Abstract
Context:
Cold exposure stimulates fibroblast growth factor 21 (FGF21) secretion in animals, enhancing the cold-induced thermogenesis (CIT) response through browning of white adipose tissue. In humans, the effects of cold exposure on circulating FGF21 levels are unknown.
Objective:
Our objective was to evaluate the effects of mild cold exposure on circulating FGF21 and its relationship with CIT and lipolysis in humans.
Design and Setting:
We conducted a randomized, single-blind, crossover intervention study at the National Institutes of Health Clinical Center.
Participants:
Participants were healthy adults.
Intervention:
Subjects were exposed to a 12-h exposure to 24 or 19 C in a whole-room indirect calorimeter.
Outcome Measures:
Energy expenditure, plasma FGF 21, nonesterified fatty acid, and adipose tissue microdialysis glycerol concentrations were evaluated.
Results:
At 24 C, plasma FGF21 exhibited a diurnal rhythm, peaking at 0800 h [110 (59–178) pg/ml], and progressively dropped to a nadir at 1700 h [41 (21–71) pg/ml, P < 0.0001] before rising at 1900 h [60 (11–81) pg/ml, P < 0.0001]. Exposure at 19 C lessened the diurnal reduction of FGF21 observed at 24 C from 0800–1700 h and augmented overall FGF21 levels by 37 ± 45% (P = 0.01). The change in area under the curve plasma FGF21 between 19 and 24 C correlated positively with the change in area under the curve adipose microdialysate glycerol (R2 = 0.35, P = 0.04) but not with nonesterified fatty acid. Cold-induced increase in FGF21 predicted greater rise in energy expenditure during cold exposure (β = 0.66, P = 0.027), independent of age, gender, fat mass, and lean mass.
Conclusions:
Mild cold exposure increased circulating FGF21 levels, predicting greater lipolysis and CIT. A small reduction in environmental temperature is sufficient to modulate FGF21 diurnal rhythm in humans, which may mediate cold-induced metabolic changes similar to those in animals.
Fibroblast growth factor-21 (FGF21) is a newly identified hormone that plays a central role in energy homeostasis and substrate metabolism in rodents (1). FGF21 increases energy expenditure (EE) and alleviates obesity when overexpressed or administered pharmacologically in animals (2, 3). FGF21 is expressed in liver, white adipose tissue (WAT), skeletal muscle, and pancreas (4), but the mechanisms underlying FGF21-mediated metabolic effects are not completely understood.
Very recently, the identification of brown adipose tissue (BAT) as a source of FGF21 provided new insight into FGF21 actions (5). In contrast to WAT, which functions as a site of energy storage, BAT is a thermogenic organ that dissipates energy as heat via the action of uncoupling protein-1 (UCP1). This unique action defines its critical role in cold-induced thermogenesis (CIT). In animal models, cold exposure increases FGF21 expression in BAT and augments its secretion, leading to UCP1 transcription and browning of WAT (6–8). In other words, FGF21 induces a BAT-like program in WAT to expand whole-body thermogenic machinery to maintain thermal balance in rodents. In humans, the effects of cold exposure, the canonical afferent pathway of BAT activation, on FGF21 levels have not been investigated. In this study, we examined the impact of mild cold exposure on plasma FGF21 levels and its contribution to CIT in healthy volunteers.
Subjects and Methods
Subjects
This is a secondary analysis of data collected from participants in a protocol that investigated the physiological effects of mild cold exposure (www.ClinicalTrials.gov identifier NCT00521729) (9). Data from volunteers with sufficient stored blood samples for FGF21 assay were included in this study. Inclusion criteria were men and women 18–60 yr old and body mass index (BMI) of 20–27 kg/m2. Exclusion criteria were blood pressure over 140/90 mm Hg, cardiovascular/endocrine disease, pregnancy/use of hormonal contraception, and use of prescription medication.
Clinical protocol
The protocol was approved by the National Institute of Diabetes and Digestive and Kidney Diseases-National Institute of Arthritis and Musculoskeletal and Skin Disease Institutional Review Board and informed consent obtained from all subjects. The study was a randomized, single-blind, crossover intervention (9). Volunteers were randomly assigned to a 12-h stay in a whole-room indirect calorimeter at either 24 C (75 F) or 19 C (68 F) for continuous EE measurement. Recording began at 0800 h after an overnight fast. Volunteers wore hospital scrubs with no additional blankets. Physical activity and diet were standardized during the study (Supplemental Data, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). The study concluded at 2000 h. Volunteers then repeated the 12-h stay at the second temperature after a 36-h recovery period.
