Abstract
Context:
Vitamin D insufficiency is associated with increased cardiovascular events in the general population. Additionally, low serum 25-hydroxyvitamin D [25(OH)D] is associated with endothelial dysfunction and arterial stiffness. However, little is known about the association between serum 25(OH)D level and myocardial blood flow.
Objective:
Our objective was to examine the association between serum 25(OH)D levels and coronary flow reserve (CFR) measured by 13N-positron emission tomography in asymptomatic middle-aged male twins.
Design:
The Emory Twin Study is a cross-sectional study of soldiers from the Vietnam Era Registry.
Setting:
The study was conducted at the General Clinical Research Center, Emory University, Atlanta, GA.
Participants:
A total of 368 middle-aged male twins were enrolled for the study. Serum 25(OH)D levels were measured in all subjects and classified as vitamin D insufficiency [25(OH)D <30 ng/ml] or sufficiency [25(OH)D ≥30 ng/ml]. Positron emission tomography with [13N]ammonia was used to evaluate myocardial blood flow at rest and after adenosine stress. CFR was measured as the ratio of maximum to rest myocardial blood flow.
Main Outcome Measure:
Primary outcome was CFR measurement.
Results:
Mean overall serum 25(OH)D concentration was 37.0 ± 21.4 ng/ml; 167 twins (45%) were vitamin D insufficient. CFR was significantly lower in subjects with vitamin D insufficiency compared with subjects with vitamin D sufficiency (2.41 vs. 2.64; P = 0.007), even after adjustment for traditional cardiovascular risk factors, serum PTH, calcium, and phosphorus levels, and season. An abnormal CFR (CFR <2) was more prevalent in subjects with vitamin D insufficiency than with vitamin D sufficiency (31 vs. 20%; P = 0.03). In addition, in vitamin D status-discordant twin pairs, CFR was significantly lower in the vitamin D-insufficient twin than in the vitamin D-sufficient co-twin (2.35 vs. 2.58; P = 0.037).
Conclusion:
Vitamin D insufficiency is associated with lower CFR in men. This association may help explain some of the increased cardiovascular risk reported in individuals with vitamin D insufficiency.
Vitamin D insufficiency is a common problem, affecting individuals across the globe. Hypovitaminosis D is associated with several chronic disease states including cardiovascular disease (CVD). Several epidemiological studies have demonstrated an association between low serum 25-hydroxyvitamin D [25(OH)D] concentrations and the risk of hypertension, left ventricular hypertrophy, heart failure, peripheral arterial disease, coronary artery disease, myocardial infarction, and cardiovascular mortality (1–6).
Vitamin D3 is produced in the skin from 7-dehydrocholesterol upon exposure to UV light. Cutaneous production of vitamin D3 represents the primary source of vitamin D for humans, although some foods have small amounts of vitamin D3 or D2. Both vitamin D3 and vitamin D2 undergo hydroxylation in the liver to 25[OH]D. Subsequently, 25(OH)D is converted to 1,25-dihydroxyvitamin D [1,25(OH)2D] by the 1-α-hydroxylase enzyme predominantly in the kidney, although this enzyme is also found in several other tissues including endothelial cells, vascular smooth muscle cells, and cardiomyocytes (7–10). 1,25(OH)2D exerts its effects through binding with the vitamin D receptor (VDR) in the nucleus of target cells (11). Like the enzyme 1α-hydroxylase, VDR is widely expressed in several human tissues besides bone, including the heart and vasculature (10, 12). Consequently there could be a local conversion of serum 25(OH)D to 1,25(OH)2D in the cardiovascular system; the active hormone, in turn, through VDR binding might act in an autocrine or paracrine fashion modulating endothelial, vascular smooth muscle, and cardiac cell function as well as inflammatory pathways (13). In fact, vitamin D deficiency has been linked with markers of abnormal vascular function such as increased arterial stiffness and abnormal vascular endothelial reactivity and vascular calcification (14–18). All of these mechanisms may result in a greater risk of ischemic heart disease and myocardial infarction. However, whether vitamin D insufficiency is associated with abnormal myocardial blood flow, an early marker of myocardial microcirculatory dysfunction and future coronary artery disease, is unknown.
The purpose of this study was to examine the association between serum 25(OH)D concentration and coronary flow reserve (CFR) measured with positron emission tomography (PET) imaging with [13N]ammonia in a national sample of middle-aged male twins. Additionally, because of the twin design, we were able to assess the potential familial or genetic confounding of the association between vitamin D status and CFR.
Subjects and Methods
Study population
The Emory Twin Study (ETS) is an investigation of the psychological, behavioral, and biological risk factors for subclinical CVD using twins (19). Middle-aged male monozygotic and dizygotic twin pairs born between 1946 and 1956 were obtained from the Vietnam Era Twin Registry (20). The ETS includes three random samples: pairs discordant for major depression, pairs discordant for posttraumatic stress disorder (PTSD), and pairs free of depression or PTSD. All twins were examined in pairs at the Emory University General Clinical Research Center, and all data collection occurred during a 24-h admission under controlled conditions. The twins resided in a range of latitudes in the United States between 21° and 49° N, except for one subject who was living at 61° N. The ETS was approved by the Emory Institutional Review Board, the research procedures used were in accordance with the ethical standards of Emory University, and all subjects signed an informed consent form to participate in the study.
Measurements
All measurements were performed in the morning after an overnight fast, and both twins in a pair were tested at the same time. A medical history was obtained and a physical examination was performed on all twins. Cigarette smoking was categorized as current or no smoking. Physical activity was assessed with a modified version of the Baecke Questionnaire of Habitual Physical Activity, with values ranging from 3.43 (minimal) to 13.04 (intense) (21). The Framingham risk score was calculated according to published criteria (22). Measured weight and height were used to calculate body mass index (BMI) as weight in kilograms divided by the height in square meters. Systolic blood pressure and diastolic blood pressure were measured by mercury sphygmomanometer in the right arm after 10 min of rest with the subject in a sitting position. The average of two measurements obtained 5 min apart was used in the analyses. Diabetes mellitus was considered present if the patients received treatment with insulin or oral hypoglycemic drugs or had a fasting glucose above 126 mg/dl.
