Abstract
Context:
PTH may be an effective treatment option for hypoparathyroidism, but long-term data are not available.
Objective:
We studied the effect of 4 yr of PTH(1–84) treatment in hypoparathyroidism.
Design:
Twenty-seven subjects were treated with PTH(1–84) for 4 yr, with prospective monitoring of calcium and vitamin D requirements, serum and urinary calcium, serum phosphorus, bone turnover markers, and bone mineral density (BMD).
Results:
Treatment with PTH(1–84) reduced supplemental calcium requirements by 37% (P = 0.006) and 1,25-dihydroxyvitamin D requirements by 45% (P = 0.008). Seven subjects (26%) were able to stop 1,25-dihydroxyvitamin D completely. Serum calcium concentration remained stable, and urinary calcium and phosphorus excretion fell. Lumbar spine BMD increased by 5.5 ± 9% at 4 yr (P < 0.0001). Femoral neck and total hip BMD remained stable. At 4 yr, distal radius BMD was not different from baseline. Bone turnover markers increased significantly, reaching a 3-fold peak from baseline values at 6–12 months (P < 0.05 for all), subsequently declining to steady-state levels at 30 months. Hypercalcemia was uncommon (11 episodes in eight subjects over 4 yr; 1.9% of all values), with most episodes occurring within the first 6 months and resolving with adjustment of supplemental calcium and vitamin D.
Conclusions:
PTH(1–84) treatment of hypoparathyroidism for up to 4 yr maintains the serum calcium concentration, while significantly reducing supplemental calcium and 1,25-dihydroxyvitamin D requirements. Lumbar spine BMD increases without significant changes at other sites. These data provide support for the safety and efficacy of PTH(1–84) therapy in hypoparathyroidism for up to 4 yr.
Hypoparathyroidism is a disorder characterized by hypocalcemia and deficient PTH. It is the only classic endocrine deficiency disease for which the missing hormone, PTH, is not yet an approved therapy. Standard treatment consists of oral calcium and vitamin D supplementation. However, with standard therapy, maintaining normal serum calcium levels often presents a therapeutic challenge. Concerns also exist regarding hypercalciuria and ectopic soft tissue calcification that can be associated with long-term use of calcium and vitamin D (1–4).
Prior investigations suggest that PTH treatment is an effective therapeutic option in hypoparathyroidism (5–8). We previously reported that treatment with PTH(1–84) for 2 yr in hypoparathyroid subjects led to reduced supplemental calcium and 1,25-dihydroxyvitamin D requirements (8), and similar observations were also reported in a 6-month trial of PTH(1–84) in hypoparathyroid subjects (7). However, given the chronic nature of hypoparathyroidism, long-term data on the safety and efficacy of PTH use in this population are essential. In contrast to osteoporosis, the potential duration of PTH therapy in hypoparathyroidism may be greater than 2 yr. In this report, we describe the effects of PTH(1–84) treatment on biochemical, densitometric, and dynamic indices in hypoparathyroid patients for 4 yr. These are the longest-term data to this point on the use of PTH therapy in this disease.
Subjects and Methods
Study design and protocol
Human recombinant PTH(1–84) (NPS Pharmaceuticals, Bedminster, NJ) was used to treat hypoparathyroid subjects for 4 yr, administered at a starting dose of 100 μg sc every other day. This dose was based upon our previous experience demonstrating that this regimen restores reduced bone turnover markers in hypoparathyroidism to levels that are within the normal range (8). A single study physician titrated calcium and vitamin D supplementation to reach a target dose of 1.5 g/d of calcium and 0.25 μg/d of 1,25-dihydroxyvitamin D. Monitoring was accomplished by review of symptomatology and regular measurements of serum and urine calcium further detailed below.
Subjects
The diagnosis of hypoparathyroidism in men and women was established by the simultaneous presence of serum calcium and PTH concentrations below the lower limits of normal on at least two occasions separated by at least 30 d. Hypoparathyroidism was present for at least 2 yr to establish a chronic hypoparathyroid state. All subjects had to be on stable regimens of supplemental calcium and vitamin D intake for at least 6 months before enrollment. Subjects were excluded if they had been on a bisphosphonate within 5 yr before study entry or for more than 6 months at any time or if they were women within 5 yr of menopause. Subjects were also excluded if they used any of the following medications: estrogens, progestins, raloxifene, calcitonin, systemic corticosteroids, fluoride, lithium, statins, loop diuretics, or methotrexate. The following potentially confounding disorders were also exclusionary criteria: Paget's disease of bone, diabetes mellitus, chronic liver or renal disease, acromegaly, Cushing's syndrome, rheumatoid arthritis, or multiple myeloma.
Patients were recruited from the Metabolic Bone Diseases Unit of Columbia University Medical Center and from the Hypoparathyroidism Association. The study was approved by the Institutional Review Board of Columbia University Medical Center. All subjects gave written informed consent.
Of the 82 patients who provided written informed consent, 27 subjects have reached the 4-year time-point and are included in this analysis. It seemed rational to present the data at this time given this substantial number of subjects followed for this long period. The other subjects are not part of this analysis for the following reasons: three withdrew consent before administration of study drug; 24 have not yet reached the 4-yr time-point; and 28 withdrew during yr 1–4 because of the logistics of travel/personal (n = 9), noncompliance/lost to follow-up (n = 8), adverse events not attributed to study drug (gastrointestinal illness, vestibular neuritis, depression, headache/syncope; n = 4), unrelated health issues (n = 4), apparent recovery from hypoparathyroidism (n = 2), and nephrolithiasis (n = 1). The majority of subjects (n = 16) withdrew within the first year.
