Abstract
One of the primary goals for tissue engineering is to induce new tissue formation by stimulating specific cell function. Human mesenchymal stem cells (hMSCs) are a particularly important cell type that has been widely studied for differentiation down the osteogenic (bone) lineage, and we recently found that simple phosphate functional groups incorporated into poly(ethylene glycol) hydrogels could induce osteogenesis without using differentiation medium by unknown mechanisms. Here, we aimed to determine whether direct or indirect cell/materials interactions were responsible for directing hMSCs down the osteogenic lineage on phosphate (PO4) functionalized poly(ethylene glycol) (PEG) hydrogels. Our results indicated that serum components adsorbed onto PO4-PEG hydrogels from media in a pre-soaking step were sufficient for attachment and spreading of hMSCs, even when seeded in serum-free conditions. Blocking antibodies for collagen and fibronectin (targeted to the hydrogel), as well as β1 and β3 integrin blocking antibodies (targeted to the cells), each reduced attachment of hMSCs to PO4-PEG hydrogels, suggesting that integrin-mediated interactions between cells and adsorbed matrix components facilitate attachment and spreading. Outside in signaling, and not merely shape change, was found to be required for osteogenesis, as alkaline phosphatase (ALP) activity and expression of CBFA1, osteopontin and collagen-1 were each significantly down regulated upon inhibition of focal adhesion kinase (FAK) phosphorylation even though focal adhesion structure or cell shape were unchanged. Our results demonstrate that complex function (i.e.,osteogenic differentiation) can be controlled using simple functionalization strategies, such as incorporation of PO4, but that the role of these materials may be due to more complex influences than has previously been appreciated.
1. INTRODUCTION
Human mesenchymal stem cells derived from bone marrow have been shown to be capable of differentiating down osteogenic, adipogenic and chondrogenic lineages [1]. Traditionally, differentiation of these cells has been achieved by adding soluble cues to the culture medium. The roles and mechanisms of various media additives in inducing differentiation of hMSCs have been extensively studied. For example, dexamethasone, bone morphogenetic protein (BMP), β-glycerol phosphate have been shown to up-regulate expression of osteogenic related genes in hMSCs [1–5]. To complement these approaches, there is growing interest in the biomaterial research community to use synthetic biomaterials as culture platforms for hMSCs and for applications in tissue regeneration. Their design has been inspired by previous work to capture and present molecular interactions that induce specific functions of hMSCs.
Specifically, synthetic scaffolds have been extensively used as culture platforms to introduce both biochemical and biophysical signals to control and promote osteogenic differentiation of hMSCs. Inorganic materials, such as tricalcium phosphate, hydroxyapatite, and bioglass, have all been widely used as coatings and bulk materials and considered osteoconductive materials for bone regeneration applications [6–10]. The main limitation when using inorganic materials is the inability to control and tune the materials properties, especially degradability, as well as the limited ability to alter the chemistry to incorporate biochemical cues that promote bone formation [9, 10]. As an alternative, materials based on organic polymers have become attractive as biomaterial scaffolds for bone tissue engineering. While their mechanical properties can be inferior compared to inorganic materials, polymers allow for tailoring of material properties and introduction of a wide range of chemistries that allow researchers to incorporate functionalities into the materials [10].
In particular, poly(ethylene glycol) (PEG) based hydrogel scaffolds have been used by our group and others to present various biochemical signals to study and control osteogenic differentiation of hMSCs [11, 12]. Because PEG minimizes non-specific protein interactions, the role and function of specific biochemical signals on hMSC function can be studied independent of non-specific protein adsorption from serum employed in cell culture. Researchers have employed PEG gels to present various biochemical cues, including peptides [13], growth factors [14], chemical functionalities [15], enzymatic degradability [16], and controlled material elasticity [17, 18], and study the role of integrin signaling, growth factor signaling, matrix degradability and matrix mechanics during osteogenic differentiation of hMSCs.
Complexity, cost and limited understanding of the most critical factors for inducing desired hMSC functions have prompted researchers to investigate alternative and simpler approaches for the design of synthetic biomaterials. For example, researchers have identified biomaterial formulations based on simple chemistries that influence critical cell functions such as proliferation, differentiation etc. Several studies have investigated the influence of small chemical functional groups on proliferation and/or differentiation of hMSCs using self-assembled monolayers [19–22] and hydrogel systems [15,23]. For example, osteogenic and chondrogenic differentiation of hMSCs depends on the choice of small molecules used to functionalize surfaces, with thiol or amine functional groups promoting osteogenic differentiation and acid or hydroxyl functional groups promoting chondrogenic differentiation [20,21]. Of interest to this work, Benoit et al. demonstrated that phosphate functional groups induced osteogenic differentiation in hMSCs on 2D gels, as well as when encapsulated in 3D hydrogel environments [15]. Interestingly, osteogenic differentiation on this otherwise inert PEG hydrogel was achieved without the addition of any soluble osteoinductive factors to the media, indicating that osteogenic differentiation was specifically due to the presence of the small molecule phosphate functional group. Collectively, these studies demonstrate the potential for simple strategies to offer simpler, cheaper and more efficient design principles for cell carriers to promote hMSC differentiation. While these phenomenological observations are quite interesting and insightful, they provide very little mechanistic understanding of how these functionalized biomaterials guide cell behavior.
Here, we attempt to elucidate some aspects of the mechanism driving osteogenic differentiation of hMSCs on phosphate-functionalized PEG gels, as they were previously shown to induce osteogenic differentiation in hMSCs without the need for osteoinduction media [15]. Several studies have previously shown the importance of adsorbed extra cellular matrix (ECM) proteins in mediating cell interaction with biomaterials and that composition, as well as orientation, of the adsorbed ECM proteins is considered to be a major determinant of cell material interactions with biomaterials [24–29]. Specifically, previous literature has shown that ECM proteins influence hMSC differentiation in in vitro cultures [30–35]. For example, collagen-1 (coll-1) and fibronectin (FN) when adsorbed onto TCPS were shown to promote mineralization and upregulate osteogenic genes, CBFA-1 and osteocalcin, in hMSCs [30]. Further, integrins that bind to these ECM proteins were demonstrated to play a key role in both promoting and inhibiting osteogenic differentiation of MSCs [31]. Previous studies also showed that interaction of α2β1 integrin with collagen-1 activated the transcription factor CBFA-1 and promoted matrix mineralization, and further, that blocking these interactions resulted in a marked decrease in matrix mineralization [32, 33]. Blocking interaction of integrins α5β1, α3β1 with FN has been shown to inhibit upregulation of osteogenic genes, ALP activity and mineral formation in osteoprogenitor cultures [35]. Specifically, FN fragments with high specificity for binding to α5β1 integrins increased ALP activity and upregulated gene expression of ALP, CBFA-1 and osteocalcin in hMSCs [35]. Interestingly, the integrin αvβ3, which also has binding sites on FN, was shown to have a negative effect on hMSC osteogenic differentiation. hMSCs when cultured on FN fragments with a high specificity for binding to αvβ3 integrins were shown to decrease gene expression of ALP, CBFA-1 and osteocalcin [35].
Here, we aimed to determine if hMSCs were directly interacting with the phosphate functional groups on PEG hydrogel or indirectly with adsorbed ECM proteins present in serum. The role of focal adhesion kinase (FAK) signaling in inducing osteogenic differentiation on these gels was also investigated. Gaining a mechanistic understanding of how these gels cause phenotypic changes in hMSCs is an important aspect to our overall understanding of matricellular signaling, as well as our ability to design improved materials for tissue engineering applications.
2. MATERIAL AND METHODS
All materials were purchased from Sigma-Aldrich unless otherwise specified.
2.1. Cell culture
hMSCs were isolated from human bone marrow (Cambrex) and cultured in growth media (low glucose Dulbecco’s Modified Eagle Medium (DMEM, Gibco) supplemented with 10% fetal bovine serum (Gibco), 1% Penicillin/Streptomycin (Gibco), and 0.2% Fungizone (GIBCO)). hMSCs at passage three were used in all the studies.