Venous blood samples were drawn for the measurement of glucose, lipid profile, creatinine, calcium, phosphorus, 25(OH)D, and PTH after an overnight fast. Serum creatinine, calcium, phosphorus, and glucose levels were measured on the Beckman CX-7 chemistry autoanalyzer (Beckman Counter Diagnosis, Fullerton, CA). Total cholesterol was determined by enzymatic methods (Beckman Coulter Diagnostics). Direct high-density lipoprotein and direct low-density lipoprotein cholesterol were obtained using homogeneous assays (Equal Diagnostics, Exton, PA). The Emory Lipid Research Laboratory, a participant in the Centers for Disease Control/National Heart, Lung, and Blood Institute Lipid Standardization Program, performed all analyses from freshly isolated EDTA plasma. Serum 25(OH)D concentrations were determined by ELISA (IDS Inc., Fountain Hills, AZ). Vitamin D insufficiency was defined as serum 25(OH)D levels less than 30 ng/ml and vitamin D sufficiency as serum 25(OH)D levels equal to or higher than 30 ng/ml. Accuracy of the 25(OH)D measurements were ensured by participation in the vitamin D external quality assessment scheme as well as the National Institute of Standards and Technology/National Institutes of Health Vitamin D Metabolites Quality Assurance Program. Serum PTH levels were determined by ELISA (Immutopics International, San Clemente, CA). Both serum 25(OH)D and PTH measurements were performed in duplicate.
PET protocol
The PET [13N]ammonia protocol employed for this study has been described before (23) and is briefly summarized here. All twins received a similar low-fat dinner the night before the PET studies were performed and remained fasting thereafter until the scans were completed. They were instructed to abstain from smoking and drinking alcoholic or caffeinated beverages and from eating any food other than what was served to them for 24 h before the studies. All medications were withheld the morning of the PET scan. The entire imaging protocol was performed by personnel blinded to the clinical status of the twins. Twins underwent PET imaging at rest and after pharmacological (adenosine) stress testing. The PET data were collected in two-dimensional mode using a CTI ECAT Exact 47 (921) camera (5-mm resolution) (Siemens, Knoxville, TN). In December 2006, this camera was decommissioned and substituted with a three-dimensional GE PET-CT Discovery LS scanner (General Electric, Milwaukee, WI). Approximately 18% of the sample was assessed with the new camera. Similar scanning methodology was used with the two cameras. We performed a repeatability study by imaging five volunteers with each of the two cameras and comparing the myocardial perfusion results. This comparison study found a good correlation coefficient of 0.72 between the two cameras (P < 0.001). However, each patient underwent imaging and myocardial blood flow quantification using only one PET scanner; hence, the repeatability study was not strictly necessary to validate the conclusions of this study. The PET study started with the injection of 2- to 3-mCi dose of [13N]ammonia followed by a 4-min static scan that was reconstructed without any attenuation correction to simply verify the subject's position. Subsequently, rest and pharmacological stress [13N]ammonia imaging was performed. The rest and stress imaging protocols were identical and required the injection of 20 mCi of [13N]ammonia; for the stress phase, adenosine (0.14 mg/kg · min) was infused for 2 min before the injection of [13N]ammonia and for 2 more minutes after the injection of the radiotracer. Data were collected in 47 planes, 3.375 mm thick, covering a range of 16 cm for the CTI ECAT 921 camera or in 35 planes, 4.25 mm thick, covering a range of 15 cm for the GE PET-CT Discovery LS scanner. After the conclusion of the dynamic sequence, a 15-min gated acquisition was started. Finally, transmission data were collected for 5 min using germanium 68 (68Ge) rods for segmented attenuation correction. The injections of [13N]ammonia were separated by at least 50 min to allow [13N]ammonia from the first injection to decay to a level where it would not interfere with the second study. Images were reconstructed with filtered back projection using a Hann filter cutoff at 1 cycle/cm and included attenuation correction. The electrocardiographic output was monitored continuously, and blood pressure and heart rate measurements were taken before, during (every minute), and after adenosine infusion.
Myocardial blood flow measures and CFR determination
Myocardial blood flow was measured at rest and after pharmacological stress with adenosine. The input function was generated by drawing a region of interest in the left ventricular chamber on a midventricular slice, and flow was calculated (expressed in milliliters per minute per gram of tissue) using established techniques (24). The left ventricle was sampled radially from 40 different angles, and 40 samples of flow were obtained for each short axis slice. The resulting hundreds of samples were grouped into 20 segments.
The CFR for the entire myocardium (across all 20 regions) was defined as the ratio of maximum myocardial blood flow during stress to myocardial blood flow at rest. Abnormal CFR was defined as CFR below 2 (25, 26).
Myocardial perfusion defect score
A summed score was constructed describing the number and severity of visible perfusion defects across 20 myocardial segments. In each segment, the defect severity was quantified on a point scale varying from 0 (normal) to 4 (absent perfusion); the points were then summed up across the 20 segments yielding a total score. Separate scores were obtained for the rest (summed rest score) and stress [summed stress score (SSS)] scans. A reversibility defect score (summed difference score) was obtained by subtracting the stress score from the rest score. A true perfusion abnormality was considered present when the SSS was at least 4 (27).
