Abstract
Although the acute toxic effects of trichothecene mycotoxin deoxynivalenol (DON or vomitoxin), a known cause of human food poisoning, have been well characterized in several animal species, much less is known about closely related 8-ketotrichothecenes that similarly occur in cereal grains colonized by toxigenic fusaria. To address this, we compared potencies of DON, 15-acetyldeoxynivalenol (15-ADON), 3-acetyldeoxynivalenol (3-ADON), fusarenon X (FX), and nivalenol (NIV) in the mink emesis model following intraperitoneal (ip) and oral administration. All five congeners dose-dependently induced emesis by both administration methods. With increasing doses, there were marked decreases in latency to emesis with corresponding increases in emesis duration and number of emetic events. The effective doses resulting in emetic events in 50% of the animals for ip exposure to DON, 15-ADON, 3-ADON, FX, and NIV were 80, 170, 180, 70, and 60 µg/kg bw, respectively, and for oral exposure, they were 30, 40, 290, 30, and 250 µg/kg bw, respectively. The emetic potency of DON determined here was comparable to that reported in analogous studies conducted in pigs and dogs, suggesting that the mink is a suitable small animal model for investigating acute trichothecene toxicity. The use of a mouse pica model, based on the consumption of kaolin, was also evaluated as a possible surrogate for studying emesis but was found unsuitable. From a public health perspective, comparative emetic potency data derived from small animal models such as the mink should be useful for establishing toxic equivalency factors for DON and other trichothecenes.
Key Words: mycotoxin, trichothecene, emesis, deoxynivalenol, vomitoxin, 15-acetyldeoxynivalenol, 3-acetyldeoxynivalenol, fusarenon X, nivalenol.
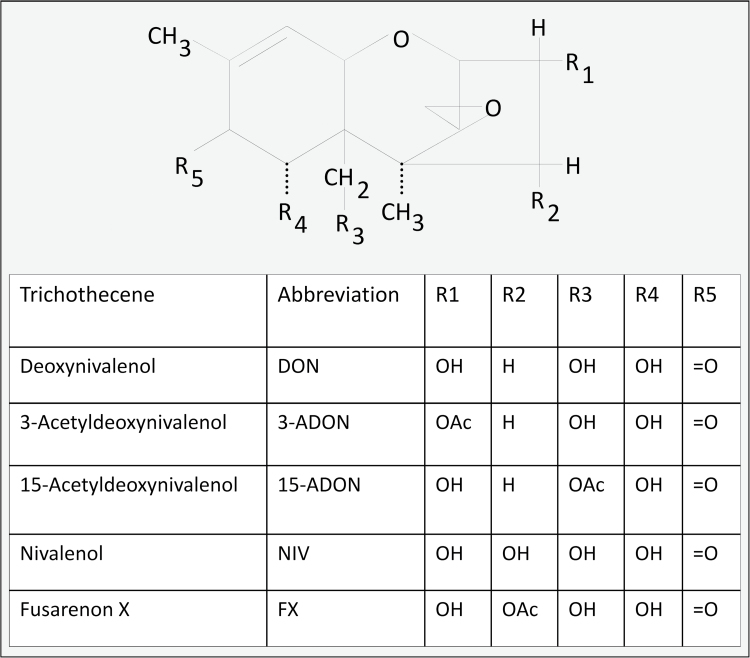
Food contamination by trichothecene mycotoxins has long been associated with human and animal toxicoses and remains a worldwide public health concern (Pestka, 2010a,b). The 8-ketotrichothecenes produced by the mold genus Fusarium are common contaminants of cereal grains. This family is characterized by having a keto group at carbon 8 of the parent epoxytrichothecene nucleus and includes five closely related congeners: (1) deoxynivalenol (DON), (2) 15-acetyldeoxynivalenol (15-ADON), (3) 3-acetyldeoxynivalenol (3-ADON), (4) fusarenon X (FX; 4-acetylnivalenol), and (5) nivalenol (NIV) (Fig. 1).
FIG. 1.
Structures of 8-ketotrichothecenes.
Adverse effects of trichothecenes that have been reported in experimental animal studies include emesis, nausea, anor- exia, growth retardation, neuroendocrine changes, and immunosuppression (Pestka, 2010a). Investigations of human food poisoning outbreaks that have been etiologically linked to trichothecene exposure have identified nausea and vomiting as primary symptoms (Luo, 1994; Ueno, 1987; Yoshizawa, 1983). Given the high relevance of the emetic response to human food poisoning, it is surprising that there have been relatively few studies on this effect in experimental animals with respect to DON and even fewer for the other 8-ketotrichothecenes. Defined as a forceful expulsion of the contents of the gastrointestinal tract though the mouth and nose, emesis serves as a reflex defense mechanism to protect the body from absorption of ingested toxins (Andrews and Hawthorn, 1988). Severe emesis can disrupt normal nutrition, hydration, and electrolyte balance, thereby having serious implications for human and animal health.
DON, the most commonly encountered 8-ketotrichothecene in food, was discovered 40 years ago (Yoshizawa and Morooka, 1973) and is colloquially referred to as “vomitoxin” because of its potent emetic effects in pigs consuming Fusarium-contaminated feed (Vesonder et al., 1973). Indeed, DON readily induces vomiting in several animal models following oral, intraperitoneal (ip), intravenous (iv), and subcutaneous (sc) exposure (Friend et al., 1982; Forsyth et al., 1977; Hughes et al., 1999; Pestka et al., 1987; Prelusky and Trenholm, 1993; Ueno et al., 1974; Yoshizawa and Morooka, 1977; Young et al., 1983). The 8-ketotrichothecenes 15-ADON, 3-ADON, FX, and NIV have also been reported to cause vomiting in experimental animals (Hedman et al., 1997; Matsuoka et al., 1979; Pestka et al., 1987; Ueno et al., 1971, 1974; Yoshizawa and Morooka, 1974 , 1977). However, to date, the emetic capacities of these latter congeners have not been systematically compared with respect to DON in a common animal model, making it impossible to accurately predict their relative potential to induce nausea and vomiting in humans and animals.
Although mice and rat models are frequently used for toxicology research, they lack an emetic reflex (Horn, 2008). Two surrogate endpoints that have been proposed to be predictive of nausea or an emetic-like sickness response in rodents are feed refusal and pica (Andrews and Horn, 2006; Takeda et al., 1993; Vera et al., 2006). We have developed a mouse anorexia bioassay for DON (Flannery et al., 2011) that has been recently applied to the comparison of the 8-ketotrichothecenes (Wu et al., 2012), however, the direct relevance of these latter findings to emesis has yet to be determined. Pica behavior, defined as ingestion of nonnutritional clay such as kaolin, is also considered as an indicator of nausea in rodents (du Sert et al., 2012). DON recently has been reported to increase kaolin intake in mice following oral exposure (Girardet et al., 2011), suggesting that pica behavior might be useful for comparing emetic potencies of trichothecenes.
Species with a vomiting reflex used to study trichothecene-induced emesis include pigs, dogs, cats, and ducklings. Pigs are of interest because of their agricultural relevance and the similarity of their gastrointestinal systems to those of humans (Szelenyi et al., 1994). However, this species offers challenges because of its size and expense. Dogs and cats are also sensitive to DON, and both species have been involved in food poisoning outbreaks via contaminated pet food (Hughes et al., 1999). Ethical issues of using companion species and maintenance costs for such studies present complications for their use as emesis models (du Sert et al., 2012). Finally, ducklings are relatively insensitive to trichothecenes (Ueno et al., 1971, 1974; Yoshizawa and Morooka, 1974 , 1977) and thus not practical for emesis studies.
Small-animal alternatives to the above-mentioned models include the mink (Neovison vison) and ferret (Mustela putorius furo), both of which belong to the Mustelidae family. These species display emetic behavior analogous to that reported for humans and thus are applicable to studying emetogenic compounds (du Sert et al., 2011; du Sert et al., 2011; Qian et al., 2009, 2010; Zhang et al., 2006). Notably, we have previously observed that mink refuse to consume food contaminated with DON (Gibson et al., 1993), suggesting that this species is sensitive to this toxin.
The intent of this study was to compare the potencies of 8-ketotrichothecenes in the mouse pica and mink emesis models. The results suggest that the mouse pica model would not be a satisfactory approach for predicting emesis in susceptible species. In contrast, studies in the mink indicated that (1) this species exhibited similar responses to DON as pigs and dogs, (2) the five major 8-ketotrichothecenes induced vomiting, (3) latency to emesis decreased and frequency of emetic events increased as doses were increased, and (4) the emetic responses to the congeners were differentially affected by ip and oral exposure.
MATERIALS AND METHODS
Toxins.
The 8-ketotrichothecenes were isolated, and purity (>98%) was verified by elemental analysis and LC-MS as previously described (Wu et al., 2012). All toxins were dissolved in filter-sterilized phosphate buffered saline (PBS).
Mouse pica study.
