Abstract
Context:
Babies of obese women are often large at birth, which is associated with perinatal complications and metabolic syndrome later in life. The mechanisms linking maternal obesity to fetal overgrowth are largely unknown.
Objective:
We tested the hypothesis that placental insulin/IGF-I and mammalian target of rapamycin (mTOR) signaling is activated and amino acid transporter activity is increased in large babies of obese women.
Design and Setting:
Pregnant women were recruited prospectively for collection of placental tissue at a university hospital and academic biomedical center.
Patients or Other Participants:
Twenty-three Swedish pregnant women with first trimester body mass index ranging from 18.5 to 44.9 kg/m2 and with uncomplicated pregnancies participated in the study.
Interventions:
There were no interventions.
Main Outcome Measures:
We determined the phosphorylation of key signaling molecules (including Akt, IRS-1, S6K1, 4EBP-1, RPS6, and AMPK) in the placental insulin/IGF-I, AMPK, and mTOR signaling pathways. The activity and protein expression of the amino acid transporter systems A and L were measured in syncytiotrophoblast microvillous plasma membranes.
Results:
Birth weights (range, 3025–4235 g) were positively correlated to maternal body mass index (P < 0.05). The activity of placental insulin/IGF-I and mTOR signaling was positively correlated (P < 0.001), whereas AMPK phosphorylation was inversely (P < 0.05) correlated to birth weight. Microvillous plasma membrane system A, but not system L, activity and protein expression of the system A isoform SNAT2 were positively correlated to birth weight (P < 0.001).
Conclusions:
Up-regulation of specific placental amino acid transporter isoforms may contribute to fetal overgrowth in maternal obesity. This effect may be mediated by activation of insulin/IGF-I and mTOR signaling pathways, which are positive regulators of placental amino acid transporters.
Women entering pregnancy overweight [body mass index (BMI), 25–29.9 kg/m2] or obese (BMI ≥ 30 kg/m2) have an increased risk to deliver a large for gestational age (LGA) baby, often defined as a birth weight above the 90th centile (1–3). Large babies have an increased risk for shoulder dystocia and plexus injury at delivery (1, 2) and are susceptible to develop obesity, diabetes, and hypertension in childhood and later in life (4). The mechanisms underlying the relationship between excess maternal adiposity and fetal overgrowth are not well established.
To grow appropriately, the fetus is critically dependent on nutrient supply across the placenta, which is determined by numerous factors including placental and umbilical blood flows, transplacental concentration gradients, and placental metabolism. In addition, the type, number, and activity of transporter proteins in the syncytiotrophoblast plasma membranes constitute an important determinant for the transplacental transport of nutrients such as glucose and amino acids. In pregnancies complicated by intrauterine growth restriction (IUGR), placental nutrient transporters for amino acids, such as the sodium-dependent system A (5, 6), are down-regulated. Women having type 1 diabetes or developing gestational diabetes mellitus (GDM) are more likely to give birth to a LGA baby (7), and an increased placental nutrient transport capacity may be one important factor contributing to fetal overgrowth in these pregnancy complications. For example, placental leucine transport has been shown to be increased in GDM/LGA (8), and system A activity was increased in microvillous plasma membrane (MVM) isolated from placentas obtained from pregnancies complicated by diabetes (8). In contrast to these findings, a previous study showed that system A amino acid transporter activity is reduced, and the activity of system L is unaltered in MVM vesicles isolated from type 1 diabetes pregnancies with LGA babies (9). Placental glucose transport and glucose transporter protein 1 protein expression were reported to be increased in type 1 diabetes (10, 11). In addition, emerging evidence suggests that fatty acid transport to the fetus may be increased in diabetes with or without obesity, providing one possible explanation for the increased adiposity observed in babies of mothers with diabetes. For example, the activity of placental lipoprotein lipase has been shown to be increased in pregnancies with type 1 diabetes and fetal overgrowth (12), and placental expression of the fatty acid binding protein 4 (13) and endothelial lipase (14) is elevated in pregnancies of obese women with diabetes. Although it is well established that high prepregnancy BMI is strongly associated to fetal overgrowth (1–3), the effect of maternal overweight and obesity on placental function in women without diabetes remains largely unknown (15, 16).
Placental nutrient transport is controlled by fetal, placental, and maternal factors. Placental mammalian target of rapamycin (mTOR) constitutes a positive regulator of trophoblast amino acid transporters (17, 18). In addition, in vitro studies have demonstrated that hormones such as insulin, IGF-I, and leptin, which are upstream regulators of mTOR, stimulate placental transporters for amino acids (19–22). Thus, placental growth and nutrient transport are under the regulation of metabolic hormones (23–25). Obesity in pregnancy is associated with perturbed maternal metabolism and circulating hormone levels. For example, obese pregnant women have higher serum levels of leptin, insulin, and IL-6 in late pregnancy compared with pregnant women with normal prepregnancy BMI (26). We recently extended these observations and reported increased circulating levels of leptin and insulin already in first trimester among overweight and obese women (27). Thus, it is possible that increased levels of maternal hormones such as insulin, leptin, and IGF-I provide a link between maternal obesity and fetal overgrowth by up-regulation of placental nutrient transport capacity. In the current study, we tested the hypothesis that placental insulin/IGF-I and mTOR signaling is activated and amino acid transporter activity is increased in large babies of obese women.