Statistical analyses
We compared demographic and clinical variables between study subjects based on vitamin D status. P values were corrected for the correlation between co-twins using generalized estimating equations for categorical variables and mixed-effects models for continuous variables. Spearman correlation was initially used to evaluate the association between 25(OH)D level and CFR. We then used regression modeling for twin pairs to examine the association between vitamin D status and CFR. In the analysis of twins as separate individuals, in which all twins were included, we initially fit an unadjusted mixed linear model adapted for twins studies (28) that examined the association of vitamin D status with CFR (base model). We then adjusted for traditional cardiovascular risk factors, including the Framingham risk score, history of CVD, medications (statins, aspirin, β-blockers, angiotensin-converting enzyme inhibitors, and angiotensin receptor antagonists), physical activity, BMI, and history of depression and/or PTSD. Because we wanted to know whether vitamin D insufficiency was associated with an abnormal CFR regardless of coronary artery disease, in a subsequent step, we included the presence of perfusion abnormalities in the model. Analyses were further adjusted for markers of mineral metabolism (serum calcium, phosphorus, and PTH) and season of blood collection (November–March vs. April–October) (29). We repeated the above analyses using a dichotomous definition of abnormal CFR (i.e. CFR <2) and a true perfusion abnormality (i.e. SSS ≥4); for these analyses, we used a generalized estimating equation logistic regression model.
Finally, we reevaluated the association of vitamin D status with CFR within twin pairs discordant for vitamin D status (one brother was insufficient and the other sufficient). The within-pair approach automatically takes into account shared familial and environmental influences. These within-pair analyses were further stratified by zygosity to determine whether the association between vitamin D status and CFR were different between monozygotic and dizygotic twins. Monozygotic twins share 100% of their genes, whereas dizygotic twins only share on average 50% of their genes. Smaller within-pair differences in myocardial perfusion among vitamin D-discordant monozygotic twins than vitamin D-discordant dizygotic pairs would suggest genetic confounding. This is formally tested by including a zygosity by vitamin D term in the model. These analyses were conducted using mixed regression modeling and examined the relationship between vitamin D status and CFR both before and after adjustment for cardiovascular risk factors, history of CVD, and severity of perfusion defects (SSS).
A two-tailed P value <0.05 was considered statistically significant. Statistical analyses were performed using the statistical software package SPSS version 19 (SPSS Inc., Chicago, IL).
Results
Demographic and clinical characteristics
In total, 368 twins underwent 25(OH)D assay and PET imaging. The mean serum 25(OH)D concentration in the whole study cohort was 37.0 ± 21.4 ng/ml (median 31 ng/ml; interquartile range 23–44.7 ng/ml) and 167 twins (45.4%) had vitamin D insufficiency (Table 1). The mean serum 25(OH)D level collected during the winter months was significantly lower compared with the samples collected during the summer months (33.3 ± 21.3 vs. 39.5 ± 21.2 ng/ml; P = 0.006). History of depression and diabetes mellitus were more prevalent in the insufficient vitamin D group compared with the sufficient vitamin D group. There was a trend toward a higher BMI and greater use of angiotensin-converting enzyme inhibitors in the group with vitamin D insufficiency.
Table 1.
Demographic and clinical characteristics in the entire sample and according to vitamin D status
| Demographic and clinical characteristics | All twins, n = 368 | Vitamin D insufficiency [25(OH)D <30 ng/ml], n = 167 | Vitamin D sufficiency [25(OH)D ≥30 ng/ml], n = 201 | P valuea |
|---|---|---|---|---|
| Demographics | ||||
| Age (yr) | 54.9 ± 3.0 | 55.1 ± 2.9 | 54.7 ± 3.0 | 0.3 |
| Caucasian [n (%)] | 344 (93.5) | 151 (90.4) | 193 (96) | 0.1 |
| History of depression [n (%)] | 88 (23.9) | 50 (29.3) | 38 (18.9) | 0.006 |
| Mineral metabolism characteristics | ||||
| Calcium (mg/dl) | 8.97 ± 0.3 | 8.98 ± 0.3 | 8.97 ± 0.3 | 0.8 |
| Phosphorus (mg/dl) | 3.09 ± 0.5 | 3.09 ± 0.5 | 3.09 ± 0.5 | 0.8 |
| 25(OH)D (ng/ml) | 37.04 ± 21.4 | 21.3 ± 5.9 | 50.1 ± 20.9 | <0.001 |
| PTH (pg/ml) | 59.5 ± 22.3 | 60.9 ± 21.5 | 58.4 ± 22.9 | 0.2 |
| Cardiovascular risk factors | ||||
| Smoking, yes (%) | 94 (25.5) | 49 (29.3) | 45 (22.4) | 0.1 |
| Physical activity score | 7.34 ± 1.8 | 7.34 ± 1.9 | 7.43 ± 1.7 | 0.3 |
| BMI (kg/m2) | 29.4 ± 4.9 | 29.9 ± 5.6 | 29 ± 4.1 | 0.07 |
| Systolic blood pressure (mm Hg) | 130.2 ± 15.3 | 129.7 ± 15.1 | 130.6 ± 15.6 | 0.6 |
| Diastolic blood pressure (mm Hg) | 81.2 ± 10.4 | 81.5 ± 10.6 | 80.9 ± 10.3 | 0.6 |
| Total cholesterol (mg/dl) | 186.4 ± 37.9 | 185.5 ± 39.6 | 187.1 ± 36.5 | 0.7 |
| High-density lipoprotein (mg/dl) | 38.6 ± 10.3 | 38.1 ± 10.9 | 38.9 ± 8.7 | 0.4 |
| Low-density lipoprotein (mg/dl) | 122.4 ± 33.6 | 122.8 ± 35.9 | 122.1 ± 31.7 | 0.8 |
| eGFR (ml/min · 1.73 m2) | 88.4 ± 12.4 | 88.8 ± 13.3 | 88.1 ± 11.5 | 0.5 |
| Diabetes, yes (%) | 37 (10.1) | 24 (14.4) | 13 (6.5) | 0.02 |
| CVD history, yes (%) | 60 (16.3) | 31 (18.6) | 29 (14.4) | 0.6 |
| Framingham risk score | 6.2 ± 2.23 | 6.24 ± 2.3 | 6.16 ± 2.1 | 0.7 |
| Concurrent medications | ||||
| ACE inhibitors, yes (%) | 51 (13.9) | 29 (17.4) | 22 (10.9) | 0.07 |
| Angiotensin receptor blockers, yes (%) | 13 (3.5) | 8 (4.8) | 5 (2.5) | 0.4 |
| Statins, yes (%) | 83 (22.6) | 38 (22.8) | 45 (22.4) | 0.7 |
| Aspirin, yes (%) | 94 (25.5) | 45 (26.9) | 49 (24.4) | 0.6 |
| β-Blockers, yes (%) | 46 (12.5) | 25 (15) | 21 (10.4) | 0.2 |
All data are reported as mean ± sd or number of subjects (percentage). ACE, Angiotensin-converting enzyme; eGFR, estimated glomerular filtration rate.