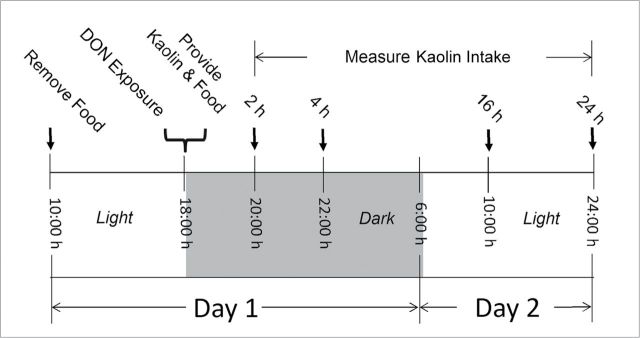
Male B6C3F1 mice (10-week old) were obtained from Charles River Breeding (Portage, MI) and housed individually in polycarbonate cages in a room with temperature at 21°C–24°C, relative humidity at 40–55%, and lights on a 12-h cycle (6:00–18:00h)/dark (18:00–6:00h). Animal treatment followed National Institutes of Health guidelines and were approved by the Michigan State University Institutional Animal Care and Use Committee (MSU-IACUC). The general experimental design for the pica study (Fig. 2) was based on previously described protocols (Flannery et al., 2011; Girardet et al., 2011). To minimize stress to the animals, all procedures were consistently and rapidly conducted. Briefly, mice were acclimated for 1 week after arriving and randomly divided into different groups according to body weight 1 day prior to the experiment. On the day of the experiment, groups of mice (n = 5) were fasted from 10:00h to 18:00h (water provided ad lib) and dosed with 0, 2.5, 5, and 10mg/kg bw DON in 100-µl PBS by oral gavage using a sterile 22 G 1.5″ disposable feeding tube (Instech Solomon, Plymouth Meeting, PA). Following DON treatment, mice were then immediately provided two preweighed kaolin pellets (≈ 4g) and six preweighed food pellets (≈ 20g) (Research Diets Inc., New Brunswick, NJ) in two separate 2″ high glass jars. Kaolin intake was measured at intervals for 24h after gavage.
FIG. 2.
Experimental design for pica bioassay in mice.
Mink emesis studies.
Experiments were performed using sixty 1- to 2-year-old, standard dark, female mink bred and housed at the Michigan State University Experimental Fur Farm. Housing of animals was according to guidelines specified in the Standard Guidelines for the Operation of Mink Farms in the United States (Fur Commission USA, 2010) and was approved by MSU-IACUC. Prior to the first experiment, the animals were acclimated for 1 week and fed the MSU Experimental Fur Farm ranch diet, which was formulated to meet the nutrient requirements of mink (Zhang et al., 2009). The mink recovered rapidly after trichothecene exposures and exhibited normal appetite within 24h after challenge. Therefore, to minimize the number of animals used, the mink were rested for a minimum of 1 week between experiments and were randomized and reused in subsequent trials.
For the ip exposure study (Fig. 3A), conducted in March and April, 2011, mink were housed individually in suspended wire cages (76-cm long × 61-cm wide × 46-cm high) in an enclosed barn. A wooden nest box (38-cm long × 28-cm wide × 27-cm high) bedded with aspen shavings and excelsior (wood wool) was attached to the outside of each cage. Ventilation was provided by two wall fans and ceiling vents. Room temperature was kept above 0°C by two thermostatically controlled heaters. For emesis trials, mink were fasted for 24h (water available ad lib) prior to conducting the experiment in order to maintain a constant volume of gastric contents in each animal. On the day of experiment, mink were provided 50-g feed at 12:00h, which was consumed rapidly. After 30min (12:30h), mink were given either toxin or PBS in a volume of 1ml/kg bw via ip injection using a sterile 20-G, 2.54-cm needle. After dosing, animals were returned to individual cages, food and water were provided ad lib, and emesis was monitored for 6h. During the observation period, the incidence of emesis, latency to emesis, emesis duration, and number of emetic events were recorded. Incidence is defined as the ratio of animals exhibiting emesis to total test animals. Latency to emesis refers to the time from dosing toxins to the first emetic event, whereas emesis duration is the time from the first occurrence of emesis to the end of the last emesis. An emetic event was characterized as either vomiting or retching. Vomiting is defined as rhythmic abdominal contraction with oral expulsion of either solid or liquid material, whereas retching refers to responses that mimicked vomiting but without any material being expelled (Hasegawa et al., 2002).
FIG. 3.
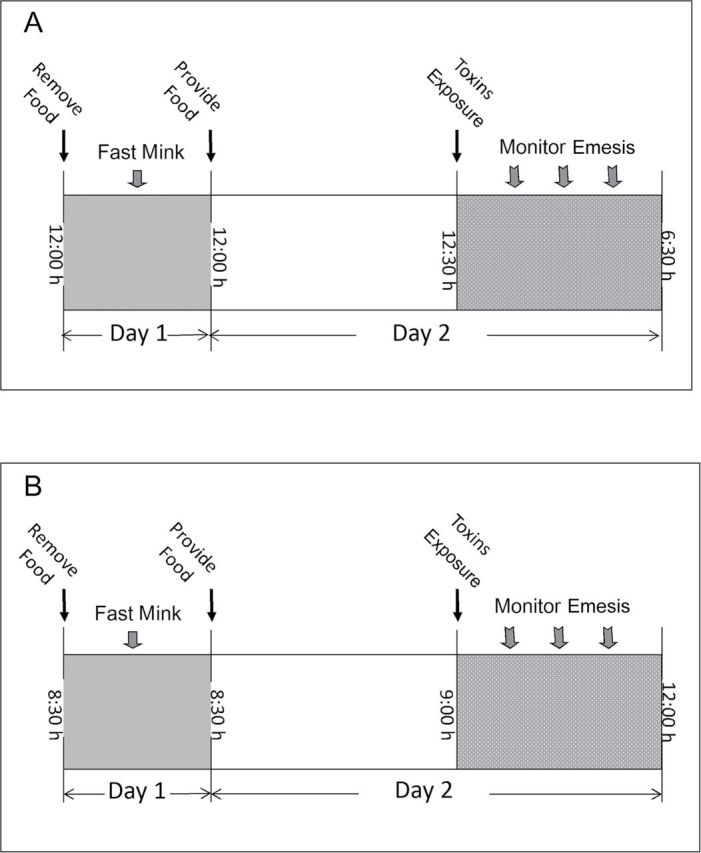
Experimental design for emesis bioassay following ip (A) and oral (B) exposure.
For the oral exposure study (Fig. 3B), conducted in June and July, 2011, the same 60 mink were housed singly in wire cages (62-cm long × 25-cm wide × 38-cm high) and provided with a nest box (24-cm long × 24-cm wide × 29-cm high) bedded with aspen shavings and excelsior within a shaded, open-sided pole barn. Temperature, humidity, and photoperiod were dependent on ambient summer conditions. In an attempt to minimize the effect of higher midday ambient temperatures during the summer, experiments were initiated in the morning (8:30a.m.). Mink were fasted for 24h prior to dosing (water available ad lib) and provided 50g of feed at 8:30h on the day of experiment. After 30min (9:00h), mink were administered either toxin or saline (1ml/kg bw) via oral gavage using a sterile 16-G, 5-cm stainless steel gavage tube. Animals were then monitored for emesis over the subsequent 3h as described above.
A follow-up study was conducted in June, 2012, to directly compare emetic responses in 30 new female mink (1-year old) following ip and oral exposure to DON as described above to assess seasonal differences.
Data analysis.
The emetic dose (ED) was determined with Proc Probit using SAS (Version 9.2, SAS, Cary, NC). All other data were plotted and statistically analyzed using SigmaPlot 11 for Windows (Jandel Scientific, San Rafael, CA). Means were considered significantly different at p < 0.05. In the mouse pica model, a two-way repeated ANOVA (dose and time) using Bonferroni t-test was used to assess significant differences in kaolin intake. For dose-dependent emesis studies, Fischer’s Exact Test was used for incidence, retching, vomiting, and total emetic events. A t-test or one-way ANOVA with Tukey test was used to determine significant differences within doses for latency and duration. A two-way ANOVA using Bonferroni t-test was used to assess significant differences in mean cumulative emetic events.
