Abstract
Cell–cell interactions within the tumour microenvironment have crucial roles in epithelial tumorigenesis. Using Drosophila genetics, we show that the oncoprotein Src controls tumour microenvironment by Jun N-terminal kinase (JNK)-dependent regulation of the Hippo pathway. Clones of cells with elevated Src expression activate the Rac-Diaphanous and Ras-mitogen-activated protein kinase (MAPK) pathways, which cooperatively induce F-actin accumulation, thereby leading to activation of the Hippo pathway effector Yorkie (Yki). Simultaneously, Src activates the JNK pathway, which antagonizes the autonomous Yki activity and causes propagation of Yki activity to neighbouring cells, resulting in the overgrowth of surrounding tissue. Our data provide a mechanism to explain how oncogenic mutations regulate tumour microenvironment through cell–cell communication.
Keywords: Src, JNK, Hippo pathway
Introduction
Cancers develop through the cooperation of oncogenic alterations within multicellular communities [1, 2]. Cell–cell interactions between oncogenic cells and surrounding normal cells also have crucial roles in driving epithelial tumorigenesis [3]. However, the mechanisms by which each genetic alteration contributes to oncogenic cell–cell communication in vivo are poorly understood.
The src gene is the first identified oncogene encoding a non-receptor tyrosine kinase that controls various signalling pathways [4, 5, 6]. While it has been documented that Src is a potent inducer of tumorigenesis and its expression is highly elevated in many types of epithelial cancers [7, 8], the mechanism by which Src drives tumorigenesis within multicellular communities in vivo has remained unclear.
The genetic mosaic techniques available in Drosophila provide an ideal model system for studying tumorigenesis through interactions of cell clones with genetically traceable oncogenic mutations [9, 10]. While there are nine Src family members in mammals [7], the Drosophila genome encodes only two src homologues, src42A and src64B [11, 12]. Intriguingly, despite its potent transforming activity found in mammalian counterparts, overexpression of either one of the fly Src homologues in the posterior part of eye imaginal disc does not cause tumorigenesis but rather induces cell death [13, 14]. Similarly, in mammalian cells and in zebrafish, cells expressing oncogenic Src are eliminated from an epithelial sheet when surrounded by normal cells [15]. These observations suggest that elevated Src expression within the normal tissue has to cooperate with other oncogenic alteration(s) to drive tumorigenesis, and that cell–cell interactions with neighbouring cells might have an important role in Src-induced tumorigenesis. Here, using the Drosophila genetic mosaic technique, we investigated the mechanism of epithelial tumorigenesis by Src in a clonal context.
Results and Discussion
Src induces non-autonomous growth through Yki
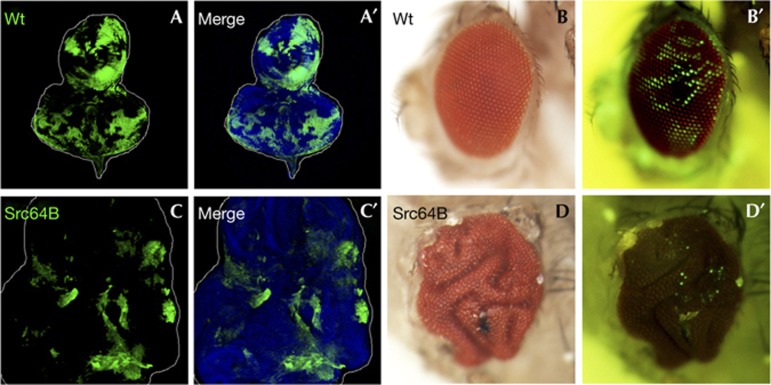
To study the mechanism by which elevated Src expression regulates tumorigenesis in vivo, we generated clones of Src-expressing cells in Drosophila imaginal epithelia. Clones of cells overexpressing Src64B did not result in overproliferation but were eliminated from the tissue during development (Fig 1C,D, compared with Fig 1A,B). This is consistent with previous observations that overexpression of Src64B causes cell death in the differentiating eye disc tissue [13, 14]. Intriguingly, we found that wild-type tissue surrounding Src-expressing clones significantly overgrew (Fig 1C, compared with Fig 1A), which resulted in a highly aberrant ‘folded-eye’ phenotype with very few green fluorescent protein (GFP)-positive Src-expressing clones in the adult (Fig 1D, compared with Fig 1B). Similar results were also obtained in the wing disc (supplementary Fig S1 online). These data indicate that elevated Src expression in clones of cells results in growth disadvantage cell-autonomously but stimulates overgrowth of surrounding tissue non-cell-autonomously.
Figure 1.
Src causes non-autonomous tissue overgrowth. (A–D) Eye-antennal discs (A, A′, C and C′) and eyes (B, B′, D and D′) of adult flies bearing GFP-labelled wild-type (A, A′, B and B′) or Src64B-expressing (C, C′, D and D′) clones are shown. Cell nuclei were stained with DAPI (blue) (A′ and C′). Clones were visualized by GFP fluorescence in the adult (B′,D′). See supplementary Information online for genotypes. DAPI, 4,6-diamidino-2-phenylindole; GFP, green fluorescent protein; wt, wild type.
