Abstract
If Narcissus could have self-renewed even once on seeing his own reflection, he would have died a happy man. Stem cells, on the other hand, have an enormous capacity for self-renewal; in other words, the ability to replicate and generate more of the same. In adult organisms, stem cells reside in specialized niches within each tissue. They replenish tissue cells that are lost during normal homeostasis, and on injury they repair damaged tissue. The ability of a stem cell to self-renew is governed by the dynamic interaction between the intrinsic proteins it expresses and the extrinsic signals that it receives from the niche microenvironment. Understanding the mechanisms governing when to proliferate and when to differentiate is vital, not only to normal stem cell biology, but also to ageing and cancer. This review focuses on elucidating conceptually, experimentally and mechanistically, our understanding of adult stem cell self-renewal. We use skin as a paradigm for discussing many of the salient points about this process, but also draw on the knowledge gained from these and other adult stem cell systems to delineate shared underlying principles, as well as highlight mechanistic distinctions among adult tissue stem cells. By doing so, we pinpoint important questions that still await answers.
Keywords: stem cells, self-renewal, homeostasis, wound-repair, regeneration
See the Glossary for abbreviations used in this article.
Glossary.
- ACF7
actin cytoskeletal crosslinking factor 7
- aPKC
atypical protein kinase C
- Bmi1
B-lymphoma Moloney murine leukaemia virus insertion region 1
- BMP
bone morphogenetic protein
- CDK
cyclin-dependent kinase
- E2F
E2 transcription factor
- Ezh
enhancer of zeste homologue
- GSK3β
glycogen synthase kinase 3 beta
- Hmga2
high mobility group AT-hook 2
- Lgn
Leu–Gly–Asn repeat-enriched protein
- Lgr5
leucine-rich repeat containing G-protein-coupled receptor 5
- Mud
mushroom body defect
- NuMA
nuclear/mitotic apparatus protein
- p16Ink4a
tumour suppressor protein encoded by the Ink4a gene locus
- p19Arf
tumour suppressor protein encoded by the Ink4 gene locus that uses a different reading frame from p16
- Par3
partitioning defective protein 3
- Pins
partner of Inscuteable
- PRC2
polycomb repressor complex 2
- Rosa26
a broadly expressed but non-essential gene
- Rb
retinoblastoma
- Runx1
runt-related transcription factor 1
- shRNA
shorthairpin RNA
- Smad
TGF-β signalling transcription factors originally defined as mutants giving small animal size
- Tbx1
T-box transcription factor 1
- TCF
transcription cell factor
- Wnt
mammalian homologues of Drosophila ‘wingless’ signalling protein
- YFP
yellow fluorescent protein
Concept of stem cell self-renewal
Self-renewal is the specific cellular action that involves proliferation accompanied by maintenance of both multipotency and tissue regenerative potential. To achieve self-renewal, two things must happen: first, the cell must enter the cell cycle and divide, and second, at least one of the progenies must be an undifferentiated cell. Failure in either one of these two aspects leads to cell depletion and eventual tissue malfunction.
Several excellent reviews have focused on self-renewal in specialized adult stem cells, including those of the intestine and haematopoietic system [1,2]. However, self-renewal is not unique to stem cells, as some progenitor cells can also self-renew [3]. The main distinction between progenitor cells and stem cells is whether their ability to self-renew is short term (progenitor) or long term (stem cell). Although this distinction might sometimes seem vague, ‘long term’ typically indicates potential that is retained throughout the lifetime of the animal. Although the lifespan of insects is markedly different to that of humans, long-term self-renewal ability of tissue stem cells truly represents the distinction between life and death for most multicellular organisms.
The ability of stem cells to survive and retain their proliferative potential throughout the lifespan of the animal does not necessarily imply that they have an endless capacity to divide, or that they undergo constant self-renewal. Rather, it means that the frequency and timing of actual stem cell self-renewal divisions are tightly regulated within the tissue to ensure the lifelong maintenance of the stem cell population. If stem cells are exhausted too quickly, or if genetic defects or damage reduce their proliferative potential, tissue atrophy and premature ageing can arise. Conversely, mutations that promote more frequent stem cell divisions without appropriate differentiation balance can result in abnormal tissue development and even cancer.
In most tissues, stem cell self-renewal is coupled with tissue regeneration. As tissues have different developmental needs and cellular hierarchy, the in vivo self-renewal frequencies of adult tissue stem cells are bound to differ. However, the underlying principle is the same: stem cells self-renew to sustain long-term tissue regeneration [4].
Several examples illustrate the differences in stem cell self-renewal frequency. Hair follicles undergo cyclical, often synchronized bouts of growth, degeneration and rest. In mice, the growth phase lasts typically about a month, whereas the degeneration phase lasts several days. By contrast, the resting phase can last from one day to a couple of months, which typically increases as the mice age [5]. The hair follicle stem cells (HFSCs) that fuel the growth phase are located in a niche called ‘the bulge’, and for much of the hair cycle they exist in a quiescent state [6,7]. They only become activated and self-renew within the bulge after initiation of each new cycle [5]. A few days later, bulge HFSCs return to quiescence, suggesting that proliferation within the bulge is aimed at replenishing the stem cells that were used to initiate regeneration (Fig 1A; [5,8,9,10]).
Figure 1.
Timing and frequency of adult stem cell self-renewal proliferation. (A) Self-renewal of mouse HFSCs. Hair follicles undergo cycles of growth (pink box), degeneration (not shown) and rest (blue box). HFSCs are located in a niche called ‘the bulge’, in which they normally exist in a quiescent state, as represented by green cells. They only become activated just after the growth phase has begun, where they self-renew (as indicated by red cells) for a few days before returning to quiescence. HFSCs that exit the bulge niche proliferate earlier and remain proliferative slightly longer than those in the bulge. This leads to generation of more HFSCs that form a new bulge as the degenerative phase ends and the follicle returns to rest. (B) Self-renewal proliferation of mouse ISCs. Intestine epithelium constantly regenerates. It takes approximately 3–5 days for cells near the base of the crypt to be shed from the villus tip. ISCs reside at the bottom of the crypt, where they self-renew continuously, dividing once per day. (C) Activation of mouse HSCs. Most HSCs reside in the bone marrow, in two separate niches: the endosteum and central marrow. The most quiescent HSCs retain nucleotide and/or fluorescent histone labelling and are sometimes referred to as LRCs (see text). They divide approximately once every 4–5 months during normal homeostasis. Although not definitively shown, some researchers have proposed that the more active HSCs divide approximately once a month and tend to localize in the central marrow, close to the vasculature, where they enter and circulate in the bloodstream. HFSC, hair follicle stem cell; HSC, haematopoietic stem cell; ISC, intestine stem cell; LRC, label retaining cell; SC, stem cell.
The small intestine undergoes rapid turnover. It takes approximately 3–5 days for cells near the base of the crypt to differentiate, move upwards and be shed from the villus tip. To sustain this constant turnover, mouse intestinal stem cells (ISCs) within the crypt base undergo nearly continuous self-renewal proliferation, dividing on estimate at least once a day (Fig 1B; [11,12,13]).