Patients and Methods
Ethical approval
These studies conformed to the standards set by the latest revision of the Declaration of Helsinki and were approved by the Committee for Research Ethics at the University of Gothenburg. Informed consent was obtained from subjects at recruitment. After obtaining all the relevant clinical information, samples were coded and deidentified. Some analyses were performed at the University of Gothenburg, and deidentified samples were subsequently transferred to the University of Texas Health Science Center San Antonio for further studies.
Subjects
Pregnant women with an early pregnancy BMI [weight (kilograms)/height (meters)2] ranging from 18.5 to 44.9 kg/m2 were enrolled in Gothenburg, Sweden, and placentas were collected after term delivery. BMI was determined based on length and weight measurements at the first prenatal visit at 8–12 wk gestation. Estimated date of delivery was determined from the last menstrual period and confirmed by ultrasound at 16–18 wk gestation. When a large fetus was suspected based on clinical signs, repeated ultrasounds were carried out to confirm acceleration of fetal growth. Study subjects were recruited either immediately before delivery (n = 7) at the Sahlgrenska University Hospital or in gestational wk 8–12 at the Lundby Prenatal Care Center (n = 16). Subjects recruited in early pregnancy were part of a prospective cohort of 49 pregnant women described in detail elsewhere (27). The 16 subjects from this cohort included in the current study represented the cases in which the placenta was obtained immediately after delivery and therefore were a random sample of the larger cohort. The same inclusion and exclusion criteria were used for all study subjects. The inclusion criteria were Scandinavian heritage, good health, and age of at least 20 yr. The exclusion criteria were smoking, vegetarianism, assisted reproduction, concurrent disease such as eating disorder, chronic hypertension and diabetes, and development of pregnancy complications such as gestational diabetes, preeclampsia, or IUGR.
Preparation of placental homogenates and syncytiotrophoblast microvillous membranes
Placentas were collected and weighed before trimming of the cord and membranes. MVM vesicles were prepared as described previously (8, 28). Briefly, placentas were immediately placed on ice after delivery and dissected. The chorionic plate, amniotic sac, and decidua were removed. Approximately 50 g of villous tissue was cut into small pieces and rinsed with ice-cold physiological saline. Tissue was placed in ice-cold buffer D [250 mm sucrose, 0.7 μm pepstatin A, 1.6 μm antipain, 80 μm aprotinin, 1 mm EDTA, 10 mm HEPES-Tris (pH 7.4)] at 4 C and homogenized on ice using a polytron (Kinematika AG, Lucerne, Switzerland). The homogenate was snap-frozen in liquid nitrogen and stored at −80 C until analysis or further processing. To prepare MVM vesicles, homogenates were thawed on ice and then centrifuged twice at 10,000 × g for 15 min, and the resulting supernatants were combined and centrifuged at 125,000 × g for 30 min. The pelleted crude membrane fraction was resuspended in buffer D, and 12 mm MgCl2 was added. The resulting suspension was subjected to slow stirring on ice for 20 min. Subsequently, the suspension was centrifuged for 10 min at 2500 × g. The supernatant, which contained the MVM, was centrifuged two times for 30 min at 125,000 × g. Vesicles were aliquoted, snap-frozen in liquid nitrogen, and stored at −80 C until use. MVM enrichment was determined as the MVM/homogenate ratio of alkaline phosphatase activity, which was assessed using standard activity assays. Enrichment of alkaline phosphatase activity in MVM was 30 ± 6-fold and was independent of maternal BMI. Protein content of the homogenates and MVM was determined by the method of Bradford.
Western blot
Protein expression of total and phosphorylated Akt (Thr-308 or Ser-473), insulin receptor substrate 1 (IRS-1; Tyr-612), AMP-activated kinase (AMPKα; Thr-172), serum and glucocorticoid-regulated kinase 1 (SGK1; Ser-422), protein kinase C-α (PKCα; Ser-657), S6 kinase 1 (S6K1; Thr-389), eukaryotic initiation factor 4E-binding protein 1 (4E-BP1; Thr-37/46 or Thr-70), and ribosomal protein S6 (RPS6; Ser-235/236) was analyzed in placental homogenates. The IRS-1 and PKCα antibodies were purchased from Millipore (Billerica, MA), the SGK1 antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and the remaining antibodies were purchased from Cell Signaling Technology (Boston, MA). Protein expression of the three system A amino acid transporter isoforms SNAT (sodium-dependent neutral amino acid transporter) 1, -2, and -4 was determined in MVM vesicles using Western blotting. A polyclonal SNAT2 antibody generated in rabbits (29) was a generous gift from Dr. P. D. Prasad at the University of Georgia. Affinity-purified polyclonal anti-SNAT1 (raised against the peptide sequence VPEDDNISNDSNDFT) and anti-SNAT4 antibodies (raised against the peptide sequence YGEVEDELLHAYSKV) were generated in rabbits by Eurogentec (Seraing, Belgium). For negative controls, the purified antigenic peptide was used in 15-fold excess to preabsorb antibody overnight at 4 C. Western blotting was performed as described previously (30). Briefly, total protein (10–20 μg) from placental homogenate/MVM was loaded and separated on Bis-Tris gels (7–12% acrylamide) and transferred onto nitrocellulose membranes. Membrane blocking and antibody incubations were performed as described in the protocol provided by the manufacturer. Subsequently, membranes were incubated with the appropriate secondary peroxidase-labeled antibodies for 1 h. After washing, bands were visualized using enhanced chemiluminescence detection reagents (GE Healthcare, Piscataway, NJ). Because protein expression of β-actin in placental samples was independent of BMI (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org), all blots were stripped and reprobed for β-actin. Analysis of the blots was performed by densitometry using α Imager (Alpha Innotech Corporation, Santa Clara, CA). To account for variation in loading, the density of the target band was divided by the corresponding β-actin band. For each target, all values were expressed in relation to the highest target/β-actin ratio, which was arbitrarily assigned a value of 1.0.