P value among 25(OH)D status.
Vitamin D status and PET results
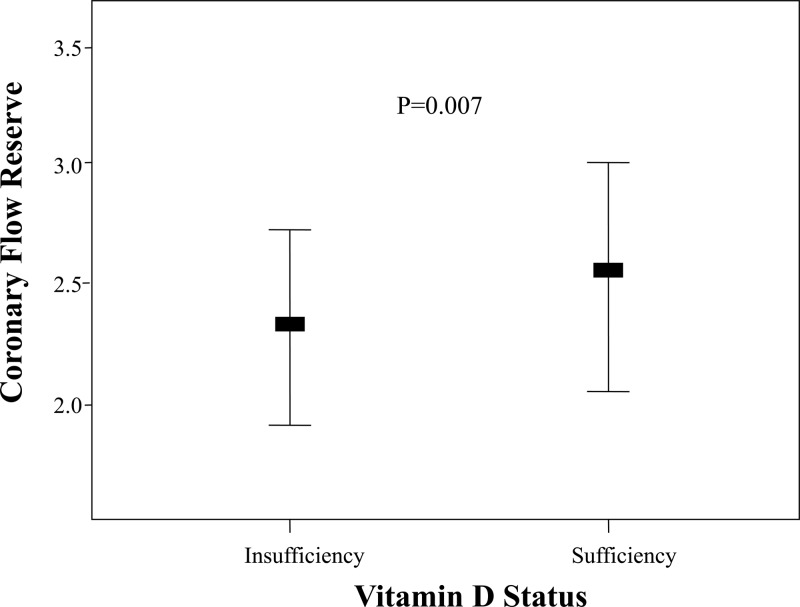
There was a weak but significant association between 25(OH)D levels and CFR (r = 0.165; P = 0.002). The mean CFR was significantly lower in the vitamin D-insufficient group compared with the vitamin D-sufficient group (2.41 vs. 2.64, respectively; P = 0.007) (Fig. 1 and Table 2). Furthermore, the prevalence of abnormal CFR (CFR <2) was significantly higher in the group with vitamin D insufficiency (P = 0.03) (Table 2). Both perfusion defect severity score at rest (summed rest score) and after stress (SSS) were significantly higher in the vitamin D-insufficient group compared with the sufficient group, but the extent of ischemia (summed difference score) was not different between groups (Table 2).
Fig. 1.
Vitamin D status and CFR. The horizontal thick lines indicate the median values; the upper and lower bounds represent the 75th and 25th percentile.
Table 2.
Myocardial perfusion imaging in the entire sample and according to 25(OH)D status
| Myocardial perfusion | All twins, n = 368 | Vitamin D insufficiency [25(OH)D <30 ng/ml], n = 167 | Vitamin D sufficiency [25(OH)D ≥30 ng/ml], n = 201 | P valuea |
|---|---|---|---|---|
| MBF at rest (ml/min · g) | 0.67 ± 0.15 | 0.68 ± 0.14 | 0.66 ± 0.16 | 0.089 |
| MBF during stress (ml/min · g) | 1.62 ± 0.47 | 1.59 ± 0.42 | 1.65 ± 0.5 | 0.2 |
| CFR | 2.54 ± 0.82 | 2.41 ± 0.72 | 2.64 ± 0.89 | 0.007 |
| CFR <2 | 93 (25.3) | 52 (31.1) | 41 (20.4) | 0.03 |
| SSS | 2.31 ± 4.9 | 2.72 ± 4.98 | 1.96 ± 4.9 | 0.04b |
| SSR | 0.59 ± 2.4 | 0.91 ± 2.98 | 0.32 ± 1.7 | 0.002b |
| SSD | 1.67 ± 4.9 | 1.72 ± 3.46 | 1.62 ± 4.6 | 0.3b |
| Abnormal perfusion (SSS ≥4) | 90 (24.5) | 49 (29.3) | 41 (20.6) | 0.07 |
Data are reported as mean ± sd or number of subjects (percentage). MBF, Myocardial blood flow; SSD, summed difference score; SSR, summed rest score.
P value among 25(OH)D status.
P values are corrected to SSS, SSR, and SSD log-transformed (to improve the distribution).
In the unadjusted model, CFR was significantly lower in the vitamin D-insufficient group than in the sufficient group. After adjustments for the Framingham risk score, CFR remained lower in the vitamin D-insufficient group. Furthermore, CFR remained significantly lower in the insufficient group after adjusting for history of CVD, use of medications that could affect CFR, physical activity, BMI, history of depression, severity of perfusion defects, serum levels of calcium, phosphorus and PTH, and season (P = 0.03, Table 3). The results remained essentially unchanged after excluding patients with previous history of CVD (data not shown). The unadjusted estimated odds ratio of vitamin D insufficiency in the presence of an abnormal CFR was 1.53 (95% confidence interval = 1.07–2.17).
