Abstract
Argonaute (Ago) proteins are typically recruited to target messenger RNAs via an associated small RNA such as a microRNA (miRNA). Here, we describe a new mechanism of Ago recruitment through the Drosophila Smaug RNA-binding protein. We show that Smaug interacts with the Ago1 protein, and that Ago1 interacts with and is required for the translational repression of the Smaug target, nanos mRNA. The Ago1/nanos mRNA interaction does not require a miRNA, but it does require Smaug. Taken together, our data suggest a model whereby Smaug directly recruits Ago1 to nanos mRNA in a miRNA-independent manner, thereby repressing translation.
Keywords: Smaug, Argonaute, nanos , translational repression, Drosophila
INTRODUCTION
Post-transcriptional regulation can be mediated by cis elements within target mRNAs that recruit trans factors to influence transcript expression. Cis elements can be recognized by RNA-binding proteins that interact with specific sequences and/or structural elements 1]. For example, Drosophila Smaug is the founding member of a conserved class of regulators that recognize target mRNAs through stem/loop structures referred to as Smaug recognition elements (SREs) [2, 3, 4, 5, 6, 7, 8]. Smaug is a multifunctional regulator that uses several mechanisms to regulate target transcripts [9, 10, 11, 12].
Cis elements can also represent binding sites for RNAs where recognition is mediated via base pairing. For example, Argonaute (Ago) proteins are recruited to mRNAs via an associated small RNA, such as a microRNA (miRNA) [13]. Ago/miRNA complexes typically repress translation and/or induce mRNA degradation.
Here, we report that Smaug functions with Ago1 protein to regulate the expression of one of Smaug’s best-characterized target mRNAs, nanos (nos), in the early Drosophila embryo. We show that Smaug interacts with Ago1, that Ago1 interacts with nos mRNA and that Ago1 is required to repress nos translation. Surprisingly, Ago1’s interaction with nos mRNA does not require the Ago1/miRNA interaction but it does require Smaug. These data suggest a model whereby Smaug recruits Ago1 to nos mRNA in a miRNA-independent manner to repress nos translation. We propose that other RNA-binding proteins likely regulate the expression of target mRNAs through direct recruitment of Ago proteins.
RESULTS
Smaug interacts with Ago1 and Ago2
Previously we identified Not1, a member of the Ccr4/Not deadenylase, as a Smaug-binding protein through purification of Smaug and we went on to demonstrate a role for the deadenylase in Smaug function [10]. Another Smaug-binding partner identified in these purifications was Ago2. Drosophila Ago2 is most closely associated with siRNA-mediated regulation while Ago1 is associated with miRNA-mediated regulation [14]. To verify the interaction with Ago2 and determine whether Ago1 also interacts with Smaug, we assayed Smaug immunoprecipitates for Ago1 and Ago2 by western blot. Both Ago1 and Ago2 specifically co-immunoprecipitated with Smaug while an irrelevant RNA-binding protein, Dp1, did not (Fig 1). The interaction with Ago1 and Ago2 persisted in the presence of RNase, arguing that the interaction between Smaug and Ago1/Ago2 is RNA-independent and that these proteins are part of the same protein complex.
Figure 1.
Smaug co-immunoprecipitates with Ago1 and Ago2. Embryo extracts collected 0–2 h post egg laying were immunoprecipitated with anti-Smaug antibody or non-immune guinea-pig serum. Western blots to assay for the indicated proteins in crude extracts and the indicated immunoprecipitates are shown. Where indicated, immunoprecipitations were performed in the presence of RNase A. Embryo extract lanes represent 5% of the material used in the immunoprecipitations. Ago, Argonaute.
Ago1 represses unlocalized nos mRNA translation
We next asked whether Ago1 and/or Ago2 might function in Smaug-mediated repression by assaying the expression of nos mRNA, one of Smaug’s best-characterized targets. nos mRNA is inefficiently localized to the posterior of the early embryo [15, 16, 17] and posteriorly localized nos mRNA is translated while nos mRNA that escapes the localization machinery and is found distributed throughout the embryo is translationally repressed [18]. This differential translational regulation results in the accumulation of Nos protein at the posterior of the embryo and is, in part, mediated by Smaug, which represses the translation of unlocalized nos mRNA through two SREs in the nos 3′-untranslated region (UTR) [2, 3, 4, 19].