Measurements of amino acid transporter activity in MVM
The activity of the amino acid transporter systems A and L was measured as previously described (8). In brief, MVM vesicles were preloaded by incubation in 300 mm mannitol and 10 mm HEPES-Tris (pH 7.4) overnight at 4 C. At time zero, 30 μl of vesicles were rapidly mixed (1:2) with the appropriate incubation buffer containing 14C-methyl-aminoisobutyric acid (MeAIB; 150 μm) or 3H-L-leucine (0.375 μm) at 37 C. Uptake of radiolabeled substrate was terminated by the addition of 2 ml of ice-cold PBS after 30 sec (MeAIB) or 8 sec (leucine) (8). Subsequently, vesicles were rapidly separated from the substrate medium by filtration on mixed ester filters (0.45-μm pore size; Millipore Corporation, Billerica, MA) and washed with 3 × 2 ml of PBS. In studies of MeAIB transport, 150 mm NaCl and 150 mm KCl were used in incubation buffers to assess total and sodium-independent uptake, respectively. In leucine transport experiments, nonmediated flux was studied in the presence of 30 mm unlabeled L-leucine.
In all uptake experiments, each condition was studied in triplicate for each vesicle preparation. Filters were dissolved in 2 ml of liquid scintillation fluid and counted, and uptakes were expressed as picomoles per milligram of protein. Na+-dependent uptake of MeAIB (corresponding to system A activity) was calculated by subtracting Na+-independent from total uptakes. Mediated leucine uptake, which in isolated MVM almost entirely represents system L activity (31), was calculated by subtracting nonmediated transport from total uptake. Uptakes were expressed as picomoles per minute per 30 sec (system A) or picomoles per minute per 8 sec (system L).
Data presentation and statistics
Summary data are presented as means ± sem. Variables were analyzed as continuous across the range of BMI and birth weights, and linear relationships between variables were determined using bivariate analysis and Pearson's correlation coefficients. A P < 0.05 value (two-tailed) was considered significant.
Results
Clinical data
Table 1 shows selected clinical data for the study subjects, divided into Normal BMI (18.5–24.9 kg/m2) and High BMI (25–44.9 kg/m2) groups according to measurements of body weight and length in early pregnancy. There were no statistical differences between BMI groups with regard to gestational weight gain or gestational age. There was a trend toward higher birth weights and placental weights in the High BMI group; however, these differences failed to reach statistical significance. However, when analyzed across the BMI range of all subjects, placental weights (r = 0.43; P < 0.05) and birth weights (r = 0.46; P < 0.05) were positively correlated to maternal BMI.
Table 1.
Selected clinical data
| Normal BMI (18.5–24.9) | High BMI (25.0–44.9) | |
|---|---|---|
| n | 11 | 12 |
| BMI (kg/m2) | 21.7 ± 0.6 | 38.8 ± 2.3a |
| Gestational weight gain (kg) | 11.3 ± 1.1 | 13.1 ± 1.4 |
| Gestational age (wk) | 40.6 ± 0.4 | 40.4 ± 0.5 |
| Birth weight (g) | 3423 ± 67 | 3635 ± 151 |
| Placental weight (g) | 599 ± 45 | 679 ± 28 |
Data are expressed as means ± sem.
P < 0.0001 vs. Normal BMI, Student's t test.
Activity of placental insulin/IGF-I signaling
Placental insulin/IGF-I signaling activity was assessed by determining phosphorylation of IRS-1 at Tyr-612 and Akt at Thr-308 (Fig. 1). IRS-1 phosphorylation was positively correlated to BMI (P < 0.05; Fig. 1B). In addition, phosphorylation of both IRS-1 and Akt was positively correlated to birth weight (P < 0.01; Fig. 1, C and D). There was no significant correlation between BMI or birth weight and total IRS-1 or Akt expression.
Fig. 1.
Placental insulin/IGF-I signaling in relation to BMI and birth weight. A, Representative Western blots for total and phosphorylated IRS-1 (Tyr-612) and Akt (Thr-308) in homogenates of placentas from pregnancies with varying maternal BMI and birth weights. There was no significant correlation between BMI or birth weight and total IRS-1 or Akt. B, Relationship between BMI and phosphorylation of placental IRS-1. C and D, Relationship between birth weight and phosphorylation of placental IRS-1 (C) or Akt (D). n = 17; r = Pearson's correlation coefficient.
