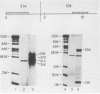
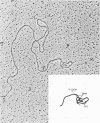
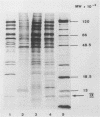
Abstract
A human cell line (293) transformed by adenovirus type 5 encodes mRNA's and proteins from the early region 1 (E1) of the viral genome. These products correspond to those synthesized early after adenovirus infection of normal cells. This pattern of expression is different from that observed at later times in the lytic cycle. We have determined whether integrated sequences can undergo the early-late transition during infection of transformed cells. Cultures of 293 cells were infected with mutants of adenovirus type 5 that have deletions in EI genes. In such infections, the integrated sequence complements the deletion mutants so that viral DNA replication, late mRNA and protein synthesis, and viral assembly occur. Because the infecting genomes lack EI sequences, the products synthesized from the integrated DNA could be analyzed. In contrast to the early-late transition that occurs with EI DNA in free viral genomes, the pattern of mRNAs and proteins made from the integrated sequences was restricted to the early pattern. Assuming that the viral sequences in 293 cells have not become altered during the history of the cells, our results suggest that regulation of integrated adenovirus genes may not be determined exclusively by nucleotide sequence recognition. Apparently, during infection certain factors prevent the integrated viral genes from responding to the regulatory signals which control late expression from free EI DNA. The distinction between integrated and free viral sequences might reflect the different fates of viral and host transcripts during the lytic cycle of adenovirus.
Full text
PDF


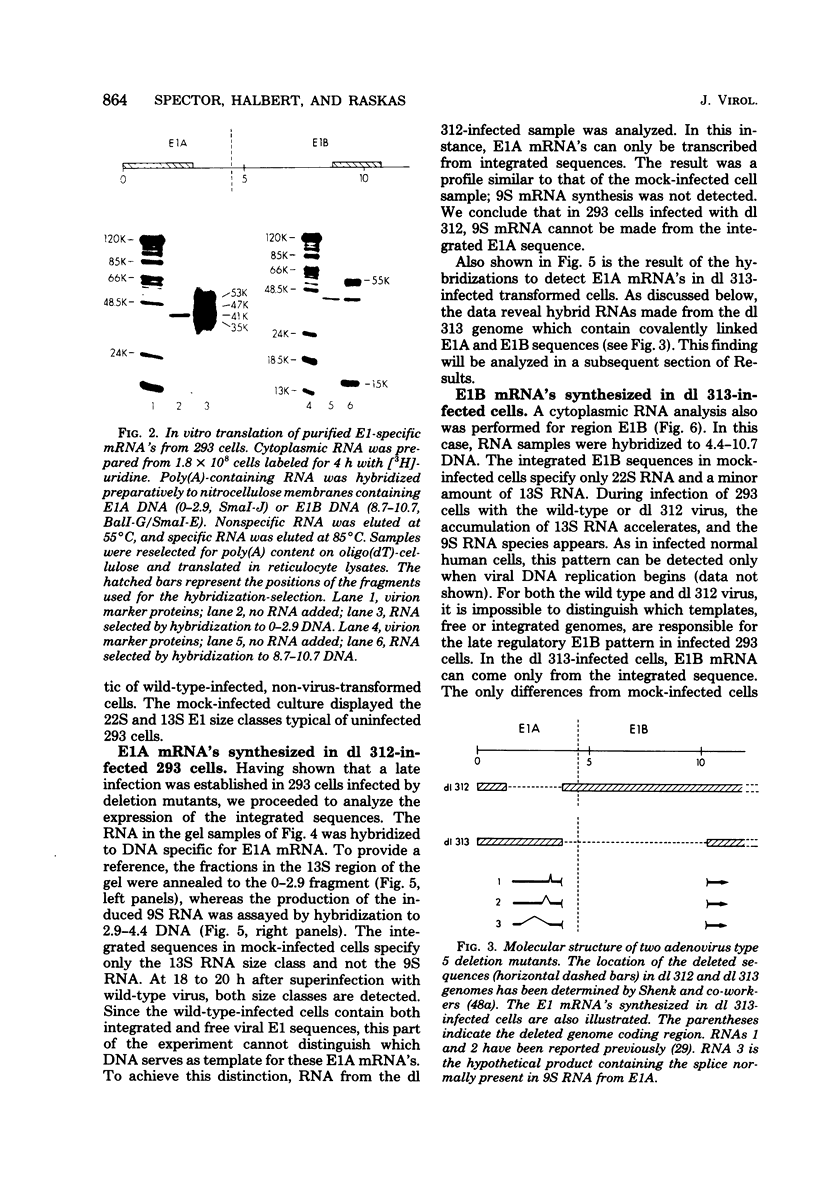
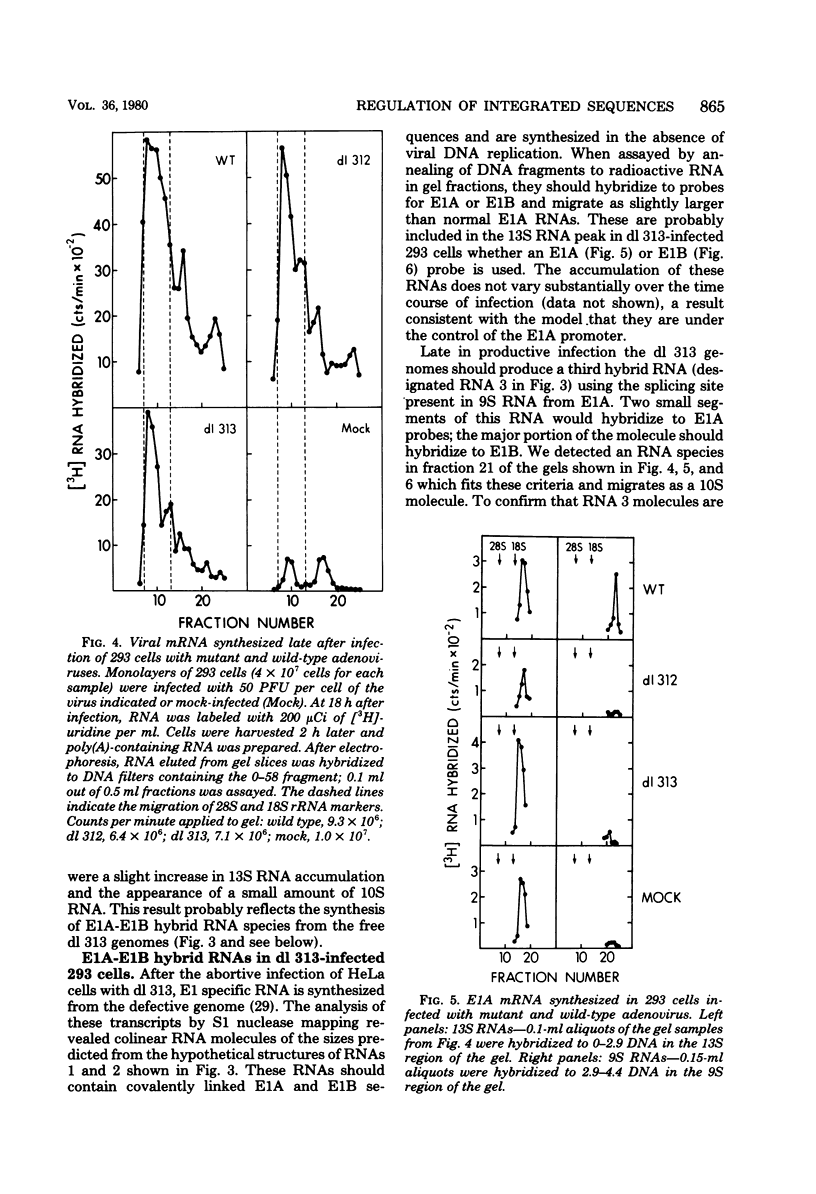
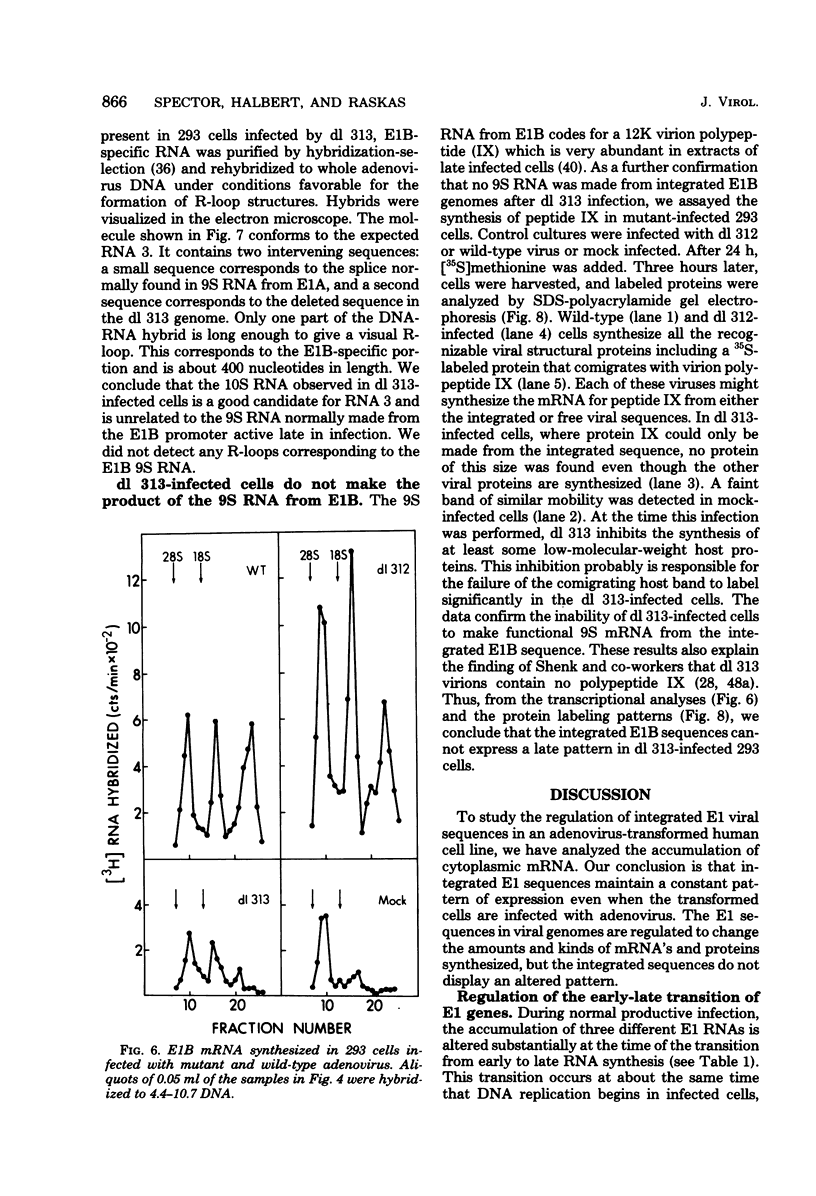
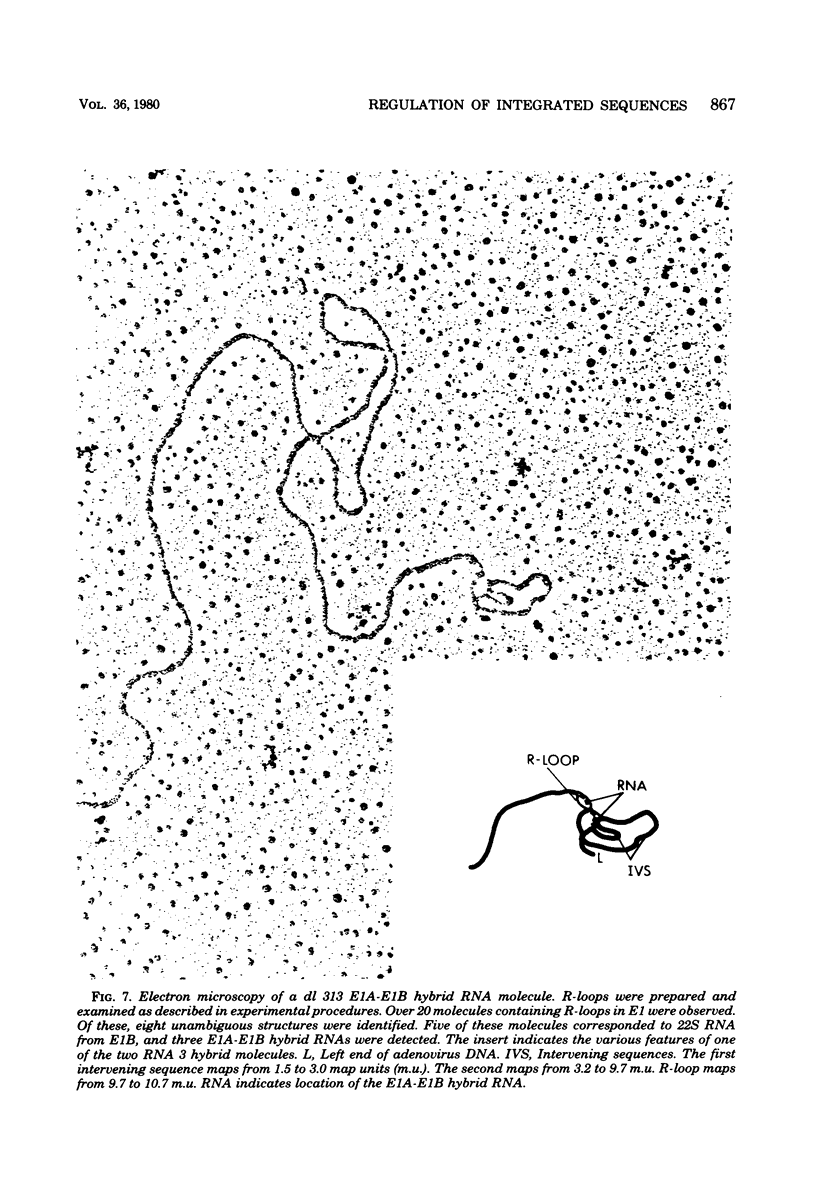