To assess whether Ago1 and/or Ago2 regulate nos, we compared the expression of Nos protein in early wild-type embryos to embryos that do not express wild-type Smaug, Ago1 or Ago2 (Fig 2A–C). These proteins are maternally contributed, therefore we assayed embryos from mutant mothers (which are hereafter denoted as smaug mutant, Ago1 mutant and Ago2 mutant embryos). Ago1 mutants are homozygous lethal and as such we used Flp/FRT-mediated mitotic recombination [20, 21] to collect embryos lacking Ago1 protein. A smaug allele that encodes a non-functional protein that lacks its RNA-binding domain and an Ago2 null allele are homozygous viable allowing us to collect mutant embryos from mutant females. In smaug mutant embryos, unlocalized nos mRNA was translated resulting in ectopic Nos protein accumulation throughout the embryo (Fig 2A). In Ago2 mutant embryos, Nos regulation appeared to be unaffected (Fig 2B); however, Ago1 mutant embryos expressed ectopic Nos protein (Fig 2A), indicating that Ago1 is required to repress the expression of unlocalized nos mRNA.
Figure 2.
Ago1 represses the translation of unlocalized nos mRNA. Wild-type, smaug, Ago1 or Ago2 embryos were fixed and stained with anti-Nos (A,B) or anti-Osk (C) antibodies. Embryos containing eight nuclei, as assayed via staining with picogreen, are shown. (D) Extracts derived from embryos collected 0–2 h post egg laying were assayed for indicated proteins by western blots. (E) Northern blot analysis was performed on total RNA extracted from wild-type or Ago1 mutant embryos collected at the indicated times post egg laying. (F,G) Northern blots were exposed to PhosphorImager screens and quantified with ImageQuant Software. nos mRNA levels from three independent experiments were quantified and normalized using RpLP2 mRNA as a loading control. Error bars indicate s.e.m. Ago, Argonaute; nos, nanos; Osk, Oskar.
Oskar (Osk) protein, which is also localized to the posterior of the embryo, is required for translation of nos mRNA, and ectopic Osk protein overrides the translational repression of unlocalized nos mRNA resulting in the expression of ectopic Nos [22]. To test whether Ago1 has a role in regulating Osk expression, Ago1 mutant embryos were stained with an anti-Osk antibody. Osk expression was unaffected indicating that the ectopic Nos protein observed in Ago1 mutant embryos was not an indirect effect of ectopic Osk expression (Fig 2C). Similarly, Smaug protein levels were unaffected in Ago1 mutant embryos, demonstrating that the ectopic Nos in Ago1 mutants was not an indirect effect of Smaug misregulation (Fig 2D).
nos mRNA is regulated at the levels of translation and mRNA stability. To determine the precise role of Ago1 in nos regulation, we assayed the levels and stability of nos mRNA in Ago1 mutant embryos by northern blot (Fig 2E). In both wild-type and Ago1 mutant embryos, nos mRNA is initially abundant at early stages and undergoes decay over a 2.5 h period with similar kinetics (Fig 2E,F). In addition, ectopic Nos protein accumulates in Ago1 mutant embryos despite a small reduction in the initial levels of nos mRNA (Fig 2E,G). Taken together, these data indicate that Ago1 is required to repress the translation of unlocalized nos mRNA.
The Ago1/nos mRNA interaction requires Smaug
To determine whether Ago1 interacts with nos mRNA in vivo, embryos were crosslinked and extracts were immunoprecipitated with an anti-Ago1 antibody. Using quantitative real-time PCR, we found that nos mRNA was enriched ∼2-fold in Ago1 immunoprecipitates compared with control immunoprecipitations with non-immune serum while bicoid mRNA was not, suggesting that Ago1 interacts with nos mRNA (Fig 3A). Interestingly, this enrichment was abrogated when extracts were prepared from smaug mutant embryos (Fig 3B), while Ago1 is expressed and immunoprecipitated at similar levels in wild-type and smaug mutant embryos (Fig 3C). Taken together, these data indicate that Smaug is necessary for the interaction between Ago1 and nos mRNA.
