Abstract
Context:
The increasing use of tyrosine kinase inhibitor therapy outside of the context of the clinical trial for treatment of advanced thyroid cancer has highlighted the need for a systematic approach to the clinical application of these agents in order to improve patient safety and monitoring promote consistency among providers, and ensure compliance with both institutional and industry standards.
Evidence:
We reviewed professional thyroid cancer guidelines, the National Comprehensive Cancer Network task force reports, American Society of Clinical Oncology safety standards, review articles, and clinical trials published within the past 10 yr and also included relevant older studies.
Conclusions:
Review of available published data and the collective experience prescribing tyrosine kinase inhibitors at The University of Texas MD Anderson Cancer Center have highlighted the need for a systematic, comprehensive, and uniform approach to managing these patients. This paper discusses the approach adopted by the Department of Endocrine Neoplasia at the MD Anderson Cancer Center and illustrates practice patterns, experience, and our standardized approach related to prescribing commercially available tyrosine kinase inhibitors outside of the context of a clinical trial for patients with advanced thyroid cancer.
With the advent of orally available and molecularly targeted antineoplastic agents, the National Comprehensive Cancer Network (NCCN) raised concern regarding new, unforeseen safety challenges with oral drugs compared with parenteral agents. These included reduced checks and balances to avoid medication errors and issues related to shifts in responsibility for managing complicated oral regimens from the practitioner to the patient, such as the potential for nonadherence, misconceptions regarding safety and side effects, profiles of oral administration, and recognition and reporting of potentially serious symptoms to physicians (1). In 2009, the American Society of Clinical Oncology (ASCO) and the Oncology Nursing Society (ONS) collaborated to develop a series of consensus-derived safety standards to provide a basis for safe administration of outpatient chemotherapy to adult cancer patients (2). These guidelines, subsequently updated in 2012 (3), represent early attempts to establish standards for education and monitoring of patients receiving orally active biological agents.
The recommended use of tyrosine kinase inhibitor (TKI) therapy outside of clinical trials in patients with progressive thyroid cancer (http://www.nccn.org/professionals/physician_gis/pdf/thyroid.pdf) (5) as well as the recognition by the National Comprehensive Cancer Network (NCCN)/ASCO/ONS of the necessity for standardized approaches to patients receiving these biological agents have highlighted the need for guidance to prescribing physicians to improve patient safety and monitoring and to promote consistency and compliance with both institutional and industry standards. Here, we describe the approach adopted at The University of Texas MD Anderson Cancer Center (MDACC) for management of TKI therapy in patients with thyroid cancer.
Thyroid Cancer
An estimated 56,000 new cases of thyroid cancer will be diagnosed in the United States in 2012, and the number continues to rise (6).
Primary thyroid cancer comprises four histopathological subtypes: papillary (accounting for >85% of cases), follicular (5–10%), medullary (MTC; 5%), and anaplastic (1%) (7). Differentiated thyroid cancer (DTC), arising from the follicular cells of the thyroid, includes papillary and follicular thyroid cancers and is by far the most common form of thyroid cancer. In most cases, treatment of DTC includes surgical resection, radioactive iodine (RAI), and TSH-suppressive therapy (8). In the 10–20% of cases who develop distant metastatic disease, RAI can be curative, but conventional therapy is ineffective in at least half of the patients (9). In patients with metastatic DTC that continues to progress despite conventional therapy, the long-term overall survival rate drops to 10% (10). Until the recent introduction of TKIs, there were few effective treatment options for progressive, unresectable DTC refractory to RAI (11). Although currently there are no U.S. Food and Drug Administration (FDA)-approved TKIs for DTC, based on clinical trial data, several drugs are being used in clinical practice for the treatment of advanced disease, and pivotal phase III studies are under way.
MTC, which arises from neuroendocrine parafollicular “C” cells, accounts for a small percentage of thyroid cancers. Like DTC, primary and regionally metastatic MTC is treated surgically, but MTC is not responsive to either RAI or TSH suppression. Unresectable, locally advanced or metastatic MTC is not curable, and chemotherapy and radiation therapy have been largely ineffective. Targeted therapies such as TKIs offer a treatment option in these patients. Vandetanib is currently the only FDA-approved TKI for MTC at this time. However, similar to DTC, several commercially available drugs are being used in clinical practice in patients who are intolerant to vandetanib or who have progressed on this agent, and additional medications are being studied in clinical trials.
Small-molecule TKIs have been prescribed for thyroid cancer at MDACC since 2006—both in clinical trials and in routine care as clinically indicated. The decision to prescribe TKIs outside the context of a clinical trial, as recommended by NCCN and American Thyroid Association (ATA) guidelines (4, 5), has been based on a lack of suitable clinical trials, patients' inability to travel to a trial site or to qualify for existing trials, insurance coverage issues, or the patients' unwillingness to participate in a clinical trial. The off-label prescription of TKIs has been undertaken in patients who have failed standard therapies and who have significantly progressive, life-threatening, or symptomatic disease. Only physicians well-versed in the management of advanced thyroid cancers should make the decision to start systemic therapy with TKIs because the risks of therapy can often outweigh potential benefits in many patients.
Small-Molecule TKIs
Discoveries of key oncogenic mutations involved in tumorigenesis and the role of angiogenesis in DTC and MTC have led to clinical trials of multikinase inhibitors over the past decade. Furthermore, success in phase II and phase III trials (12–18) has led to more widespread use of commercially available TKIs. In fact, the most recent NCCN and ATA (5) practice guidelines for thyroid carcinoma recommend that patients with “clinically progressive or symptomatic disease pursue clinical trials for non-radioiodine responsive tumors and consideration of small-molecule kinase inhibitors or systemic therapy if trial not available” (4). During the last several years, there has been a considerable increase in prescriptions of TKIs outside of clinical trials (in noninvestigational settings) a trend that will likely continue. Moreover, FDA approval of vandetanib in April 2011 marks the first small-molecule TKI approved with the primary indication of treatment of thyroid cancer.