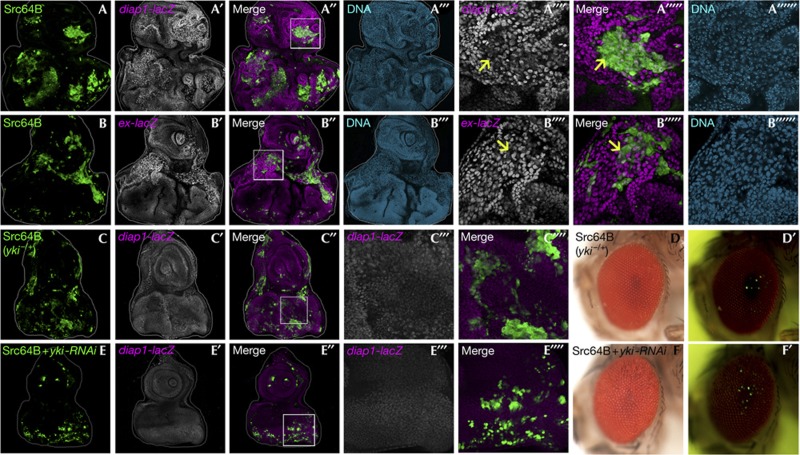
To dissect the mechanism of the non-autonomous overgrowth caused by Src, we sought to identify the molecules that regulate the non-autonomous tissue growth. It has been shown that non-autonomous overgrowth of imaginal tissue can be caused by compensatory proliferation triggered by dying cells upregulating secreted growth factors, such as Dpp (a Drosophila BMP/TGFβ homologue), Hedgehog and Wingless (a Drosophila Wnt homologue) [16, 17]. However, these growth factors were not upregulated in Src-expressing clones (data not shown). Instead, we found that activity of the transcriptional coactivator Yorkie (Yki), a downstream effector of the tumour-suppressor Hippo pathway [18, 19, 20], was elevated in both Src-expressing clones and surrounding wild-type cells, as revealed by the Yki-target gene reporters diap1-lacZ, expanded (ex)-lacZ and Cyclin E (Fig 2A,B) (data not shown), as well as by the nuclear translocation of Yki (supplementary Fig S2A online). The upregulation of Yki-target gene in both Src-expressing clones and surrounding wild-type cells was also observed in the wing disc (supplementary Fig S2B online). These results indicate that Yki regulates Src-induced non-autonomous overgrowth. Indeed, heterozygosity for the yki gene in the entire tissue significantly suppressed diap1 expression (Fig 2C) as well as the non-autonomous overgrowth (Fig 2D; quantified in supplementary Fig S3A online). Interestingly, reducing yki expression only in Src-expressing clones was sufficient to block diap1 expression in both Src-expressing clones and their neighbouring wild-type cells (Fig 2E), as well as the non-autonomous overgrowth (Fig 2F; quantified in supplementary Fig S3B online). Similarly, both autonomous and non-autonomous Yki activities (supplementary Fig S2C online), as well as non-autonomous overgrowth (supplementary Fig S2D–G online; quantified in supplementary Fig S3C,D online), by Src were blocked by coexpression of Hippo (Hpo) or Warts (Wts), two components of the Hippo kinase cascade that negatively regulate Yki activity [18, 19, 20]. These results indicate that Yki activation in Src-expressing clones causes non-autonomous Yki activation in neighbouring cells, similar to what has been observed in wing disc regeneration [21, 22].
Figure 2.
Src induces autonomous and non-autonomous activation of Yki. (A–F) Eye-antennal discs (A–A′′′′′′, Bld–B′′′′′′, C–C′′′′ and E–E′′′′) and eyes (D, D′, F and F′) of adult flies bearing GFP-labelled MARCM clones expressing Src64B in diap1-lacZ/+ background (A–A′′′′′′), Src64B in ex-lacZ/+ background (B–B′′′′′′), Src64B in yki/+ background (C–C′′′′, D and D′) and Src64B+yki-RNAi (E–E′′′′, F and F′) are shown. The reporter activity was visualized by anti-β-galactosidase staining. (A′′′′)–(A′′′′′′), (B′′′′)–(B′′′′′′), (C′′′), (C′′′′), (E′′′) and (E′′′′) are high-magnification images of the boxed area in (A′′), (B′′), (C′′) and (E′′). Arrows indicate Src64B-expressing clones with lower Yki-target gene expression compared with neighbouring wild-type cells. Cell nuclei were stained with DAPI (blue) (A′′′, B′′′, A′′′′′′ and B′′′′′′). Clones were visualized by GFP fluorescence in the adult (D′ and F′). See supplementary Information online for genotypes. DAPI, 4,6-diamidino-2-phenylindole; GFP, green fluorescent protein.
Src causes JNK-dependent propagation of Yki activity
Our observations that Yki was activated in Src-expressing clones raised the question of why Src-expressing clones do not overgrow but are instead eliminated from the tissue. Given that Yki activity within Src-expressing clones was significantly lower than that in surrounding wild-type cells (Fig 2A,B; supplementary Fig S2B online, arrows), a possible mechanism underlying the elimination of Src-expressing clones is cell competition owing to confrontation with cells with higher Yki activity [23]. Indeed, anti-cleaved Caspase-3 staining detected extensive cell death in Src-expressing cells at boundaries between Src-expressing clones and surrounding wild-type tissue (Fig 3A). Furthermore, consistent with the previous reports that blocking cell death in ‘loser’ cells prevents cell competition [24, 25, 26, 27], overexpression of baculoviral p35 in Src-expressing clones resulted in significantly larger clones (supplementary Fig S4A online, compared with Figs 1C, 2A, B and 3A), suggesting that Src-expressing cells are eliminated by cell competition.
Figure 3.