RESULTS
Effects of DON on Kaolin Consumption in the Mouse
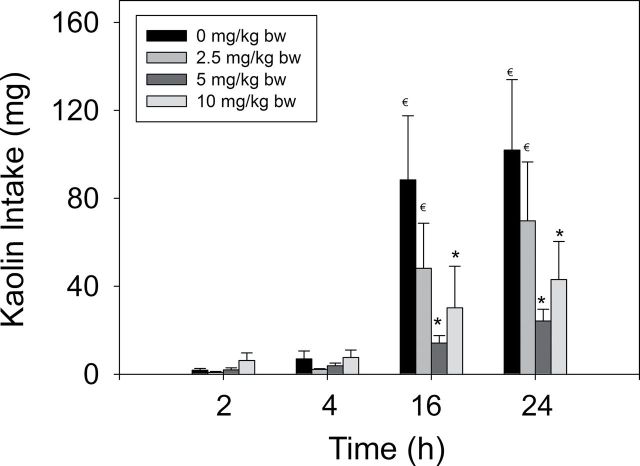
Kaolin consumption after 2 and 4h was not significantly affected by ip exposure to DON at 2.5–10mg/kg bw (p < 0.05) (Fig. 4). Rather than being increased, cumulative kaolin intakes after 16 and 24h were reduced by 60–80% following exposure to 5 and 10mg/kg bw DON, respectively. These results were not consistent with the induction of pica for kaolin by DON model. It was therefore concluded that this rodent model was not a suitable substitute for predicting nausea and emesis in other species and therefore was not employed in subsequent studies.
FIG. 4.
Effect of DON on kaolin intake. Data represent mean ± SEM (n = 8 per group). Symbols: * indicates statistically significant differences in cumulative emetic episodes compared with the control (p < 0.05). € indicates a statistically significant difference relative to the 2-h time point within a given dose (p < 0.05).
Emetic Effects of ip Exposure of Mink to 8-Ketotrichothecenes
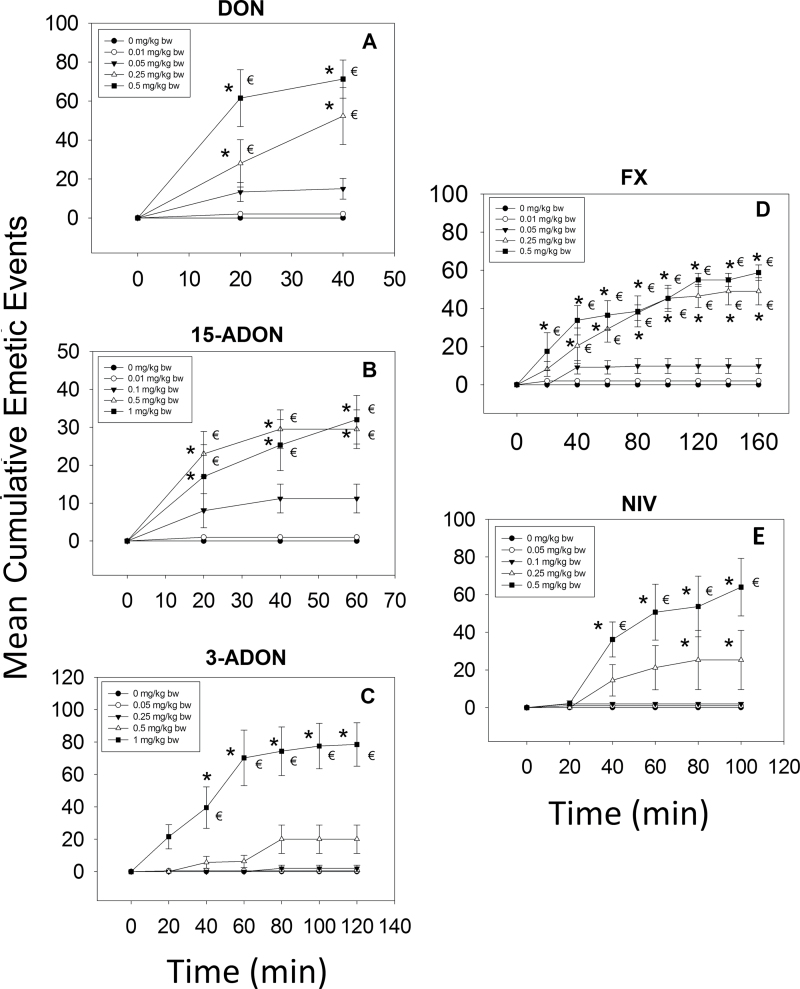
The lowest dose at which DON induced emesis in mink following ip exposure was 0.1mg/kg bw, with 50% of the animals responding (Table 1). After exposure to DON at 0.25mg/kg bw, all animals vomited. The majority of emetic episodes occurred within 20min, although new emetic events were detectable up to 80min at the 0.25-mg/kg bw dose (Fig. 5A).
TABLE 1.
Comparison of Emetic Responses in Mink Following ip Exposure to 8-Ketotrichothecenes
| Toxin | Dose (mg/kg bw) | Incidence (responding/tested) | Latency to emesis (min)a,b | Emesis duration (min)a,b | Emetic eventsc | ||
|---|---|---|---|---|---|---|---|
| Retching | Vomiting | Total | |||||
| Control | 0 | 0/26 | - | - | 0±0 | 0±0 | 0±0 |
| DON | 0.025 | 0/6 | - | - | 0±0 | 0±0 | 0±0 |
| 0.05 | 0/6 | - | - | 0±0 | 0±0 | 0±0 | |
| 0.1 | 3/6 | 14±2 | 6±3 | 14±8 | 4±2 | 17±9 | |
| 0.25* | 6/6 | 10±2 | 38±5 | 94±13 | 16±2 | 110±14 | |
| 3-ADON | 0.1 | 0/6 | - | - | 0±0 | 0±0 | 0±0 |
| 0.2 | 3/6 | 36±14 | 3±2 | 7±4 | 2±1 | 9±5 | |
| 0.3* | 5/6 | 48±9 | 26±7 | 28±8 | 6±2 | 34±9 | |
| 0.4* | 6/6 | 19±4 | 54±2 | 81±10 | 16±2 | 97±12 | |
| 15-ADON | 0.1 | 0/6 | - | - | 0±0 | 0±0 | 0±0 |
| 0.25* | 5/6 | 12±2 | 3±1 | 26±8 | 4±1 | 31±9 | |
| 0.5* | 5/6 | 11±1 | 24±8 | 38±9 | 7±2 | 45±11 | |
| 1* | 6/6 | 8±1 | 32±5 | 58±5 | 13±3 | 71±7 | |
| FX | 0.025 | 0/6 | - | - | 0±0 | 0±0 | 0±0 |
| 0.05 | 0/6 | - | - | 0±0 | 0±0 | 0±0 | |
| 0.1* | 5/6 | 63±19 | 22±4 | 18±5 | 5±1 | 23±6 | |
| 0.25* | 6/6 | 20±4 | 92±22 | 136±8 | 26±3 | 162±10 | |
| NIV | 0.01 | 0/6 | - | - | 0±0 | 0±0 | 0±0 |
| 0.05 | 0/6 | - | - | 0±0 | 0±0 | 0±0 | |
| 0.1* | 6/6 | 83±8 | 56±23 | 33±7 | 9±2 | 42±9 | |
| 0.25* | 6/6 | 29±5 | 158±12 | 132±20 | 30±2 | 162±20 | |
aAverage of positive responders only.
bIf animals failed to retch or vomit, the latency and duration of emesis are shown as “-.”
cAverage of both responders and nonresponders. Data represent the mean ± SEM. Values with an asterisk indicate insignificant differences at p < 0.05 relative to the control for incidence, retching, vomits, and total emetic events.
FIG. 5.
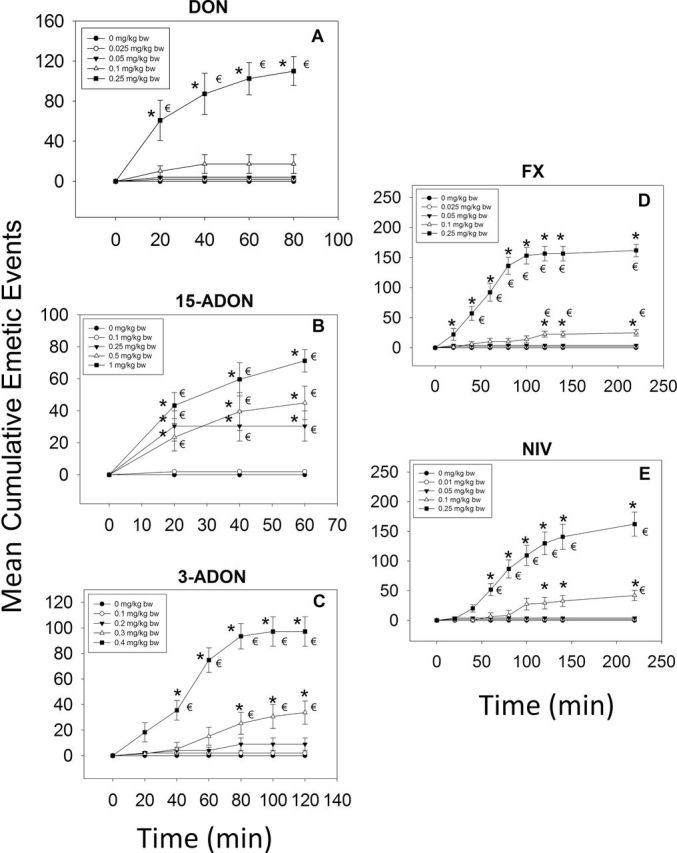
Mean cumulative emetic events in mink following ip exposure to 8-ketotrichothecenes. Data are averages for both responders and nonresponders and represent mean ± SEM (n = 6 per group). Symbols: * indicates statistically significant differences in cumulative emetic episodes compared with the control (p < 0.05). € indicates a statistically significant difference relative to the 0-min time point within a given dose (p < 0.05).