In most tumors, tyrosine kinases function as signaling intermediates, stimulating tumor proliferation, angiogenesis, invasion, metastasis, and cell autoregulation. Tyrosine kinases affect regulation of both cancer cells and noncancer cells. Small-molecule TKIs have been a focus of interest in the treatment of thyroid cancer since the discovery of the oncogenic roles of mutations in serine kinase BRAF and tyrosine kinases RET and RAS and of their ability to inhibit growth factor receptors such as vascular endothelial growth factor (VEGF) receptor (19, 20). Despite inhibition of oncogenic kinases, the primary functional role of TKIs may be their inhibition of angiogenesis.
Several multitargeted kinase inhibitors have entered clinical trials for patients with advanced or progressing metastatic thyroid cancers (21). Because of the similarity between RET and VEGF receptor kinases, most TKIs affect both. Many of the commercially available TKIs have similar targets and mechanisms of action as well as similar therapeutic and toxicity profiles. Although vandetanib is the only TKI with a primary indication of thyroid cancer, the other TKIs have shown efficacy in thyroid cancer clinical trials; however, it is important to note that use of these agents for unapproved indications may not be available in certain countries outside the United States.
Here, we briefly review available TKIs most frequently used for the treatment of thyroid cancer. This paper will not discuss off-label use of combination TKI therapy because the efficacy and potential for adverse events have not been well established in thyroid cancer patients and are beyond the scope of this paper.
Sorafenib
Perhaps the most thoroughly evaluated oral TKI thus far, sorafenib (14, 17, 22), targets VEGF receptors 2 and 3, common RET/PTC, and is a weak inhibitor of the serine kinase BRAF (23). Sorafenib is currently approved for treatment of unresectable hepatocellular carcinoma and advanced renal cell carcinoma (24). Among 27 patients enrolled in a phase II study in progressive, metastatic, RAI-refractory DTC, 26% experienced a partial response, and 56% had stabilization of disease (17). Therapeutic efficacy in RAI-refractory DTC was similar in two subsequently published phase II trials (14, 22). We observed similar response rates in our experience with off-label use of sorafenib. Cabanillas et al. (25) reported that lung lesions appeared to be more responsive to intensive treatment than lesions located in lymph nodes or bone.
Because of sorafenib's anti-RET and VEGF receptor activity, MTC is a potential target of this TKI (26). In a small pilot study involving five patients with metastatic MTC who were treated with sorafenib, two patients had a response (including one complete response) after only 6 months of treatment, and all experienced symptomatic improvement (27). In a phase II trial in 16 patients with sporadic metastatic MTC, one patient had a partial response, but 50% had stable disease beyond 15 months, and 85% had a decrease in either calcitonin or carcinoembryonic antigen level (28).
Sunitinib
Sunitinib is an orally active, multitargeted inhibitor of VEGF receptor, RET/PTC subtypes 1 and 3, platelet-derived growth factor receptors, and other receptor tyrosine kinases with direct antitumor and antiangiogenic activity (29). It is currently approved for the treatment of advanced renal cell carcinoma, gastrointestinal stromal tumor, and progressive and well-differentiated pancreatic neuroendocrine tumor (30). An open-label phase II study showed a 13% partial response rate and 68% disease stabilization rate for patients with DTC (31). A second phase II study reported partial remission or disease stabilization in two of 12 patients thus far (31). Results from the largest open-label phase II trial to date, which included 28 patients with progressive DTC and seven patients with MTC, reported complete response in one patient, partial response in 28% of patients, and disease stabilization in 46% of patients (13). Further analysis suggested that reduction in fluorodeoxyglucose uptake in positron emission tomography was a predictor of partial response or stabilization of disease.
Pazopanib
Pazopanib is an orally active angiogenesis inhibitor targeting VEGF receptors, platelet-derived growth factor, and c-Kit. Currently approved for the treatment of advanced renal cell carcinoma (32), pazopanib is under clinical development for treatment of multiple tumor types. In a phase II trial for which results were published in 2010, 37 patients with DTC resistant to therapeutic RAI with radiographically confirmed disease progression, according to the Response Evaluation Criteria in Solid Tumors (RECIST), were treated with pazopanib. Pazopanib induced partial responses in a substantial proportion of patients, with an estimated 66% likelihood of response lasting longer than 1 yr (10).
Vandetanib
Vandetanib is one of the first TKIs to be studied in thyroid cancer cell lines, and is the only TKI to date with the primary indication of symptomatic or progressive, unresectable, locally advanced or metastatic MTC (33). In an open-label phase II study for efficacy in patients with metastatic familial forms of MTC (34), 30 patients were enrolled. Confirmed partial response was reported in 20% of these patients, and an additional 53% had stable disease for 24 months. Serum calcitonin and serum carcinoembryonic antigen levels decreased 50% from baseline for at least 4 wk in 80 and 53%, respectively. A subsequent multicenter, randomized phase III trial over 24 months showed that patients randomized to receive vandetanib showed markedly longer progression-free survival than patients who received placebo, with an estimated 11-month prolongation of median progression-free survival. The progression-free survival benefit was demonstrated in both the hereditary and sporadic forms of MTC (15). Additionally, improved progression-free survival has been reported in a placebo-controlled, randomized phase II study in progressive DTC patients in which 145 patients were randomized to placebo or vandetanib. Improvement in progression-free survival was noted to be 11.1 months in the vandetanib group vs. 5.9 months in the placebo group (36).