Activity of placental AMPK signaling
As shown in Fig. 2, placental AMPK activity, as determined by Thr-172 phosphorylation, was inversely correlated to maternal BMI (P < 0.05) and birth weight (P < 0.05). There was no significant correlation between BMI or birth weight and total AMPK expression.
Fig. 2.
Placental AMPK signaling in relation to BMI and birth weight. A, Representative Western blots for total and phosphorylated AMPKα (Thr-172) in homogenates of placentas from pregnancies with varying BMI and birth weights. There was no significant correlation between BMI or birth weight and total AMPKα. B and C, Relationship between BMI (B) or birth weight (C) and phosphorylation of placental AMPKα. n = 17; r = Pearson's correlation coefficient.
Activity of placental mTOR complex (mTORC)-1 signaling
mTOR is a ubiquitously expressed serine/threonine kinase that exists as two complexes, mTORC1 and -2, with distinct regulation and function (32). S6K1, RPS6, and 4E-BP1 are key downstream targets of mTORC1. Placental 4E-BP1 phosphorylation (both at Thr-37/46 and Thr-70) was positively correlated to early pregnancy BMI (P < 0.01; Fig. 3, B and C). Phosphorylation of S6K1 (Thr-389; P < 0.01), RPS6 (Ser235/236; P < 0.05), and 4E-BP1 (Thr 37/46, P < 0.05; and Thr-70, P < 0.001) was positively correlated to birth weight (Fig. 3, D–G). There was no significant correlation between BMI or birth weight and total S6K1, RPS6, or 4E-BP1 expression.
Fig. 3.
Placental mTORC1 signaling in relation to BMI and birth weight. A, Representative Western blots for total and phosphorylated S6K1 (Thr-389), 4E-BP1 (Thr-37/46 or Thr-70), and RPS6 (Ser-235/236) in homogenates of placentas from pregnancies with varying BMI and birth weights. There was no significant correlation between BMI or birth weight and total S6K1, 4E-BP1, or RPS6. B and C, Relationship between BMI and phosphorylation of placental 4EBP-1 (Thr-37/46) (A) or 4E-BP1 (Thr-70) (B). D–G, Relationship between birth weight and phosphorylation of placental S6K1 (D), RPS6 (E), 4E-BP1 (Thr-37/46) (F), or 4EBP-1 (Thr-70) (G). n = 17; r = Pearson's correlation coefficient.
Activity of placental mTORC2 signaling
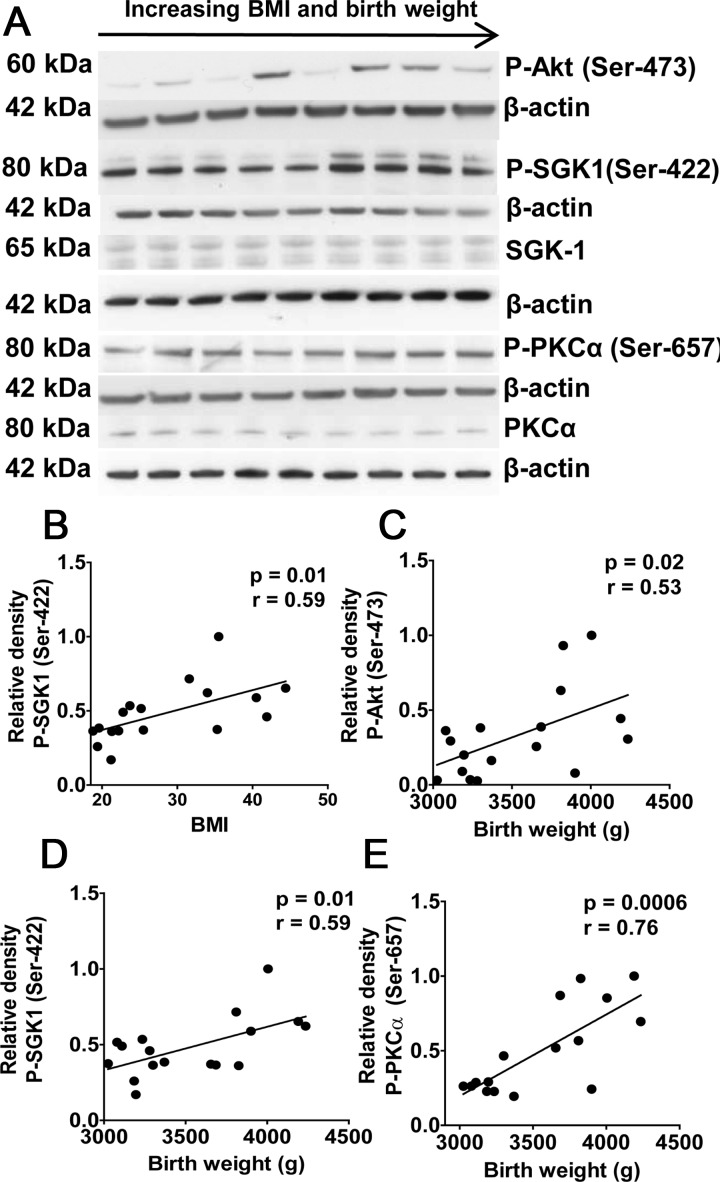
mTORC2 phosphorylates Akt (Ser-473), SGK1 (Ser-422), and PKCα (Ser-657). Phosphorylation of placental SGK1 was positively correlated to maternal early pregnancy BMI (P < 0.05; Fig. 4B). In addition, phosphorylation of all three mTORC2 targets was positively correlated to birth weight (Fig. 4, C–E). The expression of total SGK1 and PKCα was not influenced by maternal BMI or birth weight.