The mouse haematopoietic system is responsible for producing nearly a billion circulating blood cells every day [14,15]. Studies show that there appears to be a pool of long-term haematopoietic stem cells (HSCs) that divide about once a month to fuel the production of blood cells during normal homeostasis, whilst a smaller pool of dormant long-term HSCs seem to be set aside to respond during a crisis, as they divide no more than once every four to five months (Fig 1C; [15,16,17]).
Experimental demonstration of stem cell self-renewal
Stem cells are defined by their behaviour, rather than the specific genes that they express. All stem cells give rise to both differentiated progenies, as well as undifferentiated stem cells with regenerative potential. So far, however, a common set of genes has not yet been described which, by their expression alone, distinguish stem cells from non-stem cell progeny [11]. Given the plasticity and heterogeneity of most mouse adult stem cell systems [5,16,18,19,20], such a singular ‘stemness identifier’ might not exist. In its absence, researchers have resorted to other types of experimental evidence to address whether a particular cell population has the capacity to sustain long-term multiple rounds of tissue regeneration in vivo whilst maintaining the pool of undifferentiated cells.
Described over 50 years ago, HSCs were the first cells shown to self-renew and regenerate tissue in the long term. This capacity was assessed by showing that HSCs could replenish myeloid and lymphoid cell lineages over many serial transplantation assays into recipient mice, whose own HSCs had been ablated by lethally irradiating their niche, the bone marrow [21].
The cells displaying these defining features of stem cells represented only a small fraction of total untreated bone marrow. They were enriched for expression of certain cell surface markers and underwent mitosis once a month to fuel the production of blood cells during normal homeostasis (Fig 1C). However, when challenged, this tiny subpopulation ‘packed a powerful punch’. Thus, on transplantation into the bone marrow of an irradiated host recipient, the HSCs rescued and sustained the entire blood and immune system for months. Importantly, when re-isolated and transplanted serially into a new irradiated mouse, the secondary HSCs again reconstituted the entire haematopoietic system of the recipient. This has adorned them with the distinction of being long-term HSCs (LT-HSCs). The capacity for serial transplantation distinguishes LT-HSCs from their early progeny—short-term HSCs (ST-HSCs) and multipotent progenitors (MPPs)—that have considerable but transient proliferative and multipotent characteristics [22,23,24].
Across cell types, stemness cannot be defined by a specific number of cell divisions. Indeed, stem cell assignments are often relative and based on comparative behaviours. Moreover, these hierarchical assignments are still unfolding, as evidenced by the identification of an even longer-term HSC, which normally rests within the bone marrow in a deeply dormant state, only to be aroused during crisis, at which time they can repopulate the LT-HSCs and reconstitute the haematopoietic system [16,17]. Finally, in the realm of stem cell biology, HSCs are the gold standard for self-renewal capacity: neuronal stem cells would be elated with the regenerative potential of a downstream committed progenitor from the haematopoietic system.
Whilst HSCs set the paradigm for stem cells and self-renewal in vivo, epidermal stem cells set the paradigm in vitro. Pioneered over 30 years ago as the first human adult stem cells cultured and used in regenerative medicine, epidermal keratinocytes can be expanded for many generations in vitro without differentiating or losing their stemness [25]. A small skin biopsy from a badly burned patient can be cultured to produce sheets of epidermis, which can be grafted back onto the patient to reconstruct and sustain for many years the epidermal barrier function of the skin [26]. Such methods have been adapted to the corneal stem cells of the limbus, enabling patients blinded in industrial accidents to see again [27,28]. Turning to mice for their power as a genetic model, researchers have also isolated stem cells from the hair follicle bulge, cultured the stem cells in the long term and shown that they can regenerate hair follicles, epidermis and sebaceous glands when engrafted onto host recipient mice [7,29,30].
Lineage-tracing assays and stem cell self-renewal
For solid adult tissues that undergo turnover, lineage tracing is often used together with transplantation assays to document the ability of a stem cell to both self-renew and generate tissue in the long term [5,31,32,33]. In a perfect lineage-tracing experiment to investigate long-term self-renewing and tissue regenerative ability, a single stem cell is first exclusively tagged with a genetic marker, the expression of which will be sustained not only in the mother stem cell, but also her progeny. In the second step, the behaviour of the tagged stem cell is monitored long term in vivo—ideally for the lifespan of the animal—to test directly whether it can both sustain tissue formation and maintain the pool of undifferentiated cells.
Although simplistic in concept, interpretations have been clouded by tagging methods, which are often not restricted to the stem cell population. Even when they are, it is often technically impossible to conduct live imaging to monitor a single stem cell and its progeny over extended time periods. That said, more sophisticated lineage-tracing tools are being devised for tagging cells at increased resolution. Such efforts continue to help in identifying the true stem cells that have long-term self-renewal and tissue regenerative ability [5,18,19,34,35,36].
Several studies have demonstrated the elegance of lineage-tracing methods, as well as the caution that should be taken when interpreting results. By labelling slow-cycling HFSCs with nucleotide or fluorescent histones, and then monitoring the subsequent dilution of label as they become mobilized at the start of a new hair cycle, it was discovered that HFSCs at the base of the bulge (hair germ) are the first to be activated [7,9]. Although the details are still unfolding, most of these cells form a pool of rapidly proliferating matrix cells that give rise to the differentiated cells of the mature follicle and its hair [5]. However, most, if not all, are short-lived (‘transit-amplifying’), undergoing apoptosis when the regression phase ensues.
A staggered series of pulse–chase experiments and lineage tracing revealed that as the new hair follicle emerges, HFSCs from the bulge generate a trail of ‘outer root sheath’ (ORS) cells along the outer surface of the follicle (Figs 1A, 2A). Both bulge and upper ORS stem cells become briefly proliferative within a few days after the new hair cycle has begun. In the mature follicle, the upper ORS stem cells return to quiescence just after the bulge [5]. Most of these upper ORS stem cells survive, and at the regression phase they form a new bulge to be used in the next hair cycle [5].
Figure 2.