Initiation of TKIs for Thyroid Cancer
Prior to initiation of TKI therapy, it is important to identify which patients should be placed on therapy and, as importantly, which group of patients should not. Additionally, as these medications can cause life-threatening adverse effects, only physicians who have support, expertise, and experience and are well versed in the prescribing/use of these agents should initiate management. In general, patients who qualify for treatment are patients with symptomatic or progressive disease and good overall performance status (as discussed below), those patients who are not amenable to surgery or local treatment, and those who are unable to participate in clinical trials. We typically do not consider initiation of therapy in patients who are considered to be at high risk for adverse events associated with this class of medications (e.g. extremely uncontrolled hypertension or disease in areas of high risk for development of fistula or bleeding) as well as patients with poor performance status or a history of nonadherence to prescribed medications.
Before initiation, a comprehensive review is necessary to ascertain each patient's suitability for this therapy. Initial evaluation should include assessment of the patient's performance status using one of the widely reliable and valid performance scales, [the Eastern Cooperative Oncology Group Performance Status (ECOG) (37) or Karnofsky Performance Status (KPS) (38)], in addition to a detailed physical examination. Performance status gives the treating healthcare team an assessment of how the patient functions and it is a good estimate of whether or not the patient can tolerate chemotherapy. Little is known about the tolerability of TKIs in patients with poor performance status (e.g. ECOG > 2) because most clinical trials of TKIs have excluded patients with poor performance status for concerns about toxic effects.
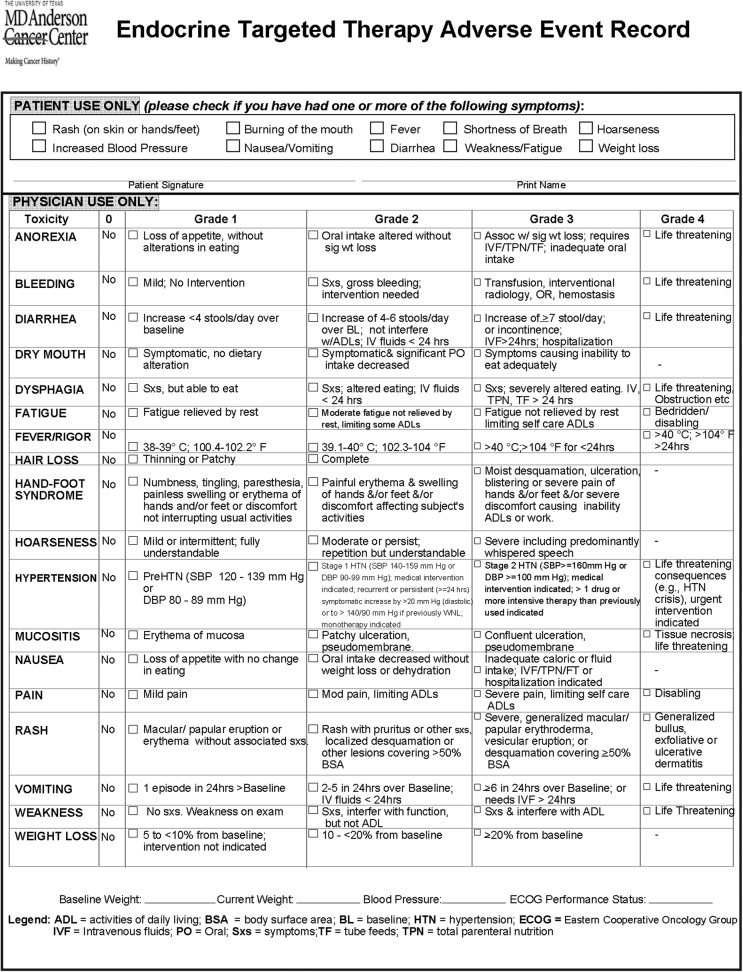
Review of medical history and laboratory data should focus on evaluation of cardiovascular, hepatic, and renal status. Because many patients may have previous exposure to a TKI or preexisting organ dysfunction, and given the wide range of toxic effects associated with this class of medications, it is necessary before prescribing a TKI to establish and document baseline symptomatology and laboratory values for each patient. We created an adverse events monitoring tool that includes the most common adverse events associated with TKIs and their grades in checklist form (Fig. 1) adapted from the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 (39). We use this tool to screen patients prior to initiation of a TKI, with the purpose of clearly documenting each patient's baseline characteristics, and again at subsequent clinic visits to monitor progression of symptoms related to therapy.
Fig. 1.
Endocrine targeted therapy adverse event record.
Specific considerations for each TKI are discussed in the sections on patient monitoring and dose modifications, but because of the shared mechanisms of action, certain general considerations can be applied to the class as a whole. Here, we briefly review the more common systemic effects shared by all commercially available TKIs used commonly for the treatment of thyroid cancer.
Cardiovascular effects
Hypertension
Hypertension is the most common cardiovascular side effect associated with antiangiogenic drugs. In general, the severity of hypertension is dose related. Although the mechanism for hypertension is not well understood, a recent study reported that elevation in blood pressure above 140/90 mm Hg was associated with significant survival benefit, even if the patient was treated with an antihypertensive agent, and questioned whether such elevation may be a pharmacodynamic biomarker of successful inhibition of the VEGF pathway (40).
Onset of hypertension can vary in both time from therapy initiation and degree of severity (ranging from moderate to hypertensive emergency), and currently there are no clear guidelines for managing TKI-induced hypertension; thus, the choice of antihypertensive agent(s) should be individualized. The Angiogenesis Task Force of the National Cancer Institute Investigational Drug Steering Committee recently published guidelines for management of hypertension in patients on a TKI, recommending that blood pressure be controlled for a goal of less than 140/90 mm Hg before initiation of the TKI (37). The blood pressure should be checked again within 1 wk after starting therapy. If the blood pressure is above goal, antihypertensive therapy should be initiated or adjusted (41). Patients should continue to check their blood pressure daily and report results regularly. Once blood pressure is at goal, monitoring on a monthly basis is recommended. Notably, interruption or dose reduction of the TKI may be indicated to achieve adequate blood pressure control.