Figure 3.
Ago1 interacts with nos mRNA in a Smaug-dependent manner. Crosslinked embryo extracts collected 0–2 h post egg laying were immunoprecipitated with anti-Ago1 antibody or normal mouse serum, and levels of specific mRNAs were quantified by quantitative real-time PCR. (A) The fold enrichment of nos and bicoid mRNAs in Ago1 immunoprecipitates versus normal mouse serum immunoprecipitates. (B) The fold enrichment of nos mRNA in Ago1 immunoprecipitates versus normal mouse serum immunoprecipitates normalized to bicoid mRNA levels (n=5). Error bars represent s.e.m. calcuated from five biological replicates, the dotted line indicates onefold (i.e., no) enrichment and ** indicates a significant difference between the indicated samples as judged by the t-test (P<0.007). (C) Crosslinked extracts from wild-type and smaug mutant embryos were collected 0–2 h post egg laying and western blots were used to assay the levels of Ago1 protein in both the starting extracts and in material immunoprecipitated from these extracts using anti-Ago1 antibody. Dp1 serves as a loading control for the embryo extracts. Note that similar levels of Ago1 protein are immunprecipitated from smaug mutant extract compared with wild-type (97.2%±7.0%, n=2). Ago, Argonaute; IP, immunoprecipitation; nos, nanos.
Ago1 recruitment to nos mRNA is miRNA-independent
The requirement of Smaug protein for Ago1’s interaction with nos mRNA suggests that miRNAs are either not required, or not sufficient, for stable recruitment of Ago1 to the nos transcript. One possibility is that Smaug recruits Ago1 to the nos mRNA to trigger translational repression, bypassing the requirement for a miRNA. Indeed, the first ∼180 nucleotides of the nos 3′UTR have been shown to be necessary and sufficient for nos translational repression in the early embryo, and sequences downstream of this region seem to have no role [19] and a search of this region using three different miRNA prediction algorithms [23,24, 25] failed to identify miRNA-binding sites for miRNAs that are known to be expressed in the early embryo.
To explore a miRNA-independent recruitment model, we first performed experiments to determine whether Smaug could recruit Ago1 to a synthetic biotinylated RNA consisting of a 15 nucleotide stem/loop SRE (a 5 base-pair stem and a 5 base loop) with a three base 3′ tail in vitro. Streptavidin beads carrying this RNA were mixed with wild-type embryo extract and bound material was eluted and assayed via western blot. We found enrichment of Smaug and Ago1 proteins on beads carrying a functional SRE compared with beads carrying an RNA with a single-nucleotide change that disrupts Smaug binding (Fig 4A). In contrast, we failed to see enrichment of an irrelevant RNA-binding protein, Dp1. Given the small size of this synthetic SRE and its limited number of unpaired bases, these results strongly argue that Smaug recruits Ago1 to this RNA in the absence of miRNA/RNA interactions.
Figure 4.
Smaug mediates miRNA-independent recruitment of Ago1 to nos mRNA. (A) Embryo extracts collected 0–3 h post egg laying were incubated with biotinylated SRE+ or SRE− RNAs immobilized on streptavidin particles. Western blots to assay for the indicated proteins in crude extracts as well as the indicated purification are shown. For Smaug Westerns, equivalent amounts of material were run in extract lanes and capture lanes while the embryo extract lanes for the other Westerns are equivalent to 2% of the material run in the capture lanes. (B) Embryo extracts collected 0–2 h post egg laying from wild-type or transgene-carrying flies were incubated with anti-FLAG beads. Western blots to assay for the indicated proteins in crude extracts as well as the indicated purification are shown. Embryo extract lanes represent 5% of the material used in the immunoprecipitations. Dp1 serves as a loading control for the embryo extracts. (C) Purifications similar to (B) using crosslinked embryo samples were assayed for indicated miRNAs by northern blot. Embryo extract lanes represent 5% of the material used in the immunoprecipitations. (D) Purifications similar to (B) using crosslinked embryo samples were assayed for the indicated mRNAs by quantitative real-time PCR. The fold enrichment of the indicated mRNAs in transgenic embryos versus wild-type embryos carrying no transgene normalized to bicoid mRNA levels is shown. Error bars represent s.e.m. calculated from five biological replicates, the dotted line indicates onefold (i.e., no) enrichment and ** indicates a significant difference between the indicated samples as judged by the t-test (P<0.0011). Ago, Argonaute; miRNA, microRNA; IP, immunoprecipitation; nos, nanos.