2.2. Phosphate functionalized poly(ethylene glycol) polymer gels
Gels were formed by polymerizing poly(ethylene glycol) dimethacrylate (PEGDMA) (Mn ~550 Da) with 0.5 wt% of the photo-initiator, 2,2-dimethoxy-1,2-di(phenyl) acetophenone (Ciba-Giegy) by exposure to ~4 mWcm−2 ultraviolet light for 10 min. Phosphate functional groups were incorporated into the gels by adding ethylene glycol methacrylate phosphate (EGMP) at a concentration of 50mM (or otherwise mentioned) to the macromer-initiator solution before polymerization. Gels were swollen in phosphate buffer saline (PBS, 1×, GIBCO) for at least 24 h. Circular disks (10 mm diameter, 120 µm thick) were cut from the gel using biopsy punches and transferred to tissue culture plates. For cell culture experiments gels were sterilized in 70% ethanol and washed thoroughly in PBS before seeding.
2.3. Quantification of protein adsorption
Phosphate functionalized gels were incubated in 10% serum containing media for overnight and washed with PBS (0.5 ml in each well of a 24 well plate, 3×) to remove weakly adsorbed proteins. Adsorbed proteins were eluted in sodium dodecyl sulfate (SDS) buffer for 2 h and concentrated using Zeba desalting columns (7k MWCO, Pierce). Protein levels were quantified by a µBCA assay (Pierce) according to manufacturer’s instructions.
2.4. Focal adhesion staining and calculating cell shape parameters
Focal adhesion staining was performed on hMSCs attached to the gels (5 × 103 cells/cm2 seeding density) using a focal adhesion staining kit (FAK100, EMD Millipore) according to manufacturer’s instructions. Cell density was chosen to avoid excessive cell-cell contacts after hMSC attachment to gels. Cells were counterstained with 4,6-diamidino-2-phenylindole (DAPI, Invitrogen, 300nM) for nuclei and stored in PBS at 4 C until imaged with a Zeiss confocal microscope.
hMSC shape parameters were quantified by immunostaining with rhodamine phalloidin (Molecular Probes, R415, 1:200 dilution). Samples were imaged using a confocal microscope (Zeiss, LSM 710), and the RGB images were converted into binary images and analyzed by an in-built ‘analyze particles’ macro in ImageJ that automatically generates an average area per cell and aspect ratio. Ten images per condition were analyzed.
2.5. Adhesion blocking and integrin blocking experiments
Phosphate gels were incubated in growth media overnight to allow for serum proteins to adsorb. Gels were washed with PBS to remove weakly attached proteins and incubated with antibody solutions containing antibodies against fibronectin (Abcam, ab2413, 1:200), collagen-1 (Abcam, ab34710, 1:1000) or IgG (Isotype control, Sigma, M5284) in PBS for 1 h. Gels were then washed three times for 10 min each with PBS to remove loosely attached antibodies. hMSCs were then seeded onto gels in serum free media (low glucose Dulbecco’s Modified Eagle Medium (DMEM, Gibco) supplemented with 1% Penicillin/Streptomycin (Gibco), and 0.2% Fungizone (GIBCO)) at a density of 5×103 cells/cm2 and allowed to attach for 12 h. Gels were washed in PBS to remove loosely attached cells and stained with calcein AM (Invitrogen) for live cells as per manufacturer’s protocols. hMSCs were imaged using an epifluorescence microscope and live cells were counted manually using ImageJ software.
For integrin blocking experiments, hMSCs were incubated with antibody solutions containing antibodies against β1 (Abcam, ab52971, 1:50 dilution), β3 (Abcam, ab47584, 1:50 dilution) integrins or IgG (Isotype control, Sigma, M5284) in serum-free media for 30 min and seeded onto serum pre-incubated gels in serum free media at 5×103 cells/cm2 density. Integrin blocked hMSCs were allowed to attach to the gels for 12 h, washed with PBS and stained with calcein AM. Stained hMSCs were imaged using an epifluorescence microscope and the number of attached cells were counted using ImageJ software.
2.6. Role of focal adhesion kinase (FAK) in osteogenesis of hMSCs on phosphate gels
To examine integrin signaling dependent osteogenic differentiation of hMSCs on phosphate gels, cells were cultured in the presence (at 3µM) and absence of a small chemical inhibitor for pFAK, PF-573228 (Tocris Biosciences). For the inhibition experiments, hMSCs were seeded onto phosphate gels or tissue culture polystyrene (TCPS) and cultured in growth or osteogenic media (high glucose Dulbecco’s Modified Eagle Medium (DMEM, Gibco) supplemented with 10% fetal bovine serum (Gibco), 1% Penicillin/Streptomycin (Gibco), and 0.2% Fungizone (GIBCO), 10mM β-glycerol phosphate (Sigma), 50 µg/ml ascorbic acid (Sigma), and 100 nM dexamethasone (Sigma)) at a density of 5×103 cells/cm2.
2.7. Alkaline phosphatase (ALP) production of hMSCs cultured on phosphate gels
ALP production was measured using an assay based on the change in absorbance of o-nitrophenol (ALP substrate, Invitrogen) as it is enzymatically cleaved by ALP. Cells were removed from culture, washed in PBS and lysed in 50 µl RIPA buffer (Invitrogen) for 15 min with gentle shaking. The sample solutions were diluted with 50 µl of PBS. 50 µl of the sample solution was then mixed with 50 µl of ALP substrate, and the absorbance of the solution at 405 nm was measured at 1-minute intervals ten times with a plate reader (Victor2, Perkin Elmer). ALP activity levels were calculated as the slope of the increase in absorbance at 405 nm with time. In parallel, gels were stained with calcein AM (Invitrogen) to stain live cells. Ten images per sample were analyzed for cell number using a cell counter plug-in in ImageJ. ALP activity levels were normalized using the total cell numbers on the gel.
2.8. Gene expression of hMSCs cultured on phosphate gels
Gene expression of hMSCs cultured on phosphate gels was analyzed using reverse-transcription polymerase chain reaction (RT-PCR). At day 14 of culture, gels were removed from culture and washed three times in PBS. RNA was isolated using TRI reagent (Sigma) and standard manufacturer’s protocols. The resulting RNA pellet was re-suspended in nuclease free water and treated with DNase (Bio-Rad) to digest any residual genomic DNA. The obtained RNA was precipitated in isopropanol after washing with phenol-chloroform, re-suspended in 20 µl nuclease free water, and quantified by measuring absorbance at 280 nm with a NanoDrop ND-1000 spectrophotometer (Thermo Scientific). Samples with a 280 nm to 260 nm absorbance ratios greater than 1.85 were considered pure and were used for the reverse transcription step.
Reverse transcription was performed using the iScript cDNA Synthesis Kit (Bio-rad). A total of 15 ng RNA was used for the single-stranded cDNA synthesis. The reverse transcription reaction tube was incubated at 25 C for 5 min, at 42 C for 30 min, and terminated at 85 C for 5 min. PCR was conducted using the iCycler Real-Time PCR machine (Bio-Rad). Primers and probes were obtained from Bio-Rad. Table 1 shows the primer sequences used in this study. The following PCR parameters were utilized: 95 C for 90 s followed by 45 cycles of 95 C for 30 s and 55 C for 60 s. Threshold cycle (CT) analysis was used to quantify PCR products, normalized to GAPDH and relative to expression of hMSCs at day 1 cultured on TCPS in growth media.
Table 1.
Primer Sequences used for qRT-PCR
| Primer | Nucleotide Sequence (5’-3’) |
|---|---|
| COLL-1a | GGGCAAGACAGTGATTGAATACA GGATGGAGGGAGTTTACAGGAA |
| CBFA1 | GGTATGTCCGCCACCACTC TGACGAAGTGCCATAGTAGAGATA |
| GAPDH | GCAAGAGCACAAGAGGAAGAG AAGGGGTCTACATGGCAACT |
| OPN | ATTCTGGGAGGGCTTGGTTG TCTGGTCCCGACGATGCT |
2.9. Statistical Analysis
Data collected throughout this study are represented as mean ± standard error of three different samples (or otherwise mentioned). For effect of serum on hMSC attachment, shape and for gene expression studies, data sets were compared using two-way ANOVA analysis with Tukey tests for comparison. For effect of PO4 concentration on serum protein adsorption and integrin or adhesion blocking studies, data sets were compared using one-way ANOVA analysis with Tukey tests for comparison. In all studies, p-values less than 0.05 were considered as significant.