Blood samples collected at 0, 3, 5, 9, and 11 h through airtight sampling ports were used for FGF21 and substrate measurements. Microdialysis of abdominal sc adipose tissue was performed using a CMA 60 (CMA Microdialysis, Stockholm, Sweden) catheter. Additional details of microdialysis sampling and laboratory and anthropometry assessment are summarized in Supplemental Data.
Statistical analysis
Statistical analysis was performed using SPSS version 17.0 (SPSS, Inc., Chicago, IL). Data are expressed as mean ± sd for normally distributed variables. Because plasma FGF21 was not distributed normally, data were reported as median (interquartile range) and log transformed before analyses. Areas under the curve (AUC) were calculated using the trapezoidal rule. Paired t test was used for comparison of measurements at 24 and 19 C. Repeated-measures ANOVA with Bonferroni correction was used to analyze plasma FGF21 time course. To evaluate the effect of cold exposure, the difference between plasma FGF21 levels, substrate concentration, and total EE measured at the two study temperatures (parameter at 19 C − parameter at 24 C) was used. A stepwise linear regression model was used to examine the impact of variables on CIT. An α-error of 0.05 was considered the threshold for statistical significance.
Results
Twelve healthy volunteers [32 ± 11 yr old, five females, with BMI of 23 ± 3 kg/m2, fat mass (FM) of 15.5 ± 5.0 kg, and lean mass (LM) of 51.2 ± 9.4 kg] with sufficient stored blood samples for FGF21 analysis were included in the current study. One EE recording was not available due to equipment failure. At 24 C, fasting FGF21 levels at 0800 h were 110 (59–178) pg/ml, with no differences between the sexes. No relationships were observed between plasma FGF21 concentrations and age, BMI, FM, or LM (data not shown).
Diurnal changes in plasma FGF21 in healthy adults
We next examined diurnal excursion of plasma FGF21 levels between 0800 and 1900 h at 24 C. FGF21 levels changed significantly (P < 0.01) during the day: highest at 0800 h [110 (59–178) pg/ml] and progressively declining to a nadir at 1700 h [41 (21–71) pg/ml] before rising at 1900 h [60 (11–81) pg/ml].
Modulation of plasma FGF21 levels by mild cold exposure
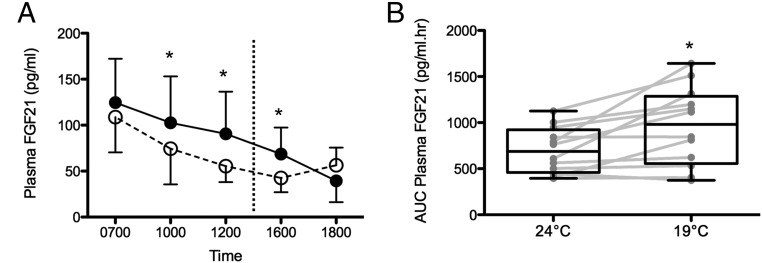
Given significant diurnal changes in plasma FGF21 concentrations, we next compared FGF21 levels in paired blood samples obtained at 24 C and at 19 C in the same volunteer. Diurnal rhythm of plasma FGF21 concentration was retained at 19 C. The highest levels were observed at 0800 h [126 (62–204) pg/ml], followed by a progressive decline reaching a nadir at 1900 h [31 (10–75) pg/ml]. The rise in the late afternoon observed at 24 C was not seen at 19 C. Log plasma FGF21 concentration was significantly higher at 19 C compared with 24 C at paired time points after 3 h (P < 0.001), 5 h (P < 0.0001), and 9 h (P < 0.01) of cold exposure (Fig. 1A). Compared with 24 C, log AUC FGF21 was significantly greater at 19 C (37 ± 45%, P = 0.01) (Fig. 1B).
Fig. 1.
Diurnal changes in plasma FGF21 concentrations at 19 and 24 C. A, FGF21 levels were significantly higher after 3, 5, and 9 h of cold exposure. Dashed line indicates liquid meal provided at 1400 h. B, AUC plasma FGF21 level (0700–1800 h) was significantly higher at 19 C. *, P < 0.05.
Association of FGF21 with lipolysis
To elucidate the physiological significance of higher FGF21 levels during cold exposure, we evaluated the association of plasma FGF21 with lipolysis during cold exposure.