JNK signalling causes propagation of Yki activity to neighbouring cells. (A–D) Eye-antennal discs bearing GFP-labelled MARCM clones expressing Src64B (A–A′′′′′ and B–B′′′′′) and Src64B+BskDN (C–C′′′′′ and D–D′′′′′) are shown. Caspase activity (A), JNK activity (B) or Yki activity (C and D) was visualized by anti-caspase-3 antibody staining (A), the puc-lacZ reporter (B), the diap1-lacZ reporter (C) or the ex-lacZ reporter (D). (A′′′′)–(D′′′′) and (A′′′′′)–(D′′′′′) are high-magnification images of the boxed area in (A′′)–(D′′). Cell nuclei were stained with DAPI (blue) (A′′′–D′′′). See supplementary Information online for genotypes. DAPI, 4,6-diamidino-2-phenylindole; GFP, green fluorescent protein.
We next examined the mechanism by which different levels of Yki activity are generated in Src-expressing clones and surrounding wild-type cells. One possible mechanism underlying this is that Src activates intracellular signalling that counters Yki activity. A strong candidate for such signalling is Jun N-terminal kinase (JNK) signalling, as it has recently been shown that JNK signalling antagonizes Yki activity in clones of cells mutant for the apico–basal polarity gene scribble (scrib) [28, 29]. Indeed, JNK signalling was strongly activated in Src-expressing clones (Fig 3B), as revealed by the JNK activity reporter puc-lacZ [30]. Strikingly, blocking JNK signalling in Src-expressing clones by a dominant-negative form of Drosophila JNK Bsk (BskDN) not only resulted in overgrowth of Src-expressing clones but also blocked non-autonomous Yki activation in surrounding wild-type cells (Fig 3C,D). Overexpression of yki-RNAi or Wts in Src/BskDN-expressing clones abolished both Yki-target gene expression and the tissue overgrowth (supplementary Fig S4B,C online). These results indicate that JNK signalling not only antagonizes Yki activity but also causes the propagation of Yki activity to neighbouring cells. Consistent with these results, we found that, while clones of Yki-overexpressing cells induce diap1 expression strictly in a cell autonomous manner (supplementary Fig S5A online), coactivation of JNK signalling in these clones by a constitutively activated form of JNK kinase Hep (HepCA) resulted in non-autonomous upregulation of diap1 in surrounding cells (supplementary Fig S5B online). Overexpression of HepCA on its own caused neither autonomous nor non-autonomous diap1 expression (supplementary Fig S5C online). Although some non-autonomous JNK activation was observed in wild-type cells surrounding Src-expressing clones (Fig 3B), the non-autonomous JNK activity seemed not to be essential for inducing non-autonomous Yki activation, as Yki/HepCA-expressing clones activated JNK signalling in a cell-autonomous manner (data not shown). These data suggest that clones of cells with elevated Src expression induce overgrowth of surrounding tissue through JNK-dependent propagation of Yki activity.
Src activates Yki through F-actin accumulation
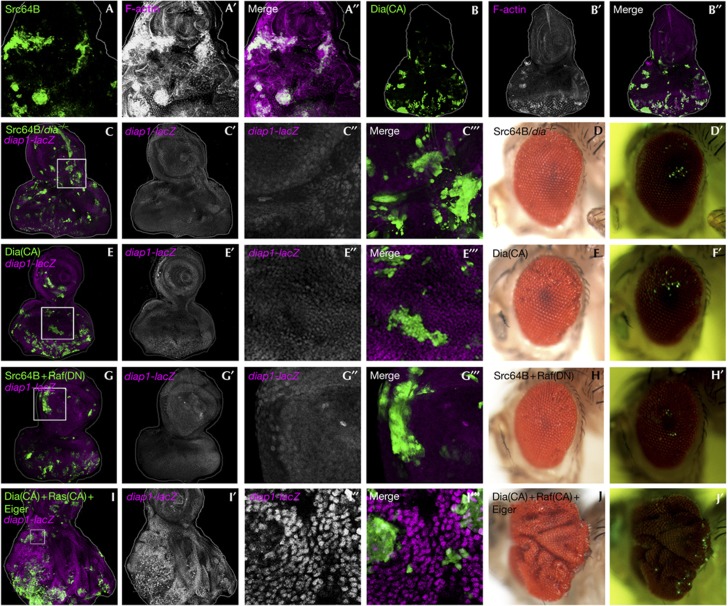
Having shown that Yki activity propagates from Src-expressing clones to neighbouring tissue, we sought to explain why Yki is activated in Src-expressing clones in the first place. It has been well documented that Src regulates cytoskeletal F-actin organization in mammalian systems [31]. Interestingly, recent studies have shown that intracellular accumulation of F-actin leads to activation of Yki/YAP (a mammalian Yki homologue) through inactivation of the Hippo pathway [32, 33, 34, 35]. We found that F-actin was highly accumulated in Src-expressing clones (Fig 4A). Furthermore, in Src-expressing clones, loss of Diaphanous (Dia), a Rho GTPase effector that promotes F-actin accumulation (Fig 4B) and has been shown to inactivate the Hippo pathway [33], significantly blocked diap1 expression (Fig 4C, compared with Fig 2A) and the non-autonomous overgrowth (Fig 4C,D; quantified in supplementary Fig S3E online), as well as F-actin accumulation (supplementary Fig S6A,B,D online). We further sought to identify the upstream RhoGTPase that regulates the Dia-mediated F-actin accumulation and found that inhibition of Rac1, but not Rho1, in Src-expressing clones strongly blocked the non-autonomous overgrowth (supplementary Fig S7A–D online; quantified in supplementary Fig S3F,G online) as well as F-actin accumulation (supplementary Fig S6C–E online). These results indicate that Src causes inactivation of the Hippo pathway through Rac1-Dia-mediated F-actin accumulation.