3. RESULTS
3.1 Serum proteins facilitate hMSC attachment and spreading on PO4-PEG hydrogels
In order to determine the importance of serum in mediating cell/matrix interactions, we first investigated hMSC attachment to phosphate functionalized gels compared to control PEG gels in the presence and absence of serum, with results presented in Figure 1. While attachment was limited in serum free media for both gel formulations (Figure 1A, grey bars), PO4-PEG gels promoted significantly higher hMSC attachment (~550 cells/cm2, Figure 1A, black bars), spreading (~104 µm2, Figure 1B, solid black bars), in the presence of serum compared to control PEG hydrogels. Aspect ratio of hMSCs on PO4-PEG gels was similar to control PEG gels in the presence of serum (~2, Figure 1B, textured bars). Next, we investigated whether the influence of serum was due to soluble or sequestered proteins. Phosphate functionalized hydrogels were incubated in media containing serum for overnight, washed thoroughly to remove weakly attached serum components, and then seeded with hMSCs in serum free media. As shown in Figure 2 (See Experimental section for details), phosphate functionalized hydrogels promoted higher protein adsorption (~500 ng/cm2) compared to control PEG control hydrogels (~100 ng/cm2) independent of phosphate concentration (Figure 2). Our results indicated that hMSCs attached to pre-treated PO4-PEG in serum-free conditions similarly to cells seeded in serum (Figure 1A, white bar textured, ~525 cells/cm2) while very few cells attached to control gels. Based on these results, we conclude that adsorbed serum components were mediating attachment and spreading of hMSCs on PO4-PEG hydrogels.
Figure 1.
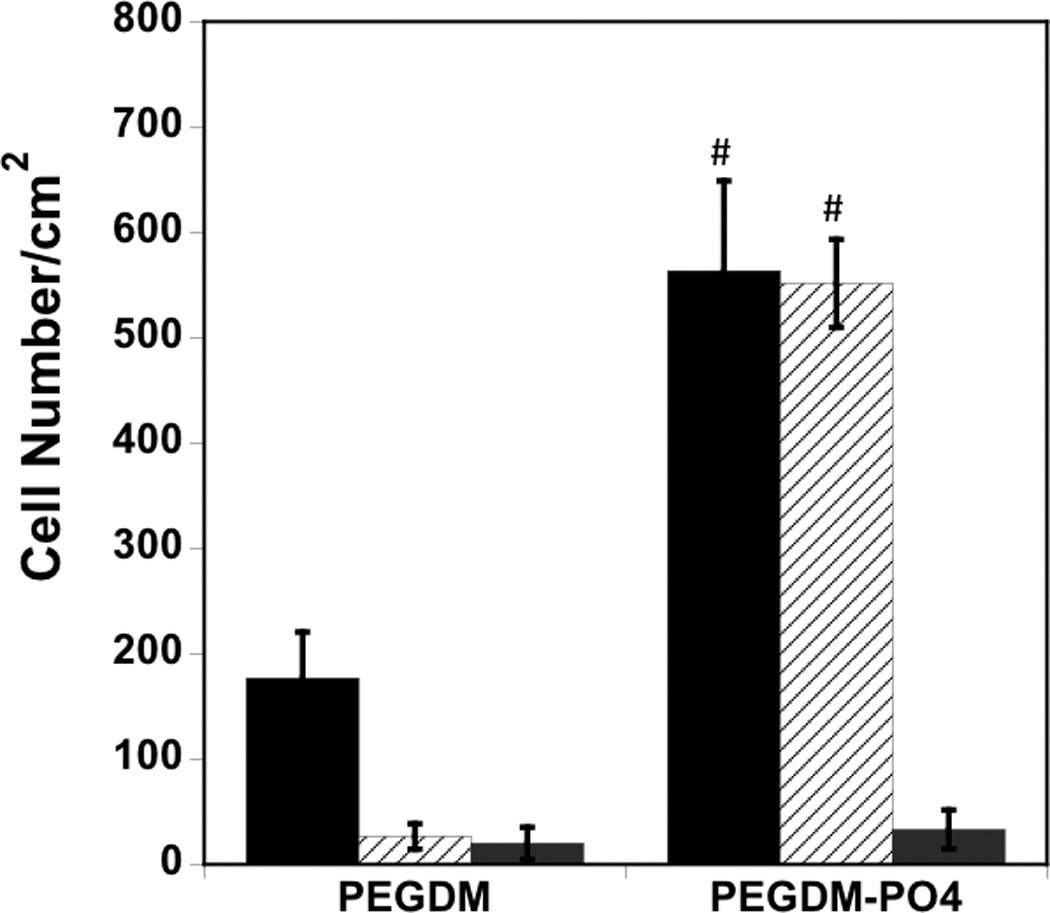
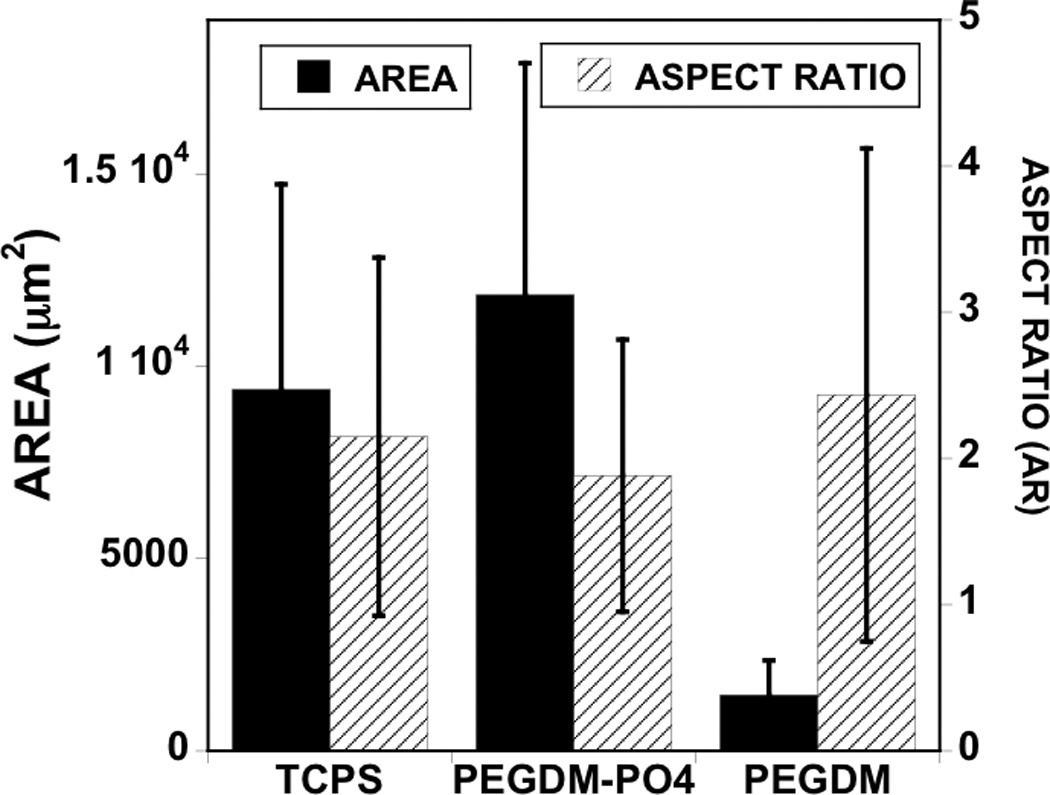
Serum components mediate hMSC attachment and spreading on PO4-PEG gels ([PO4 = 50mM) (A) Effect of seeding conditions on hMSC adhesion to gels. Average number of cells per area attached to PEGDM and PO4-PEG gels when seeded in serum containing media (black bar), pre-incubated with serum containing media and seeded in serum free media (white bar, textured), and seeded in serum free media (grey bar). (# indicates significant difference compared to that on control gels (PEGDM); p<0.05, Data represents averages of N=3 different experiments with n = 3 replicates during each experiments). (B). Cell shape parameters; average cell area (filled black bars) and average aspect ratio (striped bars) when cultured for 24 h on different gels as indicated. Cell shape parameters of hMSCs on TCPS were also included for comparison. Phosphate functional groups promote cell spreading similar to cells cultured on tissue culture polystyrene (TCPS) when seeded in growth media. Error bars represent standard deviations for N=150–250 cells.