Following ip administration of 15-ADON, the minimum emetic dose was 0.25mg/kg bw, with 83% of the treated mink experiencing emesis (Table 1). When the dose was increased to 1mg/kg bw, all mink vomited. Throughout the dose range of 0.25–1.0mg/kg bw, emetic responses began within 10–15min after treatment and ended by 60min (Fig. 5B). When 3-ADON was administered ip, doses of 0.2, 0.3, and 0.4mg/kg bw caused emesis in 50, 83, and 100% of the mink, respectively, whereas 0.1-mg 3-ADON/kg bw had no effect (Table 1). The majority of emetic events occurred within the first 60min at a dose of 0.4mg/kg bw and within the first 80min at doses of 0.2 and 0.3mg/kg bw (Fig. 5C).
Upon ip exposure to 0.1 and 0.25mg/kg bw, FX induced emesis in 83 and 100% of the mink, respectively, whereas 0.025 and 0.05mg/kg bw had no effect (Table 1). The two high doses evoked emesis within 20min with no emetic events recorded after 140min (Fig. 5D). Mink dosed ip with NIV at 0.01 and 0.05mg/kg bw did not exhibit emesis, whereas exposure to 0.1 and 0.25mg/kg bw induced emesis in all animals within these groups (Table 1). The dose of 0.1mg/kg bw induced emesis within 60min, which continued up to 220min postdosing (Fig. 5E), whereas 0.25mg/kg bw caused emesis to occur within 40min and to persist until 220min postdosing.
Emetic Effects of Oral Exposure to 8-Ketotrichothecenes
When DON was administered orally, no effects were observed at 0.01mg/kg bw, whereas the 0.05 mg/kg bw dose caused 83% of the exposed mink to vomit (Table 2). Increasing the dose to 0.25 or 0.5mg/kg bw evoked emesis in all animals. Most emetic events were observed within 20min and lasted up to 40min (Fig. 6A).
TABLE 2.
Comparison of Emetic Responses in Mink Following Oral Exposure to 8-Ketotrichothecenes
| Toxin | Dose (mg/kg bw) | Incidence (responding/tested) | Latency to emesis (min)a,b | Emesis duration (min)a,b | Emetic eventsc | ||
|---|---|---|---|---|---|---|---|
| Retching | Vomiting | Total | |||||
| Control | 0 | 0/30 | - | - | 0±0 | 0±0 | 0±0 |
| DON | 0.01 | 0/6 | - | - | 0±0 | 0±0 | 0±0 |
| 0.05* | 5/6 | 15±2 | 1±1 | 12±4 | 3±1 | 15±5 | |
| 0.25* | 6/6 | 17±2 | 6±1 | 44±13 | 8±1 | 52±15 | |
| 0.5* | 6/6 | 11±3 | 14±3 | 62±9 | 9±1 | 71±10 | |
| 3-ADON | 0.05 | 0/6 | - | - | 0±0 | 0±0 | 0±0 |
| 0.25 | 1/6 | 62±0 | 1±0 | 2±2 | 1±1 | 3±3 | |
| 0.5* | 5/6 | 53±9 | 12±9 | 16±8 | 4±1 | 20±9 | |
| 1* | 6/6 | 19±5 | 44±9 | 65±11 | 14±2 | 79±13 | |
| 15-ADON | 0.01 | 0/6 | - | - | 0±0 | 0±0 | 0±0 |
| 0.1* | 5/6 | 20±4 | 1±1 | 9±3 | 2±1 | 11±4 | |
| 0.5* | 6/6 | 18±3 | 3±1 | 24±5 | 6±1 | 30±5 | |
| 1* | 6/6 | 17±4 | 14±7 | 26±5 | 6±1 | 32±6 | |
| FX | 0.01 | 0/6 | - | - | 0±0 | 0±0 | 0±0 |
| 0.05* | 4/6 | 23±2 | 13±10 | 8±3 | 2±1 | 10±4 | |
| 0.25* | 6/6 | 36±10 | 34±11 | 38±5 | 11±2 | 49±7 | |
| 0.5* | 6/6 | 15±3 | 63±21 | 45±4 | 13±2 | 58±4 | |
| NIV | 0.05 | 0/6 | - | - | 0±0 | 0±0 | 0±0 |
| 0.1 | 0/6 | - | - | 0±0 | 0±0 | 0±0 | |
| 0.25* | 4/6 | 31±4 | 15±10 | 21±14 | 4±2 | 25±16 | |
| 0.5* | 6/6 | 29±4 | 32±10 | 55±14 | 9±2 | 64±15 | |
Average of positive responders only.
If animals failed to retch or vomit, the latency and duration of emesis are shown as “-.”
Average of both responders and nonresponders. Data represent the mean ± SEM. Values with an asterisk indicate insignificant differences at p < 0.05 relative to the control for incidence, retching, vomits, and total emetic events.
FIG. 6.
Mean cumulative emetic events in mink following oral exposure to 8-ketotrichothecenes. Data are averages for both responders and nonresponders and represent mean ± SEM (n = 6 per group). Symbols: * indicates statistically significant differences in cumulative emetic episodes compared with the control (p < 0.05). € indicates a statistically significant difference relative to the 0-min time point within a given dose (p < 0.05).
The lowest oral dose of 15-ADON that caused emesis was 0.1mg/kg bw with 83% of the mink responding to the treatment (Table 2). Doses of 0.5 and 1.0mg/kg bw 15-ADON induced emesis in all mink. Emesis typically occurred within 20min, and new emetic events were detectable only up to 60min (Fig. 6B). Oral dosing with 0.25, 0.5, and 1.0 mg/kg bw 3-ADON induced emesis in 17, 83, and 100% of the mink, respectively, whereas 0.05mg/kg bw had no effect (Table 2). New emetic events were not apparent after 80min (Fig. 6C).
Following oral dosing with FX at 0.05, 0.25, and 0.5mg/kg bw, 67, 100, and 100% mink experienced emesis, respectively (Table 2). The 0.05 mg/kg bw dose induced emesis between 40 and 80min postdosing (Fig. 6D). Doses of 0.25 and 0.5mg/kg bw both caused emesis within 20min, and emetic events continued until 140 and 160min postdosing, respectively. When mink were treated orally with NIV, no emesis occurred at doses of 0.05 and 0.1mg/kg bw, whereas doses of 0.25 and 0.5mg/kg bw induced emesis in 67 and 100% of the mink, respectively (Table 2). Dosing with 0.25 mg/kg bw NIV caused emesis between 40 and 80min postdosing (Fig. 6E). The 0.5-mg/kg bw dose also caused emesis within 40min, but emesis continued up to 100 min postdosing.
Comparison of Emetic Responses to DON Following Oral and ip Exposure
A follow-up study was conducted to compare the emetic responses following concurrent ip and oral exposures to DON at 0, 0.025, 0.05, 0.1, and 0.25mg/kg bw (Table 3). Mink exposed ip to DON to 0.05mg/kg bw had no effect, whereas exposure to 0.10 and 0.25mg/kg bw caused vomiting in 66 and 100% of the mink, respectively. In comparison, oral exposure up to 0.025 mg/kg bw DON had no effect, whereas 0.05, 0.1, and 0.25mg/kg bw caused vomiting in 33, 66 mitigating any seasonal effect, and 100% of the animals. Latency, duration, and number of emetic events for the two exposure regimens were thus comparable to those described above (Tables 1 and 2) mitigating any seasonal effect.
TABLE 3.
Comparison of Emetic Responses in Mink Following ip and Oral Exposure
| Toxin DON | Dose (mg/kg bw) | Incidence (responding/tested) | Latency to emesis (min)a,b | Emesis duration (min)a,b | Emetic eventsc | ||
|---|---|---|---|---|---|---|---|
| Retching | Vomiting | Total | |||||
| IP | 0 | 0/3 | - | - | 0±0d | 0±0d | 0±0d |
| 0.025 | 0/3 | - | - | 0±0d | 0±0d | 0±0d | |
| 0.05 | 0/3 | - | - | 0±0d | 0±0d | 0±0d | |
| 0.1 | 2/3 | 13±2 | 9±4 | 37±29e | 7±5e | 44±35e | |
| 0.25 | 3/3 | 11±1 | 48±4 | 84±4e | 20±1e | 105±3e | |
| Oral | 0 | 0/3 | - | - | 0±0d | 0±0d | 0±0d |
| 0.025 | 0/3 | - | - | 0±0d | 0±0d | 0±0d | |
| 0.05 | 1/3 | 8±0 | 7.5±0 | 24±24e | 4±4e | 28±28e | |
| 0.1 | 2/3 | 14±6 | 5±0.3 | 23±14e | 7±4e | 30±16e | |
| 0.25 | 3/3 | 11±3 | 5±2 | 44±23e | 10±1e | 55±24e | |
aAverage of positive responders only.
bIf animals failed to retch or vomit, the latency and duration of emesis is shown as “-.”
cAverage of both responders and nonresponders. Data represent the mean ± SEM. Values without the same superscript within a column differ (p < 0.05).
d,eDifferent letters indicate a statistically significant difference between dose groups.