Table 3.
Mixed-model linear regression analysis for CFR by vitamin D status, treating twins as individuals
| CFR | Vitamin D insufficiency, n = 167 [mean (95% CI)] | Vitamin D sufficiency, n = 201 [mean (95% CI)] | Difference (mean ± se) | P value |
|---|---|---|---|---|
| Model 1: Unadjusted | 2.41 (2.29–2.53) | 2.64 (2.53–2.75) | −0.23 ± 0.08 | 0.007 |
| Model 2: model 1 + adjustment for Framingham risk score,a history of CVD, and medications,b physical activity, BMI, and history of depression and PTSD | 2.42 (2.15–2.68) | 2.62 (2.35–2.90) | −0.21 ± 0.08 | 0.02 |
| Model 3: model 2 + adjustment for perfusion defectsc | 2.43 (2.17–2.69) | 2.62 (2.35–2.89) | −0.19 ± 0.08 | 0.03 |
| Model 4: model 3 + adjustment for mineral metabolismd and vitamin D season variability | 2.41 (2.14–2.68) | 2.60 (2.32–2.87) | −0.19 ± 0.09 | 0.03 |
CI, Confidence interval.
Adjusted for Framingham risk score including age, serum total cholesterol and high-density lipoprotein, systolic/diastolic blood pressure, presence of diabetes mellitus, and smoking.
Medications included use of statins, aspirin, β-blockers, angiotensin-converting enzyme inhibitor, and angiotensin receptor antagonist.
Adjusted for severity of perfusion defects (SSS).
Adjusted for serum PTH, calcium, and phosphorus.
Table 4 shows the within-pair comparisons of CFR in twin pairs discordant for vitamin D status. There were 54 twin pairs (32 monozygotic and 22 dizygotic twin pairs) who were discordant for vitamin D status. Analysis of these pairs demonstrated that CFR was lower in the twins with vitamin D insufficiency compared with their co-twins with vitamin D sufficiency even after adjustment for cardiovascular risk factors, severity of perfusion defects, and season. In zygosity-specific analyses, the association of vitamin D status and CFR was marginally significant for dizygotic twins (P = 0.06 unadjusted and P = 0.079 adjusted) but not statistically significant for monozygotic pairs (P = 0.2 unadjusted and P = 0.17 adjusted). The interaction between vitamin D status and zygosity was not statistically significant (P = 0.17).
Table 4.
Mixed-model linear regression analysis for CFR by vitamin D status within twin pairs
| Sample and analysis | Vitamin D insufficiency [mean (95% CI)] | Vitamin D sufficiency [mean (95% CI)] | Within-pair difference |
|
|---|---|---|---|---|
| Estimate ± se | P value | |||
| All pairs | ||||
| Unadjusted | 2.36 (2.16–2.56) | 2.60 (2.40–2.80) | −0.24 ± 0.10 | 0.03 |
| Adjusteda | 2.35 (2.10–2.59) | 2.58 (2.35–2.81) | −0.24 ± 0.11 | 0.037 |
| Monozygotic | ||||
| Unadjusted | 2.42 (2.13–2.71) | 2.60 (2.31–2.89) | −0.18 ± 0.14 | 0.2 |
| Adjusteda | 2.27 (1.91–2.63) | 2.48 (2.16–2.81) | −0.21 ± 0.15 | 0.17 |
| Dizygotic | ||||
| Unadjusted | 2.26 (1.98–2.55) | 2.59 (2.31–2.88) | −0.33 ± 0.17 | 0.06 |
| Adjusteda | 2.38 (2.02–2.73) | 2.69 (2.34–3.04) | −0.32 ± 0.17 | 0.079 |
For all discordant twin pairs, n = 54; for monozygotic pairs, n = 32; and for dizygotic pairs, n = 22. CI, Confidence interval.
Adjusted for Framingham risk score including age, serum total cholesterol, serum high-density lipoprotein, systolic/diastolic blood pressure, presence of diabetes mellitus, smoking, history of CVD, season, and severity of perfusion defects (SSS).
Discussion
In this cross-sectional study, middle-aged male twins with vitamin D insufficiency had a lower CFR than participants with normal serum levels of 25(OH)D even after adjustment for several confounders and history of CVD. The association persisted within twin pairs, which represents a form of control for shared early familial and genetic factors. It is possible that a genetic substrate is shared between vitamin D status and myocardial perfusion. However, a formal test of the interaction of vitamin D and zygosity on CFR was not significant. Thus, although there was some evidence of potential genetic confounding (absence of an effect in monozygotic pairs), the data are not completely supportive (lack of a significant interaction by zygosity).
PET has emerged as a very helpful imaging tool for the diagnosis and risk stratification of coronary artery disease. This imaging modality further allows an estimation of blood flow per gram of myocardial tissue, and our demonstration that a lower CFR persisted after adjustment for severity of perfusion abnormalities suggests, without proving, that the lower CFR observed in individuals with vitamin D insufficiency may be due to microvascular abnormalities rather than flow-limiting epicardial coronary artery stenoses. Abnormal myocardial microvascular function is believed to be an early marker of coronary artery disease and has adverse prognostic implications (30). Furthermore, long-term abnormalities of myocardial microcirculation may lead to functional and eventually irreversible structural changes leading to left ventricular dysfunction (31–33). Several cardiovascular risk factors such as diabetes, smoking, hypertension, inflammation, and dyslipidemia are associated with microvascular impairment (30, 33). In this study, we have further demonstrated that vitamin D insufficiency is associated with lower CFR. To date, the association between low serum 25(OH)D levels and parameters of vascular function has been assessed with brachial artery flow-mediated dilation studies, carotid-femoral pulse wave velocity, and digital reactive hyperemia index. Low vitamin D status has been associated with vascular endothelial dysfunction, arterial stiffness, and abnormal microvascular circulation in conductance and resistance arteries (14–18, 34, 35). Additionally several short-term studies demonstrated improvement of several parameters of peripheral vascular function after vitamin D replacement in individuals with suboptimal serum 25(OH)D concentration (36–39). Our results extend these findings by showing an association between serum 25(OH)D levels and reduced myocardial blood flow. Whether CFR may be improved after normalization of serum 25(OH)D levels via sun exposure or supplementation is unknown.