Balancing stem cell self-renewal and tissue regeneration. (A, A') Molecular and lineage-tracing studies have demonstrated that HFSCs (green, quiescent; red, proliferative) support follicle homeostasis and hair growth and, at the same time, maintain the pool of undifferentiated stem cells. Activation, proliferation and fate commitment of HFSCs are achieved in part through Wnt signalling and BMP inhibition (A), which reaches a crucial threshold at the transition that mobilizes the stem cell niche to make a new hair follicle (A). Activated HFSCs maintaining contact with the dermal papilla (brown) soon commit to form transit-amplifying (TA) matrix progenitors (blue), and can never again be stem cells. As the follicle grows downwards and matures, BMP inhibition and Wnt signalling at the base (hair bulb) continue to increase, and matrix cells rapidly divide several times, then terminally differentiate to produce the hair and the inner cells of the hair follicle (A'). At the end of the growth phase, HFSCs that exited the niche to the upper portion of the outer root sheath (ORS) return to form the new bulge and hair germ. Those further along in transit between the bulge and the matrix reach a cell-fate commitment ‘point of no return’. However, they too move back to the niche, where they become the keratin 6-positive (K6+) inner bulge cells (shown in orange), which further suppress HFSC self-renewal by expressing inhibitory signals such as BMPs [5]. (B) Lineage-tracing experiments have demonstrated that ISCs (shown in green) self-renew long term and generate all of the differentiated progeny in the intestinal epithelium, including Paneth cells (shown in orange), which maintain self-renewal within the stem cell niche in part by expressing Wnt signals [39]. (C) Downstream HSC progenies participate in regulating HSC activation. HSCs first generate MPPs that are similar in concept to the intermediate, unspecified proliferative matrix cells of the hair follicle. MPPs then differentiate into one of several haematopoietic lineages. Macrophages are one of the terminally differentiated cell types of the HSC lineage [15]. They circulate and return to the niche, where they provide inhibitory signals that restrict the number of HSCs that become activated for circulation. Within the bone marrow, the niche for HSCs was initially thought to be the osteoblast lining of the bone, but evidence points to a more important role for perivascular and endothelial cells in HSC maintenance [94]. BMP, bone morphogenetic protein; HFSC, hair follicle stem cell; HSC, haematopoietic stem cell; ISC, intestinal stem cell; MPP, multipotent progenitor; Wnt, mammalian homologue of Drosophila ‘wingless’ signalling protein.
In an elegant application of lineage tracing, in which the Lgr5 promoter was used to mark ISCs, it was shown that these cells can sustain the long-term maintenance of its associated villus for at least one year (Fig 2B; [37,38]). Similarly to the hair follicle, ISCs give rise to a zone of transiently proliferative cells that differentiate to form not only Paneth cells at the base of the niche, but also the columnar enterocytes, mucin-secreting goblet cells and enteroendocrine cells that together form each intestinal villus. ISCs can also be maintained in vitro, in which, under the right conditions, individual Lgr5+ stem cells grow autonomously into crypt-like structures that can be serially propagated as self-renewing organoid cultures that recapitulate much of the complex biology of the intestinal epithelium [39,40]. When grafted, these cells can incorporate and form the colon epithelium of the villus. In addition, these organoid cultures led researchers to uncover a surprising dependency of stem cells on their differentiated Paneth cell neighbours, which act not only as infection-fighting cells for the niche, but also as signalling centres that maintain stem cell proliferation (Fig 2B; [39]).
Long-term tracings, with either Lgr5 or K14 promoter tagging strategies, have also demonstrated that HFSCs self-renew in the long term and contribute to hair homeostasis for up to a year [8,41,42]. Moreover, by combining lineage tracing with nucleotide pulse–chase experiments, a series of snapshots of proliferative progeny relations were generated that, when strung together, produced a movie of cell progression during hair growth (Fig 2A). This method, in conjunction with molecular analyses, revealed that differentiated progeny (K6+) cells return to the niche at the end of each hair cycle, and participate in regulating HFSC quiescence (Fig 2A; [5]). The ability of lineage-specific progeny to provide inhibitory feedback signals to their stem cell parents also seems to apply to the haematopoietic system, in which macrophages generated from HSCs return to the bone marrow where they are important for HSC retention in the bone marrow niche (Fig 2C; [43,44]). It is interesting that the directionality of the feedback signal—positive for ISCs and negative for HFSCs and HSCs—correlates with the stem cells and whether they maintain a proliferative or quiescent state.
In adult mouse interfollicular epidermis, the presence of basal cells that can self-renew in the long term in vivo was investigated by two separate lineage-tracing studies [34,36]. In the first study, some basal epidermal cells from a Rosa26YFP reporter mouse were fortuitously marked by a cytochrome P450 promoter-driven Cre recombinase. When the behaviour of the clones was followed over the coming year, the number of clones fell to 3% of the original number, whilst the remaining clones contained basal cells that expanded at a linear rate. Mathematical modelling on the basis of these data pointed to the existence of a single type of cell that chooses proliferation or differentiation randomly, such that most divisions result in one proliferative and one differentiating daughter [34]. This idea contrasted with the long-standing model that basal epidermal stem cells give rise to transit-amplifying progenitors, which then progress to differentiate terminally into the stratified layers of the epidermis [45].
Whilst provocative, the conclusions from the Clayton study relied on the assumption that the cytochrome P450-Cre sporadically marked all basal cells equally. A study suggests that this might not have been the case. Thus, when basal epidermal cells were clonally tagged by using an inducible K14 promoter [46], many of the marked clones survived for up to a year and showed only modest expansion [36]. This time, mathematical modelling of the data fit the behaviour of a stem cell with the ability to self-renew and, at the same time, give rise to lineage-committed progeny. Moreover, when a subset of basal cells were marked by using an inducible promoter (Inv-CreER) for the early differentiation marker involucrin [47], more than 90% of clones were progressively lost within the year, whilst the remaining clones expanded linearly within the basal layer, suggesting that the K14-marked cells are bona fide stem cells, whereas the Inv-CreER cells are the transit-amplifying committed progenitors. Moreover, the modelling predicted that most of the stem cells and transit-amplifying cells divided to yield asymmetrical cell fates, whilst approximately 10% of the cells divided symmetrically [36].
Together, these lineage-tracing studies provide compelling support for the existence of a basal epidermal layer replete with long-term stem cells, as well as short-term progenitors, which maintain the differentiated layers of the stratified epithelium. These two studies serve to both highlight the power as well as the potential pitfalls involved in lineage-tracing studies.
Other cases seem simpler. In the mammary and sweat glands, unipotent stem cells are responsible for sustaining long-term homeostasis of the myoepithelial and luminal epithelial layers of the gland [31,48,49]. Despite similarities in architecture and unipotency, the frequency of use of stem cells varies markedly for these two adult glandular structures. Mammary glands respond to hormonal stimuli by undergoing massive tissue expansion to fulfil the need for milk [50,51]. By contrast, sweat glands respond to neuronal stimuli by cranking out more sweat per gland with little, if any, tissue expansion [52]. Thus, mammary gland stem cells are mobilized with every pregnancy, whereas sweat gland stem cells are only aroused when tissue is damaged, and otherwise remain largely dormant. This difference in stem cell activity probably explains why breast cancers occur so frequently and sweat gland cancers are so rare [31].
Another intriguing feature of stem cells that was revealed by lineage tracing is that when challenged to undergo de novo tissue regeneration, they can often expand their repertoire of differentiation fates. Such behaviour is illustrated by the myoepithelial stem cells of sweat and mammary glands. Whilst normally acting as unipotent progenitors, purified myoepithelial cells can unleash multipotent behaviour when engrafted, generating glands de novo that are repleted with both myoepithelial and luminal epithelial cells [31,48,50,51]. Similar behaviour is shown by HFSCs, which normally only make hair follicles, but can generate sebaceous glands and epidermis on grafting [30,53]. Such stem cell features capture the fascination of both biologist and clinician alike, as it forms the crux of what is needed for the advance of regenerative medicine.