Biochemical evaluation
Blood was obtained at baseline three times before treatment and at months 1, 2, 3, 4, 5, 6, 9, 12, 18, 24, 30, 36, 42, and 48. The average of three pretreatment serum calcium values was used as the baseline calcium value. Blood sampling was performed 48 h after the last PTH injection. Biochemistries were measured by automated techniques. Twenty-four-hour urinary calcium excretion was measured at baseline and at 3, 6, 9, 12, 18, 24, 30, 36, 42, and 48 months.
Serum markers of bone turnover
Bone turnover markers were measured at baseline and months 6, 12, 18, 24, 30, 36, 42, and 48. Bone-specific alkaline phosphatase was measured by enzyme immunoassay (Quidel, San Diego, CA); inter- and intraassay coefficients of variation (CVs) were 8 and 6%, respectively. N-mid osteocalcin was measured by ELISA [Immunodiagnostic Systems (IDS), Scottsdale, AZ]; inter- and intraassay CVs were 4 and 2%, respectively. Propeptide of type I collagen (P1NP) was measured by RIA (IDS); inter- and intraassay CVs were 8.3 and 6.5%, respectively. Collagen type 1 cross-linked C-telopeptide was measured by ELISA (IDS); inter- and intraassay CVs were 10.9 and 3%, respectively. Tartrate-resistant acid phosphatase 5b (TRAP) was measured by ELISA (IDS); inter- and intraassay CVs were 9 and 5%, respectively.
Safety outcomes
In addition to the time-points listed above, serum calcium concentration was measured 1 and 2 wk after initiation of PTH(1–84). Serum calcium was measured 1 wk after each reduction in calcium or 1,25-dihydroxvitamin D supplementation to ensure stability of the serum calcium concentration. If symptoms of hypocalcemia, such as numbness or paresthesias, developed, upward adjustments in calcium or 1,25-dihydroxyvitamin D dosing were made. Information regarding adverse events was recorded at each study visit.
Bone mineral density (BMD)
Areal BMD was measured at the lumbar spine (L1–L4), total hip, femoral neck, and distal one third radius by dual x-ray absorptiometry (Hologic, Waltham, MA). Subjects were measured on the same densitometer, using the same software, scan speed, and technologist, certified by the International Society of Clinical Densitometry. Measurements were performed twice at baseline and at months 6, 12, 18, 24, 30, 36, 42, and 48. The average value of two pretreatment BMD measurements was used for the baseline value. Short-term in vivo precision error (root-mean-square sd) was 0.026 g/cm2 for L1–L4 (1.1%), 0.041 g/cm2 for the femoral neck (2.4%), and 0.033 g/cm2 (1.8%) for the forearm.
Statistical analysis
A linear mixed model for repeated measures approach was applied with a single fixed effect of time and baseline level of the outcome entered as a continuous covariate. The autoregressive covariance structure (1) was determined before inferential testing to provide the best covariance model fit across all of the outcomes to be tested. This analysis assesses the reliability of the within-subject change from baseline (SAS Proc MIXED, version 9.2; SAS Institute, Cary, NC). Calcium and 1,25-dihydroxyvitamin D supplementation requirements were compared between the group of subjects that discontinued therapy before 4 yr and the 27 subjects included in this analysis. Subjects with at least a 50% decrease in both calcium and 1,25-dihydroxyvitamin D supplementation were also analyzed separately, consistent with the endpoints in the recently completed pivotal clinical trial (NCT00732615; Ref. 9). For the categorical determination of changes in urinary calcium, the 42-month urinary calcium values were used in three patients with missing 48-month values. Z-Scores for bone turnover markers were calculated with normal ranges for men and pre- and postmenopausal women. Data in the body of the text are reported as model-estimated means and sd values, and differences between baseline and subsequent times were tested by simultaneous confidence intervals. P values <0.05 were used to establish significance. No adjustment for multiple comparisons was made for testing different dependent variables.
Results
Baseline characteristics
Table 1 shows baseline characteristics of the 27 subjects. The mean age was 51 yr (range, 25–68), and 74% were women, consistent with the demographics of the disease. The two major etiologies of hypoparathyroidism were postsurgical and autoimmune disease. The duration of hypoparathyroidism ranged from 2 to 46 yr. Baseline biochemistries and BMD are also shown in Table 1. Serum calcium concentration was typically normal as a result of supplementation with calcium and vitamin D. BMD was normal or above average for a young, normal population.
Table 1.