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiello L., Guilfoyle R., Huebner K., Weinmann R. Adenovirus 5 DNA sequences present and RNA sequences transcribed in transformed human embryo kidney cells (HEK-Ad-5 or 293). Virology. 1979 Apr 30;94(2):460–469. doi: 10.1016/0042-6822(79)90476-8. [DOI] [PubMed] [Google Scholar]
- Aleström P., Akusjärvi G., Perricaudet M., Mathews M. B., Klessig D. F., Pettersson U. The gene for polypeptide IX of adenovirus type 2 and its unspliced messenger RNA. Cell. 1980 Mar;19(3):671–681. doi: 10.1016/s0092-8674(80)80044-4. [DOI] [PubMed] [Google Scholar]
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK P. H., ROWE W. P., TURNER H. C., HUEBNER R. J. A SPECIFIC COMPLEMENT-FIXING ANTIGEN PRESENT IN SV40 TUMOR AND TRANSFORMED CELLS. Proc Natl Acad Sci U S A. 1963 Dec;50:1148–1156. doi: 10.1073/pnas.50.6.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello L. J., Ginsberg H. S. Inhibition of host protein synthesis in type 5 adenovirus-infected cells. J Virol. 1967 Oct;1(5):843–850. doi: 10.1128/jvi.1.5.843-850.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz G. A., Flint S. J. Inhibition of HeLa cell protein synthesis during adenovirus infection. Restriction of cellular messenger RNA sequences to the nucleus. J Mol Biol. 1979 Jun 25;131(2):353–373. doi: 10.1016/0022-2836(79)90081-0. [DOI] [PubMed] [Google Scholar]
- Benjamin T. L. Virus-specific RNA in cells productively infected or transformed by polyoma virus. J Mol Biol. 1966 Apr;16(2):359–373. doi: 10.1016/s0022-2836(66)80179-1. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Structure of the adenovirus 2 early mRNAs. Cell. 1978 Jul;14(3):695–711. doi: 10.1016/0092-8674(78)90252-0. [DOI] [PubMed] [Google Scholar]
- Brison O., Kédinger C., Chambon P. Adenovirus DNA template for late transcription is not a replicative intermediate. J Virol. 1979 Oct;32(1):91–97. doi: 10.1128/jvi.32.1.91-97.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson D. P., Raskas H. J. Structure and metabolism of adenovirus RNAs containing sequences from the fiber gene. J Mol Biol. 1980 Aug 15;141(3):249–265. doi: 10.1016/0022-2836(80)90180-1. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Broker T. R., Lewis J. B. Complex splicing patterns of RNAs from the early regions of adenovirus-2. J Mol Biol. 1979 Oct 25;134(2):265–303. doi: 10.1016/0022-2836(79)90036-6. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Roberts J. M., Lewis J. B., Broker T. R. A map of cytoplasmic RNA transcripts from lytic adenovirus type 2, determined by electron microscopy of RNA:DNA hybrids. Cell. 1977 Aug;11(4):819–836. doi: 10.1016/0092-8674(77)90294-x. [DOI] [PubMed] [Google Scholar]
- Craig E. A. Analysis of early adenovirus 2 RNA using Eco R-R1 viral DNA fragments. J Virol. 1975 May;15(5):1202–1213. doi: 10.1128/jvi.15.5.1202-1213.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Gel electrophoresis of avian leukosis and sarcoma viral RNA in formamide: comparison with other viral and cellular RNA species. J Virol. 1973 Sep;12(3):594–599. doi: 10.1128/jvi.12.3.594-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S. J., Sambrook J., Williams J. F., Sharp P. A. Viral nucleic acid sequences in transformed cells. IV. A study of the sequences of adenovirus 5 DNA and RNA in four lines of adenovirus 5-transformed rodent cells using specific fragments of the viral genome. Virology. 1976 Jul 15;72(2):456–470. doi: 10.1016/0042-6822(76)90174-4. [DOI] [PubMed] [Google Scholar]
- Freeman A. E., Black P. H., Vanderpool E. A., Henry P. H., Austin J. B., Huebner R. J. Transformation of primary rat embryo cells by adenovirus type 2. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1205–1212. doi: 10.1073/pnas.58.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga K., Green M. The mechanism of viral carcinogenesis by DNA mammalian viruses: viral-specific RNA in polyribosomes of adenovirus tumor and transformed cells. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1567–1574. doi: 10.1073/pnas.55.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Galos R. S., Williams J., Binger M. H., Flint S. J. Location of additional early gene sequences in the adenoviral chromosome. Cell. 1979 Aug;17(4):945–956. doi: 10.1016/0092-8674(79)90334-9. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Abrahams P. J., Mulder C., Heijneker H. L., Warnaar S. O., De Vries F. A., Fiers W., Van Der Eb A. J. Studies on in vitro transformation by DNA and DNA fragments of human adenoviruses and simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):637–650. doi: 10.1101/sqb.1974.039.01.077. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J., Heijneker H. L. Size and location of the transforming region in human adenovirus type 5 DNA. Nature. 1974 Oct 25;251(5477):687–691. doi: 10.1038/251687a0. [DOI] [PubMed] [Google Scholar]
- Halbert D. N., Spector D. J., Raskas H. J. In vitro translation products specified by the transforming region of adenovirus type 2. J Virol. 1979 Sep;31(3):621–629. doi: 10.1128/jvi.31.3.621-629.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P. R., Darnell J. E., Jr Differential accumulation of virus-specific RNA during the cell cycle of adenovirus-transformed rat embyro cells. J Virol. 1975 Apr;15(4):806–811. doi: 10.1128/jvi.15.4.806-811.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Kitchingman G. R., Lai S. P., Westphal H. Loop structures in hybrids of early RNA and the separated strands of adenovirus DNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4392–4395. doi: 10.1073/pnas.74.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maat J., Van Ormondt H. The nucleotide sequence of the transforming HindIII-G fragment of adenovirus type 5 DNA. The region between map positions 4.5 (HpaI site) and 8.0 (HindIII site). Gene. 1979 May;6(1):75–90. doi: 10.1016/0378-1119(79)90086-6. [DOI] [PubMed] [Google Scholar]
- Maat J., van Beveren C. P., van Ormondt H. The nucleotide sequence of adenovirus type 5 early region E1: the region between map positions 8.0 (HindIII site) and 11.8 (SmaI site). Gene. 1980 Jun;10(1):27–38. doi: 10.1016/0378-1119(80)90140-7. [DOI] [PubMed] [Google Scholar]
