Beneath the surface of the Southern Ocean, in the deep waters of the continental shelf and continental slope of Antarctica, a magnificent fauna consisting of over 320 known species of fish thrives in the cold and dark. These fish are uniquely adapted to the extreme environment in which they live. Over the course of millennia, they have evolved a remarkable range of physiological and biochemical features, the most visually stunning of which is that some lack haemoglobin (Fig 1). Yet, these species are under threat from global climate change and fishing, and the secrets they might unlock for science could be lost in the next decades if action is not taken.
Figure 1.
The blackfin icefish Chaneocephalus aceratus. Photo credit: Steve Untracht.
A human can survive in freezing water for around 15 min before their organs begin to shut down. The fauna of the Southern Ocean spend their entire lives at these extreme temperatures. The fish species present are dominated by members of the Notothenioidei, which comprises 50% and 90% of the Antarctic finfish diversity and biomass, respectively [1,2,3]. The notothenioids are a suborder of perch-like fishes that are mainly benthic and highly endemic to the Antarctic region. They are divided into eight families that have radiated from an ancestral stock during the last 20 million years to fill a variety of niches. These families include the Nototheniidae—the most diverse and specious family of the notothenioids—the Bathydraconidae—the dragonfishes, so-named because of their especially long and narrow body forms—and the Channichthyidae—the icefishes, known for their colourless blood.
…the Antarctic and its spectacular fauna are suffering a fate similar to others on the planet—their survival is threatened by anthropogenic activities
The composition of today's Antarctic fish fauna has been strongly influenced by the distinctive oceanographic features of the Southern Ocean, the waters of which are delineated by the Antarctic polar front—between 50° S and 60° S depending on season and ocean sector—representing a boundary with steep physical (for example, temperature) and chemical (for example, salinity) gradients. This boundary effectively isolates the Southern Ocean biota from the warmer waters found further north. Annual temperatures in the Southern Ocean range negligibly (−1.9 °C to −1.7 °C), or by only a few degrees depending on the locale, and temperatures throughout the water column are relatively constant [4,5]. Although, only 10% of the world's oceans comprises the Southern Ocean, the Antarctic Circumpolar Current, which moves more than 130 million cubic metres per second, is the largest volume current on the planet. By contrast, all the world's rivers combined have a flow of approximately one million cubic metres per second.
Although the pelagic and benthic environments of the Southern Ocean have been relatively stable for millions of years, Antarctica has not always been cold. Prior to the separation of the Antarctic continent from the rest of the ancient supercontinent Gondwana, the fishes present during the early Eocene epoch were exceptionally diverse and included many fish groups that are no longer represented in the modern fauna. As Antarctica drifted southward 20–25 million years ago, and new patterns of oceanic circulation were established, a crash in species diversity occurred. Warm water refuges to the north were no longer accessible to many species. Those fishes able to survive in the cooling waters gained a series of biological innovations that are essential for life in the cold.
Yet, these remarkable creatures are under threat. Despite the seemingly pristine picture painted of the Antarctic environment in popular films such as March of the Penguins (2005), the Antarctic and its spectacular fauna are suffering a fate similar to others on the planet—their survival is threatened by anthropogenic activities. Among the greatest challenges faced are global warming, commercial fishing and the introduction of non-native species [6].
Much less is known about the effects of warming on fish populations because of a lack of long-term data sets on population structure and lack of knowledge of their reproductive biology
The Western Antarctic Peninsula (WAP) region is one of the three most rapidly warming regions on earth. Atmospheric temperatures have risen by nearly 3 °C and summer sea surface temperatures by more than 1 °C since 1951 [7,8,9]. One of the most dramatic events illustrating the devastating effects of warming in this region was the collapse of the central portion (B) of the Larsen Ice Shelf in 2002—3,250 km2 in size. The Larsen B is only one of several ice shelves that have disintegrated during the last 50 years. Others include the Wordie Ice Shelf during the 1980s, the Larsen A and Prince Gustav Ice Shelves in 1995, and the Wilkins Ice Shelf in 2008. Together, these collapsed shelves encompass around 28,000 km2—an area approximately the size of Belgium [10]. In addition to the melting ice shelves, thinning of the sea ice is also occurring. Winter sea ice coverage declined by 10% per decade between 1979 and 1999 in the Bellingshausen and Amundsen seas along the western shore of the Antarctic Peninsula (reviewed in [11]). In conjunction, warming is affecting the biota in the WAP. Phytoplankton biomass is declining in the northerly region of the WAP, coinciding with a decline in krill, which are being replaced by gelatinous salps [12,13]. Adélie penguin populations, which are dependent on ice, are declining precipitously by as much as 65% in some areas and are being replaced by gentoo and chinstrap penguins [14]. Much less is known about the effects of warming on fish populations because of a lack of long-term data sets on population structure and a lack of knowledge of their reproductive biology. However, data obtained from laboratory studies suggest that Antarctic fishes might not fare well under warming conditions. Evolution, by natural selection, has made Antarctic fishes superiorly suited to life in the cold but less adept at weathering life at warmer climates.