Figure 4.
Src activates Yki through F-actin reorganization. (A–J) Eye-antennal discs (A–A′′, B–B′′, C–C′′′, E–E′′′, G–G′′′ and I–I′′′) and eyes (D, D′, F, F′, H, H′, J and J′) of adult flies bearing GFP-labelled MARCM clones expressing Src64B (A–A′′), DiaCA (B–B′′, F and F′), Src64B/dia−/− in diap1-lacZ/+ background (C–C′′′), Src64B/dia−/− (D and D′), DiaCA in diap1-lacZ/+ background (E–E′′′), Src64B+RafDN in diap1-lacZ/+ background (G–G′′′), Src64B+RafDN (H and H′) and DiaCA+RasCA+Eiger in diap1-lacZ/+ background (I–I′′′, J and J′) are shown. F-actin (A, B), or Yki activity (C, E, G and I) was visualized by phalloidin staining (A,B) or the diap1-lacZ reporter (C, E, G and I). C′′, C′′′, E′′, E′′′, G′′, G′′′, I′′ and I′′′ are high-magnification images of the boxed area in C, E, G and I. Clones were visualized by GFP fluorescence in the adult (D′, F′, H′ and J′). See supplementary Information online for genotypes. GFP, green fluorescent protein.
Rac/Ras/JNK activates Yki non-autonomously
We next asked whether F-actin accumulation in Src-expressing clones is sufficient to cause non-autonomous overgrowth. We found that, when surrounded by wild-type tissue, F-actin accumulation by a constitutively activated form of Dia (DiaCA) (Fig 4B) or Rac1 (Rac1CA) (data not shown) induced neither diap1 expression nor non-autonomous overgrowth (Fig 4E,F; supplementary Fig S8A online). This indicates that elevated Src expression in clones of cells must induce an additional downstream effect(s) essential for inducing non-autonomous overgrowth. It has recently been shown that two downstream signalling pathways of Src, the JAK/STAT pathway and the Ras pathway, are involved in non-autonomous tissue growth during Drosophila intestinal regeneration [36, 37, 38, 39]. We therefore tested the involvement of these signalling pathways in Src-induced non-autonomous overgrowth. Reduction in the stat92E gene dosage, which can dominantly suppress tissue overgrowth of imaginal discs [40, 41, 42], did not affect the non-autonomous overgrowth caused by Src (supplementary Fig S8B,C online). In addition, reducing the expression level of unpaired (upd), an IL-6-like ligand that activates the JAK/STAT pathway and can induce non-autonomous overgrowth when overproduced in vps25−/− clones [40, 41, 43, 44], showed no effect on the non-autonomous overgrowth caused by Src-expressing clones (supplementary Fig S8D,E online). These data suggest that JAK/STAT signalling is not responsible for the non-autonomous overgrowth. We found that the MAP kinase pathway, one of the two main downstream pathways of Ras signalling [45], is strongly activated in Src-expressing clones (supplementary Fig S8F online). Strikingly, blocking the Raf-mitogen-activated protein kinase (MAPK) pathway by a dominant-negative form of Raf (RafDN) in Src-expressing clones suppressed both autonomous and non-autonomous diap1 expression, as well as the non-autonomous overgrowth (Fig 4G,H, quantified in supplementary Fig S3H online). In contrast, blocking the PI3K pathway, the other main downstream signalling of Ras [45], did not suppress the non-autonomous overgrowth (supplementary Fig S8G online). These results indicate that Raf-MAPK signalling, in addition to Rac-Dia signalling, is essential for causing the Src-induced non-autonomous overgrowth.
We found that clones of cells activating Ras signalling alone moderately accumulated F-actin and caused marginal upregulation of diap1 (supplementary Fig S9A,B online). Interestingly, coactivation of the Rac1-Dia and Ras-MAPK pathways synergistically induced F-actin accumulation and diap1 expression (supplementary Fig S9C online). diap1 was upregulated not only in DiaCA/RasCA-expressing clones but also in surrounding wild-type cells (supplementary Fig S9C′′′, C′′′′ online), suggesting that coactivation of Dia/Ras signalling leads to JNK activation. Indeed, blocking JNK signalling in DiaCA/RasCA-expressing clones cancelled the non-autonomous upregulation of diap1 and resulted in autonomous overgrowth (supplementary Fig S9D online). Importantly, blocking the Rac1-Dia or Ras-MAPK pathway by Rac1DN or RafDN was not sufficient to inhibit JNK activation in Src-expressing cells (supplementary Fig S9E,F online), indicating that Src signalling also feeds into JNK activation through a pathway independent of the Rac1-Dia and Ras-MAPK pathways. We therefore examined whether ectopic activation of the Rac-Dia, Ras-MAPK and JNK pathways (JNK activation was induced by Eiger [46]) could phenocopy the Src-induced non-autonomous overgrowth. Significantly, clones of cells expressing DiaCA/RasCA/Eiger caused non-autonomous tissue overgrowth accompanied by non-cell autonomous upregulation of diap1 (Fig 4I,J). Both autonomous and non-autonomous diap1 expressions were abolished when Wts was coexpressed in DiaCA/RasCA/Eiger-expressing clones (supplementary Fig S9G online). Furthermore, nuclear translocation of Yki was observed in both DiaCA/RasCA/Eiger-expressing cells and their neighbouring cells (supplementary Fig S9H online). Together, these data indicate that clones of cells with elevated Src expression cause non-autonomous tissue overgrowth through activation of the Rac-Dia, Ras-MAPK and JNK pathways, which induces propagation of Yki activity to neighbouring cells (Fig 5).