DISCUSSION
Although vomiting is a hallmark of human food poisoning by 8-ketotrichothecenes, there have been relatively few investigations of their comparative effects because of the challenges inherent in conducting emesis studies in large animal species such as pigs and dogs. This study is novel because it is the first to employ a small-animal model, the mink, to characterize trichothecene-induced emesis. In addition, it is the first to systematically compare the emetogenic potential of the 8-ketotrichothecenes in a common animal model.
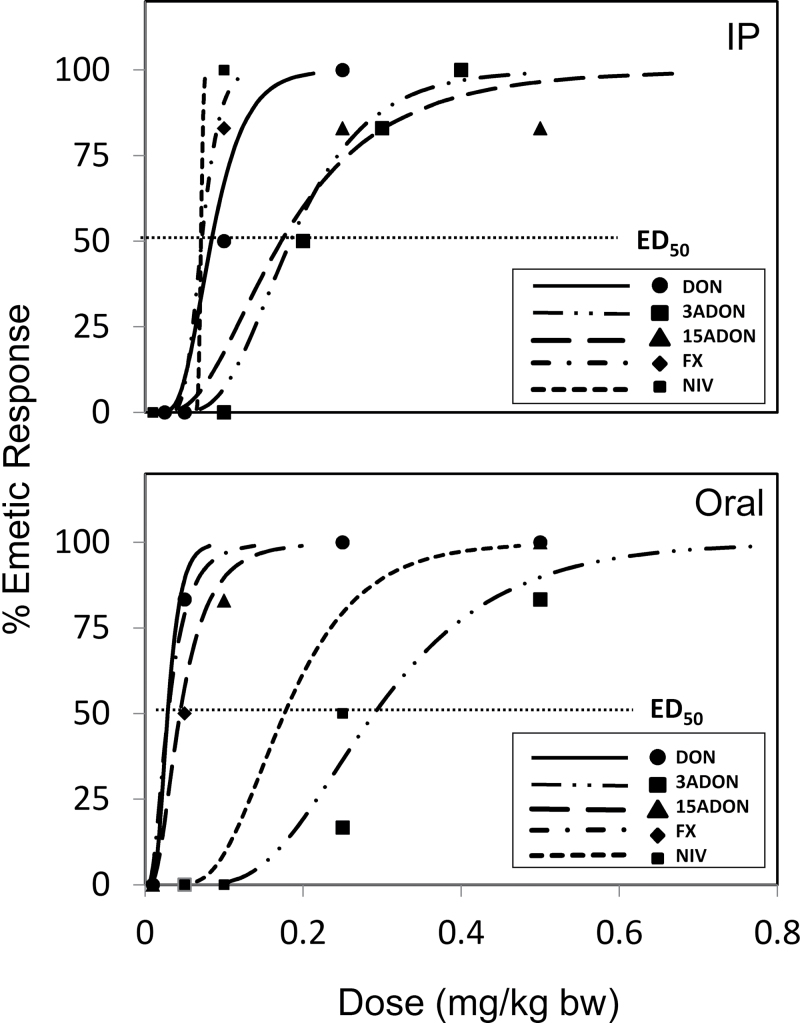
Incidence, latency, duration, and intensity of emetic events are the key considerations when characterizing potencies of emesis-inducing chemicals (Kris et al., 2006). Using incidence data, we determined each toxin’s no-observed adverse effect level (NOAEL) and lowest observed effect level (LOAEL) as well as its ED50 value (Fig. 7 and Table 4). The NOAEL and LOAEL of DON (0.05 and 0.1mg/kg bw for ip exposure, respectively, and 0.01 and 0.05mg/kg bw for oral exposure, respectively) were similar to those reported previously in studies with other species (Table 5). For example, it was reported that a NOAEL and LOAEL for DON-induced emesis following ip exposure in pigs were 0.025 and 0.05mg/kg bw, respectively, and 0.075 and 0.1mg/kg bw after oral exposure, respectively (Forsyth et al., 1977). We similarly reported a NOAEL and LOAEL of 0.025 and 0.05mg/kg bw for both ip and oral exposure of pigs to DON (Pestka et al., 1987). The EDs50 observed here for DON (0.08mg/kg bw, ip; 0.03mg/kg bw, oral) were also generally consistent with those reported for pigs (0.02, mg/kg bw, iv; 0.075mg/kg bw, oral) (Prelusky and Trenholm, 1993; Young et al., 1987).
FIG. 7.
Emetic dose curves for 8-ketotrichothecenes following ip and oral exposure.
TABLE 4.
Summary of NOAELs, LOAELs, and EDs50 for Emetic Effects of 8-Ketotrichothecenes
| Intraperitoneal | Oral | |||||
|---|---|---|---|---|---|---|
| Toxin | NOAELa | LOAELb | ED50 c | NOAELa | LOAELb | ED50 c |
| DON | 0.05 | 0.1 | 0.08 (0.05–0.18) | 0.01 | 0.05 | 0.03 (0–0.05) |
| 15-ADON | 0.1 | 0.25 | 0.17 (0.07–0.29) | 0.01 | 0.1 | 0.04 (0–0.11) |
| 3-ADON | 0.1 | 0.2 | 0.18 (0.1–0.27) | 0.05 | 0.25 | 0.29 (0.01–0.47) |
| FX | 0.05 | 0.1 | 0.07 (0.04–0.1) | 0.01 | 0.05 | 0.03 (0.01–0.08) |
| NIV | 0.05 | 0.1 | 0.06 (0–0) | 0.1 | 0.25 | 0.18 (0.1–0.29) |
aNOAEL = no observed adverse effect level.
bLOAEL = lowest observed adverse effect level.
cED50 = Dose causing emesis in 50% of the animals tested. ED50 values were determined using a Proc Probit model.
TABLE 5.
Previously Reported Emetic Effects of 8-Ketotrichothecenes
| Toxin | Species | Route | NOAEL (mg/kg bw) | LOAEL (mg/kg bw) | References |
|---|---|---|---|---|---|
| DON | Pig | ip | n.d. | 0.05 | Vesonder et al., 1973 |
| DON | Pig | oral | 0.075 | 0.1 | Forsyth et al., 1977 |
| ip | 0.025 | 0.05 | |||
| DON | Pig | ip | 0.025 | 0.05 | Pestka et al., 1987 |
| DON | Pig | oral | 0.025 | 0.05 | Prelusky et al., 1993 |
| iv | 0.015 | 0.02 | |||
| DON | Dog | sc | n.d. | 0.1 | Yoshizawa et al., 1974 |
| Dog | iv | n.d. | 0.1 | ||
| Yoshizawa et al., 1977 | |||||
| 0.42 | |||||
| 15-ADON | Pig | oral | 0.05 | 0.075 | Pestka et al., 1987 |
| ip | 0.05 | 0.075 | |||
| 15-ADON | Dog | sc | n.d | 0.2 | Yoshizawa et al., 1974 |
| 3-ADON | Dog | sc | n.d. | 0.2 | Yoshizawa et al., 1977 |
| 3-ADON | Dog | iv | n.d. | 0.2 | |
| Yoshizawa et al., 1977 | |||||
| FX | Dog | iv | n.d | 0.1 | Matsuoka et al., 1979 |
| FX | Cat | sc | n.d. | 1 | Ueno et al., 1971 |
| NIV | Dog | iv | n.d. | 0.3 | Matsuoka et al., 1979 |
ED50 values have not been reported previously for 15-ADON. The NOAELs and LOAELs for this congener were reported to be 0.05mg/kg bw and 0.075mg/kg bw, respectively, following both oral and ip exposure (Pestka et al., 1987). These values are consistent with the oral data reported here (0.01 and 0.1mg/kg bw, respectively), but different than the ip NOAEL and LOAEL in the present study (0.1 and 0.25mg/kg bw, respectively). Species differences in absorption, distribution, metabolism, and excretion of 15-ADON following ip exposure might explain the differences in these values. EDs50, NOAELs, and LOAELs for emesis induced by 3-ADON, FX, and NIV following ip and oral exposure presented here are the first published values for any species.
The latency to first emesis reflects a toxicant’s capacity to activate the emetic mechanism, whereas the duration of emesis is indicative of the rate and extent of excretion or elimination of the emetic agent. In general, DON evoked emesis more rapidly than that of the other 8-ketotrichothecenes tested. Furthermore, the emetic response was transient, suggesting that DON is absorbed and metabolized rapidly. In pigs and mice, absorption of DON is rapid, reaching a peak plasma concentration of 15–30min following oral administration and declining rapidly thereafter with 75–90% cleared after 3h (Amuzie et al., 2008; Azcona-Olivera et al., 1995; Pestka et al., 2008; Prelusky et al., 1988).