Balancing stem cell self-renewal and differentiation
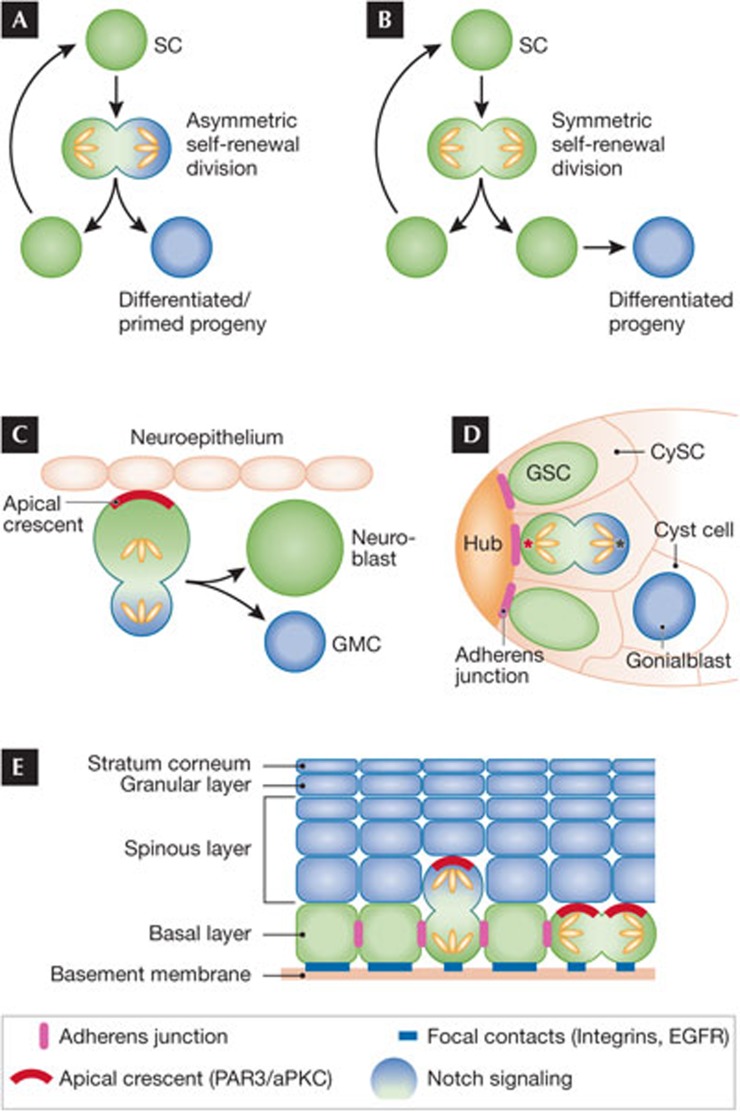
The ability of stem cells to self-renew in the long term and differentiate are their defining features. Two distinct models can explain how these universal features maintain tissue balance. In the asymmetrical division model, polarization and disproportionate segregation of cell-fate-defining components occurs during mitosis (Fig 3A). At the end of anaphase, one daughter has received an excess of stem cell factors and remains undifferentiated, whereas the second daughter receives an excess of lineage commitment factors and embarks on a differentiation programme. In the symmetrical division model, DNA, proteins, RNAs and other cellular components are distributed equally between the two daughter cells. In this scenario, cell fate specification or differentiation occurs after completion of mitosis and is not obligatorily coupled with cell division (Fig 3B).
Figure 3.
Symmetrical as opposed to asymmetrical cell division to achieve self-renewal and differentiation. (A) In asymmetrical cell divisions, cell fate specification is coupled to mitosis and involves unequal partitioning of cellular components to the resulting daughter cells. (B) In symmetrical cell divisions, cellular components are equally distributed to the two daughter cells. Although cell fate specification is not coupled to mitosis in a symmetrical division, it can be intimately linked if one of the two daughter cells is displaced to a new microenvironment. (C) Drosophila neuroblasts divide by asymmetrical cell division. To partition cellular components unequally, they use a polarized Par3/aPKC-containing apical crescent (marked in red) to establish cell polarity. As the neuroblast enters mitosis, the apical crescent recruits additional proteins that polarize the spindle and differentially partition Notch signalling components, such that on completion of mitosis, the cell retaining the apical crescent remains as a stem cell and the other becomes the differentiated GMC. (D) Drosophila male GSCs also divide asymmetrically, but through a different mechanism. GSCs surround the Hub cells located at the tip of the testis. They are interspersed with somatic CySCs. GSCs attach to the niche Hub cells through adherens junctions (marked in purple), which polarize the mother centriole (marked in red) and align the GSC spindle in a fashion such that one daughter retains the mother centriole and its association with the Hub, whilst the other becomes fated to differentiate as a gonialblast. (E) Mouse embryonic basal epidermal cells undergo asymmetrical cell divisions. These cells are polarized through intercellular adherens junctions (marked in purple), and basement membrane–substratum junctions (marked in blue). These features localize an apical crescent, which serves as a platform for recruiting some of the same spindle-orienting, asymmetrical division components as the fly neuroblast. After divisions perpendicular to the basement membrane, one daughter probably inherits integrins and associated growth factor receptors disproportionately, remaining as a basal stem cell, whereas the displaced suprabasal daughter cell differentially inherits Notch signalling. Approximately 60% of embryonic basal epidermal cells undergo asymmetrical division whilst the remaining ones undergo symmetrical cell division relative to the basement membrane. aPKC, atypical protein kinase C; CySC, cyst stem cell; GMC, ganglion mother cell; GSC, germline stem cell; Par3, partitioning defective protein 3; SC, stem cell.
Distinctions between asymmetrical and symmetrical divisions are frequently difficult to make even with genetic and biochemical evidence. Confounding these ambiguities, no single cell division mechanism has been found that applies to all stem cells. Even for a specific stem cell type and its proliferative progeny, it is often not clear whether they divide exclusively in an asymmetrical or symmetrical mode. Indeed, given the impact that the microenvironment has on cell behaviour and fate specification, it seems plausible to expect that stem cells and their proliferative progenitors use different division mechanisms to fulfil their role in tissue morphogenesis and wound repair.
Some stem cells or progenitors clearly achieve self-renewal and differentiation through mitosis-coupled asymmetrical partitioning of their cellular components. The best-studied examples are in lower eukaryotes, in which the power of genetics has unravelled many of the mechanistic details involved (Fig 3C; [54,55,56]). To generate one neuroblast and one neuron, mitotic Drosophila neuroblasts use a polarized Par3–aPKC cortical complex to recruit several conserved asymmetrical cell division proteins—Inscuteable, Pins and Mud—which, in turn, perpendicularly orient the spindle and differentially partition Notch signalling. Moreover, the process is activated through a mitosis-specific kinase, and several of the key proteins involved are stabilized and localized asymmetrically in early prophase, after nuclear envelope breakdown [55,56,57]. Interestingly, for the early Caenorhabditis elegans embryo, the first cell division is asymmetrical and involves a mechanism similar to that of the fly neuroblast [58].