Baseline characteristics of the hypoparathyroid population
| n = 27 | Range (median) | Normal range | |
|---|---|---|---|
| Age (yr) | 51 ± 12 | 25–68 (52) | |
| Sex | Female, 20 (premenopausal, 10; postmenopausal, 10) | ||
| Male, 7 | |||
| Etiology | Postoperative, 16 | ||
| Autoimmune, 10 | |||
| DiGeorge, 1 | |||
| Duration of hypoparathyroidism (yr) | 20 ± 15 | 2–46 (16) | |
| Fractures in adulthood (no. of patients) | 9a | ||
| Kidney stones (no. of patients) | 3 | ||
| Basal ganglia calcifications (no. of patients) | 3 | ||
| Calcium supplement dose (g/d) | 2.73 ± 2.9 | 0–9.0 (2.0) | |
| 1,25-dihydroxyvitamin D supplement dose (μg/d) | 0.65 ± 0.7 | 0–3.0 (0.5) | |
| Daily vitamin dose (IU/d) | 9,590 ± 28,000 | 0–100,000 (400) | |
| Thiazide dose (mg/d) [n = 7] | 32 ± 10 | 25–50 (25) | |
| Serum calcium (mg/dl)b | 8.43 ± 2.2 | 6.6–10.1 (8.5) | 8.6–10.2 |
| PTH (pg/ml) | 5 ± 4 | <1–14.2 (3) | 10–64 |
| Creatinine (mg/dl) | 1.01 ± 0.3 | 0.60–1.50 (0.95) | 0.50–1.30 |
| Phosphate (mg/dl) | 4.4 ± 2 | 2.6–5.8 (4.4) | 2.5–4.5 |
| Total alkaline phosphatase activity (U/liter) | 67 ± 40 | 40–116 (70) | 33–96 |
| Urinary calcium excretion (mg/d) | 269 ± 340 | 53–499 (249) | 50–250c |
| 25-hydroxyvitamin D (ng/ml) | 65 ± 70 | 9–323 (31) | 30–100 |
| 1,25-dihydroxyvitamin D (pg/ml) | 34 ± 20 | 14–100 (26) | 15–60 |
| Lumbar spine BMD (g/cm2) | 1.21 ± 0.1 | 0.89–1.91 | |
| Lumbar spine T-score | +1.51 ± 2.0 | −1.40 to + 7.90 | |
| Total hip BMD (g/cm2) | 1.07 ± 0.1 | 0.65–1.46 | |
| Total hip T-score | +0.84 ± 1.3 | −2.40 to + 4.20 | |
| Femoral neck BMD (g/cm2) | 0.94 ± 0.1 | 0.64–1.30 | |
| Femoral neck T-score | +0.67 ± 1.5 | −1.85 to + 3.60 | |
| One third radius BMD (g/cm2) | 0.73 ± 0.1 | 0.60–0.90 | |
| One third radius T-score | +0.15 ± 1.0 | −1.60 to + 1.55 |
Values are expressed as mean ± sd unless described otherwise.
Among the nine subjects, there were 10 digit fractures, two wrist, three hand, three rib, and one skull fracture.
Serum calcium concentration was typically normal as a result of calcium and vitamin D supplementation.
For men, 50–300 mg/d.
Calcium and vitamin D supplementation
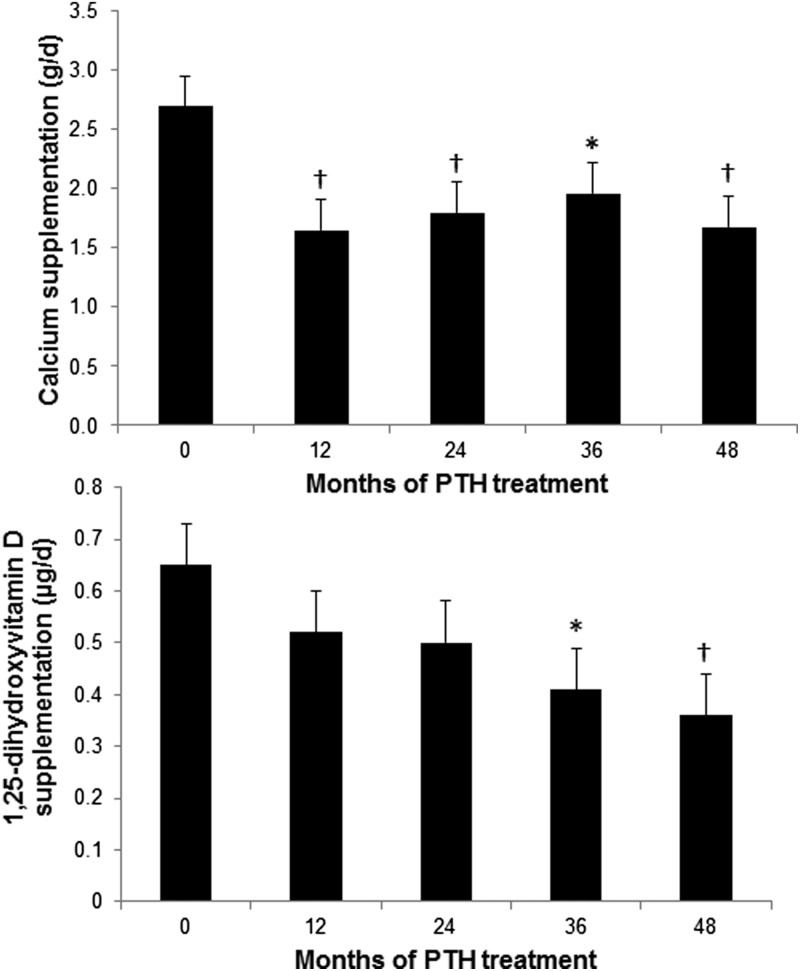
Supplemental calcium requirements fell significantly by 6 months after treatment was initiated with PTH(1–84) and remained lower throughout the study period. The average reduction in calcium supplementation was 37%, from 2.7 ± 3 g/d at baseline to 1.7 ± 3 g/d at 4 yr (P = 0.006; Fig. 1). The number of subjects who required more than 1.5 g/d of calcium supplementation fell from 19 (70%) at study entry to 12 (44%) at study conclusion. 1,25-Dihydroxyvitamin D requirements also fell significantly by 45% from 0.65 ± 0.7 μg/d at baseline to 0.36 ± 0.7 μg/d at 4 yr (P = 0.008; Fig. 1). Seven subjects (26%) were able to discontinue all 1,25-dihydroxyvitamin D supplementation. After changes to calcium and 1,25-dihydroxyvitamin D supplementation, some patients required PTH dose adjustments to 100 μg every 3 d (n = 2) or 100 μg daily (n = 5). Due to the availability of a new 50-μg dose during the 4-yr time period of the study, the dose of PTH was adjusted for some patients to 50 μg daily between yr 3 and 4 (total n = 9), the majority after month 42. There were no statistical differences at 12 months between the group that discontinued before 4 yr and the cohort included in this analysis with regard to calcium (P = 0.69) and 1,25-dihydroxyvitamin D (P = 0.35) supplementation requirements.
Fig. 1.