To test the miRNA-independent recruitment model in vivo, we produced transgenic flies that express FLAG epitope-tagged Ago1 that carries two amino-acid substitutions in the Ago1 MID domain (FLAG–Ago1K657A,K694A) that have been shown to prevent binding to miRNAs [26, 27]. Using embryo extracts, we confirmed that FLAG–Ago1K657A,K694A co-immunoprecipitated Smaug to a similar extent as a wild-type FLAG–Ago1 protein (Fig 4B). In contrast, while we could detect miR-13b and miR-305 in immunoprecipitates of the wild-type FLAG-Ago1, neither miRNA could be detected in immunoprecipitates of the FLAG–Ago1K657A,K694A mutant protein (Fig 4C).
We then captured both proteins and compared the levels of nos mRNA present in these immunoprecipitates to the levels in similar captures from embryos expressing no FLAG-tagged protein. Strikingly, nos mRNA was enriched in both FLAG–Ago1 and FLAG–Ago1K657A,K694A pulldowns while two mRNAs known to bind Ago1 through their miRNA complementarity [28] co-purified with FLAG-Ago1 but not with the FLAG-Ago1K657A,K694A mutant (Fig 4D). This demonstrates that Smaug-dependent recruitment of Ago1 to nos mRNA does not require a miRNA. Taken together, our results indicate that Smaug recruits Ago1 to nos mRNA in the absence of a targeting miRNA and that Ago1 recruitment leads to translational repression.
DISCUSSION
Our data indicate that Smaug directly recruits Ago1 to nos mRNA in a miRNA-independent fashion (Fig 5). Interestingly, previous work suggests similarities between the mechanisms that Smaug and Ago use to regulate mRNAs. For example, translationally repressed nos mRNA is polysome associated [29] similar to some transcripts that are repressed by Ago proteins [30]. However, it is unclear if Smaug protein participates in repression of nos mRNA that is polysome associated or if this is mediated the RNA-binding protein Glorund, which is another regulator of nos mRNA [31, 32]. Another similarity between Smaug and Ago-mediated mechanisms comes from in vitro translation extracts showing that both Smaug and Ago1 repress translation through steps that function downstream of translation initiation and require ATP [12, 33, 34]. While these similarities are consistent with Smaug-mediated translational repression functioning, at least to some extent, through Ago1, it is noteworthy that several other mechanisms have been shown to have roles in translational repression by Ago proteins [35, 36].
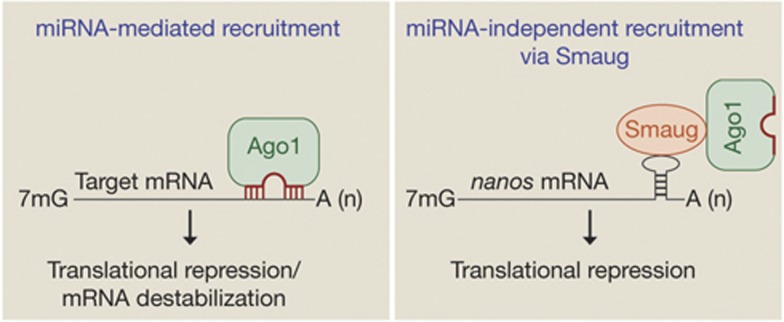
Figure 5.
Smaug recruits Ago1 to nos mRNA in a miRNA-independent manner. Ago1 is typically recruited to a target mRNA through base pairing between a miRNA and the target transcript. In contrast, our data indicate that Smaug directly recruits Ago1 to nos mRNA without the need for a targeting miRNA. While a miRNA is not required for targeting, Ago1 recruited by Smaug could be assoicated with a miRNA and, as described in more detail in the Discussion, this bound miRNA could be required for translational repression. Ago, Argonaute; miRNA, microRNA; nos, nanos.