Drosophila male germline stem cells (GSCs) undergo asymmetrical divisions, but use a different pathway. Through DE-cadherin/Armadillo (β-catenin)/adenomatous polyposis coli (APC)-mediated intercellular junctions (adherens junctions) with a central niche of Hub cells, GSCs form a single layer of polarized cells that are interspersed with cyst stem cells (CySCs) around the Hub (Fig 3D). These Hub–GSC adherens junctions also polarize the mother centriole of each GSC, and on entry into mitosis, the spindle becomes perpendicularly aligned to the Hub [59,60]. The daughter that is displaced from the Hub niche becomes fated to differentiate, whilst the one maintaining contact inherits the mother centriole and other, as yet undetermined, cellular components to maintain its stemness.
The embryonic mouse epidermis seems to draw on each of these mechanisms to assemble its own pathway to asymmetrical division. The embryonic epidermal progenitors reside in a single layer, in which they adhere through integrins to an underlying basement membrane rich in extracellular matrix (ECM) and growth factors. They adhere to each other through intercellular adherens junctions. Together, these cellular junctions confer polarity to the basal cells, which enables them to polarize apically the Par3–aPKC cortical complex and centrosome. Early in embryonic development, epidermal mitoses are mainly symmetrically relative to the underlying basement membrane, and seem to generate two undifferentiated progenitors. At a stage when the embryo is growing rapidly, and the epidermis begins to differentiate, more than 60% of mitotic basal cells display a shift in spindle orientation concomitant with apical co-localization of Inscuteable, Pins/Lgn and Mud/NuMA (Fig 3E; [61]). As these cells complete anaphase, one daughter remains attached to the basement membrane and presumably inherits most of the basally localized integrins and tyrosine kinase growth factor receptors, whilst the displaced suprabasal daughter differentially inherits Notch signalling associated with epidermal differentiation [62]. Defects in Lgn, NuMA, β1-integrin, α-catenin and cortical actin all result in alterations in the balance between growth and differentiation within the epidermis [61,62,63].
Although the mechanistic details vary, one feature common to Drosophila neuroblasts, GSCs and embryonic epidermal progenitors is that their asymmetrical cell divisions result in two geographically segregated daughter cells with different identities. Additionally, the stem cell is typically the one that stays in place, and the differentiating daughter is the one displaced from the niche. Despite the naturally attractive aspects of coupling topographical changes in niche proximity to asymmetrical cell divisions, the link does not seem as apparent in adult tissues, leading to speculation as to whether it is obligatory.
In the adult mammalian brain, progenitor divisions are asymmetrical and governed by Inscuteable/Lgn/Par3/aPKC as in the Drosophila neuroblast [64]. Although the mitotic spindle of most progenitor divisions is parallel to the ventricular ECM surface, some progenitors in developing neocortex reorient their spindle and divide in oblique orientations [65,66]. Gain- and loss-of-function studies have revealed that this decision is not only governed by Inscuteable, but is also essential for generating the correct number of neurons in all cortical layers [66,67].
For adult mouse tissues, it is not yet clear whether a unified cell division model will eventually be unveiled to explain tissue homeostasis, or whether, as it seems from the data at hand, many mechanisms are tailored to suit each particular tissue architecture and stem cell or progenitor flux [68,69]. In the adult epidermis, depending on the developmental stage, divisions can be either parallel or perpendicular to the basement membrane [36]. It has been proposed that about 80% of basal progenitors undergo asymmetrical cell divisions by asymmetrical partitioning of Notch/Numb and integrins to the resulting daughters [34,36]. If so, the mechanism of using the growth-factor/ECM-rich basement membrane to sequester tyrosine kinase receptors, integrins and other growth components within the stem cell daughter must be substituted by other, as yet unidentified, mechanisms that could similarly achieve asymmetrical partitioning of fate components.
For the intestine, the constant flux of cells is thought to be supported by a single, equipotent stem cell population that sustains homeostasis by symmetrical division, followed by random exiting of the niche and then lineage specification and differentiation [12,38]. Thus, when individual ISCs within each crypt are randomly labelled by different inheritable fluorescent markers—each intestinal unit is initially multicoloured—they become single-coloured over time. This idea, referred to as ‘neutral drift’, suggests that most individual stem cells have a finite lifetime, which is considerably shorter than the life of the animal, and that randomly over time, the ISC niche becomes replenished by freshly made ISCs. Despite the attractiveness of this model, there are still issues to resolve. ISCs are interspersed between Paneth cells in a 1:1 ratio, and it is not clear how they maintain this balance in the absence of asymmetrical division or a change in the niche environment.
The hair follicle adds yet another dimension to these models. Shortly after the start of each hair cycle, bulge cells seem to divide symmetrically to self-renew and maintain a single layer of HFSCs [5,8]. Thus, the K6+ inner bulge layer is derived not from the bulge HFSCs directly, but rather from differentiating progeny outside the niche [5]. By contrast, shorter-term proliferative progenitors within the hair germ and matrix seem to divide asymmetrically relative to the basement membrane, and in so doing, couple cell division to environmentally induced progression to differentiate [70,71].
In all of these cases involving adult epithelial stem cells and their progenitors, genetic evidence is still lacking to unequivocally resolve which divisions, if any, result in asymmetrical partitioning of protein components to generate one stem cell and one committed daughter. Moreover, as cell fate switches and proliferation are both intricately coupled to specific signal transduction pathways emanating from the niche microenvironment, it will be interesting in the future to see how these signals intersect with the mechanisms of cell divisions. Is signalling always downstream from spindle orientation and asymmetrical cell division, as it seems to be with Notch signalling, or could upstream signalling dictate these processes?
In this regard, evidence suggests that during telophase of an asymmetrical division in C. elegans, extrinsic Wnt signalling modulates spindle structures through APC, which localizes asymmetrically along the cortex, establishing asymmetrical distribution of astral microtubules [72]. Moreover, direct manipulation of this spindle asymmetry by laser irradiation altered the asymmetrical distribution of nuclear β-catenin, and in turn, laser manipulation of the spindles rescued defects in nuclear β-catenin/TCF asymmetry in Wnt mutants. This finding is particularly intriguing in the light of another study on Wnt signalling, which focuses on a different downstream effector from Wnt signalling, namely the silencing of GSK3β kinase [73]. In mouse hair follicles, if Wnt signalling comes from the base of the niche, stem cells regrow the hair follicle; if it comes from above the niche, stem cells migrate upwards towards the wound site [53]. Through localized Wnt-induced silencing of GSK3β, the cortex facing the wound edge becomes a polarizing centre for the recruitment of Par3/aPKC, APC and a functional APC relative, ACF7 [74]. Importantly, in the absence of GSK3β phosphorylation, ACF7 binds to and stabilizes microtubules—the outcome is the polarization of the microtubule–cortical complex towards the wound edge [73]. Functional studies show that this mechanism is essential for facilitating directional cell migration in a wound response.
Given the tantalizing similarities between the Wnt-induced mechanisms involved in asymmetrical polarization of microtubule–cortical complexes, in spindle orientations in the early C. elegans embryo, and in wound-related directional cell migration in mouse skin, it seems probable that the Wnt-induced activation of mouse hair follicle stem cells that occurs at the base of the bulge niche is also responsible for the asymmetrical spindle reorientation and cell fate switch that ensues at the start of the normal hair cycle [9,71]. Moreover, when considering first the diversity of ways in which Wnt signalling mechanisms are involved in regulating stem cell proliferation and cell fate commitments, and second the diversity of stem cell niches in different tissues, these factors could together explain why some stem cells divide symmetrically and why others divide asymmetrically. This combination of regulatory factors could also readily explain how stem cells might switch between asymmetrical and symmetrical divisions, depending on changes that occur within the niche microenvironment, in response to the demands for tissue regeneration.