Electrocardiographic QT interval prolongation
QT interval prolongation can occur with any of the TKIs; therefore, it should be used with caution in patients with a history of QT prolongation and in patients taking an antiarrhythmic drug. Torsades de pointes was seen, although rarely, in patients treated with sunitinib (<0.1% of patients) or pazopanib (<2% of patients). In all patients started on a TKI, an initial electrocardiogram (ECG) should be performed; electrolytes should be monitored and abnormalities corrected. Serial ECGs may be considered as dictated by the clinical scenario.
Vandetanib specifically carries a “black box” warning on QT interval prolongation, torsades de pointes, and sudden death, all of which were observed in clinical trials. Vandetanib is contraindicated in patients with a history of torsades de pointes, congenital long QT syndrome, bradyarrhythmia, or uncompensated heart failure and should not be initiated in patients whose corrected QT interval (Fridericia formula) is greater than 450 msec. Specific guidelines for monitoring of QT abnormalities, electrolytes, and thyroid function in patients taking vandetanib are detailed in the package insert (33) and are as follows: ECG at baseline and at 2–4 wk and 8–12 wk after initiation, then every 3 months afterward. QT interval also should be assessed after any dose reduction for QT prolongation or any dose interruption greater than 2 wk. Simultaneous use of drugs known to prolong the QT interval, such as amiodarone and erythromycin, should be avoided.
Congestive heart failure
Congestive heart failure (both systolic and diastolic) has been associated with TKI use, although infrequently. Congestive heart failure is associated more often with sunitinib than with other TKIs used in treatment of thyroid cancer, but it has been reported with both sorafenib and pazopanib. The clinical severity of TKI-induced heart failure varies (30). The heart failure is thought to be a result of cardiomyocyte toxicity that may be exacerbated by hypertension and perhaps preventable if treated. The TKI-induced heart failure may progress and become irreversible if standard heart failure therapy and management are not applied (30). Aggressive management of hypertension may play a role in reduction of cardiomyocyte damage. All patients beginning sunitinib therapy need to have a baseline echocardiogram and periodic monitoring while they are on therapy. Serial echocardiograms should also be considered in patients starting other antiangiogenic therapies and who have a cardiac history or have received adriamycin in the past.
In patients without cardiac risk factors, a baseline evaluation of ejection fraction should be considered. In patients with known heart failure, baseline and periodic monitoring of ejection fraction should be considered. Patients should be monitored for signs and symptoms of heart failure at each visit; should clinical manifestations of congestive heart failure appear, discontinuation of the TKI is recommended.
Renal effects
All TKIs have the potential to cause proteinuria, and therefore baseline renal function and periodic urinalysis during treatment are recommended. Patients with impaired renal function have increased exposure to TKIs, and this may necessitate dose reduction. Of all the TKIs, only sunitinib has been studied in patients with end-stage renal disease requiring peritoneal dialysis or hemodialysis (24, 30, 32, 33). No adjustment of dose was indicated, but exposure to sunitinib was approximately 50% less in dialysis patients than in nondialyzed patients. In clinical trials of pazopanib for renal cell carcinoma, proteinuria was reported in 8% of patients. Baseline renal function studies and urinalysis are recommended in addition to periodic monitoring of renal function during treatment. If the patient develops grade 4 proteinuria, discontinuation of therapy is recommended (32).
Hepatic effects
The safety and pharmacokinetics of TKIs have not been established in patients with moderate or severe hepatic impairment (24, 30, 32, 33). In clinical trials of pazopanib and sunitinib, hepatotoxicity manifested as increases in serum transaminases [alanine aminotransferase (ALT), aspartate aminotransferase (AST)] and bilirubin; this hepatotoxicity was in some cases severe or fatal. Liver failure has been observed in both clinical trials and postmarketing cases (30). Transaminase elevations generally occur early in the course of treatment (within the first 18 wk). In clinical trials of pazopanib, transaminase levels above the upper limit of normal occurred in 14% of patients. Baseline testing of serum liver functions before initiation of therapy and monitoring of these levels at least every 4 wk for the first 4 months of treatment and periodically thereafter are recommended (32). Sorafenib has been studied in patients with advanced hepatocellular carcinoma, and Child-Pugh score does not seem to influence the starting dose (42, 43). In our clinical practice, after investigation of the etiology of liver dysfunction and potential reversible causes, we generally exclude patients from therapy with elevations in transaminases greater than 2.5 times the upper limit of reference range without evidence of liver metastases, and those with known metastases to the liver are excluded if transaminases are elevated more than five times the upper limit of normal reference range. If a patient develops a grade 3 or 4 drug-related hepatic adverse event, therapy should be interrupted or discontinued.
Hematological effects
Hematological events including bone marrow suppression, thrombosis, and tumor-related hemorrhage have been reported with the use of all TKIs, and some cases were fatal. TKIs should not be initiated until surgical wounds or other lesions are fully healed, and treatment should be withheld 7 d prior to scheduled surgery. Extra caution should be taken in patients who have received external beam radiotherapy, as fistula formation can be a potentially devastating adverse event. TKIs have not been studied in patients who have a clinically significant history of cerebral or gastrointestinal hemorrhage in the past 6 months and should not be used in those patients. In patients with a history of hemoptysis (due to tracheal, bronchial, or esophageal tumor infiltration) who have received local treatment (such as external beam radiotherapy and radiofrequency ablation) as well as in patients on anticoagulants or with a history of a bleeding disorder, the risk vs. benefit of starting a TKI should be carefully weighed, and TKIs should be used with caution and regular monitoring. We recommend serial complete blood cell counts at baseline and at each clinic visit.