Our data also indicate that while Ago1 functions in nos translational repression it does not induce nos mRNA degradation. Thus, in the context of nos mRNA, Ago1 does not induce transcript decay. In contrast, Smaug has a role in both translational repression and degradation of nos message [2, 3, 4, 11, 37], indicating that some of Smaug’s functions are Ago1-independent.
The interaction of Smaug with both Ago1 and Ago2 could reflect a common binding site on Ago proteins that mediates their interaction with Smaug. However, we failed to see ectopic Nos protein in Ago2 mutant embryos. This could suggest that while the Smaug/Ago1 interaction is functional, the interaction with Ago2 is not, perhaps because of the different mechanisms that are used by Ago1 and Ago2 to repress translation [34].
The data presented here along with previous work indicate that Smaug uses several mechanisms to regulate its target mRNAs. In addition to translational repression mediated by Smaug/Ago1, Smaug interacts with the Cup protein, which in turn interacts with the cap-binding protein eIF4E [9]. eIF4E bound to the 5′ cap of an mRNA indirectly recruits the 40S ribosomal subunit to an mRNA through eIF4E’s interaction with eIF4G [38]. As Cup blocks the eIF4E/eIF4G interaction, formation of the Smaug/Cup/eIF4E complex on a target mRNA represses translation initiation [9]. Smaug also recruits the Ccr4/Not deadenylase to target mRNAs resulting in removal of the transcripts poly(A) tail, thus repressing translation and/or inducing transcript degradation [8, 10, 11]. Interestingly, Smaug has also been shown to interact with the Piwi-type Ago proteins, Aubergine and Ago3 [39]. That study proposed that a complex consisting of Smaug, Aubergine with associated piwiRNAs and the Ccr4/Not deadenylase is recruited to nos mRNA through binding of both Smaug to the nos SREs and piwiRNAs to complementary to sequences in the nos 3′UTR.
These models of Smaug function raise the question as to why one RNA-binding protein uses several mechanisms to repress its target transcripts? One possibility is that by using several mechanisms, Smaug ensures that its targets are efficiently silenced. This might be especially important for a transcript, such as nos, where even low levels of inappropriate expression are lethal to the embryo [2]. Alternatively, Smaug might use different mechanisms to regulate different target mRNAs. Indeed, while Smaug induces the degradation of Hsp83 mRNA, it does not repress Hsp83 translation [10]. In contrast, while Smaug represses nos translation [2, 3, 4], it has only a modest role in destabilizing nos mRNA [10, 37]. This differential regulation could reflect the fact that the location of the SREs in the target mRNA (e.g., 3′UTR for nos [2, 3, 4, 19] versus open reading frame for Hsp83 [8]) and/or more cis-elements within these target mRNAs along with the trans-acting factors that recognize these influence the mechanisms of Smaug function.
While our data indicate that Smaug recruits Ago1 to nos mRNA via a miRNA-independent mechanism, it is still possible that a miRNA bound to Ago1, but not base-paired with nos mRNA, could be important for nos regulation (Fig 5). In this model, the miRNA would be required for Ago1 to repress nos translation at a step downstream of recruitment. For example, miRNA binding to Ago1 might be required to induce allosteric changes in Ago1 that facilitate its interaction with other factors that are required for Ago1-mediated repression. In this model, any miRNA would be sufficient for repression as it is not base-paired with the target transcript. Indeed, it has been suggested that miRNA binding might be involved in allosteric changes that are important for Ago-mediated repression [35], while others have shown that miRNA binding confers substantial structural stability to an Ago protein [40]. We note that the Ago1 miRNA-binding mutant used in the present study also fails to interact with GW182 [27], an Ago-binding protein that is essential for translational repression [36]; therefore it could not be used to test for miRNA-independent translational repression of nos mRNA in vivo.