Several studies showed an association of low vitamin D levels with cardiovascular risk factors including hypertension, diabetes mellitus, the metabolic syndrome, obesity, hyperparathyroidism, inflammation, and depression (5, 40–44). Although vitamin D status could affect myocardial microcirculatory function and atherosclerosis through these risk factors, direct vascular effects are possible. The VDR and very likely the 25(OH)D-1α-hydroxylase enzyme are widely expressed on vascular endothelial cells (7), vascular smooth muscle cells (45), cardiomyocytes, and monocytes/macrophages (46). It is therefore plausible that active vitamin D [1,25(OH)2D] may interact with these cells, triggering specific genes that, in turn, lead to functional and morphological cardiovascular changes. Recently, Jablonski et al. (15) showed that brachial artery flow-mediated vasodilation was positively associated with serum 25(OH)D but not serum 1,25(OH)2D, suggesting a local conversion of serum 25(OH)D to its active form, acting as an autocrine or paracrine hormone.
Under physiological conditions, coronary blood flow is regulated by several mechanisms including metabolic, endothelial, myogenic, and neurohumoral pathways that influence the vascular tone. This regulation is crucial to provide an adequate supply of oxygen and substrates to the heart (30, 47). Several experimental and clinical studies have demonstrated that a low vitamin D level may trigger a number of functional and structural cardiovascular abnormalities, at least in part due to dysfunction of the vitamin D signaling pathway, such as the renin-angiotensin system activation (48–50), cardiac hypertrophy and myocardial fibrosis (51), vascular endothelial dysfunction (52), inflammation (53), vascular smooth muscle cell proliferation (45), and vascular and cardiac calcification (54). In this context, we could speculate that vitamin D insufficiency and reduced VDR activation could affect both vascular smooth muscle relaxation and release of endothelium-dependent factors ultimately blunting the normal vascular dilatory response, as reflected in a lower CFR.
There were several limitations in our study. First, the cross-sectional design limits our ability to make causal inferences between vitamin D status and CFR. Our sample was derived from a twin registry of military veterans and included only middle-aged, predominantly Caucasian men; therefore, our results should not be generalized to women, younger individuals, and other ethnic groups. We have no way to prove that a reduced CFR may have an adverse prognostic impact on patients with vitamin D insufficiency.
In conclusion, we described an association between low serum 25(OH)D level and reduced CFR in a cohort of middle-aged male twins asymptomatic for coronary artery disease. The association was independent of numerous potential confounders. This suggests that low serum 25(OH)D levels are associated with a reduction in coronary vasodilatory response. Our data contribute to the growing evidence linking vitamin D insufficiency to adverse cardiovascular outcomes.
Acknowledgments
The U.S. Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin Registry. Numerous organizations have provided invaluable assistance, including the Veterans Affairs Cooperative Study Program; Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; National Institutes of Health (NIH); National Opinion Research Center; National Research Council, National Academy of Sciences; and the Institute for Survey Research, Temple University. We gratefully acknowledge the continued cooperation and participation of the members of the Vietnam Era Twin Registry. Without their contribution, this research would not have been possible.
This work was supported by Grants K24HL077506, R01 HL68630, and R01 AG026255 (to V.V.) and Grant K23AR054334 (to V.T.) from the NIH and Grant 0245115N from the American Heart Association (to V.V.). This work was also supported in part by U.S. Public Health Service Grant UL1 RR025008 from the Clinical and Translational Science Award program, NIH, National Center for Research Resources and by the Emory University General Clinical Research Center MO1-RR00039.
Disclosure Summary: V.V., E.V., J.G., and V.T. have nothing to declare. C.K. received lecture fees from Abbott. A.B. was previously employed by Genzyme Therapeutics (2008–2009) and received lecture fees from Genzyme Therapeutics, Amgen, and Sanofi-Aventis. P.R. received research grants from Genzyme Therapeutics and Amgen.
Footnotes
- BMI
- Body mass index
- CFR
- coronary flow reserve
- CVD
- cardiovascular disease
- 1,25(OH)2D
- 1,25-dihydroxyvitamin D
- 25(OH)D
- 25-hydroxyvitamin D
- ETS
- Emory Twin Study
- PET
- positron emission tomography
- PTSD
- posttraumatic stress disorder
- SSS
- summed stress score
- VDR
- vitamin D receptor.