Dermatological effects
Hand-foot skin reactions and rash represent the most common adverse events associated with TKIs, and sorafenib in particular. Dermatological toxicities usually present as CTCAE grade 1 or 2 and generally appear within the first 6 wk of treatment. Management may include topical therapies, temporary treatment interruption, or dose modification and, in severe cases, permanent discontinuation (24). Skin evaluation for development of keratoacanthoma-type squamous cell carcinomas should be performed regularly while being treated with sorafenib. These lesions can develop as solitary or multiple lesions within weeks to months of starting therapy and are not confined to sun-exposed areas (44). These lesions should be completely excised and routine skin evaluations should be performed. Drug discontinuation is not necessarily recommended because these lesions have not been reported to metastasize.
Other considerations
Careful review of concomitant medications is necessary as TKIs have clinically significant CYP3A4-inducing and -inhibiting activities. Cytochrome P450 enzymes play a primary role in the metabolism of many drugs, and sunitinib, sorafenib, pazopanib, and vandetanib are all metabolized by cytochrome P450 3A4 (CYP3A4) (45). Simultaneous use of a CYP3A4 inducer may decrease the plasma concentration of the TKI, resulting in decreased efficacy, whereas CYP3A4 inhibitors may increase the plasma concentration, resulting in toxicity, and CYP3A4 substrates can work in both ways. Supplemental Table 1 (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org), adapted from Cabanillas et al. (46), lists the more widely used, clinically significant drugs metabolized via the CYP3A4 enzyme system.
All TKIs have the capacity to induce hypothyroidism, which is thought to be associated with poor absorption of levothyroxine due to either decreased absorption from secondary causes such as diarrhea or increased clearance (4). Patients frequently require dose increases in thyroid hormone, and thyroid function should be routinely monitored while patients are on therapy.
For ease of reference, we have constructed a system for initiation and monitoring of TKIs that incorporates manufacturer recommendations for monitoring studies as well as recommendations unique to selected therapies (Tables 1, 2). Further specific guidelines for monitoring patients taking sunitinib are specified in the package insert (30). Further specific guidelines for monitoring liver function abnormalities in patients taking pazopanib are specified in the package insert (32).
Table 1.
Suggested initiation/monitoring studies for patients receiving a TKI for treatment of thyroid cancer
| Baseline studies/imaging (monthly for the first 3 months, then every 2–3 months) | ||
|---|---|---|
| CBC | Calcium | PT/PTT |
| Electrolytes | Magnesium | INR |
| BUN | Phosphorous | TSH/Free T4 |
| Creatinine | Albumin | Urinalysis |
| Glucose | AST (SGPT)/ALT (SGOT) | B HCG (for women of childbearing potential) |
| T Bilirubin | LDH | Electrocardiogram |
Table 2.
TKI-specific monitoring
| TKI-specific monitoring | Study | Frequency |
|---|---|---|
| Pazopanib | Urinalysis | Baseline and periodically |
| AST (SGPT), ALT (SGOT), bilirubin | Baseline and monthly for 4 months | |
| Sunitinib | Echocardiogram | Baseline and as clinically indicated |
| Vandetanib | Electrocardiograma | Baseline, at 2–4 weeks and 8–12 weeks, then every 3 months |
If any dose reduction or interruption >2 weeks repeat.
Prescribing TKIs
Due to the duration of treatment, the potential for toxicities, and the need for regular monitoring, the importance of a detailed discussion and obtaining written informed consent from the patient before initiation of TKI therapy cannot be stressed enough. We have developed a set of guidelines based on safety standards derived from the consensus of ASCO and ONS (2), to be used when prescribing targeted therapy in thyroid cancer patients at MDACC (Table 3). As in the original ASCO/ONS guidelines, recommendations regarding chemotherapy planning, general chemotherapy practice standards, order standards and criteria, patient consent and education, monitoring, and assessment have been tailored for oral chemotherapy prescribing. The goal of these guidelines is improved patient safety and uniform compliance with national safety standards. The package insert for each TKI should be read and reviewed by the provider before prescribing. We suggest that other institutions review these consensus-derived safety standards as part of the TKI prescribing process, incorporating specific institutional chemotherapy prescribing mandates and individual institutional policies as warranted.
Table 3.
Standards for oral TKI use in thyroid cancer patients
General practice standards
|
Patient information/education
|
Documentation
|
Monitoring
|
Financing oral chemotherapy
The cost of TKI therapy is potentially thousands of dollars per month. For many patients, the cost of this treatment is prohibitive without assistance. Clinicians should be aware of services available through pharmaceutical-specific assistance programs that can help patients with reimbursement, alternate funding, and appeals. These programs include, but are not limited to: the vandetanib Biologics program (http://www.caprelsarems.com/), the sorafenib REACH program (http://nexavar-us.com/scripts/pages/en/hcp/hcc/patient-support/reach-program/), the sunitinib First RESOURCE program (http://pfizerhelpfulanswers.com/files/SUU00530_First_Resource_Enrollment.pdf), and the pazopanib RxAssist program (http://www.rxassist.org/pap-info/company_detail.cfm?CmpId125).
Monitoring of Patients on TKI Therapy
Although each TKI has a unique side effects profile, TKIs used commonly for treatment of thyroid cancer share many common clinical toxicities. Table 4, adapted from Cabanillas et al. (46), illustrates the most frequent of these. In our clinical practice, once medication has been initiated, for the first 3 months we follow the patient monthly in clinic and recommend weekly home blood pressure monitoring. Subsequent clinic visits (approximately every 2–3 months while on therapy) are used to assess blood pressure and correlate with home blood pressure monitoring, document overall performance status (either ECOG or KPS), screen closely for development of adverse events, review and order appropriate monitoring studies, as well as restage the patient.