In addition to the threat of a warming Southern Ocean, commercial fishing has become a serious problem for marine species in the region. The Southern Ocean has long provided a bounty of marine mammals, beginning in the late eighteenth century with non-flying seabirds (penguins) and culminating in the establishment of commercial fisheries for krill and finfish in the 1960s. With many marine finfish stocks depleted or in severe decline worldwide, fishers have had to venture further from their homeports to find suitable harvests. By the austral summer of 1969–1970, industrial-scale fishing had commenced around the island of South Georgia (Table 1; [15]). Within the first two years, more than 500,000 tons of marbled rock cod (Notothenia rossii) were harvested, followed by four years during which the catch plummeted to zero [15]. By the 1970s, fishing efforts were underway in other locales south of the Antarctic Polar Front, including the shallow shelf waters off the South Orkney and South Shetland Islands, and in areas adjacent to the islands of Kerguelen and Heard [15]. Several of the targeted species, including Champsocephalus gunnari, Pseudochaenichthys georgianus, Chaenocephalus aceratus and Chaenodraco wilsoni, were icefishes. Between 1969 and 1973, some stocks, including icefishes, were depleted to 50% of their original population size. A decade later, remaining stocks were at only 20% of their pre-fishing levels, and even today, stocks have not yet fully recovered (Table 1; reviewed in [15,16]).
Table 1. Fisheries in the Southern Ocean.
| Fishery (statistical sampling area) | Date fishery opened | Datefishery closed | Current tonnage limit | Stock remaining for closed fisheries (%) |
|---|---|---|---|---|
| South Georgia & vicinity (48.3) | ||||
| Champsocephalus gunnari | 1970 | — | 3,072 | — |
| Dissostichus eleginoides | 1976 | — | 2,600 | — |
| Gobionotothen gibberifrons | — | — | — | — |
| Pseudochaenicthys georgianus | — | — | — | — |
| Chaenocephalus aceratus | 1975 | 1999 | — | 24–40 |
| Notothenia rossii | 1969 | 1985 | — | <5 |
| Patagonotothen guntheri | 1978 | 1990 | — | ∼ 20 |
| Antarctic Peninsula & South Shetland Islands (48.1) | ||||
| C. gunnari | 1978 | 1980 | — | <5 |
| Chaenodraco wilsoni | 1978 | 1990 | — | ? |
| N. rossii | 1978 | 1990 | — | <10 |
| South Orkney Islands (48.2) | ||||
| C. gunnari | 1977 | 1990 | — | <5 |
| G. gibberifrons | 1977 | 1990 | — | 40 |
| N. rossii | 1979 | 1990 | — | <10 |
| Kerguelen Islands (58.5.1) | ||||
| C. gunnari | 1970 | — | — | ∼30 |
| D. eleginoides | 1977 | — | 5,100* | ∼15 |
| Lepidonotothen squamifrons | 1971 | 1989 | — | <5 |
| N. rossii | 1970 | — | — | <10 |
| Heard Island (58.5.2) | ||||
| C. gunnari | 1971 | 1989 | — | ∼15 |
| D. eleginoides | 1995 | — | 2,730 | ? |
| Ross Sea (88.1 & 88.2) | ||||
| Dissostichus spp. | 1996 | — | 3,812 | — |
Data from [16] and the CCAMLR fishery reports available on their website (http://www.ccamlr.org/en).
*Under French jurisdiction; ?, indicates no data.
In 1980, an international body signed the Convention for the Conservation of Antarctic Marine Living Resources, and in 1982 the Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR) was established to oversee conservation of marine life in the Southern Ocean (Fig 2). Beginning as early as 1983 and continuing into the 1990s, CCAMLR closed fisheries in various locales, but this was too late for a timely recovery for certain populations. By the time closures were enacted, some populations of fishes had already declined to less than 10% of their original stocks [15]. Even in the absence of legal fishing, most populations have not recovered in over three decades since fishing began [17]. The loss of fish stocks has been accompanied by profound declines in population abundances of various fish predators including Antarctic fur seals, gentoo and macaroni penguins and imperial shags [16]. Commercial-scale trawling in the Southern Ocean has also been extremely destructive to benthic communities, which include an exceptionally diverse and beautiful fauna of invertebrates.