A variety of RNA-binding proteins have been shown to function at numerous steps in miRNA/Ago-mediated post-transcriptional regulation [41]. For example, both a mammalian and Caenorhabditis elegans Pum form a complex that includes an Ago protein and in vitro experiments showed that the mammalian version of this complex represses translation of a reporter mRNA that carried only Pum-binding sites [42]. The lack of miRNA-binding sites within this reporter suggests that in this in vitro system Pum is able to recruit Ago to an mRNA in a miRNA-independent manner. It is, however, unclear whether this Pum/Ago complex is able to recognize target mRNAs in vivo in the absence of miRNA binding. Indeed, the authors suggested that in vivo targeting of the Pum/Ago complex might involve the presence of both Pum and miRNA-binding sites within a transcript. Thus, our data indicating that, in vivo, Smaug can recruit Ago1 to an mRNA in a miRNA-independent fashion, combined with their in vitro data suggesting that Pum can also directly recruit an Ago protein, suggests that other RNA-binding proteins might also function in a similar manner.
METHODS
Drosophila stocks. Drosophila stocks included w1118, ago2414 [43], FRTG13 Ago1EMS [44] and smaug1 [3].
Transgene construction. The base vector for expression of Ago1 transgenes was CaSpeR-Hsp83 [45], into which we cloned the Ago1 open reading frame from EST SD153909, followed by a carboxy-terminal 3 × FLAG tag-Strep tag II-His tag sequence and the alpha-tubulin 84B 3' region. Transgenes were inserted in the attP2 site on the third chromosome by Genetic Services (Cambridge, MA) using PhiC31 integrase-mediated transgenesis [46].
Immunoprecipitations. Immunoprecipitations were performed as previously described [9] with the modifications described in supplementary Information online.
Immunoprecipitation from crosslinked embryo extract. Embryos were collected 0–2 h post egg laying and crosslinked using formaldehyde as previously described [47]. Extract preparation, immunoprecipitation, RNA extraction, quantitative real-time PCR and miRNA northerns are as described in supplementary Information online.
RNA-capture experiments. This protocol is based on the work described in Jeske et al [12]. Synthetic RNAs with 3′ biotin labels were purchased from Thermo Scientific; SRE+ sequence: 5′-AGGCUCUGGCAGUCUAAA-3′, SRE− sequence: 5′-AGGCUCUcGCAGUCUAAA-3′. RNAs were captured on Streptavidin MagneSphere Paramagnetic Particles (Promega) and mixed with extract prepared from embryos collected 0–3 h post egg laying as described in supplementary Information online.
Immunohistochemistry. Embryos collected 0–3 h post egg laying were fixed and then stained using anti-Nos and anti-Osk antibodies, which were gifts from Paul MacDonald, as described in supplementary Information online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank Heli Vari for her excellent technical expertise; Peng Jin, Paul Macdonald, Akira Nakamura and Haruhiko Siomi for their gifts of reagents; Julie Claycomb for her advice on miRNA northerns; and Amy Caudy, Julie Claycomb, John Laver, Howard Lipshitz, Alexander Marsolais and Alexander Palazzo for helpful comments on this manuscript. This research was funded by Canadian Cancer Society through an operating grant to C.A.S.
Author contributions: B.D.P. and C.A.S. conceived the study, designed and performed the experiments, analysed the data and wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Hentze MW, Gebauer F, Preiss T (2007) Cis-regulatory sequences and trans-acting factors in translational control. In Translational Control in Biology and Medicine Mathews MB, Sonenberg N, Hershey JWB (eds), pp 269–295New York, USA: Cold Spring Harbor Laboratory Press, [Google Scholar]
- Smibert CA, Wilson JE, Kerr K, Macdonald PM (1996) Smaug protein represses translation of unlocalized nanos mRNA in the Drosophila embryo. Genes Dev 10: 2600–2609 [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Walker JA, Wharton RP (1999) Smaug, a novel RNA-binding protein that operates a translational switch in Drosophila. Mol Cell 4: 209–218 [DOI] [PubMed] [Google Scholar]