Fig. 4.
Placental mTORC2 signaling in relation to BMI and birth weight. A, Representative Western blots for phosphorylated Akt (Ser-473), total and phosphorylated SGK1 (Ser-422), and PKCα (Ser-657) in homogenates of placentas from pregnancies with varying BMI and birth weights. There was no significant correlation between BMI or birth weight and total SGK1 or PKCα. B, Relationship between BMI and phosphorylation of placental SGK1. C–E, Relationship between birth weight and phosphorylation of placental Akt (Ser-473) (C), SGK1 (D), or PKCα (E). n = 17; r = Pearson's correlation coefficient.
MVM system A and L transport activity
The activity of system A and system L in MVM isolated from women with normal BMI and appropriate fetal growth were similar to what we have reported previously (8). MVM system A activity in the High BMI group (69.7 ± 9.3 pmol/mg × 30 sec) was not significantly different from the normal BMI group (57.5 ± 9.4 pmol/mg × 30 sec). Similarly, MVM system L activity was not altered in the High BMI group (0.055 ± 0.013 pmol/mg × 8 sec) compared with the Normal BMI group (0.069 ± 0.013 pmol/mg × 8 sec). However, MVM system A transport activity (Fig. 5A), but not system L activity (data not shown), showed a strong positive correlation to birth weight (r = 0.60; P < 0.01), but not to maternal BMI (data not shown).
Fig. 5.
MVM system A activity and SNAT 2 expression in relation to BMI and birth weight. A, Relationship between birth weight and system A amino acid transport activity was measured in vitro in MVM isolated from placentas of pregnancies with varying BMI and birth weights (n = 23). B, Representative Western blots for MVM expression of SNAT1, -2, and -4 isoforms of the system A amino acid transporter. C and D, Relationship between BMI (C) or birth weight (D) and MVM SNAT 2 expression. n = 22; r = Pearson's correlation coefficient.
Protein expression of system A amino acid transporter isoforms in MVM
MVM SNAT1 or SNAT4 protein expression was not significantly correlated to birth weight or BMI (data not shown). In contrast, SNAT2 expression was positively correlated to maternal early pregnancy BMI (P < 0.05; Fig. 5C) and birth weight (P < 0.01; Fig. 5D).
Discussion
To the best of our knowledge, this is the first report to study placental signaling and amino acid transport in women with high BMI without pregnancy complications such as gestational diabetes. We demonstrate that the activity of the insulin/IGF-I and mTOR signaling pathways, system A amino acid transporter activity, and protein expression of the SNAT2 isoform are increased in placentas of obese women giving birth to large babies. We propose that up-regulation of specific placental amino acid transporter isoforms may contribute to fetal overgrowth in obese women. This effect may be mediated by activation of insulin and mTOR signaling pathways, which are positive regulators of placental amino acid transporters.
The microvillous plasma membrane of the syncytiotrophoblast, which is bathed in maternal blood, expresses a number of hormone receptors, including receptors for insulin (33) and IGF-I (34), suggesting that trophoblast function is regulated by maternal hormones. Maternal circulating IGF-I concentrations are positively correlated to fetal growth in normal pregnancy (35), and maternal serum concentrations of IGF-I have consistently been shown to be decreased in IUGR (36). These findings are in agreement with reports of inhibition of placental insulin/IGF-I signaling in IUGR (37, 38). In addition, fasting insulin is increased in obese pregnant women (26, 27). These observations are consistent with the increased phosphorylation of IRS-1 and Akt that we found in the placenta of high BMI women giving birth to large babies, indicating activation of insulin/IGF-I signaling.
mTOR signaling constitutes a master regulator of protein translation, thereby controlling cell growth and metabolism in response to a large number of upstream regulators, including growth factors, nutrient, oxygen, and energy levels (32). We found that both placental mTORC1 and mTORC2 signaling pathways were activated in association to high BMI and increased fetal growth. AMPK is the primary cellular energy sensor and is phosphorylated at Thr-172 in response to increased AMP/ATP ratio associated with energy deprivation. In this study we demonstrated that the activity of placental AMPK, which inhibits mTORC1, was decreased and that IGF-I/insulin signaling, which stimulates mTORC1 and -2, was activated in association to increased BMI and fetal growth. The observed changes in AMPK and insulin/IGF-I signaling are therefore likely to contribute to mTOR activation in placentas of obese women giving birth to large babies.