Self-renewal: in it for the long haul
Similarly with any cell division, stem cell self-renewal operates through the principle cell cycle machinery [75]. The p16Ink4a–CDK4/6–Rb–E2F pathway is one of the main regulators of the G1- to S-phase transition. As a potent cell cycle inhibitor, p16Ink4a can bind to and inactivate the cyclin D–CDK4/6 complex [76]. In young mice (10 weeks old), p16Ink4a is not expressed by HSCs [77]. Deletion of its locus, alone or together with another cell cycle inhibitor p19Arf—generated as an alternative reading frame of the p16Ink4a locus—results in, at best, a modest advantage in the serial transplantation efficiency of young HSCs [77,78]. However, p16Ink4a expression is often increased in old (approximately 2 years old) mouse stem cells, including HSCs and neuronal progenitors in the subventricular zone [77,79,80].
The decline in self-renewal capacity with age is partly because of increased expression of the tumour suppressor p16Ink4a. One of the major p16Ink4a suppressors in adult stem cells and progenitors is Bmi1, a component of the polycomb repressor complex 1 (PRC1; Fig 4A; [81,82,83,84]). Loss of Bmi1 leads to stem cell self-renewal defects that can be partly rescued by deletion of p16Ink4a [79,85,86]. Additional p16Ink4a suppressors include Ezh1/2 (the H3K27me3 methylases required for PRC2-mediated chromatin repression), HDAC1/2 (histone deacetylases 1/2 promoting transcriptional repression) and the p53 relative p63. In mouse skin, their loss results in severe defects including elevated p16Ink4a and diminished proliferation [87,88], and Ezh1/2-deficient HFSCs can be partly rescued by repressing the Ink4 locus [87].
Figure 4.
Intrinsic factors regulating stem cell self-renewal. (A) p16Ink4a is a potent inhibitor of the G1- to S-phase transition in the cell cycle. To allow cell cycle entry, p16Ink4a must be repressed. Many factors have been reported to silence p16Ink4a gene expression in adult stem cells including Bmi1, Hmga2, p63, Ezh1/2 and HDAC1/2. (B) Some self-renewal factors seem to be tailored to suit the context-dependent needs of a stem cell niche. One example is HFSC-enriched factor Tbx1, which suppresses BMP signalling to allow HFSC self-renewal in the BMP-high microenvironment of the bulge niche. Bmi1, B-lymphoma Moloney murine leukaemia virus insertion region 1; BMP, bone morphogenetic protein; Ezh1/2, enhancer of zeste homologue 1/2; HDAC1/2, histone deacetylase 1/2; HFSC, hair follicle stem cell; Hmga2, high mobility group AT-hook 2; p16Ink4a, tumour suppressor protein encoded by the Ink4a gene locus; Tbx1, T-box transcription factor 1.
Besides Bmi1, few genes have been shown to be involved in long-term self-renewal of stem cells. Chromatin regulator Hmga2 is one of these, which is highly expressed in fetal neural stem cells but then declines with age (Fig 4A; [89]). This decrease is partly caused by the increasing expression of let-7b microRNA, which is known to target HMGA2 protein expression and also repress p16Ink4a and p19Arf. Hmga2-deficient mice show reduced stem cell numbers and self-renewal throughout the central and peripheral nervous systems of fetal and young-adult mice, and deletion of p16Ink4a and p19Arf partly restores self-renewal capacity.
We set up an RNA interference screen to identify genes involved in hair follicle stem cell self-renewal [10]. Taking advantage of the differential expression of about 400 genes in long-term HFSCs, but not their short-lived proliferative progeny, we infected HFSCs with a pool of lentiviruses encoding shRNAs corresponding to these genes. We reasoned that if one of the 400 genes is essential for long-term self-renewal, its repression would cripple the ability of the HFSC to survive after many passages in culture, but would not interfere with short-term proliferation. In comparing the hairpins lost in the culture after long-term but not short-term passaging, we unearthed a small set of candidate self-renewal genes, one of which was Hmga2 [10]. Another was Runx1, the loss of which in adult HFSCs has previously been shown to cause proliferation defects due to elevation of p21, another cell cycle inhibitor [90]. Analogous to studies of p16Ink4a with Bmi1, suppression of p21 can rescue the proliferation defects in Runx1-deficient mice.
Among the new candidates surfacing in our self-renewal screen, the transcription factor Tbx1 was particularly intriguing. It is expressed by HFSCs in vivo and in vitro, downregulated in early progenitors and absent from transient-amplifying and terminally differentiated hair cells [10]. Loss of function studies further suggested its importance for long-term self-renewal. Without Tbx1, bulge niches became depleted of HFSCs, gradually extinguishing hair follicle regeneration on repetitive stimulation.
In contrast to Bmi1, Hmga2 and Runx1, Tbx1 does not seem to repress cell cycle inhibitors directly, but rather enables self-renewal in the niche by lowering intrinsic BMP signalling, which otherwise keeps HFSCs in a quiescent state. Inhibiting BMP signalling in the Tbx1-deficient HFSC niche can partly rescue the proliferation defect (Fig 4B). Tbx1 is also important for heart progenitor proliferation, in which it similarly functions by affecting BMP signalling [91,92]. That said, interfollicular basal epidermal stem cells do not express Tbx1, nor do they reside in an environment as rich in BMP as HFSCs. As they do undergo long-term renewal, this suggests that context dependency of this self-renewal protein is important. In the future, as more details unfold regarding the genes involved in stem cell self-renewal, it will be interesting to see whether, when and how stem cells couple self-renewal to asymmetrical as well as symmetrical cell divisions.
Open questions and future directions
The two main aspects of achieving stem cell self-renewal are entry into the cell cycle and maintenance of the undifferentiated state in at least one of the two daughters. The ability of stem cells to do both in the long term is what defines them and enables them to regenerate life-long tissues. Increasing evidence suggests that differences in stem cell niche architecture, environment and signalling confer tissue-specific constraints on when stem cells divide, how frequently and in response to what signals. These factors also seem to have an impact on whether and when stem cells divide symmetrically or asymmetrically. As researchers have dug deeper into the underlying mechanisms involved in stem cell self-renewal, it has become increasingly clear that no single rule or set of molecules applies to every stem cell. But, because of shared underlying principles, there are several common players that can exert the same effect on a variety of adult stem cells, as we have discussed here.
Although the picture of stem cell self-renewal is certainly much more vivid than a decade ago, there are still many details and questions that remain to be addressed (see Sidebar A). Can a stem cell really choose between symmetrical and asymmetrical divisions, and if so, what is the constellation of environmental signals that have an impact on such a choice? What determines whether one or both daughter cells will remain undifferentiated, and what are the underlying mechanisms involved? Although beyond the scope of the present review, research over the past several years has begun to solidify the view that stem cell maintenance is dependent on stem-cell-type-specific master transcriptional regulators, and that signalling-induced transcription factors such as β-catenin/TCF and pSmad2/Smad4 can change the status of the key genes that master regulator control [93]. Although tantalizing, many of the crucial details are still lacking and await future studies.