- Smibert CA, Lie YS, Shillinglaw W, Henzel WJ, Macdonald PM (1999) Smaug, a novel and conserved protein, contributes to repression of nanos mRNA translation in vitro. RNA 5: 1535–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv T, Lin Z, Lau S, Rendl LM, Sicheri F, Smibert CA (2003) The RNA-binding SAM domain of Smaug defines a new family of post-transcriptional regulators. Nat Struct Biol 10: 614–621 [DOI] [PubMed] [Google Scholar]
- Baez MV, Boccaccio GL (2005) Mammalian Smaug is a translational repressor that forms cytoplasmic foci similar to stress granules. J Biol Chem 280: 43131–43140 [DOI] [PubMed] [Google Scholar]
- Aviv T, Lin Z, Ben-Ari G, Smibert CA, Sicheri F (2006) Sequence-specific recognition of RNA hairpins by the SAM domain of Vts1p. Nat Struct Mol Biol 13: 168–176 [DOI] [PubMed] [Google Scholar]
- Semotok JL, Luo H, Cooperstock RL, Karaiskakis A, Vari HK, Smibert CA, Lipshitz HD (2008) Drosophila maternal Hsp83 mRNA destabilization is directed by multiple SMAUG recognition elements in the open reading frame. Mol Cell Biol 28: 6757–6772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MR, Leidal AM, Smibert CA (2004) Drosophila Cup is an eIF4E-binding protein that functions in Smaug-mediated translational repression. EMBO J 23: 150–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semotok JL, Cooperstock RL, Pinder BD, Vari HK, Lipshitz HD, Smibert CA (2005) Smaug recruits the CCR4/POP2/NOT deadenylase complex to trigger maternal transcript localization in the early Drosophila Embryo. Curr Biol 15: 284–294 [DOI] [PubMed] [Google Scholar]
- Zaessinger S, Busseau I, Simonelig M (2006) Oskar allows nanos mRNA translation in Drosophila embryos by preventing its deadenylation by Smaug/CCR4. Development 133: 4573–4583 [DOI] [PubMed] [Google Scholar]
- Jeske M, Moritz B, Anders A, Wahle E (2011) Smaug assembles an ATP-dependent stable complex repressing nanos mRNA translation at multiple levels. EMBO J 30: 90–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammell CM (2008) The microRNA-Argonaute complex: a platform for mRNA modulation. RNA Biol 5: 123–127 [DOI] [PubMed] [Google Scholar]
- Hutvagner G, Simard MJ (2008) Argonaute proteins: key players in RNA silencing. Nat Rev Mol Cell Biol 9: 22–32 [DOI] [PubMed] [Google Scholar]
- Gavis ER, Lehmann R (1992) Localization of nanos RNA controls embryonic polarity. Cell 71: 301–313 [DOI] [PubMed] [Google Scholar]
- Wang C, Dickinson LK, Lehmann R (1994) Genetics of nanos localization in Drosophila. Dev Dyn 199: 103–115 [DOI] [PubMed] [Google Scholar]
- Bergsten S, Gavis E (1999) Role for mRNA localization in translational activation but not spatial restriction of nanos RNA. Development 126: 659–669 [DOI] [PubMed] [Google Scholar]
- Gavis ER, Lehmann R (1994) Translational regulation of nanos by RNA localization. Nature 369: 315–318 [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Wharton RP (1996) The nanos gradient in Drosophila embryos is generated by translational regulation. Genes Dev 10: 2610–2620 [DOI] [PubMed] [Google Scholar]
- Chou TB, Perrimon N (1996) The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics 144: 1673–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T-B, Perrimon N (1992) Use of a yeast site-specific recombinase to produce female germline chimeras in Drosophila. Genetics 131: 643–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm JE, Smibert CA (2005) Mechanisms of translational regulation in Drosophila. Biol Cell 97: 235–252 [DOI] [PubMed] [Google Scholar]
- Betel D, Koppal A, Agius P, Sander C, Leslie C (2010) Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol 11: R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheradpour P, Stark A, Roy S, Kellis M (2007) Reliable prediction of regulator targets using 12 Drosophila genomes. Genome Res 17: 1919–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun D, Wang YL, Langenberger D, Gunsalus KC, Rajewsky N (2005) MicroRNA target predictions across seven Drosophila species and comparison to mammalian targets. PLoS Comput Biol 1: e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JB, Yuan YR, Meister G, Pei Y, Tuschl T, Patel DJ (2005) Structural basis for 5'-end-specific