Figure 2.
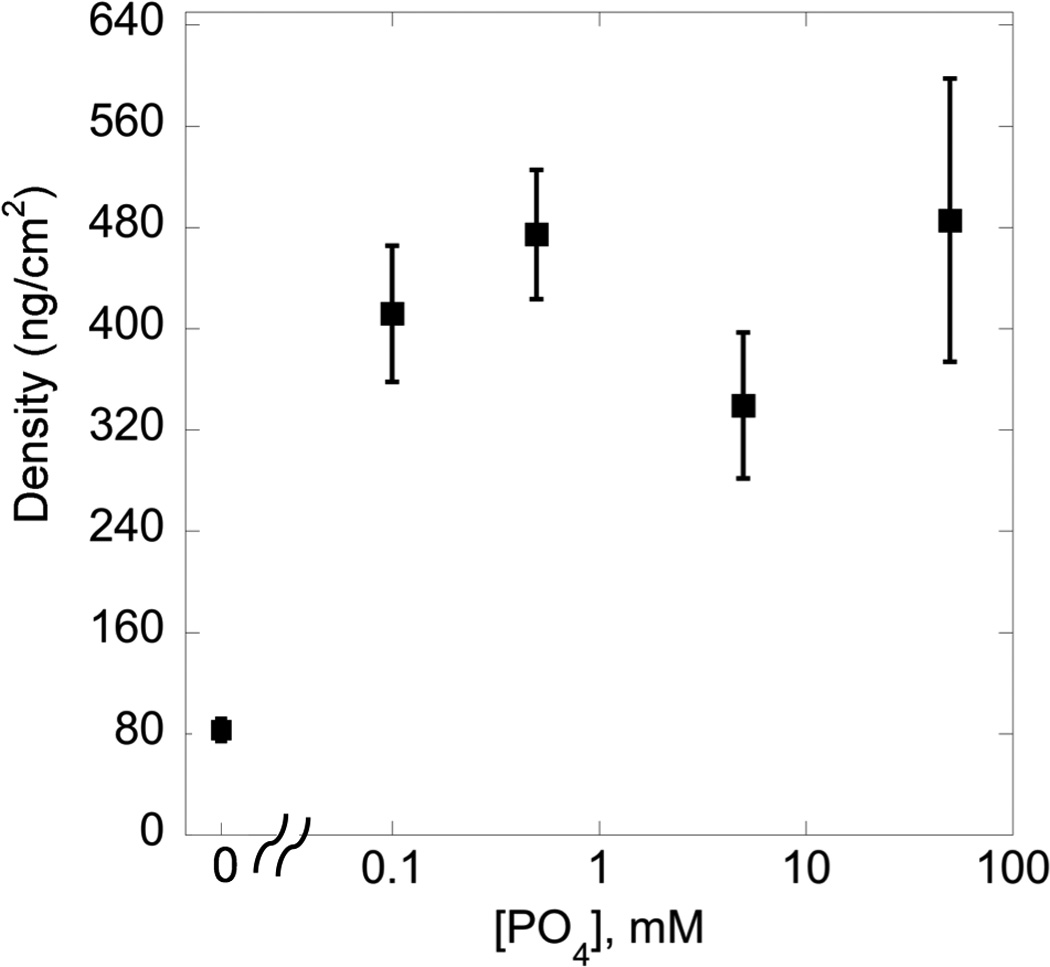
Effect of [PO4] concentration on total protein adsorbed from serum. Total protein adsorbed was significantly higher on phosphate gels compared to the control ([PO4] = 0 mM) gels. Increasing the concentration of [PO4] in the gels did not result in any significant increase in protein adsorption. Total protein adsorbed on TCPS under similar conditions is 0.5 µg/cm2(#, indicates significant difference compared to the control ([PO4] = 0 mM) gel; Data represent averages of N=3 different experiments with 4 replicates in each experiment. p<0.05)
3.2 hMSC attachment is mediated by ECM protein-integrin interactions
The ECM plays a role in influencing cell function by interacting with integrins, which can lead to “outside-in” signaling. Previous studies have indicated that collagen I (Coll-1) and fibronectin (FN) enhanced osteogenic differentiation in hMSCs. Interaction of hMSCs with Coll-1 and FN with specific integrins has been previously shown to control ALP activity, gene expression of CBFA1 and bone sialoprotein [26, 27]. Here, we investigated potential roles for Coll-1 and FN in mediating hMSC attachment to PO4-PEG hydrogels, with results illustrated in Figure 3. To determine possible roles for Coll-1 and FN in mediating hMSC attachment to PO4-PEG hydrogels, we blocked cell adhesive sites present on fibronectin and collagen-1 using specific antibodies. As shown in Figure 3, cell attachment was significantly reduced when either matrix component was blocked using antibodies, suggesting that both Coll-1 and FN were present on PO4-PEG hydrogels and that each provided binding sites for hMSCs.
Figure 3.
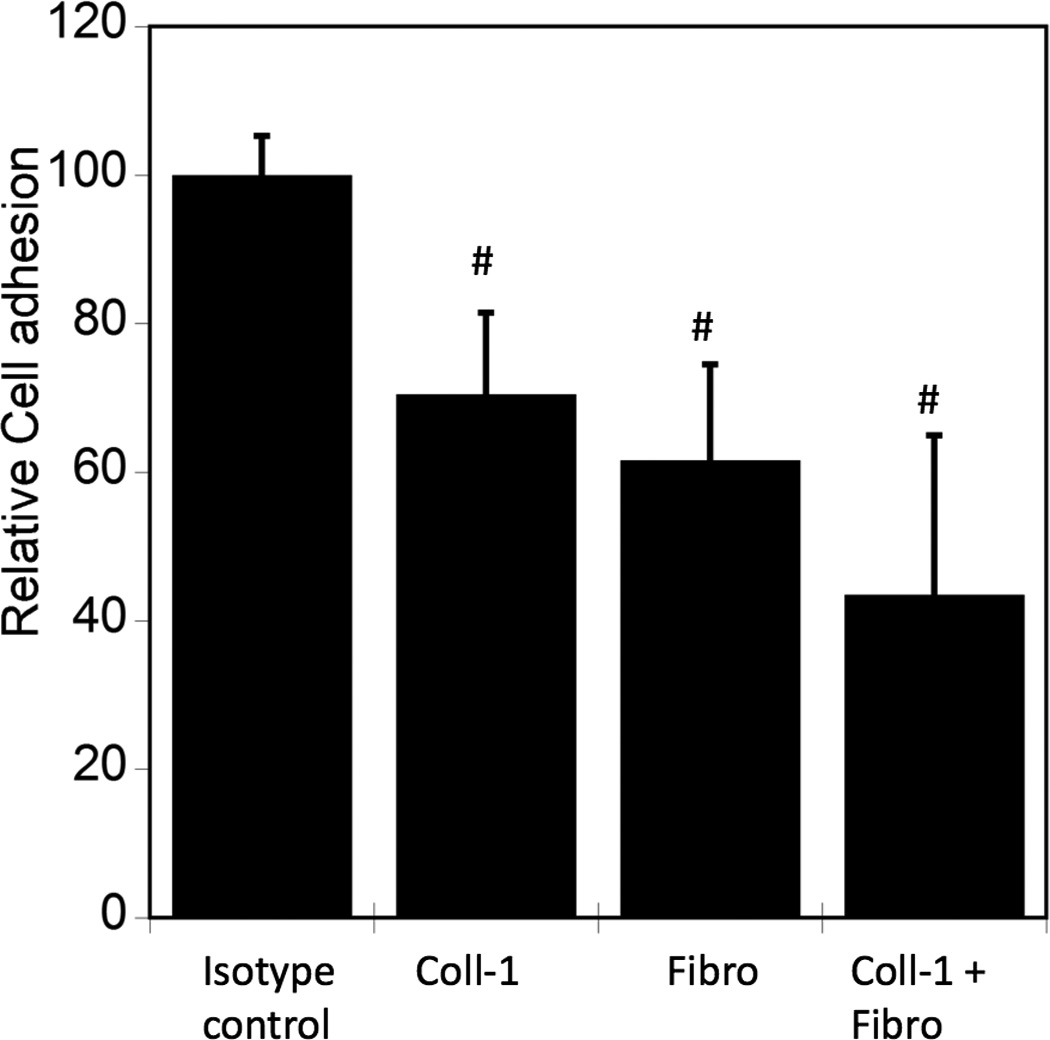
ECM proteins are differentially adsorbed on to PO4-PEG gels ([PO4 = 50mM). Blocking cell adhesion sites corresponding to specific ECM proteins (Fibro = Fibronectin, Coll-1 = Collagen-1) adsorbed onto the PO4-PEG gel influences cell attachment. Average number of cells that remained attached to PO4-PEG gels upon blocking were counted and reported as a percentage compared to control. # indicates significant difference compared to control p<=0.05. (Data represent averages of N=3 different experiments with 3 replicates in each experiment.)