- Mackey J. K., Wold W. S., Rigden P., Green M. Transforming region of group A, B, and C adenoviruses: DNA homology studies with twenty-nine human adenovirus serotypes. J Virol. 1979 Mar;29(3):1056–1064. doi: 10.1128/jvi.29.3.1056-1064.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrogan M., Raskas H. J. Species identification and genome mapping of cytoplasmic adenovirus type 2 RNAs synthesized late in infection. J Virol. 1977 Aug;23(2):240–249. doi: 10.1128/jvi.23.2.240-249.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrogan M., Raskas H. J. Two regions of the adenovirus 2 genome specify families of late polysomal RNAs containing common sequences. Proc Natl Acad Sci U S A. 1978 Feb;75(2):625–629. doi: 10.1073/pnas.75.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrogan M., Spector D. J., Goldenberg C. J., Halbert D., Raskas H. J. Purification of specific adenovirus 2 RNAs by preparative hybridization and selective thermal elution. Nucleic Acids Res. 1979 Feb;6(2):593–607. doi: 10.1093/nar/6.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R., Darnell J. E. Groups of adenovirus type 2 mRNA's derived from a large primary transcript: probable nuclear origin and possible common 3' ends. J Virol. 1978 Mar;25(3):811–823. doi: 10.1128/jvi.25.3.811-823.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perricaudet M., Akusjärvi G., Virtanen A., Pettersson U. Structure of two spliced mRNAs from the transforming region of human subgroup C adenoviruses. Nature. 1979 Oct 25;281(5733):694–696. doi: 10.1038/281694a0. [DOI] [PubMed] [Google Scholar]
- Persson H., Pettersson U., Mathews M. B. Synthesis of a structural adenovirus polypeptide in the absence of viral DNA replication. Virology. 1978 Oct 1;90(1):67–79. doi: 10.1016/0042-6822(78)90334-3. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Mathews M. B. The gene and messenger RNA for adenovirus polypeptide IX. Cell. 1977 Nov;12(3):741–750. doi: 10.1016/0092-8674(77)90274-4. [DOI] [PubMed] [Google Scholar]