Table 4.
Common adverse events associated with TKI therapies used in thyroid cancer
| Adverse event | Sorafenib (%) |
Sunitinib (%) |
Pazopanib (%) |
Vandetanib (%) |
||||
|---|---|---|---|---|---|---|---|---|
| All grades | ≥Grade 3 | All grades | ≥Grade 3 | All grades | ≥Grade 3 | All grades | ≥Grade 3 | |
| Anorexia | 16 | <1 | 34 | 2 | 22 | 2 | 21 | 4 |
| ALT elevation | NR | NR | 51 | 2.5 | 53 | 12 | 51 | 2 |
| AST elevation | NR | NR | 56 | 2 | 53 | 7.5 | NR | NR |
| Arterial thromboembolism | 2.9 | NR | NR | NR | 3 | 2 | NR | NR |
| CHF or LVEF decline | 1.7 | NR | 13 | 3 | <1% | NR | <1 | NR |
| Diarrhea | 43 | 2 | 61 | 9 | 52 | 3.5 | 57 | 11 |
| Fatigue | 37 | 5 | 54 | 11 | 19 | 2 | 24 | 6 |
| Hand-foot skin reaction | 30 | 6 | 29 | 6 | 6 | NR | NR | NR |
| Hemorrhage/bleeding (all sites) | 15 | 3 | 30 | 3 | 13 | 2 | NR | NR |
| Hypothyroidism | NR | NR | 14 | 2 | 7 | NR | NR | NR |
| Hypertension | 17 | 4 | 30 | 12 | 40 | 4 | 33 | 9 |
| Proteinuria | NR | NR | NR | NR | 9 | <1 | 10 | 0 |
| QT prolongation | NR | NR | NR | NR | NR | <2 | 36 | 4.3 |
| Stomatitis | NR | NR | 30 | 1 | 4 | NR | NR | NR |
| Weight loss | 10 | <1 | 12 | <1 | 52 | 3.5 | 10 | 1 |
[Adapted from M. E. Cabanillas et al.: Challenges associated with tyrosine kinase inhibitor therapy for metastatic thyroid cancer. J Thyroid Res 2011:985780, 2011 (46), with permission. © Hindawi Publishing Corporation.] CHF, Congestive heart failure; LVEF, left ventricular ejection fraction; NR, not reported.
To better enable a uniform and comprehensive approach to monitoring our patients receiving a TKI, we have systematically incorporated into our clinical practice general and medication-specific recommendations for monitoring (Tables 1, 2). The adverse events monitoring tool that we use to screen patients during the initial prescribing phase also is used at each subsequent visit to assess the adverse events experienced by that patient (Fig. 1). This CTCAE (39) tool allows accurate grading of each event, uses universal definitions, and allows more consistent documentation amongst providers. Utilization of this tool increases accuracy in monitoring patient symptomatology and in adherence to criteria for dose modifications or holds. We believe that the more objective grading parameters and standardized approach to diagnostic studies enabled by this system optimize patient safety.
Dose Modification/Discontinuation
Product labeling of most commercially available TKIs contains few dose reduction recommendations; adverse events are instead managed by treatment interruption or discontinuation. The decision to hold or modify the dosage of a TKI is often dictated by a combination of patient quality of life/tolerability of the medication and physician judgment.
Nonhematological adverse events
Patients with a grade 1 or 2 nonhematological adverse event may continue TKI therapy as long as treatment for the adverse event is maximized (e.g. management of grade 2 hypertension with an antihypertensive agent). When treatment for the adverse event is only minimally effective (e.g. hand-foot syndrome) and the adverse event is not tolerated well by the patient, decrease in treatment dose or interruption in therapy may be indicated. In general, recurrent, intolerable grade 2 adverse events attributed to the TKI require drug hold and potentially dose modification if the adverse event does not respond to supportive therapy. Most grade 3 toxic effects, unless easily managed (e.g. diarrhea that can be controlled), require a drug hold until the effect improves significantly. Resuming the TKI at a lower dose is often helpful; for some adverse events, the drug occasionally may be resumed at the same dose. Recurrence of a grade 3 toxic effect should be managed with the same strategy, but a third occurrence often requires discontinuation or dose reduction. All related grade 4 adverse events are by nature life threatening and require immediate drug hold or discontinuation of the TKI (46).
Hematological adverse events
Although rare in thyroid cancer patients, grade 3 and 4 hematological toxic effects should prompt a workup for alternative causes (e.g. myelodysplasia). Patients with DTC who have received a high dose of RAI may be at higher risk for bone marrow suppression, and consideration should be given to early consultation with a hematologist to assist in management. As is common to most solid tumor TKI protocols, grade 2 hematological toxic effects do not require immediate dose reduction. Grade 3–4 neutropenia and thrombocytopenia and grade 4 anemia require dose reduction after the first two occurrences.
Intolerance
Cabanillas et al. (46) proposed that TKI intolerance in thyroid cancer be defined as for chronic myeloid leukemia (35), with some modifications, as follows: 1) any grade 3–4 nonhematological toxic effect related to TKI therapy that has recurred despite dose reduction; 2) any grade 2 nonhematological toxic effect related to TKI therapy that is intolerable or persists for more than 1 month despite treatment; 3) any grade 3–4 hematological toxic effect related to TKI therapy that is unresponsive to supportive measures or would require dose reduction below the accepted minimal effective dose to increase tolerability; or 4) any grade 4 nonhematological toxic effect related to TKI therapy.
Although other less common toxicities are not discussed, they have been well described (Table 4) and may warrant dose reduction or discontinuation.