Both ADONs are identical in structure to DON with the exception of being acetylated at the 15- or 3- positions. Although no data exist yet on the toxicokinetics of 15-ADON, it is generally assumed that the rate and extent of absorption and elimination of 15-ADON are similar to those of DON because the latency to and duration of emesis were similar for the two chemicals after oral exposure (Pestka et al., 1987). The duration of emesis after ip exposure was less for 15-ADON compared with DON, suggesting that the former might be metabolized at a faster rate than the latter.
3-ADON is generally considered to be equivalent to DON in terms of causing anorexia, growth retardation, and other similar effects (Yoshizawa and Morooka, 1977). However, the emetic response induced by 3-ADON here was very different compared with DON in that oral exposure to 3-ADON evoked an emetic effect with a longer latency as well as duration. Because 3-ADON must be deacetylated to DON before appreciable absorption occurs in the gut following consumption (Eriksen et al., 2003), emesis induction might require more time due to the need for metabolic conversion of 3-ADON to DON.
Latency to emesis induced by FX was similar to that induced by DON. When radiolabeled FX was administered to mice by gavage, like DON, plasma radioactivity reached a maximum concentration at 30min (Poapolathep et al., 2003). NIV had a longer latency to emesis than DON. After oral exposure of mice to radiolabeled NIV, peak plasma radioactivity was detected after 60min. Slower absorption of NIV might be a reason for a greater latency to emesis compared with DON.
The duration of emesis induced by both FX and NIV was longer compared with DON, which might relate to differences in their respective elimination rates. Clearance of radio- labeled DON in the mouse following administration follows two-compartment kinetics with an initial rapid phase of elimination (t 1/2α =0.36h) and a slower terminal elimination phase (t 1/2β = 7.62h) (Azcona-Olivera et al., 1995). A study employing ELISA similarly reported t 1/2α and t 1/2β of 0.29h and 11.8h, respectively. (Pestka et al., 2008). In contrast, although following two-compartment kinetics clearance in mice, longer half lives were observed for FX (t 1/2α = 0.878h, t 1/2β = 37.63h) and NIV (t 1/2α = 2.5h, t 1/2β = 14.34h) (Poapolathep et al., 2003). Longer elimination half-life may lead to greater durations of emetic events compared with DON. The duration of emesis induced by NIV was longer compared with FX following ip exposure, but shorter compared with FX via oral administration. Absorption of FX is more rapid and efficient compared with NIV, as a large proportion of NIV may have passed through the gastrointestinal lumen without being absorbed following oral exposure (Poapolathep et al., 2003). This implies that limited absorption of NIV after oral exposure might have diminished its emetic effect.
Oral exposure to DON, 15-ADON, and FX evoked stronger emetic effects than ip exposure based on NOAEL, LOAEL, and ED50 values (Tables 1–3). This observation is not consistent with previous reports in pigs for DON and 15-ADON (Forsyth et al., 1977; Pestka et al., 1987), which was suggestive of less absorption of these toxins by the oral route. Similarly, ED50 values for DON based on emesis were 0.020 and 0.085 μg/kg bw following intravenous and oral exposure in pigs, respectively (Prelusky and Trenholm, 1993), again suggesting that oral exposure was less effective in emesis induction. It should be noted that, unlike previous studies in pigs that did not control food intake prior to toxin exposure, we fasted mink for 24h in this study and provided a measured amount (50g) of food, which might have facilitated efficient uptake. Other possible reasons might relate to species differences in absorption, metabolism, distribution, and bioavailability of 8-ketotrichothecenes for the different exposure routes (ip vs. oral). In addition, it is plausible that DON and its congeners bind to one or more unidentified chemoreceptors with varying affinities located in the gastrointestinal tract. Differences in the distribution of such receptors throughout the GI tract or systemically as well as differences in receptor–ligand affinity may be responsible for the observed differences in potency of the emetic response following oral and ip exposure to DON, 15-ADON, and FX. Our lab is currently conducting research to identify receptors through which DON and its congeners may act to elicit an emetic response.
It is further important to note that there were differences in environmental conditions under which the initial ip (Table 1) and oral (Table 2) studies were carried out. Specifically, the ip study was conducted in March and April, 2011, which had average temperatures of 1.1°C and 8.1°C, respectively, whereas the oral study was carried out in June and July, 2011, with average temperatures being 19.4°C and 21.4°C, respectively. Behavior of animals can be affected by heat stress, which can cause effects such as feed refusal, emesis, nausea, and low blood pressure (Lugo-Amador et al., 2004). As thermoregulation by mink at high ambient temperatures is difficult (Hansen and Jeppesen, 2003), conducting the oral study in the summer with mink being housed in an open-sided shed could possibly have enhanced the emetic response. To address this concern, we conducted a follow-up study in a new cohort of mink in June 2012 (19.5°C, average ambient temperature) in the latter environment and verified that emesis incidence was indeed greater for oral exposure than for ip exposure (Table 3). Thus differences in sensitivity to ip and oral challenges were not attributable to the temperature or housing conditions.
Conversely to the three aforementioned compounds, 3-ADON and NIV were more effective in inducing emesis following ip exposure compared with oral exposure. It is likely that 3-ADON and NIV were more completely absorbed following ip exposure. Although no data on absorption, distribution, excretion, and metabolism following oral administration of 3-ADON are available, a large proportion of NIV passes through the gastrointestinal tract without being absorbed after oral exposure in mice (Poapolathep et al., 2003). This would likely reduce the emetic effect of NIV when administered by this route. Moreover, intestinal microorganisms further attenuate the emetic response by de-epoxidation of 8-ketotrichothecenes after oral exposure (Eriksen et al., 2003; Onji et al., 1989). These possibilities will require further study.
Interestingly, the average duration and intensity of emesis after oral exposure was generally less than that for ip exposure. Salivation and vomiting induced by 8-ketotrichothecenes at relatively high doses probably reduced the amount of toxin available for absorption, leading to an overall decrease in the average duration of emesis, particularly because absorption of orally administered doses may start in the stomach or from the upper part of the duodenum (Eriksen et al., 2003; Hedman et al., 1997). The average duration of emesis directly affected the average number of emetic events because the duration of the emetic response induced by oral exposure was less compared with ip exposure.
The neurologic and physiologic basis for emesis involves a complex reflex pathway involving neurotransmitters, hormones, and afferent fibers and coordination of neural, respiratory, and digestive systems (Carpenter, 1990). The mechanisms for trichothecene-induced vomiting are not fully understood but appear to involve activation of the chemoresponsive area postrema of the brain, as suggested by studies of T-2 toxin-induced emesis in the cat (Borison and Goodheart, 1989) and DON-induced conditioned taste aversion in the rat (Ossenkopp et al., 1994). Furthermore, DON’s emetic action is likely to be at least partially due to release of the neurotransmitter 5-hydroxytryptamine (5-HT, serotonin) (Prelusky and Trenholm, 1993). 5-HT is synthesized and secreted by enterochromaffin cells and plays an important role in emesis caused by cytotoxic chemicals (Endo et al., 2000). Increased 5-HT concentration will trigger the 5-HT3 receptors on the vagal afferent nerves, activating the emesis reflex via vagal afferent nerve depolarization (Andrews et al., 1990; Hillsley and Grundy, 1998; Hillsley et al., 1998).
Although the Joint FAO/WHO Expert Committee on Food Additives (JECFA) recognized that DON potentially causes human acute illness, this group concluded that there were insufficient data to determine an acute reference dose for this effect (Canady et al., 2001). An even greater paucity of data on other 8-ketotrichothecenes makes such estimates for these congeners even more problematic (Pestka, 2010a). The findings of this study provide new insight into the relative potential of DON and its congeners to contribute to human food poisoning. The estimated mean and 95th percentile per capita grain intakes in the United States for 1- to 6-year olds are 6.3 and 12g/kg bw/d and for 16- to 21-year olds are 2.5 and 5.1g/kg bw/d, respectively (EPA, 2011). If it is assumed that humans have a similar threshold for oral DON-induced emesis (0.05mg/kg bw) and a worst case scenario it is further assumed in which an entire day’s grain intake is eaten at a single meal (e.g., breakfast) by those in the 95th percentile groups, it could be speculated that contamination levels of 4 and 10 ppm could cause vomiting episodes in the 1- to 6-year and the 16- to 21-year-old groups, respectively. Consistent with these estimates, a very large Chinese outbreak of gastrointestinal illness that included vomiting in 1984 was associated with consumption of maize contaminated with DON at levels between 3.8 and 93mg/kg (Luo, 1994). Assuming consumption of 560g of grain and 50kg bw, as estimated in another Chinese study (Gao and Yoshizawa, 1997), the LOAEL could be expected to fall between 0.04 and 1.04mg/kg bw. Similar calculations for high grain consumers can be extrapolated from the mink data for 15-ADON (8 and 20 ppm), 3-ADON (20 and 50 ppm), FX (4 and 10 ppm), and NIV (20 and 50 ppm). It must be cautioned that these estimates are preliminary at best because they do not take into account potential differences in absorption and metabolism between species or possible differences among humans in sensitivities to these toxins. Nevertheless, this research represents a first step in identifying threshold doses for DON and its congeners for induction of vomiting and noninfectious gastroenteritis.