Changes in calcium and 1,25-dihydroxyvitamin D supplementation. Calcium requirements decreased by 6 months from baseline whereas 1,25-dihydroxyvitamin D requirements decreased by 36 months. Data are expressed as mean ± se. *, P < 0.05 compared with baseline; †, P < 0.01 compared with baseline.
Twelve subjects (44%) were able to reduce their supplementation of both calcium and 1,25-dihydroxyvitamin D by at least 50%. In these subjects, calcium supplementation fell by an average of 61%, from 3.3 ± 3 g/d at baseline to 1.3 ± 3 g/d at 4 yr (P = 0.002). 1,25-Dihydroxyvitamin D requirements fell by 64%, from 0.73 ± 0.5 μg/d at baseline to 0.26 ± 0.5 μg/d at 4 yr (P = 0.001).
Serum and urinary calcium levels, other indices of mineral metabolism
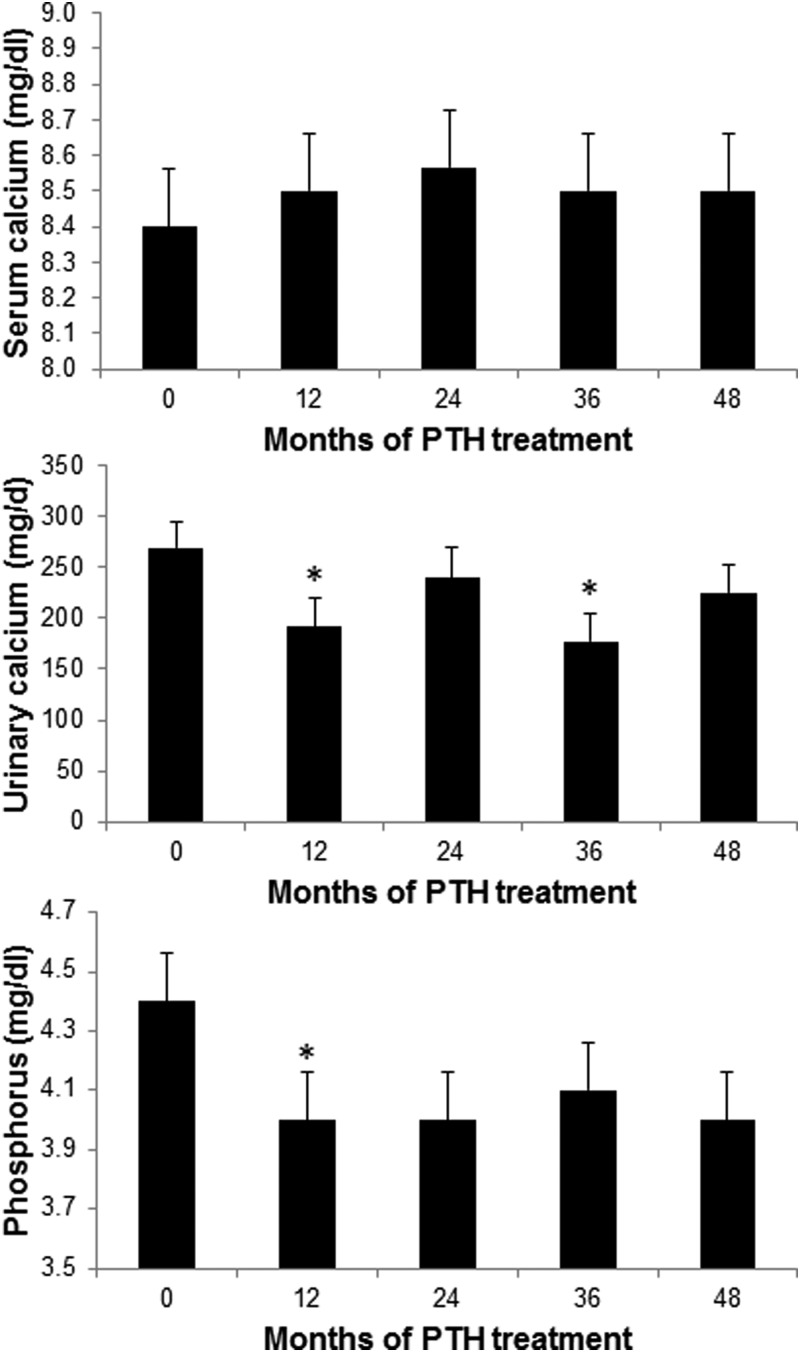
During the first 6 months of PTH(1–84) treatment, while oral calcium and 1,25-dihydroxyvitamin D supplementation was titrated downward, the serum calcium concentration increased by 0.6 mg/dl but remained well within the normal range (8.4 ± 2 to 9.0 ± 2 mg/dl; P = 0.006). The serum calcium then returned to the baseline value and did not change thereafter (Fig. 2). Urinary calcium excretion was significantly decreased over the course of 4 yr (P = 0.003) and fell significantly below baseline during yr 1, 2 (month 30; data not shown), and 3. Although urinary calcium excretion at yr 4 was still below pretreatment values, the difference did not achieve statistical significance. The group seemed to divide itself into those whose urinary calcium excretion fell by at least 10% (n = 15) over the 4 yr, those who demonstrated an increase in urine calcium excretion (n = 6), and those without a significant change in urinary calcium excretion (n = 3; data missing for n = 3). Among those whose calcium and 1,25-dihydroxyvitamin D requirements both fell by at least 50%, urinary calcium excretion also fell by 50%, from 324 ± 360 mg/d at baseline to 162 ± 390 mg/d at 4 yr (P = 0.03). In the entire cohort, the trend for serum phosphorus was a significant decline over the course of the study (P = 0.006).
Fig. 2.