Summary
During the past decade, biological discoveries have sparked trials testing novel therapies for advanced thyroid carcinomas. In contrast to the disappointing historical results of systemic cytotoxic chemotherapies in these cancers, TKIs that target angiogenesis as well as other tumor-promoting functions have produced impressive clinical results in both DTC and MTC. The FDA approval of vandetanib with the primary indication of progressive or metastatic MTC marks the first of what likely will be more targeted therapies approved for treatment of thyroid carcinoma. This, in addition to the rising number of TKIs being prescribed in noninvestigational settings, has highlighted the need for a systematic, comprehensive, and uniform approach to managing patients receiving TKI therapy.
We share our experience with using TKIs in noninvestigational settings at MDACC to illustrate the reasoning behind the creation of clinical prescribing parameters and how we use those parameters to better guide our approach to patient care. We believe that by using these tools, we will improve patient safety and monitoring, promote consistency among providers, and ensure compliance with consensus-derived safety standards for prescribing oral chemotherapy.
Acknowledgments
This paper is supported in part by the National Institutes of Health through the MD Andreson Cancer Center Support Grant, CA016672.
Disclosure Summary: A.A.C., M.E.C., C.J., S.G.W., M.A.H., A.Y., and R.V.-S. have nothing to declare. M.H. has research support from AstraZeneca. R.F.G. has research support from AstraZeneca. S.I.S. has research support from Pfizer and consulting relationships with Pfizer, Bayer, and AstraZeneca. N.L.B. has grant/research support from Bayer.
Footnotes
- ALT
- Alanine aminotransferase
- AST
- aspartate aminotransferase
- DTC
- differentiated thyroid cancer
- ECG
- electrocardiogram
- MTC
- medullary thyroid cancer
- RAI
- radioactive iodine
- TKI
- tyrosine kinase inhibitor
- VEGF
- vascular endothelial growth factor.
References
- 1. Weingart SN, Brown E, Bach PB, Eng K, Johnson SA, Kuzel TM, Langbaum TS, Leedy RD, Muller RJ, Newcomer LN, O'Brien S, Reinke D, Rubino M, Saltz L, Walters RS. 2008. NCCN Task Force Report: oral chemotherapy. J Natl Compr Canc Netw 6(Suppl 3):S1–S14 [PubMed] [Google Scholar]
- 2. Jacobson JO, Polovich M, McNiff KK, Lefebvre KB, Cummings C, Galioto M, Bonelli KR, McCorkle MR. 2009. American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards. J Clin Oncol 27:5469–5475 [DOI] [PubMed] [Google Scholar]
- 3. Jacobson JO, Polovich M, Gilmore TR, Schulmeister L, Esper P, Lefebvre KB, Neuss MN. 2012. Revisions to the 2009 American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards: expanding the scope to include inpatient settings. Oncol Nurs Forum 39:31–38 [DOI] [PubMed] [Google Scholar]
- 4. Kamba T, McDonald DM. 2007. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer 96:1788–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cooper DS, Doherty GM, Haugen BR, Hauger BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. 2009. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 6. 2012. Cancer facts and figures 2012. Atlanta: American Cancer Society [Google Scholar]
- 7. DeLellis RA, Lloyd RV, Heitz PU, Eng C. 2004. World Health Organization classification of tumors. Pathology and genetics of tumours of endocrine organs. Lyon, France: IARC Press [Google Scholar]
- 8. Sherman SI. 2003. Thyroid carcinoma. Lancet 361:501–511 [DOI] [PubMed] [Google Scholar]
- 9. Shaha AR, Shah JP, Loree TR. 1996. Patterns of nodal and distant metastasis based on histologic varieties in differentiated carcinoma of the thyroid. Am J Surg 172:692–694 [DOI] [PubMed] [Google Scholar]
- 10. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire F, Schlumberger M. 2006. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 91:2892–2899 [DOI] [PubMed] [Google Scholar]
- 11. Haugen BR. 1999. Management of the patient with progressive radioiodine non-responsive disease. Semin Surg Oncol 16:34–41 [DOI] [PubMed] [Google Scholar]
- 12. Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME, Rubin J, Sideras K, Morris JC, 3rd, McIver B, Burton JK, Webster KP, Bieber C, Traynor AM, Flynn PJ, Goh BC, Tang H, Ivy SP, Erlichman C. 2010. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol 11:962–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carr LL, Mankoff DA, Goulart BH, Eaton KD, Capell PT, Kell EM, Bauman JE, Martins RG. 2010. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res 16:5260–5268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kloos RT, Ringel MD, Knopp MV, Hall NC, King M, Stevens R, Liang J, Wakely PE, Jr, Vasko VV, Saji M, Rittenberry J, Wei L, Arbogast D, Collamore M, Wright JJ, Grever M, Shah MH. 2009. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol 27:1675–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wells SA, Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JR, Read J, Langmuir P, Ryan AJ, Schlumberger MJ. 2012. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 30:134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brose MS, Nutting CM, Sherman SI, Shong YK, Smit JW, Reike G, Chung J, Kalmus J, Kappeler C, Schlumberger M. 2011. Rationale and design of decision: a double-blind, randomized, placebo-controlled phase III trial evaluating the efficacy and safety of sorafenib in patients with locally advanced or metastatic radioactive iodine (RAI)-refractory, differentiated thyroid cancer. BMC Cancer 11:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gupta-Abramson V, Troxel AB, Nellore A, Puttaswamy K, Redlinger M, Ransone K, Mandel SJ, Flaherty KT, Loevner LA, O'Dwyer PJ, Brose MS. 2008. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol 26:4714–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wells SA, Jr, Gosnell JE, Gagel RF, Moley J, Pfister D, Sosa JA, Skinner M, Krebs A, Vasselli J, Schlumberger M. 2010. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol 28:767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang J, Yang PL, Gray NS. 2009. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer 9:28–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krause DS, Van Etten RA. 2005. Tyrosine kinases as targets for cancer therapy. N Engl J Med 353:172–187 [DOI] [PubMed] [Google Scholar]