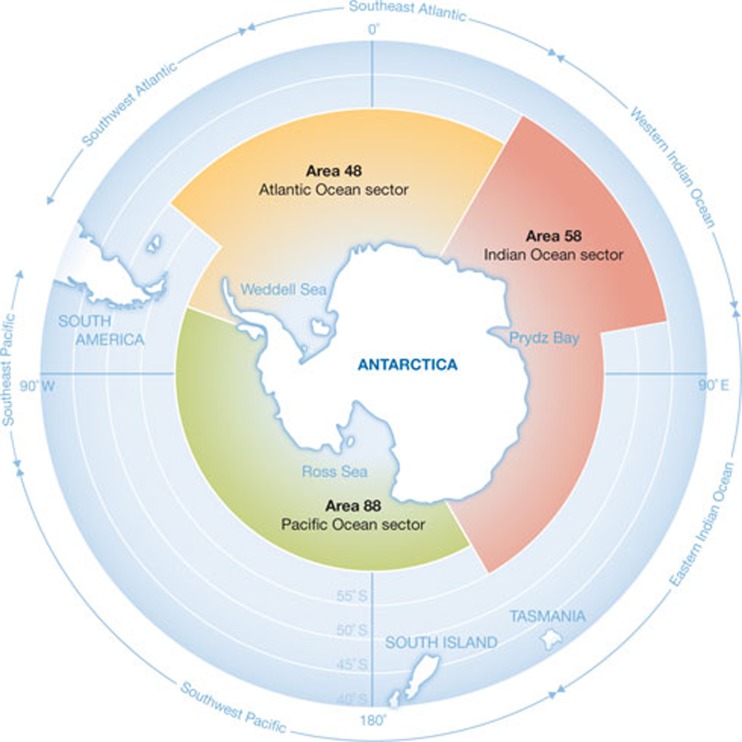
Figure 2.
The Conservation of Antarctic Marine Living Resources Convention area, depicting fishing statistical areas within the Southern Ocean. The area governed by the convention covers around 10% of the Earth's surface. It is divided into statistical areas: Area 48, Atlantic Ocean sector (yellow); Area 58, Indian Ocean sector (red); Area 88, Pacific Ocean sector (green). Each of these is further subdivided to improve the information gathered about the constituent ecosystems. Figure redrawn from http://www.ccamlr.org/en/organization/convention-area.
Unfortunately, lessons have not been learned from the mistakes made in the management of these fisheries. In 1996, CCAMLR approved commercial exploratory fishing for the Antarctic toothfish (Dissostichus mawsoni) in the Ross Sea, despite a lack of data on its reproductive biology and ecology. What is known suggests that these animals are extremely vulnerable to overfishing. They are ‘k-selected species’, meaning they are long-lived—as long as 50 years—slow growing and developing, and probably low in fecundity; no eggs, larvae or small adults have been found in the Ross Sea (reviewed in [18]). Not surprisingly, intrinsic vulnerability indices reveal that D. mawsoni is at the top of the vulnerability list among deep-sea fishes worldwide [19]. Commercial fishing this far south has been driven, in part, by overfishing of D. mawsoni's relative, the Patagonian toothfish, D. eleginoides, in more northerly regions of the Southern Ocean, which was also sanctioned by CCAMLR. Today, both species are marketed as ‘Chilean sea-bass’.
Even in the absence of legal fishing, most populations have not recovered in the more than three decades since fishing began
The large demand for toothfish has fuelled illegal, unregulated and unreported (IUU) fishing. As much as 70% of all landings are probably IUU [20]. One ‘ghost’ gillnet—not sanctioned by CCAMLR—recovered recently, spanned 130 km and had ensnared 29 tons of Antarctic toothfish [21]. According to the estimates of CCAMLR, in the 1990s, over 100,000 tons per year of toothfish (Dissostichus spp.) were harvested illegally, which was six times the reported catch from legal operators (CCAMLR website). Scientists who use D. mawsoni for their research in McMurdo Sound (located in the southwestern Ross Sea) have kept extensive records of their catch from 1972 to 2011 [22]. In the nearly 40-year period, catch per unit effort was consistent until at least 1997, and a precipitous decline began in 2001. The condition of individual fish also declined after the mid-1990s (coincident with the start of the commercial fishery), as evidenced by the greater proportion of animals displaying an ‘axe-handle’ morphology—a term coined by fishermen for fish having a shrunken head and greater length-to-body mass ratio than healthy fish [22,23]. In the absence of any correlative environmental factor, and the lack of any similar effect on other mesopredators in the area—for example, Weddell seals or Adélie penguins—it is safe to say that commercial fishing has reduced the prevalence of these animals in McMurdo Sound. As a major predator in the Ross Sea, the decline in D. mawsoni will probably have a widespread impact on the Ross Sea ecosystem.