Changes in serum calcium, urinary calcium, and serum phosphorus. Serum calcium was no different from baseline after 6 months through study conclusion. During the first 6 months of the study, there were small but significant increases from baseline within the normal range. Urinary calcium decreased significantly at months 12 and 36 and tended to be lower at 48 months. Serum phosphorus decreased and remained in the normal range throughout the study period. Data are expressed as mean ± se. *, P < 0.05 compared with baseline.
Bone mineral density
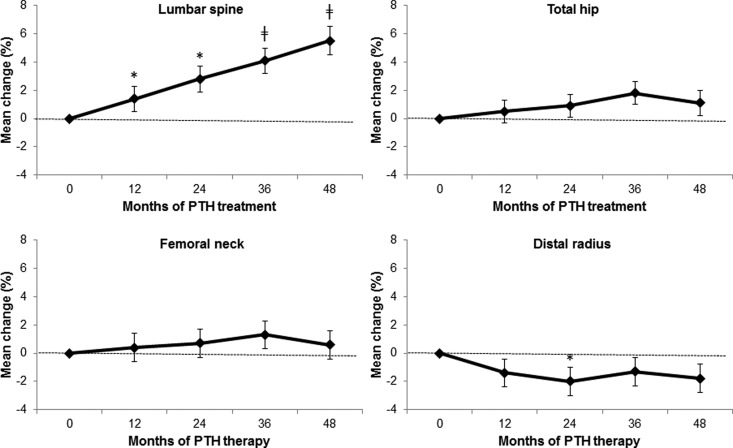
Lumbar spine BMD increased by 2.0 ± 7% at 18 months (P = 0.01) and continued to increase throughout the study period, reaching a mean gain of 5.5 ± 9% at 4 yr (P < 0.0001) (Fig. 3). Femoral neck (+0.6 ± 12% at 4 yr; P = 0.71) and total hip (+1.1 ± 9% at 4 yr; P = 0.37) BMD remained essentially unchanged. BMD at the distal one third radius, which had shown a modest but significant decline at 2 yr (−2.0 ± 6%; P = 0.018), did not progress further and was not significantly different from baseline at the end of the 4-yr period (−1.8 ± 8% from baseline; P = 0.063). The BMD response was not dependent on the extent to which calcium and 1,25-dihydroxyvitamin D requirements fell.
Fig. 3.
Changes in BMD. Lumbar spine BMD increased, whereas the total hip and femoral neck did not change and the distal one third radius BMD decreased. Data are expressed as mean ± se. *, P < 0.05 compared with baseline; ‡, P < 0.0001 compared with baseline.
Bone turnover markers
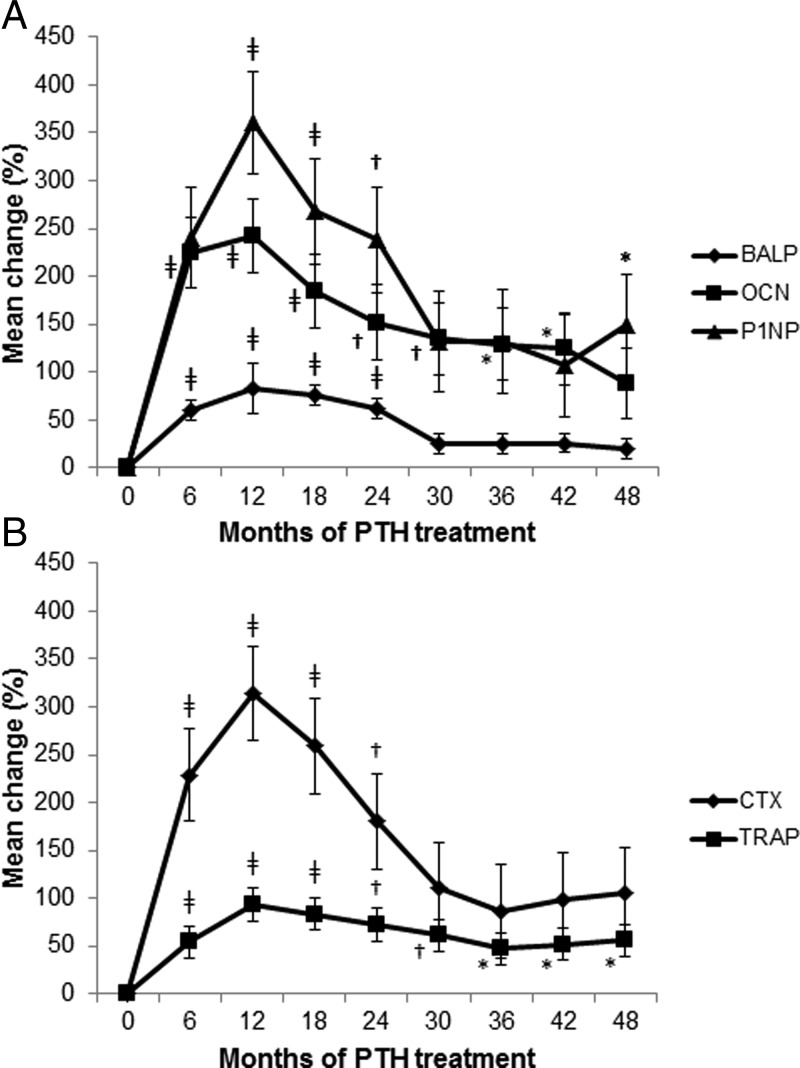
All bone turnover markers were in the low- to mid-normal range at baseline (Z-score range, −1.7 to +0.3). With PTH(1–84) treatment, all bone turnover markers increased significantly, peaking up to 3-fold above baseline values by 6–12 months (Fig. 4). Thereafter, bone turnover markers declined to steady-state levels by 30 months. This steady state was higher than pretreatment values (Z-score, −0.7 to +2.0), although statistically significant only for TRAP and P1NP (P = 0.016 and P = 0.046 from baseline to 4 yr, respectively). For the next 18 months, bone turnover markers were consistently measured at these values.