Sidebar A | in need of answers.
Can a stem cell really choose between symmetrical and asymmetrical divisions, and if so, what is the constellation of environmental signals that has an impact on such a choice?
How is the self-renewal timing and frequency determined in different tissues? What are the start and stop signals?
Is there a specific clock that ticks off cell divisions for stem cells and defines the distinction between stemness and their transit-amplifying progeny? If so, how does it work?
There are additional important questions. How is the self-renewal timing and frequency determined in different tissues? What are the start and stop signals? In many adult tissues, such as the haematopoietic system and the hair follicle, stem cells are quiescent and used sparingly, sometimes going for long periods at a time without self-renewal proliferation. But for other tissues, such as the small intestine, stem cells proliferate continuously and are supported by an active niche environment. Whether frequent or cycling slowly, the perpetual ability of stem cells to crank out tissue has led to the long-held view that stem cells have an endless capacity to self-renew. However, studies on the intestine and the hair follicle suggest that most individual stem cells might have a more finite lifetime than initially thought. Is there a specific clock that ticks off cell divisions for the stem cells? For their transit-amplifying progeny? If so, how does it work? The answers to these fascinating questions lie ahead of us. However, the foundation laid during the past decade of research provides us with a clearer glimpse into the depths of the crystal ball of stem cell self-renewal and tissue homeostasis.

Elaine Fuchs

Ting Chen
Acknowledgments
We are grateful to our colleagues in the field for their many fine research contributions that served as the inspiration to write this review. In particular, we thank Ya-Chieh Hsu for her comments on the manuscript. E.F. is an investigator of the Howard Hughes Medical Institute and has received funding from the National Institutes of Health (R01-050452) to explore stem cell self-renewal in the skin. T.C. is now an assistant investigator at the National Institute of Biological Sciences in Beijing, China. Whilst in the Fuchs’ laboratory, she was funded by the New York Stem Cell Foundation (NYSCF) Druckenmiller Fellowship, as well as the NYSTEM Scholar Award (C026722). E.F. and T.C. wrote the review. T.C. prepared the figures.
Footnotes
The authors declare that they have no conflict of interest.
References
- Copley MR, Beer PA, Eaves CJ (2012) Hematopoietic stem cell heterogeneity takes center stage. Cell Stem Cell 10: 690–697 [DOI] [PubMed] [Google Scholar]
- Barker N, van Oudenaarden A, Clevers H (2012) Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell 11: 452–460 [DOI] [PubMed] [Google Scholar]
- He S, Nakada D, Morrison SJ (2009) Mechanisms of stem cell self-renewal. Annu Rev Cell Dev Biol 25: 377–406 [DOI] [PubMed] [Google Scholar]
- O'Brien LE et al. (2011) Altered modes of stem cell division drive adaptive intestinal growth. Cell 147: 603–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Pasolli HA, Fuchs E (2011) Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 144: 92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM (1990) Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61: 1329–1337 [DOI] [PubMed] [Google Scholar]
- Tumbar T et al. (2004) Defining the epithelial stem cell niche in skin. Science 303: 359–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YV et al. (2010) Stem cell dynamics in mouse hair follicles: a story from cell division counting and single cell lineage tracing. Cell Cycle 9: 1504–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco V et al. (2009) A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 4: 155–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T et al. (2012) An RNA interference screen uncovers a new molecule in stem cell self-renewal and long-term regeneration. Nature 485: 104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Bartfeld S, Clevers H (2010) Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell 7: 656–670 [DOI] [PubMed] [Google Scholar]
- Simons BD, Clevers H (2011) Strategies for homeostatic stem cell self-renewal in adult tissues. Cell 145: 851–862 [DOI] [PubMed] [Google Scholar]
- Yilmaz OH et al. (2012) mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 486: 490–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seita J, Rossi DJ, Weissman IL (2010) Differential DNA damage response in stem and progenitor cells. Cell Stem Cell 7: 145–147 [DOI] [PubMed] [Google Scholar]
- Jaiswal S et al. (2010) Macrophages as mediators of tumor immunosurveillance. Trends Immunol 31: 212–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A et al. (2008) Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135: 1118–1129 [DOI] [PubMed] [Google Scholar]
- Foudi A et al. (2009) Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat Biotechnol 27: 84–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H et al. (2011) A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478: 255–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N et al. (2011) Interconversion between intestinal stem cell populations in distinct niches. Science 334: 1420–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T et al. (2010) Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science 328: 62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till JE, McCulloch EA (1961) A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res 14: 213–222 [PubMed] [Google Scholar]
- Morrison SJ, Weissman IL (1994) The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity 1: 661–673 [DOI] [PubMed] [Google Scholar]
- Yang L et al. (2005) Identification of Lin−Sca1+kit+CD34+Flt3− short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood 105: 2717–2723 [DOI] [PubMed] [Google Scholar]
- Majeti R, Park CY, Weissman IL (2007) Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell 1: 635–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrandon Y, Green H (1987) Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA 84: 2302–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H (1991) Cultured cells for the treatment of disease. Sci Am 265: 96–102 [DOI] [PubMed] [Google Scholar]
- Rama P et al. (2010) Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med 363: 147–155 [DOI] [PubMed] [Google Scholar]
- Ezhkova E, Fuchs E (2010) Regenerative medicine: An eye to treating blindness. Nature 466: 567–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RJ et al. (2004) Capturing and profiling adult hair follicle stem cells. Nat Biotechnol 22: 411–417 [DOI] [PubMed] [Google Scholar]
- Blanpain C et al. (2004) Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118: 635–648 [DOI] [PubMed] [Google Scholar]
- Lu CP et al. (2012) Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell 150: 136–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar K, Watt FM (2012) Lineage tracing. Cell 148: 33–45 [DOI] [PubMed] [Google Scholar]
- Snippert HJ, Clevers H (2011) Tracking adult stem cells. EMBO Rep 12: 113–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E et al. (2007) A single type of progenitor cell maintains normal epidermis. Nature 446: 185–189 [DOI] [PubMed] [Google Scholar]
- Barker N, Clevers H (2010) Lineage tracing in the intestinal epithelium. Curr Protoc Stem Cell Biol 13: 5A.4.1–5A.4.11 [DOI] [PubMed] [Google Scholar]
- Mascre G et al. (2012) Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 489: 257–262 [DOI] [PubMed] [Google Scholar]
- Barker N et al. (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007 [DOI] [PubMed] [Google Scholar]
- Snippert HJ et al. (2010) Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143: 134–144 [DOI] [PubMed] [Google Scholar]
- Sato T et al. (2011) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yui S et al. (2012) Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med 18: 618–623 [DOI] [PubMed] [Google Scholar]
- Jaks V et al. (2008) Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet 40: 1291–1299 [DOI] [PubMed] [Google Scholar]
- Zhang YV, Cheong J, Ciapurin N, McDermitt DJ, Tumbar T (2009) Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell 5: 267–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler IG et al. (2010) Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 116: 4815–4828 [DOI] [PubMed] [Google Scholar]