These declining fish numbers not only are tragic for biodiversity, they also represent an impediment to scientific progress. Evolution in the most frigid, stable environment on earth has resulted in a suite of physiological and biochemical adaptations in Antarctic fishes that offer unparalleled learning opportunities. Studies of Antarctic fishes are revealing the molecular requirements for life in the cold. In addition, many of the unique traits that have arisen in these animals can serve as models for understanding basic biological processes common to all organisms, and for elucidating the molecular mechanisms of some human diseases.
One of the most startling characteristics among the Antarctic fish fauna is the nearly translucent, white blood of Antarctic icefishes due to their unique lack of haemoglobin expression (Fig 1). The Southern Ocean is oxygen-rich, as oxygen solubility is inversely related to temperature, so that oxygen levels are approximately 1.6-fold higher in the Southern Ocean compared with temperate 20 °C seawater [5]. This has allowed icefishes to thrive despite a mutational event that took place between 2.2 and 5 million years ago and led to the loss of haemoglobin in an ancestral notothenioid [24]. Today, there are 16 species of icefishes, boasting a circum-Antarctic distribution and occupying both benthic and pelagic niches. One member of the family (Champsocephalus esox) has even strayed as far north as the Straits of Magellan.
However, whilst the high oxygen content of the ocean is beneficial to haemoglobinless icefishes, it also promotes the formation of free radicals that wreak havoc on biological macromolecules, damaging proteins, DNA and lipids. Thus, it is not surprising that Antarctic fishes are fortified with antioxidants that protect against these damaging effects. Transcript levels of antioxidants, as well as proteins that mediate iron metabolism—because iron also promotes free-radical production—are higher in Antarctic fishes compared with warm-bodied ones [25].
Another remarkable adaptation—and one which has potential practical applications in medicine—is the presence of antifreeze glycoproteins (AFGPs) in notothenioid fishes living in the Antarctic region. Without antifreeze protection, these animals would not survive, as ice crystals would propagate in their intestinal fluid and blood. AFGPs protect the fish by adsorbing onto the surface of small ice crystals where the hydroxyl and amino groups of the AFGPs form hydrogen bonds with the oxygen atoms in the ice crystals, interrupting their growth [26]. This involvement of glycoproteins in depressing the freezing point was first described in the 1960s [27]. Since then, AFGPs have become a textbook example of a ‘resistance’ adaptation for life in extreme cold environments. Interestingly, the transformation of a functionally unrelated, pancreatic protease (trypsinogen) gave rise to the evolution of the AFGP gene family [28,29]. The nucleotide identity between notothenioid AFGP and trypsinogen genes is 93–96%, which represents a divergence time between the two genes of approximately 5–14 million years—a timeframe that corresponds with the cooling of the Southern Ocean [28]. This time period is also consistent with the phyletic divergence of the five Antarctic notothenioid families that have AFGPs [28].
In addition to AFGPs, other proteins in Antarctic fishes have become retooled to function more efficiently in the cold. Perhaps the best-known example is that of microtubules, which are known to depolymerize at cold temperature in mammals. In Antarctic fishes, amino acid substitutions within the α-tubulin and β-tubulin building-blocks of microtubules stabilize interfilament interactions and promote polymerization at cold temperatures [30].
Cold has a similarly detrimental effect on proteins. Studies have found that Antarctic fishes have high levels of chaperones, which are crucial for assisting in protein folding [25,31,32]. Similarly, components of the ubiquitin-mediated pathway of protein degradation are higher in D. mawsoni compared with fishes living at warm temperatures [25]. These findings are consistent with previous studies in other species of Antarctic notothenioids and invertebrates that show collectively that cold temperature perturbs protein structure, warranting greater levels of protein chaperones, rates of protein degradation and presumably protein synthesis as well [33,34].
A long-standing question in Antarctic and more general thermal biology is whether life at cold body temperatures is accompanied by an elevation in metabolic rate to offset—partly or completely—the slowing of metabolic processes by low temperature alone. It has been recognized for more than a century that Antarctic fishes are a feisty group, as remarked by Captain Scott in his 1905 diaries from the Voyage of the Discovery: “Besides what we may call the Antarctic whiting, our people caught a quantity of a surface fish that frequented the pools and cracks in the ice during the summer […] I heard that it gave very good sport” (Fig 3).
Figure 3.
Raymond E. Priestley, Harry Dickason and Frank V. Browning use a cylindrical fishing net as part of Robert F. Scott's 1910–1913 Antarctic expedition. Reprinted with permission from the Scott Polar Research Institute.