- Philipson L., Pettersson U., Lindberg U., Tibbetts C., Vennström B., Persson T. RNA synthesis and processing in adenovirus-infected cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):447–456. doi: 10.1101/sqb.1974.039.01.057. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Skehel J. J. The polypeptides of adenovirus-infected cells. J Gen Virol. 1972 Apr;15(1):45–57. doi: 10.1099/0022-1317-15-1-45. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Botchan M., Gallimore P., Ozanne B., Pettersson U., Williams J., Sharp P. A. Viral DNA sequences in cells transformed by simian virus 40, adenovirus type 2 and adenovirus type 5. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):615–632. doi: 10.1101/sqb.1974.039.01.075. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant A., Tigges M. A., Raskas H. J. Nucleosome-like structural subunits of intranuclear parental adenovirus type 2 DNA. J Virol. 1979 Mar;29(3):888–898. doi: 10.1128/jvi.29.3.888-898.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Gallimore P. H., Flint S. J. Mapping of adenovirus 2 RNA sequences in lytically infected cells and transformed cell lines. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):457–474. doi: 10.1101/sqb.1974.039.01.058. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Pettersson U., Sambrook J. Viral DNA in transformed cells. I. A study of the sequences of adenovirus 2 DNA in a line of transformed rat cells using specific fragments of the viral genome. J Mol Biol. 1974 Jul 15;86(4):709–726. doi: 10.1016/0022-2836(74)90348-9. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Shenk T., Jones N., Colby W., Fowlkes D. Functional analysis of adenovirus-5 host-range deletion mutants defective for transformation of rat embryo cells. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):367–375. doi: 10.1101/sqb.1980.044.01.041. [DOI] [PubMed] [Google Scholar]
- Spector D. J., Crossland L. D., Halbert D. N., Raskas H. J. A 28K polypeptide is the translation product of 9 S RNA encoded by region 1A of adenovirus 2. Virology. 1980 Apr 15;102(1):218–221. doi: 10.1016/0042-6822(80)90084-7. [DOI] [PubMed] [Google Scholar]