- Chow A et al. (2011) Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med 208: 261–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PH, Watt FM (1993) Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 73: 713–724 [DOI] [PubMed] [Google Scholar]
- Vasioukhin V et al. (1999) The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci USA 96: 8551–8556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM, Green H (1982) Stratification and terminal differentiation of cultured epidermal cells. Nature 295: 434–436 [DOI] [PubMed] [Google Scholar]
- Van Keymeulen A et al. (2011) Distinct stem cells contribute to mammary gland development and maintenance. Nature 479: 189–193 [DOI] [PubMed] [Google Scholar]
- Visvader JE, Smith GH (2011) Murine mammary epithelial stem cells: discovery, function, and current status. Cold Spring Harb Perspect Biol 3: a004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton M et al. (2006) Generation of a functional mammary gland from a single stem cell. Nature 439: 84–88 [DOI] [PubMed] [Google Scholar]
- Stingl J et al. (2006) Purification and unique properties of mammary epithelial stem cells. Nature 439: 993–997 [DOI] [PubMed] [Google Scholar]
- Lobitz WC Jr, Holyoke JB, Brophy D (1956) Response of the human eccrine sweat duct to dermal injury. J Invest Dermatol 26: 247–259 [DOI] [PubMed] [Google Scholar]
- Ito M et al. (2007) Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447: 316–320 [DOI] [PubMed] [Google Scholar]
- Spradling A et al. (2011) Germline stem cells. Cold Spring Harb Perspect Biol 3: a002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller KH, Doe CQ (2009) Spindle orientation during asymmetric cell division. Nat Cell Biol 11: 365–374 [DOI] [PubMed] [Google Scholar]
- Knoblich JA (2008) Mechanisms of asymmetric stem cell division. Cell 132: 583–597 [DOI] [PubMed] [Google Scholar]
- Johnston CA et al. (2009) Identification of an Aurora-A/PinsLINKER/Dlg spindle orientation pathway using induced cell polarity in S2 cells. Cell 138: 1150–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begasse ML, Hyman AA (2011) The first cell cycle of the Caenorhabditis elegans embryo: spatial and temporal control of an asymmetric cell division. Results Probl Cell Differ 53: 109–133 [DOI] [PubMed] [Google Scholar]
- Yamashita YM, Jones DL, Fuller MT (2003) Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science 301: 1547–1550 [DOI] [PubMed] [Google Scholar]
- Yamashita YM et al. (2007) Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science 315: 518–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T, Fuchs E (2005) Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437: 275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE et al. (2011) Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature 470: 353–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxenburg C et al. (2011) Developmental roles for Srf, cortical cytoskeleton and cell shape in epidermal spindle orientation. Nat Cell Biol 13: 203–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Knoblich JA (2012) Spindle orientation in mammalian cerebral cortical development. Curr Opin Neurobiol 22: 733–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz M, Huttner WB (2005) The cell biology of neurogenesis. Nat Rev Mol Cell Biol 6: 777–788 [DOI] [PubMed] [Google Scholar]
- Postiglione MP et al. (2011) Mouse inscuteable induces apical-basal spindle orientation to facilitate intermediate progenitor generation in the developing neocortex. Neuron 72: 269–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulson ND, Lechler T (2010) Robust control of mitotic spindle orientation in the developing epidermis. J Cell Biol 191: 915–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulas S, Conder R, Knoblich JA (2012) The par complex and integrins direct asymmetric cell division in adult intestinal stem cells. Cell Stem Cell 11: 529–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich JA (2010) Asymmetric cell division: recent developments and their implications for tumour biology. Nat Rev Mol Cell Biol 11: 849–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E (2009) Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 10: 207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P et al. (2012) Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature 487: 496–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugioka K, Mizumoto K, Sawa H (2011) Wnt regulates spindle asymmetry to generate asymmetric nuclear beta-catenin in C. elegans. Cell 146: 942–954 [DOI] [PubMed] [Google Scholar]
- Wu X et al. (2011) Skin stem cells orchestrate directional migration by regulating microtubule-ACF7 connections through GSK3beta. Cell 144: 341–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama A et al. (2003) ACF7: an essential integrator of microtubule dynamics. Cell 115: 343–354 [DOI] [PubMed] [Google Scholar]
- He S et al. (2009) Bmi-1 over-expression in neural stem/progenitor cells increases proliferation and neurogenesis in culture but has little effect on these functions in vivo. Dev Biol 328: 257–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM (2004) Living with or without cyclins and cyclin-dependent kinases. Genes Dev 18: 2699–2711 [DOI] [PubMed] [Google Scholar]
- Janzen V et al. (2006) Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature 443: 421–426 [DOI] [PubMed] [Google Scholar]
- Stepanova L, Sorrentino BP (2005) A limited role for p16Ink4a and p19Arf in the loss of hematopoietic stem cells during proliferative stress. Blood 106: 827–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV et al. (2006) Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature 443: 448–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler S et al. (2006) p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell 5: 379–389 [DOI] [PubMed] [Google Scholar]
- Park IK et al. (2003) Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 423: 302–305 [DOI] [PubMed] [Google Scholar]
- Molofsky AV et al. (2003) Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 425: 962–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharek SJ et al. (2011) Lung stem cell self-renewal relies on BMI1-dependent control of expression at imprinted loci. Cell Stem Cell 9: 272–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvageau M, Sauvageau G (2010) Polycomb group proteins: multi-faceted regulators of somatic stem cells and cancer. Cell Stem Cell 7: 299–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggeman SW et al. (2005) Ink4a and Arf differentially affect cell proliferation and neural stem cell self-renewal in Bmi1-deficient mice. Genes Dev 19: 1438–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akala OO et al. (2008) Long-term haematopoietic reconstitution by Trp53−/−p16Ink4a−/−p19Arf−/− multipotent progenitors. Nature 453: 228–232 [DOI] [PubMed] [Google Scholar]
- Ezhkova E et al. (2011) EZH1 and EZH2 co-govern histone H3-K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes Dev 25: 485–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBoeuf M et al. (2010) Hdac1 and Hdac2 act redundantly to control p63 and p53 functions in epidermal progenitor cells. Dev Cell 19: 807–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino J et al. (2008) Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf expression. Cell 135: 227–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoi CS et al. (2010) Runx1 directly promotes proliferation of hair follicle stem cells and epithelial tumor formation in mouse skin. Mol Cell Biol 30: 2518–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcoli FG et al. (2009) Tbx1 regulates the BMP-Smad1 pathway in a transcription independent manner. PLoS ONE 4: e6049. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chen L et al. (2009) Tbx1 regulates proliferation and differentiation of multipotent heart progenitors. Circ Res 105: 842–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompouki E et al. (2011) Lineage regulators direct BMP and Wnt pathways to cell-specific programs during differentiation and regeneration. Cell 147: 577–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ (2012) Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481: 457–462 [DOI] [PMC free article] [PubMed] [Google Scholar]