Yet, the veracity of metabolic cold adaptation has been a tricky problem to approach scientifically and has been a source of considerable controversy. Logically, higher rates of metabolism in Antarctic fishes should confer a means of countering the reduction in metabolic rates that would otherwise be associated with low body temperatures, but this ability is probably not useful at basal or resting metabolic rates, only during periods of high metabolic output.
The most successful approach to addressing these questions has been to focus on particular physiological and biochemical functions at different levels of biological organization. For example, the motility of epithelial wound-healing cells (keratocytes) is exceptionally slow in Antarctic fishes and not thermally compensated to any extent [35]. On the other hand, Antarctic notothenioids have exceptionally high mitochondrial densities in skeletal and cardiac muscles [36] that allow the fish to produce ATP in large quantities during sustained activities, and that occupy a greater extent of cell volume to minimize diffusion distances and enhance oxygen flux [37]. Furthermore, Antarctic nototheniod fishes have the enzymatic capacities necessary to elevate the rate of ATP generation by the central oxidative pathways of metabolism compared with non-Antarctic counterparts [38,39,40].
These declining fish numbers are not only tragic for biodiversity, they also represent an impediment to scientific progress
Given these remarkable adaptations to life in the cold, what can science and medicine learn from Antarctic fishes, and icefishes in particular? One of the first questions often asked about the peculiar biology of icefishes, is how and why they have survived alongside their red-blooded relatives. One answer is that the Southern Ocean has relatively few species compared with other oceans in the world, so there is little competition for food and habitat. Second, it has long been thought that although there might be no advantage to being an icefish, there is no disadvantage either; the loss of haemoglobin is a neutral mutation, due in part to the oxygen-rich environment of the icefish. Some have even suggested that the loss of red blood cells is advantageous, as it reduces blood viscosity and, thus, the work of the heart circulating blood throughout the cardiovascular system of icefishes [41]. However, back-of-the-envelope calculations have suggested that the large blood volume of icefishes offsets this potential benefit and that the work of the icefish heart is greater than that of red-blooded species [42].
Recent work, however, has revealed that there might be some genuine benefits to being white-blooded. Haemoglobin is an iron-containing protein, and iron promotes the formation of free radicals. Iron dysregulation and corresponding oxidative stress are associated with several diseases including Alzheimer and Parkinson disease. It turns out that icefish have significantly lower levels of oxidized proteins and lipids compared with closely related red-blooded fishes [43]. Fewer damaged proteins are correlated with lower rates of protein synthesis. Moreover, having lower levels of free radicals means that icefishes can lower their antioxidant defences and, perhaps, their levels of iron-binding proteins. Studies have shown there are fewer hepcidin genes—which encode iron-binding proteins—in icefishes compared with red-blooded fishes [44]. Together, these modifications amount to less energy expended to defend against free radical production and their damaging effects.
…many of the unique traits that have arisen in these animals can serve as models […] for elucidating the molecular mechanisms of some human diseases
Icefishes also provide a natural genetic knockout for studying the effects of the loss of expression of haemoglobin and myoglobin. Geneticists have long used a forward approach to studying gene function: knocking out a gene of interest and analysing its effect on phenotype. However, there are many advantages to using a naturally occurring knockout mutant over a lab-generated one to study disease. First, many diseases are caused by mutations within the regulatory regions of a gene, sometimes affecting gene expression late during development and in specific tissues. Knockout mutations are often lethal during early stages of development—making it difficult to assess the impact they have in adults—and have an impact on cells throughout the body. Second, many diseases are pleiotropic, which makes them impossible to study in single-knockout mutants.
Icefishes can also be used as a model for studying bone development. Notothenioids, including icefishes, evolved from a benthic-dwelling ancestor. The invasion of notothenioids into the water column as benthopelagic or pelagic species required the evolution of an array of features including lipid-rich tissues and decreased bone mineralization to enhance buoyancy. Investigators have used icefishes as a model for investigating osteopenia—the reduction in bone mineral density that affects 46 million Americans. These studies have shown that the expression of key genes involved in bone development is delayed in icefishes, resulting in a cartilaginous-rich skeleton [45].
The Antarctic and the surrounding Southern Ocean is a region that has intrigued and inspired humans for centuries. This area continues to offer priceless value as a laboratory for scientific endeavours, as a wilderness for intrepid explorers and as the last large intact marine ecosystem in the world.
Most of us are familiar with the 1910–1912 expedition to the Antarctic led by Robert Falcon Scott during the ‘heroic age’ of polar exploration. 2012 marked the 100th anniversary of his remarkable trek to the South Pole and the thoughts and images that come to mind are of Scott's men fighting against the odds, their dedication to scientific discovery and their bravery in the face of adversity. We all know the story of Captain Oates deliberately walking to his death in a blizzard so that the others might survive, and the remaining three men trapped by a storm in their final encampment, starving to death on the Ross Ice Shelf only 11 miles from supplies at ‘One Ton Depot’.