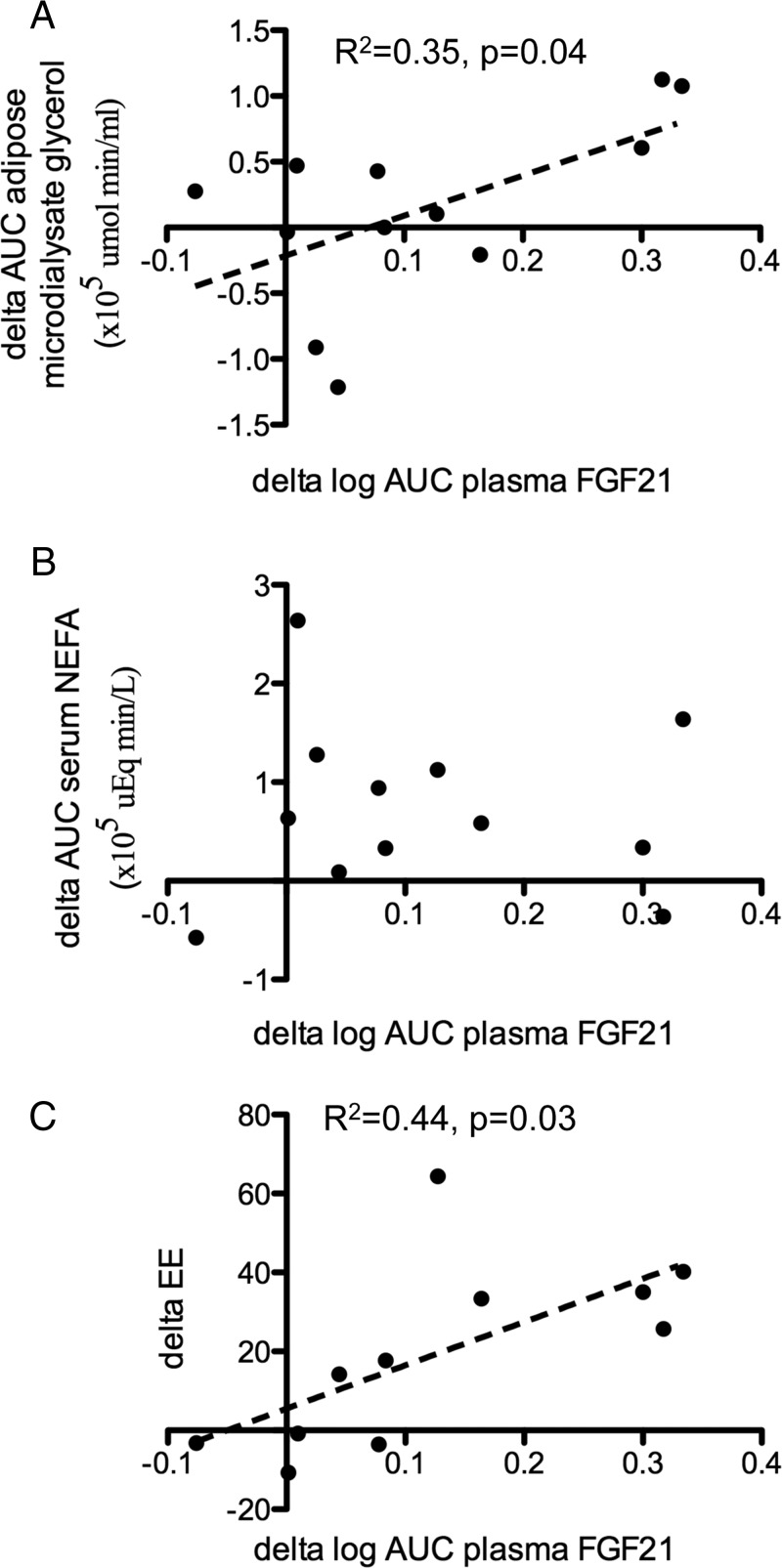
Compared with 24 C, mild cold exposure resulted in significantly increased AUC serum nonesterified fatty acids (NEFAs) (3.6 × 105 vs. 2.9 × 105 μEq·min/liter, P = 0.02) but not adipose microdialysate glycerol (1.2 × 105 vs. 1.0 × 105 μmol·min/ml, P = 0.50). On the other hand, Δ AUC adipose microdialysate glycerol concentrations between 19 and 24 C (r2 = 0.35, P = 0.04) (Fig. 2A) correlated positively with Δ log AUC plasma FGF21 levels, whereas Δ AUC serum NEFA did not (Fig. 2B). No temporal relationships were observed between diurnal NEFA and adipose microdialysate glycerol changes with FGF21 levels (Supplemental Fig. 1). Serum β-hydroxybutyrate did not correlate with FGF21 concentrations (data not shown).
Fig. 2.
Relationship between changes in plasma FGF21 and changes in adipose microdialysate glycerol concentrations (A), serum NEFA levels (B), and cold-induced increase in EE (C). Delta refers to the difference between values measured at 19 and 24 C.