System A is a sodium-dependent transporter mediating the uptake of nonessential neutral amino acids into the cell. System A activity establishes the high intracellular concentration of amino acids like glycine, which is used to exchange for extracellular essential amino acids via system L. Thus, system A activity is critical for placental transport of both nonessential and essential amino acids. System L is a sodium-independent exchanger mediating cellular uptake of essential amino acids including leucine. We found that system A activity is increased in MVM isolated from large babies of obese women, which may contribute to increased fetal amino acid availability and fetal growth. We reported previously that MVM system A activity was increased in pregnancies complicated by GDM or type 1 diabetes independently of fetal overgrowth. However, MVM system A activity was unaffected in lean nondiabetic women giving birth to LGA fetuses (8). This is consistent with the possibility that obesity and diabetes in pregnancy have common underlying metabolic disturbances that can result in increased placental nutrient transport and fetal growth.
It is well established that IGF-I, insulin, and mTOR signaling stimulate placental amino acid transport (17–19, 21, 22). The activation of insulin/IGF-I and mTOR signaling that we observed in placentas of obese women giving birth to large babies is likely to contribute to the observed increase in system A activity. However, system A amino acid transporter activity is also regulated by other signaling pathways, such as leptin, that we have not directly addressed in the present study. This is relevant because leptin stimulates trophoblast system A amino acid transport in vitro (19) and maternal leptin levels are elevated in pregnant women with high BMI (27). All three known isoforms of system A, SNAT1 (SLC38A1), SNAT2 (SLC38A2), and SNAT4 (SLC38A4), are expressed in the placenta (39). The effect of obesity on system A was isoform-specific because protein expression of SNAT2, but not SNAT1 and SNAT4, was up-regulated in MVM isolated from placentas of large babies of obese women. Little is known with respect to regulation of placental SNATs; however, SNAT4 appears to be gestationally regulated in the human placenta (39), and SNAT1 and -2 expression has been reported to be differentially regulated in response to amino acid deprivation in BeWo cells (40). Furthermore, placental IGF-II has been shown to specifically regulate SNAT4 gene expression in mice (41).
Despite a small sample size, birth weight was positively correlated to maternal BMI, in agreement with the literature (1–3). Some of the outcome variables in our study (phosphorylation of IRS-1, 4EBP-1, AMPK and SGK1, and SNAT2 protein expression) were significantly correlated to both birth weight and maternal early pregnancy BMI, whereas others (phosphorylation of Akt, S6K1, RPS6, and PKCα, and system A activity) were significantly correlated to birth weight only. This may reflect that factors unrelated to BMI regulate placental signaling and amino acid transport and contribute to increased fetal growth. Alternatively, these findings may be explained by the small sample size, which is a limitation of our study. Our results should be confirmed in larger studies, which will have the power to allow multiple regression modeling. Furthermore, our sample included a wide range of BMIs, and it cannot be excluded that a few subjects with a BMI over 40 kg/m2 may have skewed results in the obese group. Future studies may therefore benefit from stratifying study subjects by BMI category. Placental system A and L transport activity in pregnancies complicated by diabetes and fetal overgrowth has been reported to be different in a Swedish (8) and a British population (9). This suggests that there may be ethnic or population differences in the placental response to maternal metabolic disease. Thus, exploration of the impact of maternal obesity on placental signaling and transport in populations other than the one studied in the current report appears warranted. This information will increase our understanding of the mechanisms linking maternal obesity to large size at birth and may facilitate the development of novel intervention strategies to alleviate fetal overgrowth.
Acknowledgments
In memory of Margareta Wennergren (1948–2011), to whom we are profoundly indebted for her support and inspiration over many years.
We thank the midwives at Lundby Prenatal Care Center for helping us to recruit pregnant women to our study and Ellen Samuelsson and staff at Östra KKÖ and Mölndals hospitals who made it possible for us to collect the placentas.
This work was supported by grants from the Swedish Research Council (10838 to T.J. and 14555 to T.L.P.) and the National Institutes of Health (HD68370 to T.J. and DK89989 to T.L.P.).
Disclosure Summary: The authors declare that there are no conflicts of interest in relation to this study.
Footnotes
- AMPK
- AMP-activated kinase
- BMI
- body mass index
- 4E-BP1
- eukaryotic initiation factor 4E-binding protein 1
- GDM
- gestational diabetes mellitus
- IRS-1
- insulin receptor substrate 1
- IUGR
- intrauterine growth restriction
- LGA
- large for gestational age
- MeAIB
- 14C-methyl-aminoisobutyric acid
- mTOR
- mammalian target of rapamycin
- mTORC
- mTOR complex
- MVM
- microvillous plasma membrane
- PKCα
- protein kinase C-α
- RPS6
- ribosomal protein S6
- SGK1
- serum and glucocorticoid-regulated kinase 1
- S6K1
- S6 kinase 1
- SNAT
- sodium-dependent neutral amino acid transporter.