recognition of guide RNA by the A. fulgidus Piwi protein. Nature 434: 666–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland A, Huntzinger E, Schmidt S, Izaurralde E, Weichenrieder O (2011) Crystal structure of the MID-PIWI lobe of a eukaryotic Argonaute protein. Proc Natl Acad Sci USA 108: 10466–10471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong X, Hammell M, Ambros V, Cohen SM (2009) Immunopurification of Ago1 miRNPs selects for a distinct class of microRNA targets. Proc Natl Acad Sci USA 106: 15085–15090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark IE, Wyckoff D, Gavis ER (2000) Synthesis of the posterior determinant Nanos is spatially restricted by a novel cotranslational regulatory mechanism. Curr Biol 10: 1311–1314 [DOI] [PubMed] [Google Scholar]
- Meister G (2007) miRNAs get an early start on translational silencing. Cell 131: 25–28 [DOI] [PubMed] [Google Scholar]
- Kalifa Y, Huang T, Rosen LN, Chatterjee S, Gavis ER (2006) Glorund, a Drosophila hnRNP F/H homolog, is an ovarian repressor of nanos translation. Dev Cell 10: 291–301 [DOI] [PubMed] [Google Scholar]
- Andrews S, Snowflack DR, Clark IE, Gavis ER (2011) Multiple mechanisms collaborate to repress nanos translation in the Drosophila ovary and embryo. RNA 17: 967–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske M, Meyer S, Temme C, Freudenreich D, Wahle E (2006) Rapid ATP-dependent deadenylation of nanos mRNA in a cell-free system from Drosophila embryos. J Biol Chem 281: 25124–25133 [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Kawamata T, Tomari Y (2009) Drosophila Argonaute1 and Argonaute2 employ distinct mechanisms for translational repression. Mol Cell 34: 58–67 [DOI] [PubMed] [Google Scholar]
- Djuranovic S, Nahvi A, Green R (2011) A parsimonious model for gene regulation by miRNAs. Science 331: 550–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian MR, Sonenberg N (2012) The mechanics of miRNA-mediated gene silencing: a look under the hood of miRISC. Nat Struct Mol Biol 19: 586–593 [DOI] [PubMed] [Google Scholar]
- Semotok JL, Lipshitz HD (2007) Regulation and function of maternal mRNA destabilization during early Drosophila development. Differentiation 75: 482–506 [DOI] [PubMed] [Google Scholar]
- Gingras A-C, Raught B, Sonenberg N (1999) eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem 68: 913–963 [DOI] [PubMed] [Google Scholar]
- Rouget C, Papin C, Boureux A, Meunier AC, Franco B, Robine N, Lai EC, Pelisson A, Simonelig M (2010) Maternal mRNA deadenylation and decay by the piRNA pathway in the early Drosophila embryo. Nature 467: 1128–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkayam E, Kuhn CD, Tocilj A, Haase AD, Greene EM, Hannon GJ, Joshua-Tor L (2012) The structure of human Argonaute-2 in complex with miR-20a. Cell 150: 100–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kouwenhove M, Kedde M, Agami R (2011) MicroRNA regulation by RNA-binding proteins and its implications for cancer. Nat Rev Cancer 11: 644–656 [DOI] [PubMed] [Google Scholar]
- Friend K, Campbell ZT, Cooke A, Kroll-Conner P, Wickens MP, Kimble J (2012) A conserved PUF-Ago-eEF1A complex attenuates translation elongation. Nat Struct Mol Biol 19: 176–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura K, Ishizuka A, Siomi H, Siomi MC (2004) Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev 18: 1655–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Chen D, Duan R, Xia L, Wang J, Qurashi A, Jin P (2007) Argonaute 1 regulates the fate of germline stem cells in Drosophila. Development 134: 4265–4272 [DOI] [PubMed] [Google Scholar]
- Govind S, Brennan L, Steward R (1993) Homeostatic balance between dorsal and cactus proteins in the Drosophila embryo. Development 117: 135–148 [DOI] [PubMed] [Google Scholar]
- Bischof J, Maeda RK, Hediger M, Karch F, Basler K (2007) An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci USA 104: 3312–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth J, Biggin MD (2000) The specificity of protein-DNA crosslinking by formaldehyde: in vitro and in drosophila embryos. Nucleic Acids Res 28: e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.