References
- 1. Melamed ML, Muntner P, Michos ED, Uribarri J, Weber C, Sharma J, Raggi P. 2008. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol 28:1179–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. 2008. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med 168:1340–1349 [DOI] [PubMed] [Google Scholar]
- 3. Pilz S, März W, Wellnitz B, Seelhorst U, Fahrleitner-Pammer A, Dimai HP, Boehm BO, Dobnig H. 2008. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab 93:3927–3935 [DOI] [PubMed] [Google Scholar]
- 4. Kendrick J, Targher G, Smits G, Chonchol M. 2009. 25-Hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis 205:255–260 [DOI] [PubMed] [Google Scholar]
- 5. Burgaz A, Orsini N, Larsson SC, Wolk A. 2011. Blood 25-hydroxyvitamin D concentration and hypertension: a meta-analysis. J Hypertens 29:636–645 [DOI] [PubMed] [Google Scholar]
- 6. Giovannucci E, Liu Y, Hollis BW, Rimm EB. 2008. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med 168:1174–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Merke J, Milde P, Lewicka S, Hügel U, Klaus G, Mangelsdorf DJ, Haussler MR, Rauterberg EW, Ritz E. 1989. Identification and regulation of 1,25-dihydroxyvitamin D3 receptor activity and biosynthesis of 1,25-dihydroxyvitamin D3. Studies in cultured bovine aortic endothelial cells and human dermal capillaries. J Clin Invest 83:1903–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zehnder D, Bland R, Chana RS, Wheeler DC, Howie AJ, Williams MC, Stewart PM, Hewison M. 2002. Synthesis of 1,25-dihydroxyvitamin D3 by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol 13:621–629 [DOI] [PubMed] [Google Scholar]
- 9. Somjen D, Weisman Y, Kohen F, Gayer B, Limor R, Sharon O, Jaccard N, Knoll E, Stern N. 2005. 25-Hydroxyvitamin D3-1α-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation 111:1666–1671 [DOI] [PubMed] [Google Scholar]
- 10. Chen S, Glenn DJ, Ni W, Grigsby CL, Olsen K, Nishimoto M, Law CS, Gardner DG. 2008. Expression of the vitamin D receptor is increased in the hypertrophic heart. Hypertension 52:1106–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holick MF. 2007. Vitamin D deficiency. N Engl J Med 357:266–281 [DOI] [PubMed] [Google Scholar]
- 12. Merke J, Hofmann W, Goldschmidt D, Ritz E. 1987. Demonstration of 1,25(OH)2 vitamin D3 receptors and actions in vascular smooth muscle cells in vitro. Calcif Tissue Int 41:112–114 [DOI] [PubMed] [Google Scholar]
- 13. Cozzolino M, Ketteler M, Zehnder D. 2010. The vitamin D system: a crosstalk between the heart and kidney. Eur J Heart Fail 12:1031–1041 [DOI] [PubMed] [Google Scholar]
- 14. Al Mheid I, Patel R, Murrow J, Morris A, Rahman A, Fike L, Kavtaradze N, Uphoff I, Hooper C, Tangpricha V, Alexander RW, Brigham K, Quyyumi AA. 2011. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. J Am Coll Cardiol 58:186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 2011. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension 57:63–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mayer O, Jr, Filipovský J, Seidlerová J, Vanìk J, Dolejšová M, Vrzalová J, Cífková R. 2012. The association between low 25-hydroxyvitamin D and increased aortic stiffness. J Hum Hypertens 26:650–655 [DOI] [PubMed] [Google Scholar]
- 17. Yiu YF, Chan YH, Yiu KH, Siu CW, Li SW, Wong LY, Lee SW, Tam S, Wong EW, Cheung BM, Tse HF. 2011. Vitamin D deficiency is associated with depletion of circulating endothelial progenitor cells and endothelial dysfunction in patients with type 2 diabetes. J Clin Endocrinol Metab 96:E830–E835 [DOI] [PubMed] [Google Scholar]
- 18. Chitalia N, Recio-Mayoral A, Kaski JC, Banerjee D. 2012. Vitamin D deficiency and endothelial dysfunction in non-dialysis chronic kidney disease patients. Atherosclerosis 220:265–268 [DOI] [PubMed] [Google Scholar]
- 19. Vaccarino V, Lampert R, Bremner JD, Lee F, Su S, Maisano C, Murrah NV, Jones L, Jawed F, Afzal N, Ashraf A, Goldberg J. 2008. Depressive symptoms and heart rate variability: evidence for a shared genetic substrate in a study of twins. Psychosom Med 70:628–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goldberg J, Curran B, Vitek ME, Henderson WG, Boyko EJ. 2002. The Vietnam Era Twin Registry. Twin Res 5:476–481 [DOI] [PubMed] [Google Scholar]
- 21. Richardson MT, Ainsworth BE, Wu HC, Jacobs DR, Jr, Leon AS. 1995. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke Questionnaire to assess leisure-time physical activity. Int J Epidemiol 24:685–693 [DOI] [PubMed] [Google Scholar]
- 22. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. 1998. Prediction of coronary heart disease using risk factor categories. Circulation 97:1837–1847 [DOI] [PubMed] [Google Scholar]
- 23. Vaccarino V, Votaw J, Faber T, Veledar E, Murrah NV, Jones LR, Zhao J, Su S, Goldberg J, Raggi JP, Quyyumi AA, Sheps DS, Bremner JD. 2009. Major depression and coronary flow reserve detected by positron emission tomography. Arch Intern Med 169:1668–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hutchins GD, Schwaiger M, Rosenspire KC, Krivokapich J, Schelbert H, Kuhl DE. 1990. Noninvasive quantification of regional blood flow in the human heart using N-13 ammonia and dynamic positron emission tomographic imaging. J Am Coll Cardiol 15:1032–1042 [DOI] [PubMed] [Google Scholar]
- 25. Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, Burkhard N, Wyss CA, Kaufmann PA. 2009. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol 54:150–156 [DOI] [PubMed] [Google Scholar]
- 26. Camici PG, Crea F. 2007. Coronary microvascular dysfunction. N Engl J Med 356:830–840 [DOI] [PubMed] [Google Scholar]
- 27. Hachamovitch R, Berman DS, Kiat H, Cohen I, Cabico JA, Friedman J, Diamond GA. 1996. Exercise myocardial perfusion SPECT in patients without known coronary artery disease: incremental prognostic value and use in risk stratification. Circulation 93:905–914 [DOI] [PubMed] [Google Scholar]