- 21. Schlumberger M, Sherman SI. 2009. Clinical trials for progressive differentiated thyroid cancer: patient selection, study design, and recent advances. Thyroid 19:1393–1400 [DOI] [PubMed] [Google Scholar]
- 22. Hoftijzer H, Heemstra KA, Morreau H, Stokkel MP, Corssmit EP, Gelderblom H, Weijers K, Pereira AM, Huijberts M, Kapiteijn E, Romijn JA, Smit JW. 2009. Beneficial effects of sorafenib on tumor progression, but not on radioiodine uptake, in patients with differentiated thyroid carcinoma. Eur J Endocrinol 161:923–931 [DOI] [PubMed] [Google Scholar]
- 23. Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. 2004. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64:7099–7109 [DOI] [PubMed] [Google Scholar]
- 24. Package insert 2005. Sorafenib (Nexavar). South San Francisco, CA: Bayer HealthCare and Onyx Pharmaceuticals [Google Scholar]
- 25. Cabanillas ME, Waguespack SG, Bronstein Y, Williams MD, Feng L, Hernandez M, Lopez A, Sherman SI, Busaidy NL. 2010. Treatment with tyrosine kinase inhibitors for patients with differentiated thyroid cancer: the M.D. Anderson experience. J Clin Endocrinol Metab 95:2588–2595 [DOI] [PubMed] [Google Scholar]
- 26. Ball DW. 2007. Medullary thyroid cancer: therapeutic targets and molecular markers. Curr Opin Oncol 19:18–23 [DOI] [PubMed] [Google Scholar]
- 27. Kober F, Hermann M, Handler A, Krotla G. 2007. Effect of sorafenib in symptomatic metastatic medullary thyroid cancer. J Clin Oncol 25:14065 [Google Scholar]
- 28. Lam ET, Ringel MD, Kloos RT, Prior TW, Knopp MV, Liang J, Sammet S, Hall NC, Wakely PE, Jr, Vasko VV, Saji M, Snyder PJ, Wei L, Arbogast D, Collamore M, Wright JJ, Moley JF, Villalona-Calero MA, Shah MH. 2010. Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. J Clin Oncol 28:2323–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, Armand JP, Scigalla P, Raymond E. 2006. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 24:25–35 [DOI] [PubMed] [Google Scholar]
- 30. Package insert 2006. Sunitinib (Sutent). New York: Pfizer Labs Inc [Google Scholar]
- 31. Ravaud A, Fouchardiere C, Courbon F, Asselineau J. 2008. Sunitinib in patients with refractory advanced thyroid cancer: the THYSU phase II trial. J Clin Oncol 26:6058 [Google Scholar]
- 32. Package insert 2009. Pazopanib (Votrient). Philadelphia: GlaxoSmithKline [Google Scholar]
- 33. Package insert 2011. Vandetanib (Caprelsa). Wilmington, DE: AstraZeneca Pharmaceuticals [Google Scholar]
- 34. Wells SA, Jr, Gosnell JE, Gagel RF, Moley J, Pfister D, Sosa JA, Skinner M, Krebs A, Vasselli J, Schlumberger M. 2010. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol 28:767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jabbour E, Deininger M, Hochhaus A. 2011. Management of adverse events associated with tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia. Leukemia 25:201–210 [DOI] [PubMed] [Google Scholar]
- 36. Leboulleux S, Bastholt L, Krause T, de la Fouchardiere C, Tennvall J, Awada A, Gómez JM, Bonichon F, Leenhardt L, Soufflet C, Licour M, Schlumberger MJ. 2012. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol 13:897–905 [DOI] [PubMed] [Google Scholar]
- 37. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. 1982. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655 [PubMed] [Google Scholar]
- 38. Karnofsky D, Burchenal J. 1949. The clinical evaluation of chemotherapeutic agents in cancer. Evaluation of chemotherapeutic agents. New York: Columbia University Press; 199–205 [Google Scholar]
- 39. U.S. Department of Health and Human Services 2010. Common terminology criteria for adverse events (CTCAE). From http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf
- 40. Rini BI, Cohen DP, Lu DR, Chen I, Hariharan S, Gore ME, Figlin RA, Baum MS, Motzer RJ. 2011. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst 103:763–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Escalante CP, Zalpour A. 2011. Vascular endothelial growth factor inhibitor-induced hypertension: basics for primary care providers. Cardiol Res Pract 2011:816897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lencioni R, Kudo M, Ye SL, Bronowicki JP, Chen XP, Dagher L, Furuse J, Geschwind JF, Ladrón de Guevara L, de Guevara LL, Papandreou C, Sanyal AJ, Takayama T, Yoon SK, Nakajima K, Cihon F, Heldner S, Marrero JA. 2012. First interim analysis of the GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib) non-interventional study. Int J Clin Pract 66:675–683 [DOI] [PubMed] [Google Scholar]
- 43. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. 2008. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390 [DOI] [PubMed] [Google Scholar]
- 44. Robert C, Arnault JP, Mateus C. 2011. RAF inhibition and induction of cutaneous squamous cell carcinoma. Curr Opin Oncol 23:177–182 [DOI] [PubMed] [Google Scholar]
- 45. Maitland ML, Bakris GL, Black HR, Chen HX, Durand JB, Elliott WJ, Ivy SP, Leier CV, Lindenfeld J, Liu G, Remick SC, Steingart R, Tang WH. 2010. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst 102:596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cabanillas ME, Hu MI, Durand JB, Busaidy NL. 2011. Challenges associated with tyrosine kinase inhibitor therapy for metastatic thyroid cancer. J Thyroid Res 2011:985780. [DOI] [PMC free article] [PubMed] [Google Scholar]