google Scholar]
- 21. Markoe AM, Serber W, Micaily B, Brady LW. 1991. Radiation therapy for adjunctive treatment of adrenal cortical carcinoma. Am J Clin Oncol 14:170–174 [DOI] [PubMed] [Google Scholar]
- 22. Percarpio B, Knowlton AH. 1976. Radiation therapy of adrenal cortical carcinoma. Acta Radiol Ther Phys Biol 15:288–292 [DOI] [PubMed] [Google Scholar]
- 23. Magee BJ, Gattamaneni HR, Pearson D. 1987. Adrenal cortical carcinoma: survival after radiotherapy. Clin Radiol 38:587–588 [DOI] [PubMed] [Google Scholar]
- 24. Venkatesh S, Hickey RC, Sellin RV, Fernandez JF, Samaan NA. 1989. Adrenal cortical carcinoma. Cancer 64:765–769 [DOI] [PubMed] [Google Scholar]
- 25. Didolkar MS, Bescher RA, Elias EG, Moore RH. 1981. Natural history of adrenal cortical carcinoma: a clinicopathologic study of 42 patients. Cancer 47:2153–2161 [DOI] [PubMed] [Google Scholar]
- 26. Gicquel C, Bertagna X, Gaston V, Coste J, Louvel A, Baudin E, Bertherat J, Chapuis Y, Duclos JM, Schlumberger M, Plouin PF, Luton JP, Le Bouc Y. 2001. Molecular markers and long-term recurrences in a large cohort of patients with sporadic adrenocortical tumors. Cancer Res 61:6762–6767 [PubMed] [Google Scholar]
- 27. Morimoto R, Satoh F, Murakami O, Suzuki T, Abe T, Tanemoto M, Abe M, Uruno A, Ishidoya S, Arai Y, Takahashi K, Sasano H, Ito S. 2008. Immunohistochemistry of a proliferation marker Ki67/MIB1 in adrenocortical carcinomas: Ki67/MIB1 labeling index is a predictor for recurrence of adrenocortical carcinomas. Endocr J 55:49–55 [DOI] [PubMed] [Google Scholar]