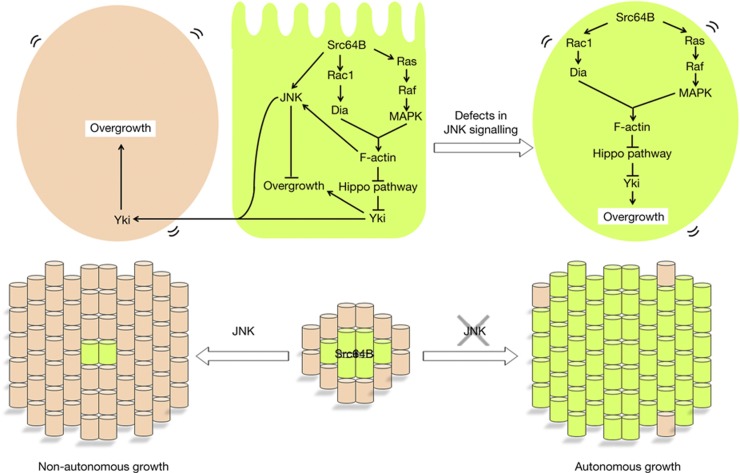
Figure 5.
A model for Src-induced tumorigenesis by JNK-dependent Hippo pathway regulation. A cell with elevated Src expression activates the Rac-Dia and the Ras-MAPK pathways, which cooperate to cause Yki activation through the Hippo pathway. At the same time, Src activates JNK signalling that causes propagation of Yki activity to neighbouring cells (left, non-autonomous growth). Blocking JNK signalling in a Src-expressing cell cancels the propagation of Yki activity and leads to autonomous activation of Yki (right, autonomous growth). See text for details. MAPK, mitogen-activated protein kinase.
Our data show that elevated Src expression inactivates the Hippo pathway and thus promotes Yki activity through F-actin accumulation. It has recently been shown that activation of Dia in large territories of Drosophila imaginal disc leads to inactivation of the Hippo pathway [33]. However, intriguingly, we found that Dia activation in small patches of cells surrounded by wild-type tissue did not cause upregulation of Yki-target gene (Fig 4E). This could be owing to the fact that clones of cells accumulating F-actin are subjected to cell competition, which has been shown to inhibit Yki activity [28]. Interestingly, we found that co-activation of Rac and Ras signalling causes synergistic accumulation of F-actin and thus upregulation of the Yki-target gene even when surrounded by wild-type tissue (supplementary Fig S9C online), suggesting that Ras signalling reverses cell competition, as it has been shown for scrib clones [47, 48].
An intriguing phenomenon observed in Src-expressing cells is the JNK-dependent switch of the autonomy of Yki activation; JNK inhibits Yki activity in Src-expressing clones, while at the same time JNK causes propagation of Yki activity to neighbouring cells (Fig 5). Indeed, it has been shown that JNK suppresses Yki activity in polarity-deficient cells [28, 29]. However, it has also been shown that JNK positively contributes to Yki activation during regeneration of wing imaginal disc [21, 22]. Thus, JNK signalling seems to exert opposite effects on Yki activity depending on the cellular contexts. We have recently found that coactivation of JNK and Ras signalling leads to inactivation of the Hippo pathway [49]. Intriguingly, however, we found that JNK suppresses Yki activity even in the presence of Ras signalling if Src is activated. It is possible that Src signalling creates a specific cellular context that switches JNK’s pro-Yki activity to anti-Yki effect. Intriguingly, Yki is activated in both apoptotic cells and surrounding wild-type cells during the process of regenerative tissue growth [21, 22], suggesting that tissue regeneration is regulated by a signalling mechanism similar to that triggered by Src.
Our findings that cells with elevated Src expression are eliminated from normal epithelium but contribute non-autonomously to overgrowth of surrounding tissue might help explain how cell–cell communication drives Src-mediated tumorigenesis in vivo. Given the potent non-autonomous induction of Yki targets by Src-expressing cells, it would be interesting to investigate whether other oncogenic mutations induced in surrounding wild-type tissue cooperate with nearby Src-expressing clones to drive tumour progression. Such non-autonomous interclonal oncogenic cooperation has recently been shown using the Drosophila genetic mosaic technique [50].
Our findings also reveal a high oncogenic potential of Src acting cell-autonomously when JNK signalling is reduced. Blocking JNK signalling in Src-expressing clones results in autonomous tumour overgrowth through the Hippo pathway, suggesting that more mutations that block JNK in Src-activated cells could cause clonal expansion and tumorigenesis (Fig 5). Interestingly, mutations in genes encoding JNK pathway components have been implicated in human cancers. For instance, loss-of-function mutations for MKK4 gene (a hep homologue in mammals) have been identified in many types of cancer [51]. In addition, missense mutations in the kinase domain of MKK7 (another hep homologue in mammals) have been reported in colorectal cancers [52]. Furthermore, somatic mutations for jnk1 and jnk2 have been identified in glioma, colorectal, head and neck cancers [52]. The development of such cancers is generally associated with a high level of Src expression [7, 8]. Given that the regulatory components of Src, JNK and Hippo signalling are highly conserved in mammals, it is possible that similar JNK-dependent Hippo pathway regulation is involved in Src-induced tumour progression in humans.