…Antarctic nototheniod fishes have the enzymatic capacities necessary to elevate the rate of ATP generation by the central oxidative pathways of metabolism…
It is a remarkable story of determination, survival and tragedy. It is also a story of physiological limits. The physiological stresses, costs and requirements associated with the march to the South Pole by Scott and his four compatriots are discussed in a review [46]. The conclusion reached is that whilst it is possible that the fate of these men might have been different had they had modern knowledge and technology, it is still nevertheless uncertain.
Similarly to Scott and his team, Antarctic fishes live near the limits of their physiological tolerance. Unfortunately, we simply do not know enough about their biology to be confident that Antarctic fishes will cope with the many challenges ahead of them. Rising seawater temperatures and ocean acidity will probably have a direct impact on the survival of Antarctic fishes and have indirect effects on other organisms within the Antarctic food web that affect fishes. How life in the Southern Ocean responds to these changes will, in part, be determined by its phenotypic plasticity. The survival of Antarctic fishes also hinges on the pressures of industrial-scale fishing and the ability to successfully manage the fisheries. As more easily accessible sources of fish continue to be depleted, the drive to exploit the rich resources of Antarctica will intensify, mandating greater oversight. The increasing exploration and exploitation of Antarctica also heightens threats imposed by introduced, non-native species.
…the Antarctic toothfish fishery should be closed until sufficient scientific data on the reproductive biology and ecology of toothfish has been gathered…
We strongly agree with assessments put forth by others in the scientific community and support the conclusion that the harvesting of living resources in the Southern Ocean “has had the most devastating effect on natural systems” [47]. We believe that the Antarctic toothfish fishery should be closed until sufficient scientific data on the reproductive biology and ecology of toothfish has been gathered, so that truly sustainable harvest limits can be enacted. In addition, IUU fishing must be more effectively curtailed and a network of marine protected areas (MPAs) should be established within the Southern Ocean, as is being done elsewhere. Marine reserves have been shown to be highly effective in increasing species diversity, biomass and health [48] and the highest priority should be placed on establishing the Ross Sea, considered ‘The Last Ocean,’ as a MPA (see http://www.lastocean.org/). The Southern Ocean is the most pristine ecosystem on earth and home to a remarkable diversity of organisms, many of which are found nowhere else on earth. It is imperative that we protect it, and we are hopeful that the Antarctic Treaty System and CCAMLR will do so.
Since preparing the first draft of this essay, the CCAMLR met in October 2012 to discuss establishing the Ross Sea as a MPA. CCAMLR was unable to reach a consensus and the topic never went to a vote. A second meeting will be held in July 2013 in Bremerhaven, Germany, to discuss the proposal again.

Kristin M O'Brien

Elizabeth L Crockett
Acknowledgments
Support was provided by the National Science Foundation (ANT 0741301 and ANT 1043781 to K.O'B. and ANT 0739637 and ANT 1043576 to E.L.C.). We thank Joseph Eastman for reading a draft of this manuscript and providing many helpful comments.
Footnotes
The authors declare that they have no conflict of interest.