Fig. 4.
Changes in markers of bone formation (P1NP, BALP, OCN; A) and resorption (CTX, TRAP; B) over 4 yr of PTH(1–84). With PTH(1–84) treatment, all bone turnover markers increased significantly, peaking up to 3-fold from baseline values at 6–12 months and subsequently declining to steady-state levels at 30 months. BALP, Bone-specific alkaline phosphatase; OCN, N-mid osteocalcin; CTX, collagen type 1 cross-linked C-telopeptide. Data are expressed as mean ± se. *, P < 0.05 compared with baseline; †, P < 0.01 compared with baseline; ‡, P < 0.0001 compared with baseline.
Adverse events
There were 11 episodes of mild hypercalcemia in eight subjects over 4 yr (1.9% of all values), most occurring within the first 6 months and resolving with adjustment of supplemental calcium and vitamin D. No hypercalcemic events required hospitalization. The most common adverse events were musculoskeletal, gastrointestinal, and genitourinary complaints (Table 2). Other adverse events over 4 yr included two fractures (yr 1, elbow; yr 4, toe) and one episode of nephrolithiasis (yr 4). The subject that developed nephrolithiasis had 14 normal serum calcium values documented from month 2 through month 48 of the study and demonstrated a greater than 10% reduction in urine calcium excretion.
Table 2.
Adverse events in subjects treated with PTH(1–84) over 4 yr
| Symptoms/complaints | Year 1 | Year 2 | Year 3 | Year 4 |
|---|---|---|---|---|
| Adverse events | ||||
| Musculoskeletal | 59 (78) | 22 (44) | 15 (37) | 20 (52) |
| Gastrointestinal | 21 (48) | 3 (7) | 3 (11) | 1 (4) |
| Genitourinary | 16 (48) | 3 (11) | 3 (11) | 4 (11) |
| Mental and mood | 21 (44) | 5 (11) | 3 (7) | 2 (4) |
| Fatigue | 15 (44) | 1 (4) | 1 (4) | 5 (15) |
| Infections | 17 (37) | 5 (19) | 4 (11) | 5 (19) |
| Headache | 12 (37) | 4 (15) | 0 | 1 (4) |
| Dermatologic | 11 (33) | 1 (4) | 3 (11) | 3 (11) |
| Nausea | 11 (30) | 2 (4) | 3 (11) | 0 |
| Hypercalcemia | 9 (26) | 2 (7) | 0 | 0 |
| Dizziness | 7 (26) | 2 (7) | 1 (4) | 0 |
| Paresthesias | 14 (22) | 2 (7) | 1 (4) | 2 (7) |
| Insomnia | 8 (22) | 1 (4) | 0 | 3 (11) |
| Thirst | 5 (19) | 0 | 0 | 0 |
| Othera | 37 (52) | 16 (44) | 7 (26) | 12 (26) |
| Serious adverse eventsb | 2 (4) | 4 (11) | 2 (4) | 2 (7) |
Data are expressed as number of events (percentage of subjects).
Includes two fractures (yr 1, elbow; yr 4, toe) and one episode of nephrolithiasis (yr 4).
Year 1, Hospital admissions for right flank pain, concussion after motor vehicle accident (same patient); yr 2, hospital admissions for hypocalcemia (three patients); yr 3, hospital admissions for hypocalcemia (same patient); yr 4, hospital admissions for hypocalcemia, dehydration.
Discussion
This is the first study to report the extended use of any PTH therapy in a well-described cohort over 4 yr, in hypoparathyroidism or any other metabolic bone disease. PTH(1–84) therapy returned the abnormally low bone turnover state characteristic of hypoparathyroidism, as measured by bone turnover markers, to more normal levels after a period of heightened responsiveness. The results also confirm that PTH(1–84) therapy in hypoparathyroidism significantly reduces calcium and 1,25-dihydroxyvitamin D requirements, maintains stable serum calcium concentrations, and tends to reduce urinary calcium excretion. This long-term experience also documents that treatment with PTH(1–84) was well tolerated. In particular, hypercalcemia was very uncommon.
It is of interest to compare our results to those of Winer et al. (5) and Sikjaer et al. (7), using PTH(1–34) and PTH(1–84), respectively, in adults with hypoparathyroidism. Winer et al. (5) randomized hypoparathyroid subjects to receive either open-label PTH(1–34) in divided doses averaging 37 μg or 1,25-dihydroxyvitamin D for 3 yr. Sikjaer et al. (7) studied hypoparathyroid subjects randomized to PTH(1–84) 100 μg daily vs. placebo for 6 months. Both forms of PTH were shown to have salutary effects on the management of hypoparathyroidism with respect to calcium and 1,25-dihydroxyvitamin D supplementation requirements. PTH(1–34) normalized urine calcium excretion (5), and our study as well demonstrated a trend toward a decline in urine calcium excretion with PTH(1–84). An increase in renal tubular absorption of calcium indicates that PTH therapy may help to restore normal renal PTH physiology. It is also evident that comorbidities in hypoparathyroid patients are due, at least in part, to the high calcium and vitamin D supplement requirements with subsequent risk for ectopic soft tissue calcification. It is reasonable to expect, therefore, that the reduction in calcium and vitamin D supplement requirements when PTH is used in hypoparathyroidism may well be associated over time with a reduction in complications. The bioavailability of PTH(1–84) is longer than PTH(1–34) (10, 11), which may help to explain why dosing with PTH(1–34) has required multiple injections per day whereas with PTH(1–84), single daily dosing and every other day dosing appears to provide good results.