METHODS
Fly strains and generation of clones. Fluorescently labelled mitotic clones were produced in larval imaginal discs using the following strains: eyFLP5, Act>y+>Gal4, UAS-GFP; FRT82B, Tub-Gal80 (82B-tester), Tub-Gal80, FRT40A; eyFLP6, Act>y+>Gal4, UAS-GFP (40A-tester), eyFLP5, Act>y+>Gal4, UAS-GFP; diap1-lacZ, FRT82B, Tub-Gal80 (diap1-lacZ 82B-tester), and Tub-Gal80, FRT19A; eyFLP5, Act>y+>Gal4, UAS-GFP; puc-lacZ/TM6B (19A puc-lacZ-tester). Yki localization was determined in posterior compartment of wing disc using the following strain; en-Gal4, UAS-GFP; Tub-Gal80ts. Additional strains used are the following: dia5 [53], diap1-lacZ (thj5C8) [54], ex-lacZ (ex697) [55], stat92E06346 [56], ykiB5 [57], UAS-BskDN [58], UAS-DiaCA [59], UAS-HepCA [60], UAS-Hpo [61], UAS-Lats (a gift from Tian Xu), UAS-PI3KDN [62], UAS- Rac1CA [63], UAS-Rac1DN [63], UAS-RafDN [64], UAS-RasCA [65], UAS-Rho1DN [66], UAS-Src64B [67], UAS-upd1-RNAi5993R-2 (National Institute of Genetics, NIG), UAS-Yki [68] and UAS-yki-RNAi4005R-2 [32].
Histology. Larval tissues were stained with standard immunochemical procedures using mouse anti-β-galactosidase antibody (Sigma; 1:250), rabbit anti-cleaved Caspase-3 antibody (Cell Signalling; 1:100), rabbit anti-phospho ERK antibody (Cell Signalling; 1:100), chick anti-GFP antibody (Aves Labs; 1:200), rabbit anti-Yki antibody (1:400), Alexa-546 or Alexa-647-conjugated phalloidin (Molecular Probes; 1:50) and mounted with 4,6-diamidino-2-phenylindole-containing Slow Fade Gold Antifade Reagent (Molecular Probes). Anti-phospho-ERK antibody staining was performed with Can Get Signal immunoreactions enhancer solution (Toyobo). Images were taken with a Zeiss LSM510 META and a Leica TCS-SP5 confocal microscopes.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank J. Pastor-Pareja for comments on the manuscript; T. Sawada, Y. Kanemoto, D. Kizawa and Y. Yamamoto for technical support; S. Ohsawa for diap1-lacZ 82B-tester; T. Orr-Weaver for guinea pig anti-Cyclin E antibody; H. Richardson for rat anti-Cyclin E antibody; K.D. Irvine for rabbit anti-Yki antibody; and T. Adachi-Yamada, M. Miura, D.J. Pan, T. Xu, the Bloomington Stock Centre, the National Institute of Genetics Stock Centre (NIG-FLY) and the Drosophila Genetic Resource Centre (DGRC, Kyoto Institute of Technology) for fly stocks. We also thank members of the TI Laboratory for discussions. This work was supported by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Science, Sports, Culture and Technology (MEXT) to T.I.; the Japan Society for the Promotion of Science (JSPS) to M.E. and T.I.; PRESTO, Japan Science and Technology Agency (JST) to T.I.; the G-COE programme for Global Centre for Education and Research in Integrative Membrane Biology to T.I.; the Takeda Science Foundation to T.I.; the Uehara Memorial Foundation to T.I.; and the International Human Frontier Science Programme Career Development Award to T.I. M.E. is supported by the JSPS Postdoctoral Fellowship.
Author Contributions: M.E. and T.I. designed the research, M.E. performed experiments, M.E. and T.I. analysed the data, and M.E. and T.I. wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646–674 [DOI] [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B (1996) Lessons from hereditary colorectal cancer. Cell 87: 159–170 [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Radisky D (2001) Putting tumours in context. Nat Rev Cancer 1: 46–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame MC (2002) Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta 1602: 114–130 [DOI] [PubMed] [Google Scholar]
- Parsons SJ, Parsons JT (2004) Src family kinases, key regulators of signal transduction. Oncogene 23: 7906–7909 [DOI] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS (1997) Cellular functions regulated by Src family kinases. Ann Rev Dev Biol 13: 513–609 [DOI] [PubMed] [Google Scholar]
- Yeatman TJ (2004) A renaissance for SRC. Nat Rev Cancer 4: 470–480 [DOI] [PubMed] [Google Scholar]
- Summy JM, Gallick GE (2003) Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev 22: 337–358 [DOI] [PubMed] [Google Scholar]
- Brumby AM, Richardson HE (2005) Using Drosophila melanogaster to map human cancer pathways. Nat Rev Cancer 5: 626–639 [DOI] [PubMed] [Google Scholar]
- Potter CJ, Turenchalk GS, Xu T (2000) Drosophila in cancer research: an expanding role. Trends Genet 16: 33–39 [DOI] [PubMed] [Google Scholar]
- Simon MA, Drees B, Kornberg T, Bishop JM (1985) The nucleotide sequence and the tissue-specific expression of Drosophila c-src. Cell 42: 831–840 [DOI] [PubMed] [Google Scholar]
- Takahashi F, Endo S, Kojima T, Saigo K (1996) Regulation of cell-cell contacts in developing Drosophila eyes by Dsrc41, a new, close relative of vertebrate c-src. Genes Dev 10: 1645–1656 [DOI] [PubMed] [Google Scholar]
- Pedraza LG, Stewart RA, Li DM, Xu T (2004) Drosophila Src-family kinases function with Csk to regulate cell proliferation and apoptosis. Oncogene 23: 4754–4762 [DOI] [PubMed] [Google Scholar]