References
- DeWitt HH (1971) Coastal and deep-water benthic fishes of the Antarctic. In Antarctic Map Folio Series (ed Bushnell VC), pp 1–10. New York, New York, USA: American Geographical Society [Google Scholar]
- Eastman JT (1993) Antarctic Fish Biology: Evolution in a Unique Environment. San Diego, California, USA: Academic [Google Scholar]
- Gon O, Heemstra PC (1990) Fishes of the Southern Ocean, first edn. Grahamstown, Eastern Cape, South Africa: JLB Smith Institute of Icthyology [Google Scholar]
- Knox GA (1994) The Biology of the Southern Ocean. Cambridge, UK: Cambridge University Press [Google Scholar]
- Littlepage JL (1965) Oceanographic Observations in McMurdo Sound, Antarctica (ed. Llano GA), pp 1–37. Washington, DC, USA: American Geophysical Union [Google Scholar]
- Chown SL et al. (2012) Conservation. Challenges to the future conservation of the Antarctic. Science 337: 158–159 [DOI] [PubMed] [Google Scholar]
- King JC (1994) Recent climate variability in the vicinity of the Antarctic Peninsula. Int J Climatol 14: 357–369 [Google Scholar]
- Meredith MP, King JC (2005) Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys Res Lett 32: L19604 [Google Scholar]
- Vaughan DG, Marshall GJ, Connolley WM, Parkinson C, Mulvaney R, Hodgson DA, King JC, Pudsey CJ, Turner J (2003) Recent rapid regional climate warming on the Antarctic Peninsula. Climatic Change 60: 243–274 [Google Scholar]
- British Antarctic Survey: Natural Environment Resource Council (2008) Science briefing—The Antarctic Peninsula's retreating ice shelves. http://www.antarctica.ac.uk/press/journalists/resources/science/antarctic_peninsula_retreating_ice_shelves.php
- Clarke A, Murphy EJ, Meredith MP, King JC, Peck LS, Barnes DK, Smith RC (2007) Climate change and the marine ecosystem of the western Antarctic Peninsula. Philos Trans R Soc Lond B Biol Sci 362: 149–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson A, Siegel V, Pakhomov E, Rothery P (2004) Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432: 100–103 [DOI] [PubMed] [Google Scholar]
- Montes-Hugo M, Doney SC, Ducklow HW, Fraser W, Martinson D, Stammerjohn SE, Schofield O (2009) Recent changes in phytoplankton communities associated with rapid regional climate change along the western Antarctic Peninsula. Science 323: 1470–1473 [DOI] [PubMed] [Google Scholar]
- Ducklow HW, Baker K, Martinson DG, Quetin LB, Ross RM, Smith RC, Stammerjohn SE, Vernet M, Fraser W (2007) Marine pelagic ecosystems: the west Antarctic Peninsula. Philos Trans R Soc Lond B Biol Sci 362: 67–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kock KH (1992) Antarctic Fish and Fisheries. Cambridge, UK: Cambridge University Press [Google Scholar]
- Ainley DG, Blight LK (2009) Ecological repercussions of historical fish extraction from the Southern Ocean. Fish & Fisheries 10: 13–38 [Google Scholar]
- Marschoff ER, Barrera-Oro ER, Alescio NS, Ainley DG (2012) Slow recovery of previously depleted demersal fish at the South Shetland Islands, 1983–2010. Fish Res 125–126: 206–213 [Google Scholar]
- Ainley DG, Brooks CM, Eastman JT, Massaro M (2012) Unnatural selection of Antarctic toothfish in the Ross Sea, Antarctica. In Protection of Three Poles (ed. Huettmann F), pp 53–75. Tokyo, Japan: Springer [Google Scholar]
- Norse EA et al. (2012) Sustainability of deep-sea fisheries. Mar Policy 36: 307–320 [Google Scholar]
- Österblom H, Sumaila UR, Bodin Ö, Sundberg JH, Press AJ (2010) Adapting to regional enforcement: fishing down the governance index. PLoS ONE 5: e12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAFFIC (2009) Australia confiscates 130 km long deepwater gillnet. http://www.traffic.org/home/2009/11/6/australia-confiscates-130-km-long-deepwater-gillnet.html
- Ainley DG, Nur N, Eastman JT, Ballard G, Parkinson CL, Evans CW, DeVries AL (2012) Decadal trends in abundance, size and condition of Antarctic toothfish in McMurdo Sound, Antarctic, 1972–2011. Fish & Fisheries [Epub ahead of print] doi:; DOI: [DOI] [Google Scholar]
- Fenaughty JM, Eastman JT, Sidell BD (2008) Biological implications of low condition factor ‘axe handle’ specimens of the Antarctic toothfish, Dissostichus mawsoni, from the Ross Sea. Antarct Sci 20: 537–551 [Google Scholar]
- Bargelloni L, Marcato S, Zane L, Patarnello T (2000) Mitochondrial phylogeny of notothenioids: a molecular approach to Antarctic fish evolution and biogeography. Syst Biol 49: 114–129 [DOI] [PubMed] [Google Scholar]