Association of FGF21 with CIT
In view of increased FGF21 concentration observed after mild cold exposure, we next investigated the relationship between CIT and plasma FGF21 levels in the 11 subjects of whom EE results were available. Total EE was significantly higher at 19 C compared with 24 C (847 ± 116 vs. 802 ± 104 kcal, P = 0.002). The Δ log AUC plasma FGF21 concentration correlated positively with Δ EE (Fig. 2C). In a forward stepwise multivariate analysis, Δ log AUC plasma FGF21 concentrations predicted Δ total EE (β = 0.66, P = 0.027), independent of age, gender, FM, LM, Δ AUC serum NEFA, and Δ AUC microdialysate glycerol (Supplemental Table).
Discussion
Our study demonstrated that mild cold exposure, well within the range of climate-controlled buildings, resulted in an increase of circulating FGF21 levels in humans. A reduction in environmental temperature from 24 C to 19 C led to a rise in overall circulating FGF21 by over a third. Augmented FGF21 levels correlated positively with increments of adipose microdialysate glycerol and total EE during cold exposure. To our knowledge, this is the first study revealing potential regulatory links between FGF21, lipolysis, and CIT in humans.
The impact of cold exposure on FGF21 has been examined only recently in rodents (6–8). Mice exposed to 4 C manifested a significant increase in FGF21 expression in BAT (6–8), accompanied by near doubling of circulating FGF21 levels after 24 h of cold exposure (8), indicating that severe cold exposure (thermoneutral temperature for mice is approximately 30 C) is a potent afferent pathway of FGF21 production. Our study has translated these discoveries to humans, demonstrating a minimal reduction in environmental temperature is sufficient to raise circulating FGF21 levels. Collectively, these results strongly suggest that FGF21 secretion is induced by cold exposure to ultimately augment CIT. This is supported by a known enhancing effect of FGF21 on cellular respiration (10) and our identification of cold-induced FGF21 increment as an independent predictor of CIT response.
The role of FGF21 on lipid metabolism is complex, and whether FGF21 stimulates (11) or attenuates (12) lipolysis is controversial. In our study, cold-induced increase in FGF21 levels correlated positively with changes in adipose microdialysate glycerol but not serum NEFA levels. It is possible that cold-modulated lipolysis in FGF21-secreting tissues is heterogeneous, rendering the overall relationships between FGF21 and circulating NEFA levels inconspicuous. We did not observe the previously reported temporal relationship between FGF21 and NEFA (13), likely a result of our less frequent FGF21 sampling, which limits the sensitivity of trend detection. Although acute nutrient intake does not alter FGF21 levels (13), the consumption of a meal during the study may have also clouded NEFA excursion interpretation. It is tempting to speculate a scenario whereby cold-induced FGF21 production stimulates lipolysis in adipose tissue, fueling whole-body thermogenic requirements to mount an adequate CIT response.
There are several limitations in this study. The sample size is small and consists of young lean individuals; thus, the effects of cold exposure on FGF21 levels in older and/or overweight adults cannot be ascertained. Although we observed robust associations between FGF21 levels and energy metabolism parameters, we emphasize that causality cannot be proven. FGF21 is known to regulate ketosis in mice (14). We did not observe similar relationships, likely due to our measurement of β-hydroxybutyrate alone but not other ketone bodies, which may be better markers for ketosis. Because the liver, muscle, and pancreas are known to secrete FGF21 (1), and only circulating FGF21 levels were measured in the current study, the origin of FGF21 secretion cannot be verified. Given the absence of overnight sampling, we cannot explore the effect of cold exposure on nocturnal FGF21 rhythm. However, a notable strength in our study is the measurement of FGF21 levels at multiple time points. In light of the recent discovery of diurnal rhythm in FGF concentrations in humans (13), also confirmed in our study, the modulatory effect of cold exposure on FGF21 could only be uncovered by paired-sample analysis at identical time points under different environmental temperatures in a controlled setting.
The mechanisms underlying FGF21-modulated CIT are unclear and may occur in an autocrine-paracrine manner in BAT (acute response) or in an endocrine fashion via promoting differentiation of WAT into BAT (long-term response). These are clinically relevant in view of the recent rediscovery of cold-activated BAT in adults (15) that is highly prevalent and inducible (16, 17), the widespread metabolic benefits of WAT browning exemplified in animal models (18), and the identification of FGF21 expression as a key marker in adipocytes with high browning potential in vitro (19). Future studies are merited to explore the relationship between FGF21 and BAT activity in humans as well as therapeutic potential of FGF21 in obesity treatment.