- Vidal M, Warner S, Read R, Cagan RL (2007) Differing Src signaling levels have distinct outcomes in Drosophila. Cancer Res 67: 10278–10285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajita M, Hogan C, Harris AR, Dupre-Crochet S, Itasaki N, Kawakami K, Charras G, Tada M, Fujita Y (2010) Interaction with surrounding normal epithelial cells influences signalling pathways and behaviour of Src-transformed cells. J Cell Sci 123: 171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FA, Perez-Garijo A, Morata G (2009) Apoptosis in Drosophila: compensatory proliferation and undead cells. Int J Dev Biol 53: 1341–1347 [DOI] [PubMed] [Google Scholar]
- Fan Y, Bergmann A (2008) Apoptosis-induced compensatory proliferation. The cell is dead. Long live the cell!. Trends Cell Biol 18: 467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D (2010) The Hippo signaling pathway in development and cancer. Dev Cell 19: 491–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder G, Johnson RL (2011) Hippo signaling: growth control and beyond. Development 138: 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Lei Q, Guan KL (2010) The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev 24: 862–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Irvine KD (2010) Regulation of Hippo signaling by Jun kinase signaling during compensatory cell proliferation and regeneration, and in neoplastic tumors. Dev Biol 350: 139–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusche FA, Degoutin JL, Richardson HE, Harvey KF (2011) The Salvador/Warts/Hippo pathway controls regenerative tissue growth in Drosophila melanogaster. Dev Biol 350: 255–266 [DOI] [PubMed] [Google Scholar]
- Tyler DM, Li W, Zhuo N, Pellock B, Baker NE (2007) Genes affecting cell competition in Drosophila. Genetics 175: 643–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Baker NE (2007) Engulfment is required for cell competition. Cell 129: 1215–1225 [DOI] [PubMed] [Google Scholar]
- Moreno E, Basler K, Morata G (2002) Cells compete for decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature 416: 755–759 [DOI] [PubMed] [Google Scholar]
- Moreno E, Basler K (2004) dMyc transforms cells into super-competitors. Cell 117: 117–129 [DOI] [PubMed] [Google Scholar]
- de la Cova C, Abril M, Bellosta P, Gallant P, Johnston LA (2004) Drosophila Myc regulates organ size by inducing cell competition. Cell 117: 107–116 [DOI] [PubMed] [Google Scholar]
- Chen CL, Schroeder MC, Kango-Singh M, Tao C, Halder G (2012) Tumor suppression by cell competition through regulation of the Hippo pathway. Proc Natl Acad Sci USA 109: 484–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggett K, Grusche F, Richardson H, Brumby A (2011) Loss of the Drosophila cell polarity regulator scribbled promotes epithelial tissue overgrowth and cooperation with oncogenic Ras-Raf through impaired Hippo pathway signaling. BMC Dev Biol 11: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Blanco E, Gampel A, Ring J, Virdee K, Kirov N, Tolkovsky AM, Martinez-Arias A (1998) Puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev 12: 557–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame MC, Fincham VJ, Carragher NO, Wyke JA (2002) v-SRC'S hold over actin and cell adhesions. Nat Rev Mol Cell Biol 3: 233–245 [DOI] [PubMed] [Google Scholar]
- Fernández BG, Gaspar P, Brás-Pereira C, Jezowska B, Rebelo SR, Janody F (2011) Actin-capping protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development 138: 2337–2346 [DOI] [PubMed] [Google Scholar]
- Sansores-Garcia L, Bossuyt W, Wada KI, Yonemura S, Tao C, Sasaki H, Halder G (2011) Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J 30: 2325–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada KI, Itoga K, Okano T, Yonemura S, Sasaki H (2011) Hippo pathway regulation by cell morphology and stress fibers. Development 138: 3907–3914 [DOI] [PubMed] [Google Scholar]
- Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL (2012) Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev 26: 54–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RL, Kohlmaier A, Polesello C, Veelken C, Edgar BA, Tapon N (2010) The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development 137: 4147–4158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Grenley MO, Bravo MJ, Blumhagen RZ, Edgar BA (2011) EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell stem cell 8: 84–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N, Wang SQ, Tan D, Gao Y, Lin G, Xi R (2011) EGFR, wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev Biol 354: 31–43 [DOI] [PubMed] [Google Scholar]
- Karpowicz P, Perez J, Perrimon N (2010) The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development 137: 4135–4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari T, Bilder D (2005) The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating Notch trafficking. Dev Cell 9: 687–698 [DOI] [PubMed] [Google Scholar]
- Moberg KH, Schelble S, Burdick SK, Hariharan IK (2005) Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit non-cell-autonomous overgrowth. Dev Cell 9: 699–710 [DOI] [PubMed] [Google Scholar]