Because emesis is stressful, it would be desirable to have an alternative rodent model based on anorexia or pica. In previous studies, the NOAELs and LOAELs for 8-ketotrichothecene-induced feed refusal in mice were approximately 10-fold greater than corresponding values based here on emesis (Flannery et al., 2011; Wu et al., 2012). Furthermore, the anorectic potencies of 8-ketotrichothecenes followed rank orders of NIV > FX > DON ≈ 3-ADON ≈ 15-ADON for ip exposure and FX > NIV > DON ≈ 3-ADON ≈ 15-ADON for oral exposure. Possible reasons for the observed species differences in sensitivity based on anorexia and emesis might be due to differences in sensitivity to and toxicokinetics of these toxins between mouse and mink, but also differences in the mechanisms involved in anorexia and emesis. Nevertheless, anorectic studies in the mouse cannot be fully relied on for prediction of emetic potencies of 8-ketotrichothecenes. It is further notable that DON caused significant reduction of kaolin consumption, instead of increased consumption associated with pica behavior as observed in a recent study (Girardet et al., 2011). Although mice displayed a trend toward pica behavior early after oral exposure to DON in that study, we found that it was rather difficult to measure kaolin as it was consumed in very small amounts. Other researchers have found difficulty in determining exact kaolin consumption in mice after exposure to emetic stimuli, suggesting that it is not useful to use pica to predict nausea in this species (Yamamoto et al., 2002).
In conclusion, the mink emesis model described herein was robust and should be applicable to further investigation of the mechanisms of 8-ketotrichothecene-induced emesis. Such studies will improve our ability to predict specific thresholds for the emetic response during trichothecene food poisoning as well as the persistence and reversibility of the emetic effect in the human population. From a public health perspective, comparative emetic potency data derived from this model should be useful for establishing toxic equivalency factors for DON and other trichothecenes.
FUNDING
USDA NIFA Award (2011-0635), USDA Wheat and Barley SCAB Initiative Award 59-0206-9-058, and Public Health Service Grant ES03553 from the National Institutes of Health.
ACKNOWLEDGMENTS
We would like to acknowledge the assistance of Andrew Cohen-Barnhouse, Angelo Napolitano, Hui-Ren Zhou, Kaiyu He, and Mary Rosner.
REFERENCES
- Amuzie C. J., Harkema J. R., Pestka J. J. (2008). Tissue distribution and proinflammatory cytokine induction by the trichothecene deoxynivalenol in the mouse: Comparison of nasal vs. oral exposure. Toxicology 248, 39–44. [DOI] [PubMed] [Google Scholar]
- Andrews P. L., Davis C. J., Bingham S., Davidson H. I., Hawthorn J., Maskell L. (1990). The abdominal visceral innervation and the emetic reflex: Pathways, pharmacology, and plasticity. Can. J. Physiol. Pharmacol. 68, 325–345. [DOI] [PubMed] [Google Scholar]
- Andrews P. L., Hawthorn J. (1988). The neurophysiology of vomiting. Baillieres Clin. Gastroenterol. 2, 141–168. [DOI] [PubMed] [Google Scholar]
- Andrews P. L., Horn C. C. (2006). Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Auton. Neurosci. 125, 100–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcona-Olivera J. I., Ouyang Y., Murtha J., Chu F. S., Pestka J. J. (1995). Induction of cytokine mRNAs in mice after oral exposure to the trichothecene vomitoxin (deoxynivalenol): Relationship to toxin distribution and protein synthesis inhibition. Toxicol. Appl. Pharmacol. 133, 109–120. [DOI] [PubMed] [Google Scholar]
- Borison H. L., Goodheart M. L. (1989). Neural factors in acute emetic, cardiovascular, and respiratory effects of T-2 toxin in cats. Toxicol. Appl. Pharmacol. 101, 399–413. [DOI] [PubMed] [Google Scholar]
- Canady R. A., Coker R. D., Rgan S. K., Krska R., Kuiper-Goodman T., Olsen M., Pestka J. J., Resnik S., Schlatter J. (2001). Deoxynivalenol, Safety Evaluation of Certain Mycotoxins in Food.Fifty-sixth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Food Additives Series 47. International Programme on Chemical Safety, World Health Organization; Geneva, pp. 420–555. [Google Scholar]
- Carpenter D. O. (1990). Neural mechanisms of emesis. Can. J. Physiol. Pharmacol. 68, 230–236. [DOI] [PubMed] [Google Scholar]
- du Sert N. P., Holmes A. M., Wallis R., Andrews P. L. (2012). Predicting the emetic liability of novel chemical entities: A comparative study. Br. J. Pharmacol. 165, 1848–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Sert N. P., Rudd J. A., Apfel C. C., Andrews P. L. (2011). Cisplatin-induced emesis: Systematic review and meta-analysis of the ferret model and the effects of 5-HT receptor antagonists. Cancer Chemother. Pharmacol. 67, 667–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T., Minami M., Hirafuji M., Ogawa T., Akita K., Nemoto M., Saito H., Yoshioka M., Parvez S. H. (2000). Neurochemistry and neuropharmacology of emesis: The role of serotonin. Toxicology 153, 189–201. [DOI] [PubMed] [Google Scholar]
- EPA. (2011). Intake of Grain Products. Exposure Factors Handbook National Center for Environmental Assessment, Washington, DC. Available at: http://www.epa.gov/ncea/efh/pdfs/efh-chapter12.pdf. Accessed October 9, 2012.
- Eriksen G. S., Pettersson H., Lindberg J. E. (2003). Absorption, metabolism and excretion of 3-acetyl DON in pigs. Arch. Tierernahr. 57, 335–345. [DOI] [PubMed] [Google Scholar]
- Flannery B. M., Wu W., Pestka J. J. (2011). Characterization of deoxynivalenol-induced anorexia using mouse bioassay. Food Chem. Toxicol. 49, 1863–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth D. M., Yoshizawa T., Morooka N., Tuite J. (1977). Emetic and refusal activity of deoxynivalenol to swine. Appl. Environ. Microbiol. 34, 547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend D. W., Trenholm H. L., Elliot J. I., Thompson B. K., Hartin K. E. (1982). Effect of feeding vomitoxin-contaminated wheat to pigs. Can. J. Animal Sci. 62, 1211–1222. [Google Scholar]
- Fur Commission USA. 2010. Standard Guidelines for the Operation of Mink Farms in the United States Available at http://www.maninnature.com/FCUSA/Members/Resources/Minkguide.pdf. Accessed October 9, 2012.