References
- 1. Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, Regan L, Robinson S. 2001. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord 25:1175–1182 [DOI] [PubMed] [Google Scholar]
- 2. Baeten JM, Bukusi EA, Lambe M. 2001. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Public Health 91:436–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ehrenberg HM, Mercer BM, Catalano PM. 2004. The influence of obesity and diabetes on the prevalence of macrosomia. Am J Obstet Gynecol 191:964–968 [DOI] [PubMed] [Google Scholar]
- 4. Boney CM, Verma A, Tucker R, Vohr BR. 2005. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes. Pediatrics 115:e290–e296 [DOI] [PubMed] [Google Scholar]
- 5. Mahendran D, Donnai P, Glazier JD, D'Souza SW, Boyd RD, Sibley CP. 1993. Amino acid (system A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr Res 34:661–665 [DOI] [PubMed] [Google Scholar]
- 6. Glazier JD, Cetin I, Perugino G, Ronzoni S, Grey AM, Mahendran D, Marconi AM, Pardi G, Sibley CP. 1997. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr Res 42:514–519 [DOI] [PubMed] [Google Scholar]
- 7. Evers IM, de Valk HW, Mol BW, ter Braak EW, Visser GH. 2002. Macrosomia despite good glycaemic control in type I diabetic pregnancy; results of a nationwide study in The Netherlands. Diabetologia 45:1484–1489 [DOI] [PubMed] [Google Scholar]
- 8. Jansson T, Ekstrand Y, Björn C, Wennergren M, Powell TL. 2002. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes 51:2214–2219 [DOI] [PubMed] [Google Scholar]
- 9. Kuruvilla AG, D'Souza SW, Glazier JD, Mahendran D, Maresh MJ, Sibley CP. 1994. Altered activity of the system A amino acid transporter in microvillous membrane vesicles from placentas of macrosomic babies born to diabetic women. J Clin Invest 94:689–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jansson T, Wennergren M, Powell TL. 1999. Placental glucose transport and GLUT 1 expression in insulin dependent diabetes. Am J Obstet Gynecol 180:163–168 [DOI] [PubMed] [Google Scholar]
- 11. Gaither K, Quraishi AN, Illsley NP. 1999. Diabetes alters the expression and activity of the human placental GLUT1 glucose transporter. J Clin Endocrinol Metab 84:695–701 [DOI] [PubMed] [Google Scholar]
- 12. Magnusson AL, Waterman IJ, Wennergren M, Jansson T, Powell TL. 2004. Triglyceride hydrolase activities and expression of fatty acid binding proteins in human placenta in pregnancies complicated by IUGR and diabetes. J Clin Endocrinol Metab 89:4607–4614 [DOI] [PubMed] [Google Scholar]
- 13. Scifres CM, Chen B, Nelson DM, Sadovsky Y. 2011. Fatty acid binding protein 4 regulates intracellular lipid accumulation in human trophoblasts. J Clin Endocrinol Metab 96:E1083–E1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gauster M, Hiden U, van Poppel M, Frank S, Wadsack C, Hauguel-de Mouzon S, Desoye G. 2011. Dysregulation of placental endothelial lipase in obese women with gestational diabetes mellitus. Diabetes 60:2457–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins L, Greenwood SL, Wareing M, Sibley CP, Mills TA. 2011. Obesity and the placenta: a consideration of nutrient exchange mechanisms in relation to aberrant fetal growth. Placenta 32:1–7 [DOI] [PubMed] [Google Scholar]
- 16. Dubé E, Gravel A, Martin C, Desparois G, Moussa I, Ethier-Chiasson M, Forest JC, Giguère Y, Masse A, Lafond J. 2012. Modulation of fatty acid transport and metabolism by obesity in the human full-term placenta. Biol Reprod 87:14, 1–11 [DOI] [PubMed] [Google Scholar]
- 17. Roos S, Kanai Y, Prasad PD, Powell TL, Jansson T. 2009. Regulation of placental amino acid transporter activity by mammalian target of rapamycin. Am J Physiol Cell Physiol 296:C142–C150 [DOI] [PubMed] [Google Scholar]
- 18. Roos S, Jansson N, Palmberg I, Säljö K, Powell TL, Jansson T. 2007. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted foetal growth. J Physiol 582:449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jansson N, Greenwood SL, Johansson BR, Powell TL, Jansson T. 2003. Leptin stimulates the activity of the system A amino acid transporter in human placental villous fragments. J Clin Endocrinol Metab 88:1205–1211 [DOI] [PubMed] [Google Scholar]
- 20. Kniss DA, Shubert PJ, Zimmerman PD, Landon MB, Gabbe SG. 1994. Insulin growth factors: their regulation of glucose and amino acid transport in placental trophoblasts isolated from first-trimester chorionic villi. J Reprod Med 39:249–256 [PubMed] [Google Scholar]
- 21. Karl PI, Alpy KL, Fisher SE. 1992. Amino acid transport by the cultured human placental trophoblast: effect of insulin on AIB transport. Am J Physiol 262:C834–C839 [DOI] [PubMed] [Google Scholar]
- 22. Karl PI. 1995. Insulin-like growth factor-1 stimulates amino acid uptake by the cultured human placental trophoblast. J Cell Physiol 165:83–88 [DOI] [PubMed] [Google Scholar]