- Classen AK, Bunker BD, Harvey KF, Vaccari T, Bilder D (2009) A tumor suppressor activity of Drosophila Polycomb genes mediated by JAK-STAT signaling. Nat Genet 41: 1150–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz HM, Chen Z, Scherr H, Lackey M, Bolduc C, Bergmann A (2006) vps25 mosaics display non-autonomous cell survival and overgrowth, and autonomous apoptosis. Development 133: 1871–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BJ, Mathieu J, Sung HH, Loeser E, Rørth P, Cohen SM (2005) Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev Cell 9: 711–720 [DOI] [PubMed] [Google Scholar]
- Shields JM, Pruitt K, McFall A, Shaub A, Der CJ (2000) Understanding Ras: ‘it ain’t over ‘til it’s over’. Trends Cell Biol 10: 147–154 [DOI] [PubMed] [Google Scholar]
- Ohsawa S, Sugimura K, Takino K, Xu T, Miyawaki A, Igaki T (2011) Elimination of oncogenic neighbors by JNK-mediated engulfment in Drosophila. Dev Cell 20: 315–328 [DOI] [PubMed] [Google Scholar]
- Brumby AM, Richardson HE (2003) Scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J 22: 5769–5779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarini RA, Xu T (2003) A genetic screen in Drosophila for metastatic behavior. Science 302: 1227–1231 [DOI] [PubMed] [Google Scholar]
- Ohsawa S, Sato Y, Enomoto M, Nakamura M, Betsumiya A, Igaki T (2012) Mitochondrial defect drives non-autonomous tumour progression through Hippo signalling in Drosophila. Nature 490: 547–551 [DOI] [PubMed] [Google Scholar]
- Wu M, Pastor-Pareja JC, Xu T (2010) Interaction between RasV12 and scribbled clones induces tumour growth and invasion. Nature 463: 545–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmarsh AJ, Davis RJ (2007) Role of mitogen-activated protein kinase kinase 4 in cancer. Oncogene 26: 3172–3184 [DOI] [PubMed] [Google Scholar]
- Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, Davies H, Teague J, Butler A, Stevens C et al. (2007) Patterns of somatic mutation in human cancer genomes. Nature 446: 153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillon DH, Wasserman SA (1994) Diaphanous is required for cytokinesis in Drosophila and shares domains of similarity with the products of the limb deformity gene. Development 120: 3367–3377 [DOI] [PubMed] [Google Scholar]
- Hay BA, Wassarman DA, Rubin GM (1995) Drosophila homologs of baculovirus inhibitor of apoptosis proteins function to block cell death. Cell 83: 1253–1262 [DOI] [PubMed] [Google Scholar]
- Boedigheimer M, Laughon A (1993) Expanded: a gene involved in the control of cell proliferation in imaginal discs. Development 118: 1291–1301 [DOI] [PubMed] [Google Scholar]
- Hou XS, Melnick MB, Perrimon N (1996) Marelle acts downstream of the Drosophila HOP/JAK kinase and encodes a protein similar to the mammalian STATs. Cell 84: 411–419 [DOI] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D (2005) The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell 122: 421–434 [DOI] [PubMed] [Google Scholar]
- Adachi-Yamada T, Gotoh T, Sugimura I, Tateno M, Nishida Y, Onuki T, Date H (1999) De Novo synthesis of sphingolipids is required for cell survival by down-regulating c-Jun N-Terminal kinase in Drosophila imaginal discs. Mol Cell Biol 19: 7276–7286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi K, Rørth P (2004) Evidence for tension-based regulation of Drosophila MAL and SRF during invasive cell migration. Dev Cell 7: 85–93 [DOI] [PubMed] [Google Scholar]
- Weber U, Paricio N, Mlodzik M (2000) Jun mediates Frizzled-induced R3/R4 cell fate distinction and planar polarity determination in the Drosophila eye. Development 127: 3619–3629 [DOI] [PubMed] [Google Scholar]
- Wu S, Huang J, Dong J, Pan D (2003) Hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114: 445–456 [DOI] [PubMed] [Google Scholar]
- Cliffe ST, Kramer JM, Hussain K, Robben JH, de Jong EK, de Brouwer AP, Nibbeling E, Kamsteeg EJ, Wong M, Prendiville J et al. (2009) SLC29A3 gene is mutated in pigmented hypertrichosis with insulin-dependent diabetes mellitus syndrome and interacts with the insulin signaling pathway. Hum Mol Genet 18: 2257–2265 [DOI] [PubMed] [Google Scholar]
- Luo L, Liao YJ, Jan LY, Jan YN (1994) Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev 8: 1787–1802 [DOI] [PubMed] [Google Scholar]
- de Celis JF (1997) Expression and function of decapentaplegic and thick veins during the differentiation of the veins in the Drosophila wing. Development 124: 1007–1018 [DOI] [PubMed] [Google Scholar]
- Karim FD, Rubin GM (1998) Ectopic expression of activated Ras1 induces hyperplastic growth and increased cell death in Drosophila imaginal tissues. Development 125: 1–9 [DOI] [PubMed] [Google Scholar]
- Strutt DI, Weber U, Mlodzik M (1997) The role of RhoA in tissue polarity and frizzled signalling. Nature 387: 292–295 [DOI] [PubMed] [Google Scholar]
- Wills Z, Bateman J, Korey CA, Comer A, Van Vactor D (1999) The tyrosine kinase Abl and its substrate enabled collaborate with the receptor phosphatase Dlar to control motor axon guidance. Neuron 22: 301–312 [DOI] [PubMed] [Google Scholar]
- Oh H, Irvine KD (2009) In vivo analysis of Yorkie phosphorylation sites. Oncogene 28: 1916–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.