- 28. Carlin JB, Gurrin LC, Sterne JA, Morley R, Dwyer T. 2005. Regression models for twin studies: a critical review. Int J Epidemiol 34:1089–1099 [DOI] [PubMed] [Google Scholar]
- 29. Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. 2008. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr 88:1519–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Camici PG, Rimoldi OE. 2009. The clinical value of myocardial blood flow measurement. J Nucl Med 50:1076–1087 [DOI] [PubMed] [Google Scholar]
- 31. Camici PG, Dutka DP. 2001. Repetitive stunning, hibernation, and heart failure: contribution of PET to establishing a link. Am J Physiol Heart Circ Physiol 280:H929–H936 [DOI] [PubMed] [Google Scholar]
- 32. Maron MS, Olivotto I, Maron BJ, Prasad SK, Cecchi F, Udelson JE, Camici PG. 2009. The case for myocardial ischemia in hypertrophic cardiomyopathy. J Am Coll Cardiol 54:866–875 [DOI] [PubMed] [Google Scholar]
- 33. Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. 2010. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging 3:623–640 [DOI] [PubMed] [Google Scholar]
- 34. Rezai MR, Wallace AM, Sattar N, Finn JD, Wu FC, Cruickshank JK. 2011. Ethnic differences in aortic pulse wave velocity occur in the descending aorta and may be related to vitamin D. Hypertension 58:247–253 [DOI] [PubMed] [Google Scholar]
- 35. Ertek S, Akgül E, Cicero AF, Kütük U, Demirtaş S, Cehreli S, Erdoğan G. 2012. 25-Hydroxy vitamin D levels and endothelial vasodilator function in normotensive women. Arch Intern Sci 8:47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. 2008. Vitamin D improves endothelial function in patients with type 2 diabetes mellitus and low vitamin D levels. Diabet Med 25:320–325 [DOI] [PubMed] [Google Scholar]
- 37. Tarcin O, Yavuz DG, Ozben B, Telli A, Ogunc AV, Yuksel M, Toprak A, Yazici D, Sancak S, Deyneli O, Akalin S. 2009. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab 94:4023–4030 [DOI] [PubMed] [Google Scholar]
- 38. Witham MD, Dove FJ, Sugden JA, Doney AS, Struthers AD. 2012. The effect of vitamin D replacement on markers of vascular health in stroke patients: a randomised controlled trial. Nutr Metab Cardiovasc Dis 22:864–870 [DOI] [PubMed] [Google Scholar]
- 39. Harris RA, Pedersen-White J, Guo DH, Stallmann-Jorgensen IS, Keeton D, Huang Y, Shah Y, Zhu H, Dong Y. 2011. Vitamin D3 supplementation for 16 weeks improves flow-mediated dilation in overweight African-American adults. Am J Hypertens 24:557–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harkness L, Cromer B. 2005. Low levels of 25-hydroxy vitamin D are associated with elevated parathyroid hormone in healthy adolescent females. Osteoporos Int 16:109–113 [DOI] [PubMed] [Google Scholar]
- 41. Holick MF. 2008. The vitamin D deficiency pandemic and consequences for nonskeletal health: mechanisms of action. Mol Aspects Med 29:361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hoogendijk WJ, Lips P, Dik MG, Deeg DJ, Beekman AT, Penninx BW. 2008. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry 65:508–512 [DOI] [PubMed] [Google Scholar]
- 43. Knekt P, Laaksonen M, Mattila C, Härkänen T, Marniemi J, Heliövaara M, Rissanen H, Montonen J, Reunanen A. 2008. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology 19:666–671 [DOI] [PubMed] [Google Scholar]
- 44. Oosterwerff MM, Eekhoff EM, Heymans MW, Lips P, van Schoor NM. 2011. Serum 25-hydroxyvitamin D levels and the metabolic syndrome in older persons: a population-based study. Clin Endocrinol (Oxf) 75:608–613 [DOI] [PubMed] [Google Scholar]
- 45. Mitsuhashi T, Morris RC, Jr, Ives HE. 1991. 1,25-Dihydroxyvitamin D3 modulates growth of vascular smooth muscle cells. J Clin Invest 87:1889–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kreutz M, Andreesen R, Krause SW, Szabo A, Ritz E, Reichel H. 1993. 1,25-Dihydroxyvitamin D3 production and vitamin D3 receptor expression are developmentally regulated during differentiation of human monocytes into macrophages. Blood 82:1300–1307 [PubMed] [Google Scholar]
- 47. Schelbert HR. 2010. Anatomy and physiology of coronary blood flow. J Nucl Cardiol 17:545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 2002. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 110:229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vaidya A, Forman JP, Hopkins PN, Seely EW, Williams JS. 2011. 25-Hydroxyvitamin D is associated with plasma renin activity and the pressor response to dietary sodium intake in Caucasians. J Renin Angiotensin Aldosterone Syst 12:311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ullah MI, Uwaifo GI, Nicholas WC, Koch CA. 2010. Does vitamin d deficiency cause hypertension? Current evidence from clinical studies and potential mechanisms. Int J Endocrinol 2010:579640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen S, Law CS, Grigsby CL, Olsen K, Hong TT, Zhang Y, Yeghiazarians Y, Gardner DG. 2011. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation 124:1838–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wong MS, Delansorne R, Man RY, Vanhoutte PM. 2008. Vitamin D derivatives acutely reduce endothelium-dependent contractions in the aorta of the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol 295:H289–H296 [DOI] [PubMed] [Google Scholar]
- 53. Ngo DT, Sverdlov AL, McNeil JJ, Horowitz JD. 2010. Does vitamin D modulate asymmetric dimethylarginine and C-reactive protein concentrations? Am J Med 123:335–341 [DOI] [PubMed] [Google Scholar]
- 54. Zittermann A, Koerfer R. 2008. Protective and toxic effects of vitamin D on vascular calcification: clinical implications. Mol Aspects Med 29:423–432 [DOI] [PubMed] [Google Scholar]