We found that BMD at the lumbar spine was above average at baseline and continued to increase throughout the study period, whereas BMD at the hip was unchanged. BMD at the distal one third radius, which showed early declines at 2 yr (8), did not progress further. These densitometric results are compatible with the differential effects of PTH at sites that are predominantly cortical (distal one third radius) or trabecular (lumbar spine) (12–16). Although bone density continued to increase at the lumbar spine, the microstructural basis for this change is not yet clear. Based upon the bone turnover marker data and what is known about the osteoanabolic effect of PTH, the increase in BMD at the lumbar spine might reflect not simply more bone mineral but also improvement in the microstructural features of the accrued bone. The lack of change in BMD at the distal one third radius does not imply that cortical bone is not enhanced by PTH(1–84) because expected salutary effects on microarchitecture and bone size could provide biomechanical advantages despite stable BMD. These expectations are supported by a histomorphometric analysis of bone biopsies from 64 subjects with hypoparathyroidism who were treated with PTH(1–84). After 2 yr, structural and dynamic properties of bone were improved by PTH(1–84) (17). Although the results of the current study suggest that these improved microstructural features of bone are maintained over a longer period of time, further direct analyses of bone by bone biopsy after 4 yr of PTH(1–84) will be needed to substantiate this likelihood.
In contrast to our results, Sikjaer et al. (7) showed a very small but significant reduction in lumbar spine BMD (−1.76 ± 1.0%) with PTH(1–84), but these results are not easily comparable because of the very short 6-month duration of the trial by Sikjaer et al. (7). Winer et al. (5) found no increase in lumbar spine BMD after 3 yr. In their study, bone turnover markers rose slowly, reaching a peak only after 2.5 yr, after which they declined over the next 6 months. These kinetics are different from our observations with PTH(1–84) in which bone turnover markers peaked much earlier and then declined to steady-state levels by 30 months, with P1NP and TRAP remaining statistically higher than baseline at 4 yr. Bone biopsies at baseline, 3, 12, and 24 months from our earlier work (17) substantiate these circulating bone turnover kinetics with increases and then decreases in histomorphometrically determined skeletal dynamics over a similar time course. These observations may indicate that the relatively short-acting PTH(1–34) at a dose approximately three times our equivalent dose of PTH(1–84) (18) is not as beneficial to bone dynamics as PTH(1–84), the natural hormone.
The strengths of this investigation include the unusually large cohort of subjects with hypoparathyroidism that was studied, the use of PTH(1–84), the natural secretory product of the parathyroid glands, and its longitudinal design. The limitations of the study include the open-label protocol and the lack of a control group not treated with PTH(1–84). However, each patient served as his/her own control with which specific changes could be carefully monitored. The stability of calcium and 1,25-dihydroxyvitamin D requirements before therapy with PTH(1–84) supports the conclusion that PTH-associated reductions in those supplements are unlikely to have occurred spontaneously. Moreover, a randomized, placebo-controlled study of PTH(1–84) is being reported in which these data are most consistent. In that study (9), the percentage of subjects who were able to reduce their requirements by more than 50% (53.5%), as well as the percentage of subjects who could entirely eliminate their need for 1,25-dihydroxyvitamin D (41.1%) are all similar to the results of this study.
Every other day injectable PTH(1–84) does not simulate normal PTH tonic and pulsatility dynamics. In the future, perhaps, it might be possible to mimic more closely the dynamics of constant PTH secretion (6). The dose regimen for a number of subjects was adjusted during the duration of the study, which indicates that this therapy will likely have to be titrated on an individual basis to achieve the best results. Because the study design called for serum and urine measurements after the first 24 h of PTH administration, earlier changes in serum and urinary analytes, if they occurred, would have been missed. In a study investigating the pharmacodynamics of PTH(1–84) 100 μg daily in hypoparathyroid patients (19), 41% of subjects developed mild asymptomatic hypercalcemia. However, the dose of PTH was higher than that used in our study, and the baseline serum calcium was substantially higher than in our subjects. Based upon the small increments in serum calcium after administration of PTH(1–84) in healthy postmenopausal women (20) and serum and urine calcium excursion in subjects with hypoparathyroidism (19), we do not believe that our subjects would have experienced significant hypercalcemia or hypercalciuria in the time period preceding our measurement.
These limitations do not alter the main conclusions of the study that demonstrate clearly that PTH(1–84) is safe and effective as a long-term treatment of hypoparathyroidism for up to 4 yr. Longer-term studies will help elucidate whether the major comorbidities associated with hypoparathyroidism are reduced or reversed with PTH therapy.
Acknowledgments
The authors acknowledge Elzbieta Dworakowski, Laura Anderson, Wendy Fan, and Zachary Lenane.
This work was supported by National Institutes of Health Grants DK069350 and DK095944, Food and Drug Administration Grant 002525, and NPS Pharmaceuticals.
Disclosure Summary: J.P.B. is a consultant for Amgen, Eli Lilly, Radius, NPS Pharmaceuticals, Merck, Warner Chilcott, and GSK and receives research support from NPS Pharmaceuticals and Amgen. M.R.R. receives research support from NPS Pharmaceuticals. S.C.C. is a consultant for Roche Diagnostics, Immunodiagnostics, Brains-Online, and Thar Pharmaceuticals and receives research support from Roche Diagnostics. No conflicts of interest are reported for the remaining authors.
Footnotes
- BMD
- Bone mineral density
- CV
- coefficient of variation
- P1NP
- propeptide of type I collagen
- TRAP
- tartrate-resistant acid phosphatase 5b.