- 23. Jones HN, Powell TL, Jansson T. 2007. Regulation of placental nutrient transport. A review. Placenta 28:763–774 [DOI] [PubMed] [Google Scholar]
- 24. Jansson T, Powell TL. 2006. IFPA 2005 Award in Placentology Lecture. Human placental transport in altered fetal growth: does the placenta function as a nutrient sensor? A review. Placenta 27(Suppl A):S91–S97 [DOI] [PubMed] [Google Scholar]
- 25. Sferruzzi-Perri AN, Owens JA, Pringle KG, Robinson JS, Roberts CT. 2006. Maternal insulin-like growth factors I and II act via different pathways to promote fetal growth. Endocrinology 147:3344–3355 [DOI] [PubMed] [Google Scholar]
- 26. Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. 2002. Maternal obesity is associated with dysregulation of metabolic, vascular and inflammatory pathways. J Clin Endocrinol Metab 87:4231–4237 [DOI] [PubMed] [Google Scholar]
- 27. Jansson N, Nilsfelt A, Gellerstedt M, Wennergren M, Rossander-Hulthén L, Powell TL, Jansson T. 2008. Maternal hormones linking maternal body mass index and dietary intake to birth weight. Am J Clin Nutr 87:1743–1749 [DOI] [PubMed] [Google Scholar]
- 28. Illsley NP, Wang ZQ, Gray A, Sellers MC, Jacobs MM. 1990. Simultaneous preparation of paired, syncytial, microvillous and basal membranes from human placenta. Biochim Biophys Acta 1029:218–226 [DOI] [PubMed] [Google Scholar]
- 29. Ling R, Bridges CC, Sugawara M, Fujita T, Leibach FH, Prasad PD, Ganapathy V. 2001. Involvement of transporter recruitment as well as gene expression in the substrate-induced adaptive regulation of amino acid transport system A. Biochim Biophys Acta 1512:15–21 [DOI] [PubMed] [Google Scholar]
- 30. Rosario FJ, Jansson N, Kanai Y, Prasad PD, Powell TL, Jansson T. 2011. Maternal protein restriction in the rat inhibits placental insulin, mTOR, and STAT3 signaling and down-regulates placental amino acid transporters. Endocrinology 152:1119–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jansson T, Scholtbach V, Powell TL. 1998. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr Res 44:532–537 [DOI] [PubMed] [Google Scholar]
- 32. Alessi DR, Pearce LR, García-Martínez JM. 2009. New insights into mTOR signaling: mTORC2 and beyond. Sci Signal 2:pe27. [DOI] [PubMed] [Google Scholar]
- 33. Desoye G, Hartmann M, Blaschitz A, Dohr G, Hahn T, Kohnen G, Kaufmann P. 1994. Insulin receptors in syncytiotrophoblast and fetal endothelium of human placenta. Immunohistochemical evidence for developmental changes in distribution pattern. Histochemistry 101:277–285 [DOI] [PubMed] [Google Scholar]
- 34. Fang J, Furesz TC, Lurent RS, Smith CH, Fant ME. 1997. Spatial polarization of insulin-like growth factor receptors on the human syncytiotrophoblast. Pediatr Res 41:258–265 [DOI] [PubMed] [Google Scholar]
- 35. Chellakooty M, Vangsgaard K, Larsen T, Scheike T, Falck-Larsen J, Legarth J, Andersson AM, Main KM, Skakkebaek NE, Juul A. 2004. A longitudinal study of intrauterine growth and the placental growth hormone (GH)-insulin-like growth factor I axis in maternal circulation: association between placental GH and fetal growth. J Clin Endocrinol Metab 89:384–391 [DOI] [PubMed] [Google Scholar]
- 36. Bhatia S, Faessen GH, Carland G, Balise RL, Gargosky SE, Druzin M, El-Sayed Y, Wilson DM, Giudice LC. 2002. A longitudinal analysis of maternal serum insulin-like growth factor I (IGF-I) and total and nonphosphorylated IGF-binding protein-1 in human pregnancies complicated by intrauterine growth restriction. J Clin Endocrinol Metab 87:1864–1870 [DOI] [PubMed] [Google Scholar]
- 37. Laviola L, Perrini S, Belsanti G, Natalicchio A, Montrone C, Leonardini A, Vimercati A, Scioscia M, Selvaggi L, Giorgino R, Greco P, Giorgino F. 2005. Intrauterine growth restriction in humans is associated with abnormalities in placental insulin-like growth factor signaling. Endocrinology 146:1498–1505 [DOI] [PubMed] [Google Scholar]
- 38. Yung HW, Calabrese S, Hynx D, Hemmings BA, Cetin I, Charnock-Jones DS, Burton GJ. 2008. Evidence of translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am J Pathol 173:451–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Desforges M, Lacey HA, Glazier JD, Greenwood SL, Mynett KJ, Speake PF, Sibley CP. 2006. The SNAT4 isoform of the system A amino acid transporter is expressed in human placenta. Am J Physiol Cell Physiol 290:C305–C312 [DOI] [PubMed] [Google Scholar]
- 40. Jones HN, Ashworth CJ, Page KR, McArdle HJ. 2006. Expression and adaptive regulation of amino acid transport system A in a placental cell line under amino acid restriction. Reproduction 131:951–960 [DOI] [PubMed] [Google Scholar]
- 41. Constância M, Angiolini E, Sandovici I, Smith P, Smith R, Kelsey G, Dean W, Ferguson-Smith A, Sibley CP, Reik W, Fowden A. 2005. Adaptation of nutrient supply to fetal demand in the mouse involves interaction between the Igf2 gene and placental transporter systems. Proc Natl Acad Sci USA 102:19219–19224 [DOI] [PMC free article] [PubMed] [Google Scholar]