Both β1- and β3- integrins have been shown to have binding ligands on Coll-1 and FN. Also, these integrins have been previously shown to regulate hMSC osteogenic differentiation [31]. We investigated the role of integrin binding for hMSCs on phosphate-functionalized hydrogels using blocking antibodies against β1 and β3 integrins. hMSCs were incubated with antibodies against β1 and β3 integrins in serum free medium and then seeded onto serum media pre-soaked phosphate functionalized gels (still in the presence of serum free medium). As shown in Figure 4A, hMSC attachment was reduced to about 60% and 50% upon blocking β1 and β3 integrins, respectively, while blocking both did not lead to additional statistically significant inhibition. These results indicate that both β1 and β3 integrins facilitate hMSC attachment to phosphate-functionalized hydrogels.
Figure 4.
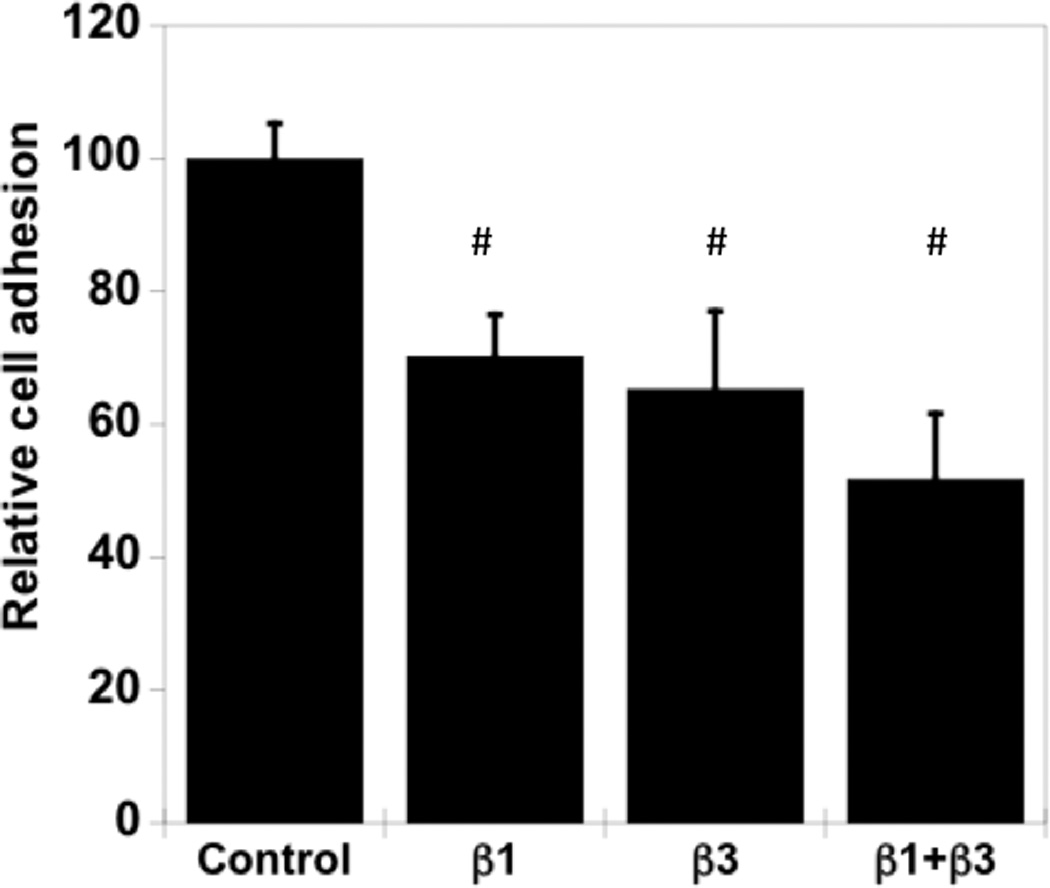
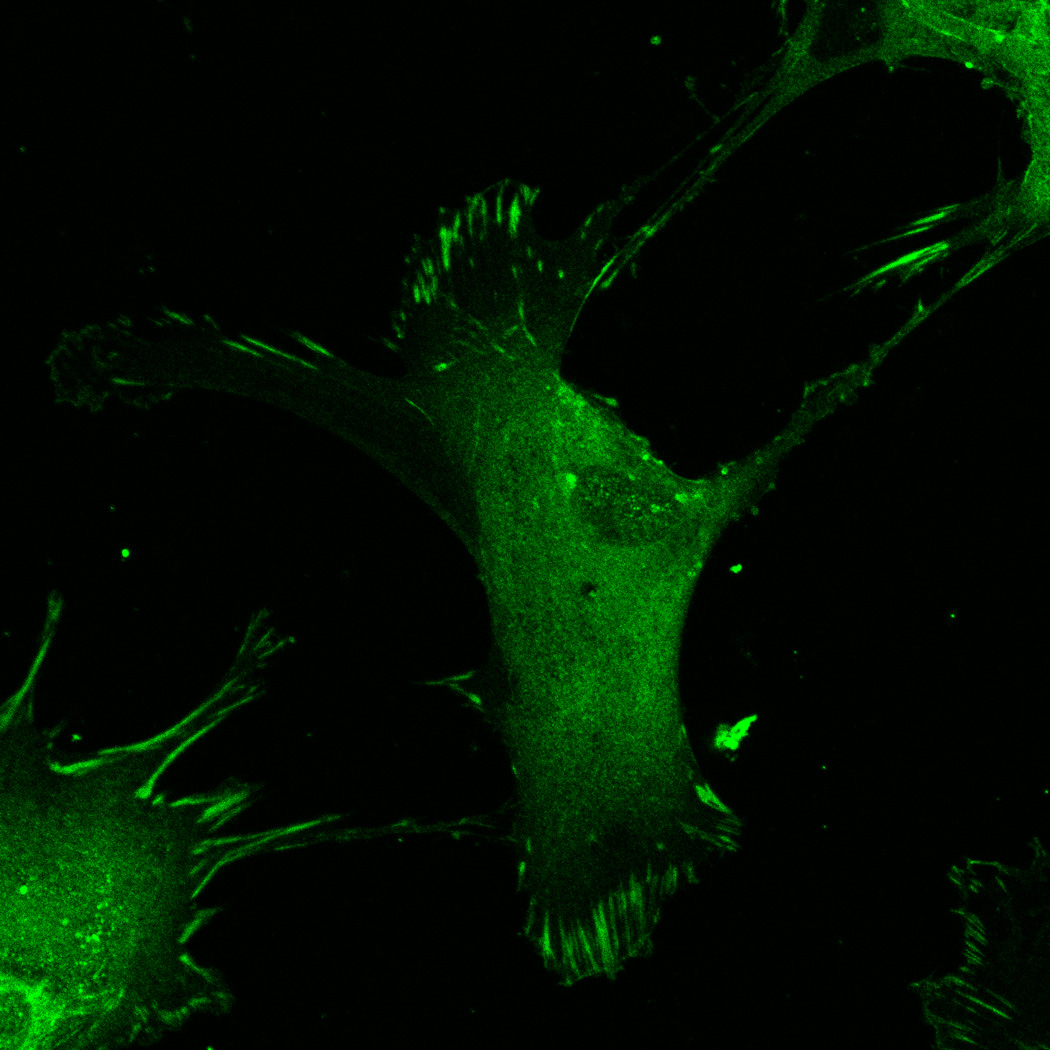
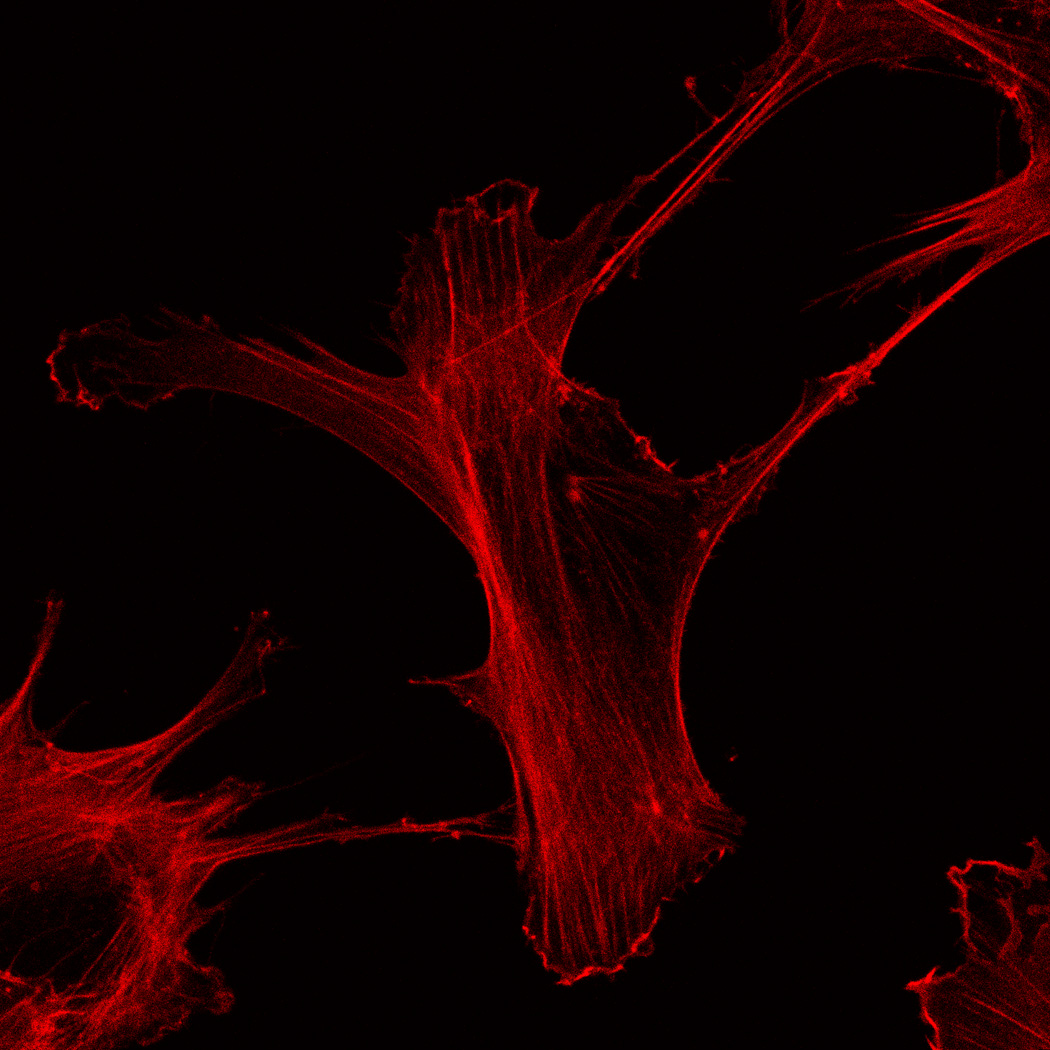
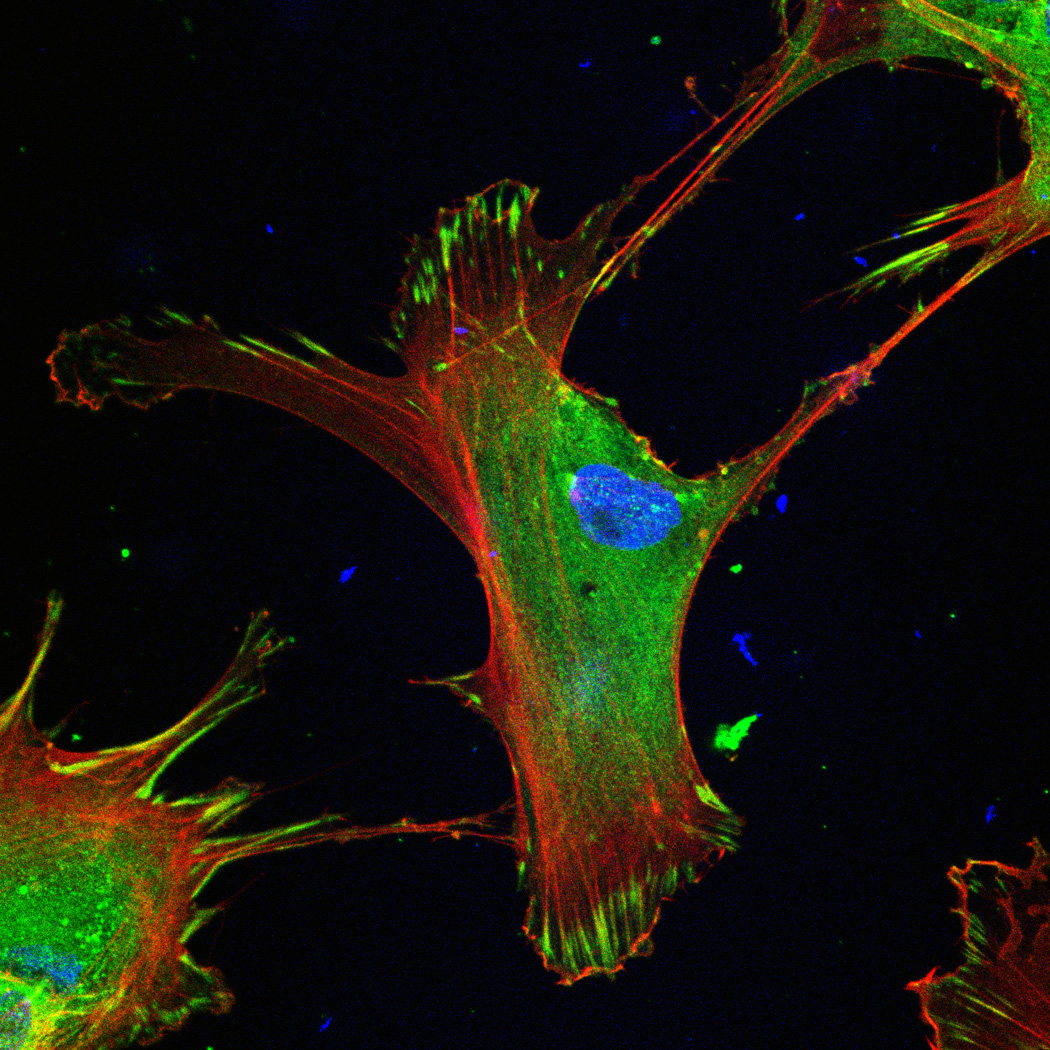
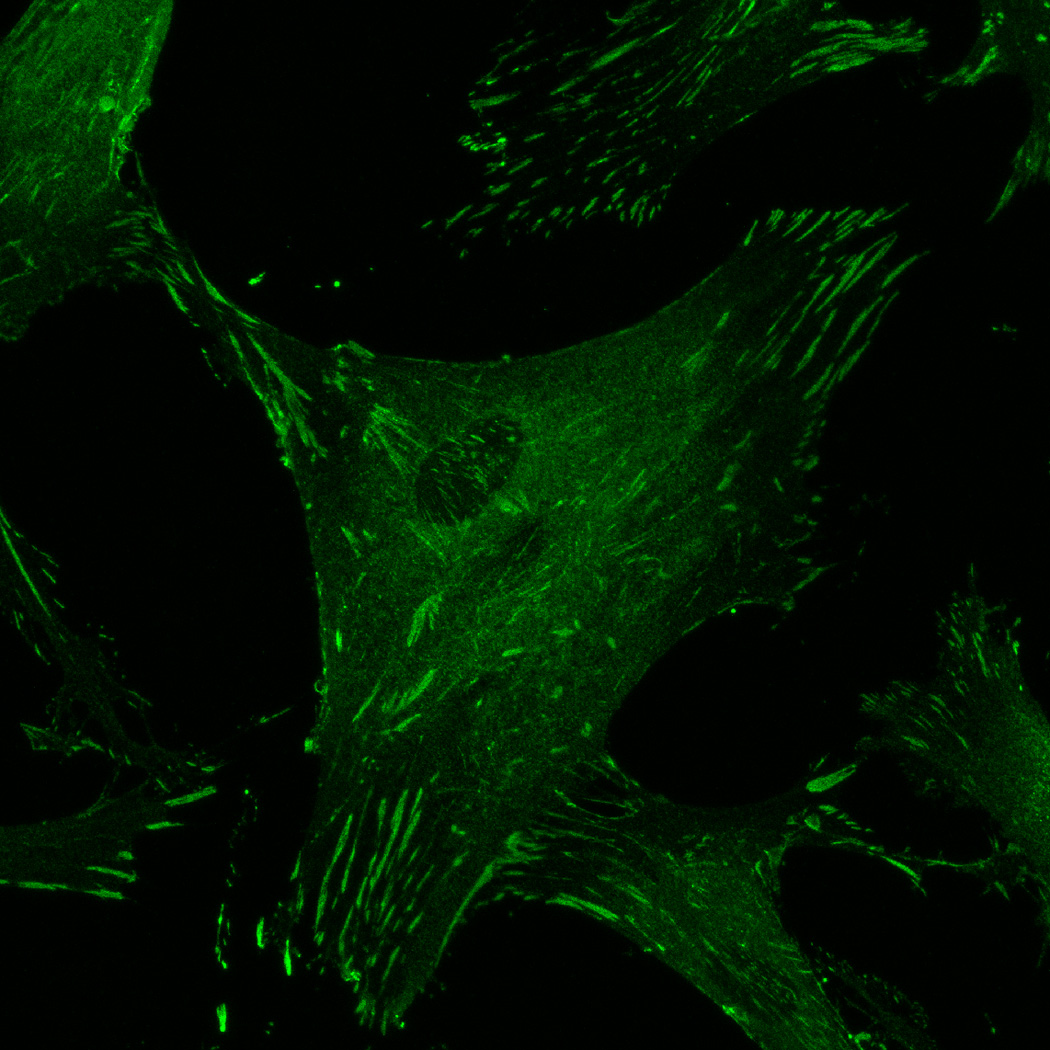
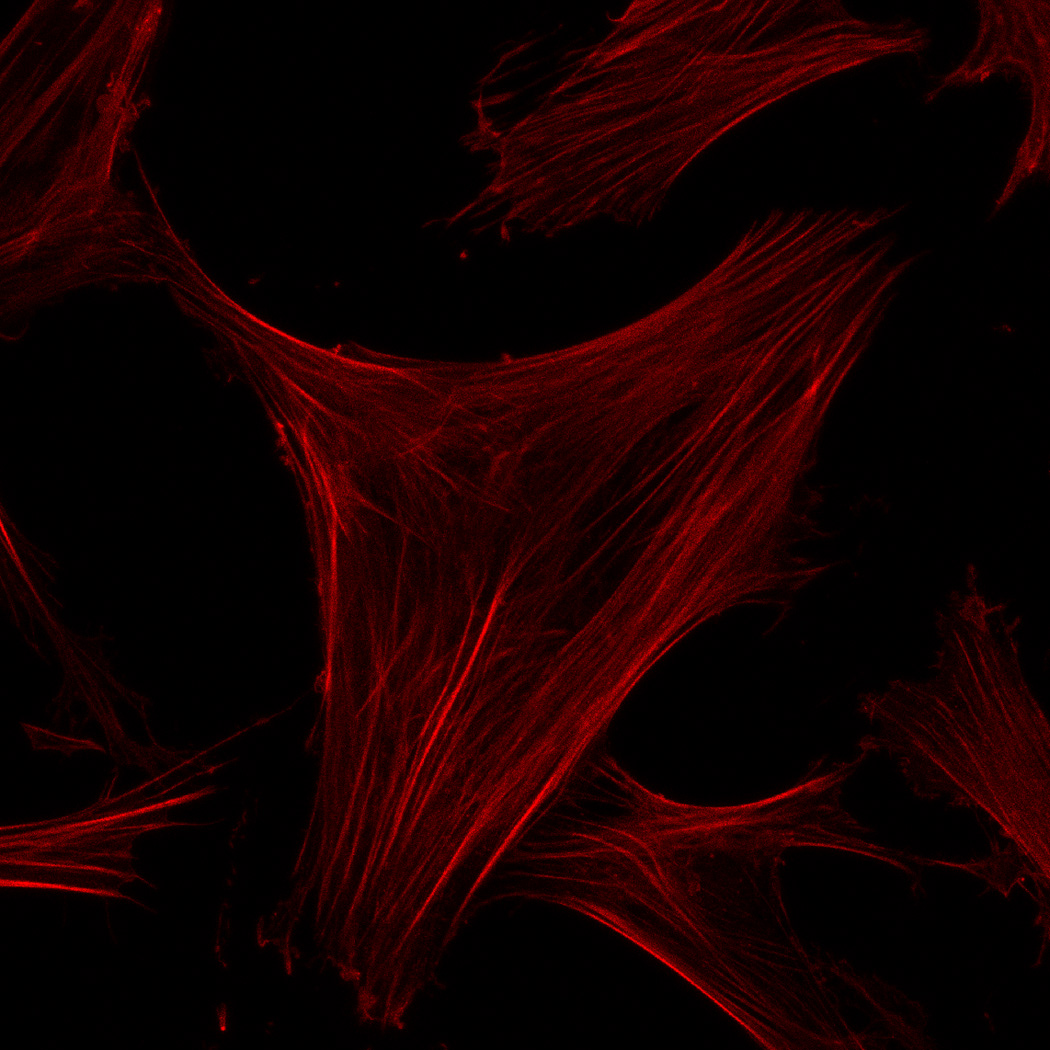
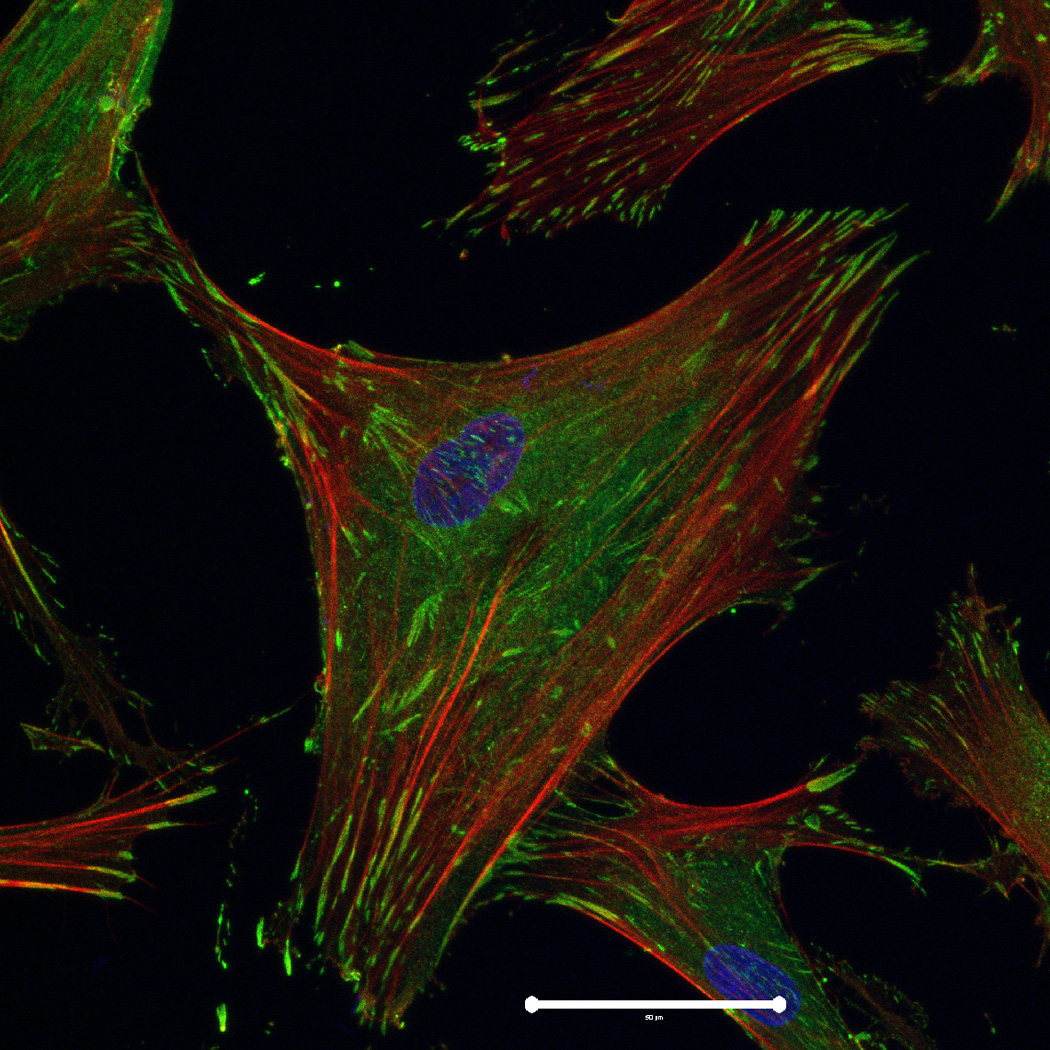
hMSC interaction with the PO4-PEG gels ([PO4] = 50mM) is mediated through β1 and β3 integrins. (A) Integrin blocking studies. Cells were incubated with β1, β3, β1+ β3 or control (IgG) antibodies for 30 min and seeded onto phosphate gels. Average number of cells attached were counted and reported as a percentage compared to control. Data represent averages of N=3 different experiments with 3 replicates in each experiment. (B–I). Immunostaining for vinculin (green, B, F), actin (red, C, G), nuclei (blue, D, H) and overlay (E, I) of hMSCs seeded on PO4-PEG gels (B–E) and on TCPS (F–I) shows the presence of well-developed focal adhesion plaques. (Scale bar = 50µm). # indicates significant difference compared to control, p<=0.05.