References
- 1. Bilezikian JP, Khan A, Potts JT, Jr, Brandi ML, Clarke BL, Shoback D, Jüppner H, D'Amour P, Fox J, Rejnmark L, Mosekilde L, Rubin MR, Dempster D, Gafni R, Collins MT, Sliney J, Sanders J. 2011. Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res 26:2317–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cusano NE, Rubin MR, Sliney J, Jr, Bilezikian JP. 2012. Mini-review: new therapeutic options in hypoparathyroidism. Endocrine 41:410–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shoback D. 2008. Clinical practice. Hypoparathyroidism. N Engl J Med 359:391–403 [DOI] [PubMed] [Google Scholar]
- 4. Mitchell DM, Regan S, Cooley MR, Lauter KB, Vrla MC, Becker CB, Burnett-Bowie SM, Mannstadt M, Long-term follow-up of patients with hypoparathyroidism—a cohort study. Program of the 94th Annual Meeting of The Endocrine Society, Houston, TX, 2012 (Abstract OR27-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Winer KK, Ko CW, Reynolds JC, Dowdy K, Keil M, Peterson D, Gerber LH, McGarvey C, Cutler GB., Jr 2003. Long-term treatment of hypoparathyroidism: a randomized controlled study comparing parathyroid hormone-(1–34) versus calcitriol and calcium. J Clin Endocrinol Metab 88:4214–4220 [DOI] [PubMed] [Google Scholar]
- 6. Winer KK, Zhang B, Shrader JA, Peterson D, Smith M, Albert PS, Cutler GB., Jr 2012. Synthetic human parathyroid hormone 1–34 replacement therapy: a randomized crossover trial comparing pump versus injections in the treatment of chronic hypoparathyroidism. J Clin Endocrinol Metab 97:391–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sikjaer T, Rejnmark L, Rolighed L, Heickendorff L, Mosekilde L. 2011. The effect of adding PTH(1–84) to conventional treatment of hypoparathyroidism: a randomized, placebo-controlled study. J Bone Miner Res 26:2358–2370 [DOI] [PubMed] [Google Scholar]
- 8. Rubin MR, Bilezikian JP. 2010. Hypoparathyroidism: clinical features, skeletal microstructure and parathyroid hormone replacement. Arq Bras Endocrinol Metabol 54:220–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bilezikian JP, Mosekilde L, Shoback D, Vokes T, Mannstadt M, Clarke B, Lagast H, Garceau R, Efficacy of recombinant human parathyroid hormone (rhPTH[1–84]) for the treatment of adults with hypoparathyroidism: a randomized, double-blind, placebo-controlled study. Program of the 94th Annual Meeting of The Endocrine Society, Houston, TX, 2012 (Abstract S18-3) [Google Scholar]
- 10. Schwietert HR, Groen EW, Sollie FA, Jonkman JH. 1997. Single-dose subcutaneous administration of recombinant human parathyroid hormone [rhPTH(1–84)] in healthy postmenopausal volunteers. Clin Pharmacol Ther 61:360–376 [DOI] [PubMed] [Google Scholar]
- 11. Lindsay R, Nieves J, Henneman E, Shen V, Cosman F. 1993. Subcutaneous administration of the amino-terminal fragment of human parathyroid hormone-(1–34): kinetics and biochemical response in estrogenized osteoporotic patients. J Clin Endocrinol Metab 77:1535–1539 [DOI] [PubMed] [Google Scholar]
- 12. Silverberg SJ, Shane E, de la Cruz L, Dempster DW, Feldman F, Seldin D, Jacobs TP, Siris ES, Cafferty M, Parisien MV, Lindsay R, Clemens TL, Bilezikian JP. 1989. Skeletal disease in primary hyperparathyroidism. J Bone Miner Res 4:283–291 [DOI] [PubMed] [Google Scholar]
- 13. Bilezikian JP, Brandi ML, Rubin M, Silverberg SJ. 2005. Primary hyperparathyroidism: new concepts in clinical, densitometric and biochemical features. J Intern Med 257:6–17 [DOI] [PubMed] [Google Scholar]
- 14. Dempster DW, Müller R, Zhou H, Kohler T, Shane E, Parisien M, Silverberg SJ, Bilezikian JP. 2007. Preserved three-dimensional cancellous bone structure in mild primary hyperparathyroidism. Bone 41:19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. 2001. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441 [DOI] [PubMed] [Google Scholar]
- 16. Rubin MR, Cosman F, Lindsay R, Bilezikian JP. 2002. The anabolic effects of parathyroid hormone. Osteoporos Int 13:267–277 [DOI] [PubMed] [Google Scholar]
- 17. Rubin MR, Dempster DW, Sliney J, Jr, Zhou H, Nickolas TL, Stein EM, Dworakowski E, Dellabadia M, Ives R, McMahon DJ, Zhang C, Silverberg SJ, Shane E, Cremers S, Bilezikian JP. 2011. PTH(1–84) administration reverses abnormal bone-remodeling dynamics and structure in hypoparathyroidism. J Bone Miner Res 26:2727–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clausen JO. 2009. Comment on Kanis et al: “European guidance for the diagnosis and management of osteoporosis in postmenopausal women.” Osteoporos Int 20:1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sikjaer T, Rejnmark L, Rolighed L, Mosekilde L, PTH(1–84) replacement therapy in hypoparathyroidism (HypoPT): a randomized controlled trial on pharmacokinetics and dynamic effects following 24 weeks of treatment. Program of the 33rd Annual Meeting of the American Society of Bone and Mineral Research, San Diego, CA, 2011 (Abstract 1098) [Google Scholar]
- 20. Fox J, Wells D, Garceau R, Relationships between pharmacokinetic profile of human PTH(1–84) and serum calcium response in postmenopausal women following 4 different methods of administration. Program of the 33rd Annual Meeting of the American Society of Bone and Mineral Research, San Diego, CA, 2011 (Abstract MO0173) [Google Scholar]