- Spector D. J., Halbert D. N., Crossland L. D., Raskas H. J. Expression of genes from the transforming region of adenovirus. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):437–445. doi: 10.1101/sqb.1980.044.01.047. [DOI] [PubMed] [Google Scholar]
- Spector D. J., McGrogan M., Raskas H. J. Regulation of the appearance of cytoplasmic RNAs from region 1 of the adenovirus 2 genome. J Mol Biol. 1978 Dec 15;126(3):395–414. doi: 10.1016/0022-2836(78)90048-7. [DOI] [PubMed] [Google Scholar]
- Tal J., Craig E. A., Zimmer S., Raskas H. J. Localization of adenovirus 2 messenger RNA's to segments of the viral genome defined by endonuclease R-R1. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4057–4061. doi: 10.1073/pnas.71.10.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate V. E., Philipson L. Parental adenovirus DNA accumulates in nucleosome-like structures in infected cells. Nucleic Acids Res. 1979 Jun 25;6(8):2769–2785. doi: 10.1093/nar/6.8.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., White R. L., Davis R. W. Hybridization of RNA to double-stranded DNA: formation of R-loops. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2294–2298. doi: 10.1073/pnas.73.7.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts C., Johansson K., Philipson L. Hydroxyapatite chromatography and formamide denaturation of adenovirus DNA. J Virol. 1973 Aug;12(2):218–225. doi: 10.1128/jvi.12.2.218-225.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ormondt H., Maat J., De Waard A., Van der Eb A. J. The nucleotide sequence of the transforming HpaI-E fragment of adenovirus type 5 DNA. Gene. 1978 Dec;4(4):309–328. doi: 10.1016/0378-1119(78)90048-3. [DOI] [PubMed] [Google Scholar]
- Wilson M. C., Sawicki S. G., Salditt-Georgieff M., Darnell J. E. Adenovirus type 2 mRNA in transformed cells: map positions and difference in transport time. J Virol. 1978 Jan;25(1):97–103. doi: 10.1128/jvi.25.1.97-103.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]