- Gao H. P., Yoshizawa T. (1997). Further study on fusarium mycotoxins in corn and wheat from a high-risk area for human esophageal cancer in China. Mycotoxins. 45, 51–55. [Google Scholar]
- Gibson M. K., Bursian S. J., Aulerich R. J. (1993). Effects of deoxynivalenol on feed consumption and body weight gains in mink (Mustela vison). Bull. Environ. Contam. Toxicol. 51, 6–11. [DOI] [PubMed] [Google Scholar]
- Girardet C., Bonnet M. S., Jdir R., Sadoud M., Thirion S., Tardivel C., Roux J., Lebrun B., Wanaverbecq N., Mounien L, et al. (2011). The food-contaminant deoxynivalenol modifies eating by targeting anorexigenic neurocircuitry. PLoS ONE 6, e26134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C. P. B., Jeppesen L. L. (2003). The influence of temperature on the activity and water use of farmed mink (Mustela vison). Animal Sci. 76, 111–118. [Google Scholar]
- Hasegawa M., Sasaki T., Sadakane K., Tabuchi M., Takeda Y., Kimura M., Fujii Y. (2002). Studies for the emetic mechanisms of ipecac syrup (TJN-119) and its active components in ferrets: Involvement of 5-hydroxytryptamine receptors. Jpn. J. Pharmacol. 89, 113–119. [DOI] [PubMed] [Google Scholar]
- Hedman R., Pettersson H., Lindberg J. E. (1997). Absorption and metabolism of nivalenol in pigs. Arch. Tierernahr. 50, 13–24. [DOI] [PubMed] [Google Scholar]
- Hillsley K., Grundy D. (1998). Sensitivity to 5-hydroxytryptamine in different afferent subpopulations within mesenteric nerves supplying the rat jejunum. J. Physiol. 509(Pt 3), 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillsley K., Kirkup A. J., Grundy D. (1998). Direct and indirect actions of 5-hydroxytryptamine on the discharge of mesenteric afferent fibres innervating the rat jejunum. J. Physiol. 506(Pt 2), 551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn C. C. (2008). Why is the neurobiology of nausea and vomiting so important? Appetite. 50, 430–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes D. M., Gahl M. J., Graham C. H., Grieb S. L. (1999). Overt signs of toxicity to dogs and cats of dietary deoxynivalenol. J. Anim. Sci. 77, 693–700. [DOI] [PubMed] [Google Scholar]
- Kris M. G., Hesketh P. J., Somerfield M. R., Feyer P., Clark-Snow R., Koeller J. M., Morrow G. R., Chinnery L. W., Chesney M. J., Gralla R. J, et al. (2006). American Society of Clinical Oncology guideline for antiemetics in oncology: Update 2006. J. Clin. Oncol. 24, 2932–2947. [DOI] [PubMed] [Google Scholar]
- Lugo-Amador N. M., Rothenhaus T., Moyer P. (2004). Heat-related illness. Emerg. Med. Clin. North Am. 22, 315–3–27, viii. [DOI] [PubMed] [Google Scholar]
- Luo X. (1994). Food poisoning caused by Fusarium toxins. In Proceedings of the Second Asian Conference on Food Safety 18–23 September 1994 Bangkok, Thailand, pp. 129–136. [Google Scholar]
- Matsuoka Y., Kubota K., Ueno Y. (1979). General pharmacological studies of fusarenon-X, a trichothecene mycotoxin from Fusarium species. Toxicol. Appl. Pharmacol. 50, 87–94. [DOI] [PubMed] [Google Scholar]
- Onji Y., Dohi Y., Aoki Y., Moriyama T., Nagami H., Uno M., Tanaka T., Yamazoe Y. (1989). Deepoxynivalenol—a new metabolite of nivalenol found in the excreta of orally-administered rats. J. Agric. Food Chem. 37, 478–481. [Google Scholar]
- Ossenkopp K. P., Hirst M., Rapley W. A. (1994). Deoxynivalenol (vomitoxin)-induced conditioned taste aversions in rats are mediated by the chemosensitive area postrema. Pharmacol. Biochem. Behav. 47, 363–367. [DOI] [PubMed] [Google Scholar]
- Pestka J. J. (2010a). Toxicological mechanisms and potential health effects of deoxynivalenol and nivalenol. World Mycotoxin J. 3, 323–347. [Google Scholar]
- Pestka J. J. (2010b). Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 84, 663–679. [DOI] [PubMed] [Google Scholar]
- Pestka J. J., Islam Z., Amuzie C. J. (2008). Immunochemical assessment of deoxynivalenol tissue distribution following oral exposure in the mouse. Toxicol. Lett. 178, 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka J. J., Lin W. S., Miller E. R. (1987). Emetic activity of the trichothecene 15-acetyldeoxynivalenol in swine. Food Chem. Toxicol. 25, 855–858. [DOI] [PubMed] [Google Scholar]
- Poapolathep A., Sugita-Konishi Y., Doi K., Kumagai S. (2003). The fates of trichothecene mycotoxins, nivalenol and fusarenon-X, in mice. Toxicon. 41, 1047–1054. [DOI] [PubMed] [Google Scholar]
- Prelusky D. B., Hartin K. E., Trenholm H. L., Miller J. D. (1988). Pharmacokinetic fate of 14C-labeled deoxynivalenol in swine. Fundam. Appl. Toxicol. 10, 276–286. [DOI] [PubMed] [Google Scholar]
- Prelusky D. B., Trenholm H. L. (1993). The efficacy of various classes of anti-emetics in preventing deoxynivalenol-induced vomiting in swine. Nat. Toxins. 1, 296–302. [DOI] [PubMed] [Google Scholar]
- Qian Q., Chen W., Yue W., Yang Z., Liu Z., Qian W. (2010). Antiemetic effect of Xiao-Ban-Xia-Tang, a Chinese medicinal herb recipe, on cisplatin-induced acute and delayed emesis in minks. J. Ethnopharmacol. 128, 590–593. [DOI] [PubMed] [Google Scholar]
- Qian Q. H., Yue W., Wang Y. X., Yang Z. H., Liu Z. T., Chen W. H. (2009). Gingerol inhibits cisplatin-induced vomiting by down regulating 5-hydroxytryptamine, dopamine and substance P expression in minks. Arch. Pharm. Res. 32, 565–573. [DOI] [PubMed] [Google Scholar]
- Szelenyi I., Herold H., Göthert M. (1994). Emesis induced in domestic pigs: A new experimental tool for detection of antiemetic drugs and for evaluation of emetogenic potential of new anticancer agents. J. Pharmacol. Toxicol. Methods. 32, 109–116. [DOI] [PubMed] [Google Scholar]
- Takeda N., Hasegawa S., Morita M., Matsunaga T. (1993). Pica in rats is analogous to emesis: An animal model in emesis research. Pharmacol. Biochem. Behav. 45, 817–821. [DOI] [PubMed] [Google Scholar]
- Ueno Y. (1987). Trichothecenes in food. In Mycotoxins in Food (Krogh P. Ed.), pp. 123–147. Academic Press, New York, NY. [Google Scholar]
- Ueno Y., Ishii K., Sato N., Otsubo K. (1974). Toxicological approaches to the metabolites of Fusaria. VI. Vomiting factor from moldy corn infected with Fusarium spp. Jpn. J. Exp. Med. 44, 123–127. [PubMed] [Google Scholar]
- Ueno Y., Ueno I., Iitoi Y., Tsunoda H., Enomoto M. (1971). Toxicological approaches to the metabolites of Fusaria. III. Acute toxicity of fusarenon-X. Jpn. J. Exp. Med. 41, 521–539. [PubMed] [Google Scholar]
- Vera G., Chiarlone A., Martín M. I., Abalo R. (2006). Altered feeding behaviour induced by long-term cisplatin in rats. Auton. Neurosci. 126–127, 81–92. [DOI] [PubMed] [Google Scholar]
- Vesonder R. F., Ciegler A., Jensen A. H. (1973). Isolation of the emetic principle from Fusarium-infected corn. Appl. Microbiol. 26, 1008–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Flannery B. M., Sugita-Konishi Y., Watanabe M., Zhang H., Pestka J. J. (2012). Comparison of murine anorectic responses to the 8-ketotrichothecenes 3-acetyldeoxynivalenol, 15-acetyldeoxynivalenol, fusarenon X and nivalenol. Food Chem. Toxicol. 50, 2056–2061. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Matsunaga S., Matsui M., Takeda N., Yamatodani A. (2002). Pica in mice as a new model for the study of emesis. Methods Find. Exp. Clin. Pharmacol. 24, 135–138. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T. (1983). Trichothecenes—Chemical, biological, and toxicological aspects.In Developments in Food Science (Ueno Y. Ed.), pp. 195–209. Kodansha Ltd; Tokyo, Japan. [Google Scholar]
- Yoshizawa T., Morooka N. (1973). Deoxynivalenol and its monoacetate: New mycotoxins from Fusaruium roseum and moldy barley. Agric. Biol. Chem. 37, 2933–2934. [Google Scholar]
- Yoshizawa T., Morooka N. (1974). Studies on the toxic substances in the infected cereals. Acute toxicities of new trichothecene mycotoxins: Deoxynivalenol and its monoacetate. J. Food Hyg. Soc. Jpn. 15, 261–269. [Google Scholar]
- Yoshizawa T., Morooka N. (1977). Trichothecenes from mold-infested cereals in Japan. In Mycotoxins in Human and Animal Health (Rodricks J. V., Hesseltine C. W., Mehlman M. A. Eds.; see FSTA (1979) 11 5C268), pp. 309–321. Pathotox; Park Forest South, IL. [Google Scholar]
- Young J. C., Trenholm H. L., Friend D. W., Prelusky D. B. (1987). Detoxification of deoxynivalenol with sodium bisulfite and evaluation of the effects when pure mycotoxin or contaminated corn was treated and given to pigs. J. Agric. Food Chem. 35, 259–261. [Google Scholar]
- Young L. G., McGirr L., Valli V. E., Lumsden J. H., Lun A. (1983). Vomitoxin in corn fed to young pigs. J. Anim. Sci. 57, 655–664. [DOI] [PubMed] [Google Scholar]
- Zhang F., Wang L., Yang Z. H., Liu Z. T., Yue W. (2006). Value of mink vomit model in study of anti-emetic drugs. World J. Gastroenterol. 12, 1300–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Bursian S. J., Martin P. A., Chan H. M., Tomy G., Palace V. P., Mayne G. J., Martin J. W. (2009). Reproductive and developmental toxicity of a pentabrominated diphenyl ether mixture, DE-71, to ranch mink (Mustela vison) and hazard assessment for wild mink in the Great Lakes region. Toxicol. Sci. 110, 107–116. [DOI] [PubMed] [Google Scholar]