In summary, mild cold exposure augmented circulating FGF21 levels, which in turn predicted the degree of CIT in humans. We conclude that a small reduction in environmental temperature, achievable in climate-controlled buildings, was able to modulate FGF21 diurnal rhythm with possible beneficial effects on whole-body energy metabolism.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases National Institutes of Health Intramural Research Program Z01-DK047057-01 and Z01-DK047057-02; P.L. was supported by an Australian National Health Medical Research Council Early Career Fellowship, the Diabetes Australia Fellowship and Bushell Traveling Fellowship, Foundation, Royal Australasian College of Physicians.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- Areas under the curve
- BAT
- brown adipose tissue
- BMI
- body mass index
- CIT
- cold-induced thermogenesis
- EE
- energy expenditure
- FGF21
- fibroblast growth factor-21
- FM
- fat mass
- LM
- lean mass
- NEFA
- nonesterified fatty acid
- WAT
- white adipose tissue.
References
- 1. Adams AC, Kharitonenkov A. 2012. FGF21: the center of a transcriptional nexus in metabolic regulation. Curr Diabetes Rev 8:285–293 [DOI] [PubMed] [Google Scholar]
- 2. Wente W, Efanov AM, Brenner M, Kharitonenkov A, Köster A, Sandusky GE, Sewing S, Treinies I, Zitzer H, Gromada J. 2006. Fibroblast growth factor-21 improves pancreatic β-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes 55:2470–2478 [DOI] [PubMed] [Google Scholar]
- 3. Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. 2005. FGF-21 as a novel metabolic regulator. J Clin Invest 115:1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Potthoff MJ, Kliewer SA, Mangelsdorf DJ. 2012. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev 26:312–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cantó C, Auwerx J. 2012. Cell biology. FGF21 takes a fat bite. Science 336:675–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chartoumpekis DV, Habeos IG, Ziros PG, Psyrogiannis AI, Kyriazopoulou VE, Papavassiliou AG. 2011. Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Mol Med 17:736–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM. 2012. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26:271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T, Villarroya F. 2011. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem 286:12983–12990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Celi FS, Brychta RJ, Linderman JD, Butler PW, Alberobello AT, Smith S, Courville AB, Lai EW, Costello R, Skarulis MC, Csako G, Remaley A, Pacak K, Chen KY. 2010. Minimal changes in environmental temperature result in a significant increase in energy expenditure and changes in the hormonal homeostasis in healthy adults. Eur J Endocrinol 163:863–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chau MD, Gao J, Yang Q, Wu Z, Gromada J. 2010. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1α pathway. Proc Natl Acad Sci USA 107:12553–12558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA. 2007. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab 5:415–425 [DOI] [PubMed] [Google Scholar]
- 12. Arner P, Pettersson A, Mitchell PJ, Dunbar JD, Kharitonenkov A, Rydén M. 2008. FGF21 attenuates lipolysis in human adipocytes: a possible link to improved insulin sensitivity. FEBS Lett 582:1725–1730 [DOI] [PubMed] [Google Scholar]
- 13. Yu H, Xia F, Lam KS, Wang Y, Bao Y, Zhang J, Gu Y, Zhou P, Lu J, Jia W, Xu A. 2011. Circadian rhythm of circulating fibroblast growth factor 21 is related to diurnal changes in fatty acids in humans. Clin Chem 57:691–700 [DOI] [PubMed] [Google Scholar]
- 14. Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. 2007. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5:426–437 [DOI] [PubMed] [Google Scholar]
- 15. Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, Nuutila P. 2009. Functional brown adipose tissue in healthy adults. N Engl J Med 360:1518–1525 [DOI] [PubMed] [Google Scholar]
- 16. Lee P, Zhao JT, Swarbrick MM, Gracie G, Bova R, Greenfield JR, Freund J, Ho KK. 2011. High prevalence of brown adipose tissue in adult humans. J Clin Endocrinol Metab 96:2450–2455 [DOI] [PubMed] [Google Scholar]
- 17. Lee P, Swarbrick MM, Zhao JT, Ho KK. 2011. Inducible brown adipogenesis of supraclavicular fat in adult humans. Endocrinology 152:3597–3602 [DOI] [PubMed] [Google Scholar]
- 18. Cinti S. 2009. Transdifferentiation properties of adipocytes in the adipose organ. Am J Physiol Endocrinol Metab 297:E977–E986 [DOI] [PubMed] [Google Scholar]
- 19. Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerbäck S, Schrauwen P, Spiegelman BM. 2012. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150:366–376 [DOI] [PMC free article] [PubMed] [Google Scholar]