- Chen Z et al. (2008) Transcriptomic and genomic evolution under constant cold in Antarctic notothenioid fish. Proc Natl Acad Sci USA 105: 12944–12949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AL, Cheng C-HC (2005) Antifreeze proteins in polar fishes. In Fish Physiology (eds Farrell AP, Steffensen JF), pp 155–201. San Diego, California, USA: Academic [Google Scholar]
- DeVries AL, Wohlschlag DE (1969) Freezing resistance in some Antarctic fishes. Science 163: 1073–1075 [DOI] [PubMed] [Google Scholar]
- Chen L, DeVries AL, Cheng CH (1997) Evolution of antifreeze glycoprotein gene from a trypsinogen gene in Antarctic notothenioid fish. Proc Natl Acad Sci USA 94: 3811–3816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CH, Chen L (1999) Evolution of an antifreeze glycoprotein. Nature 401: 443–444 [DOI] [PubMed] [Google Scholar]
- Detrich HW 3rd (1997) Microtubule assembly in cold-adapted organisms: functional properties and structural adaptations of tubulins from antarctic fishes. Comp Biochem Physiol A Physiol 118: 501–513 [DOI] [PubMed] [Google Scholar]
- Detrich HW 3rd, Amemiya CT (2010) Antarctic notothenioid fishes: genomic resources and strategies for analyzing an adaptive radiation. Integr Comp Biol 50: 1009–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne MA, Burns G, Fraser KP, Hillyard G, Clark MS (2010) Transcription profiling of acute temperature stress in the Antarctic plunderfish Harpagifer antarcticus. Mar Genomics 3: 35–44 [DOI] [PubMed] [Google Scholar]
- Clark MS, Thorne MA, Toullec JY, Meng Y, Guan LL, Peck LS, Moore S (2011) Antarctic krill 454 pyrosequencing reveals chaperone and stress transcriptome. PLoS ONE 6: e15919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place SP, Zippay ML, Hofmann GE (2004) Constitutive roles for inducible genes: evidence for the alteration in expression of the inducible hsp70 gene in Antarctic notothenioid fishes. Am J Physiol Regul Integr Comp Physiol 287: R429–R436 [DOI] [PubMed] [Google Scholar]
- Ream RA, Theriot JA, Somero GN (2003) Influences of thermal acclimation and acute temperature change on the motility of epithelial wound-healing cells (keratocytes) of tropical, temperate and Antarctic fish. J Exp Biol 206: 4539–4551 [DOI] [PubMed] [Google Scholar]
- O'Brien KM (2011) Mitochondrial biogenesis in cold-bodied fishes. J Exp Biol 214: 275–285 [DOI] [PubMed] [Google Scholar]
- Egginton S, Skilbeck C, Hoofd L, Calvo J, Johnston IA (2002) Peripheral oxygen transport in skeletal muscle of Antarctic and sub-Antarctic notothenioid fish. J Exp Biol 205: 769–779 [DOI] [PubMed] [Google Scholar]
- Crockett E, Sidell B (1990) Some pathways of energy metabolism are cold adapted in Antarctic fishes. Phys Zool 63: 472–488 [Google Scholar]
- Kawall H, Torres J, Sidell BD, Somero G (2002) Metabolic cold adaptation in Antarctic fishes: evidence from enzymatic activities of brain. Mar Genomics 140: 279–286 [Google Scholar]
- Torres JJ, Somero GN (1988) Metabolism, enymatic activities and cold adaptation in Antarctic mesopelagic fishes. Mar Biol 98: 169–180 [Google Scholar]
- di Prisco G, Cocca E, Parker S, Detrich H (2002) Tracking the evolutionary loss of hemoglobin expression by the white-blooded Antarctic icefishes. Gene 295: 185–191 [DOI] [PubMed] [Google Scholar]
- Sidell BD, O'Brien KM (2006) When bad things happen to good fish: the loss of hemoglobin and myoglobin expression in Antarctic icefishes. J Exp Biol 209: 1791–1802 [DOI] [PubMed] [Google Scholar]
- Mueller IA, Devor DP, Grim JM, Beers JM, Crockett EL, O'Brien KM (2012) Exposure to critical thermal maxima causes oxidative stress in hearts of white- but not red-blooded Antarctic notothenioid fishes. J Exp Biol 215: 3655–3664 [DOI] [PubMed] [Google Scholar]
- Xu Q, Cheng CH, Hu P, Ye H, Chen Z, Cao L, Chen L, Shen Y, Chen L (2008) Adaptive evolution of hepcidin genes in antarctic notothenioid fishes. Mol Biol Evol 25: 1099–1112 [DOI] [PubMed] [Google Scholar]
- Albertson RC, Yan YL, Titus TA, Pisano E, Vacchi M, Yelick PC, Detrich HW 3rd, Postlethwait JH (2010) Molecular pedomorphism underlies craniofacial skeletal evolution in Antarctic notothenioid fishes. BMC Evol Biol 10: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsey LG, Stroud MA (2012) 100 years since Scott reached the pole: a century of learning about the physiological demands of Antarctica. Physiol Rev 92: 521–536 [DOI] [PubMed] [Google Scholar]
- Croxall JP, Nicol S (2004) Management of Southern Ocean fisheries: global forces and future sustainability. Antarct Sci 16: 569–584 [Google Scholar]
- Lester SE, Halpern BS, Grorud-Colvert K, Lubchenco J, Ruttenberg BI, Gaines SD, Airame S, Warner RR (2009) Biological effects within no-take marine reserves: a global synthesis. Mar Ecol Prog Ser 384: 33–46 [Google Scholar]