3.3 Focal Adhesion Kinase (FAK) signaling is required for PO4-PEG hydrogel induced osteogenesis
FAK induction of the MAP kinase pathway has been shown to be a critical component in osteogenesis due to activation of CBFA-1 transcription factors [27]. Upon integrin binding and clustering, focal adhesions facilitate the recruitment and phosphorylation of FAK, which subsequently induces a cascade of downstream signaling events that ultimately control cell fate, including osteogenic differentiation of hMSCs when stimulated in conjunction with other signals found in osteogenic supplements [30]. Our results indicated that hMSC attachment to PO4-PEG hydrogels involved integrin binding to adsorbed ECM proteins. Figure 4B–I shows representative images of vinculin (Figure 4B,F) and actin (Figure 4C,G) staining of hMSCs on PO4-PEG gels in growth media (Figure 4B–E) and on TCPS in OS media (Figure 4F–I). hMSCs seeded on PO4-PEG hydrogels expressed vinculin, as well-defined fibrillar structures (10–20 µm) that were consistent with focal complexes and well developed actin stress fibers. Qualitatively, the number of fibrillar structures of vinculin and amount of actin stress fibers in hMSCs was lower on PO4-PEG gels compared to that on TCPS, indicating that the interaction of hMSCs with the adsorbed protein layer is different in both conditions. We therefore hypothesized that PO4-PEG hydrogels induced osteogenic differentiation of hMSCs through integrin recruitment of focal complexes and subsequent FAK signaling.
To verify the involvement of FAK signaling in osteogenesis for hMSCs on PO4-PEG gels, we measured the expression of osteogenic markers for cells treated with the small chemical inhibitor PF-573228, which prevents downstream signaling by interrupting phosphorylation of FAK at Tyr397 [37]. hMSCs were cultured on phosphate functionalized gels in growth media or TCPS with OS supplements over a period of 14 days in the absence or presence of PF-573228. The effect of pFAK inhibition on fold change in cell numbers on PO4-PEG gels in growth media and on TCPS in OS media are shown in Figure 5A. The concentration of pFAK inhibitor used did not significantly affect the proliferation of hMSCs on both PO4-PEG and TCPS throughout the time scale of the study. ALP activity (Figure 5B), an early osteogenic marker, at days 1, 7, and 14, or gene expression levels of Coll-1A, CBFA1, osteopontin (OPN) (Figure 6) at day 14 of hMSCs were studied. ALP activity of hMSCs cultured on phosphate functionalized gels and TCPS was increased by 2.5 and 4 fold by day 7, respectively, compared to day 1. Inhibition of phosphorylation of FAK abrogated the increase in ALP activity of hMSCs cultured on both phosphate functionalized gels and TCPS at day 7 and day 14. At day 7, ALP was increased for both TCP and PO4-PEG hydrogels, an effect that was abrogated by treatment with FAK inhibitor. While TCP had elevated expression of ALP at day 14 that was prevented by FAK inhibitor, there was no difference observed for PO4-PEG hydrogels.
Figure 5.
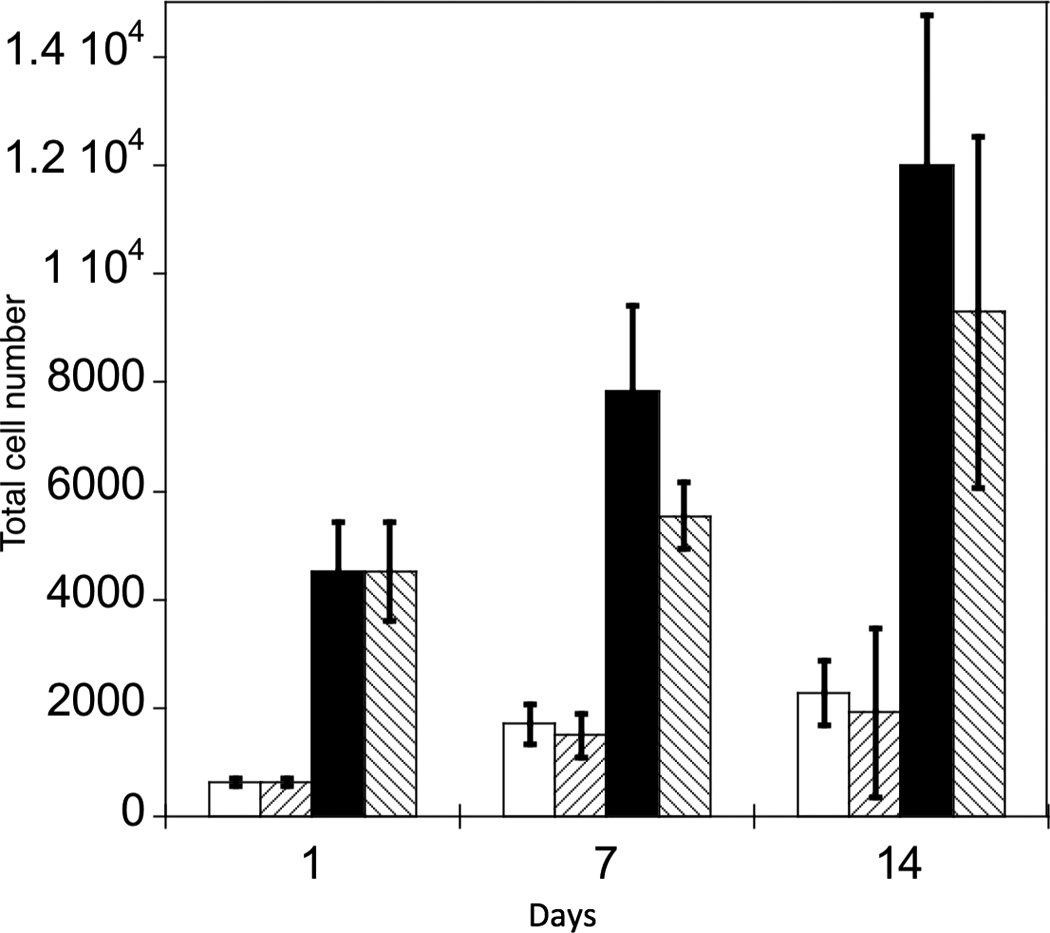
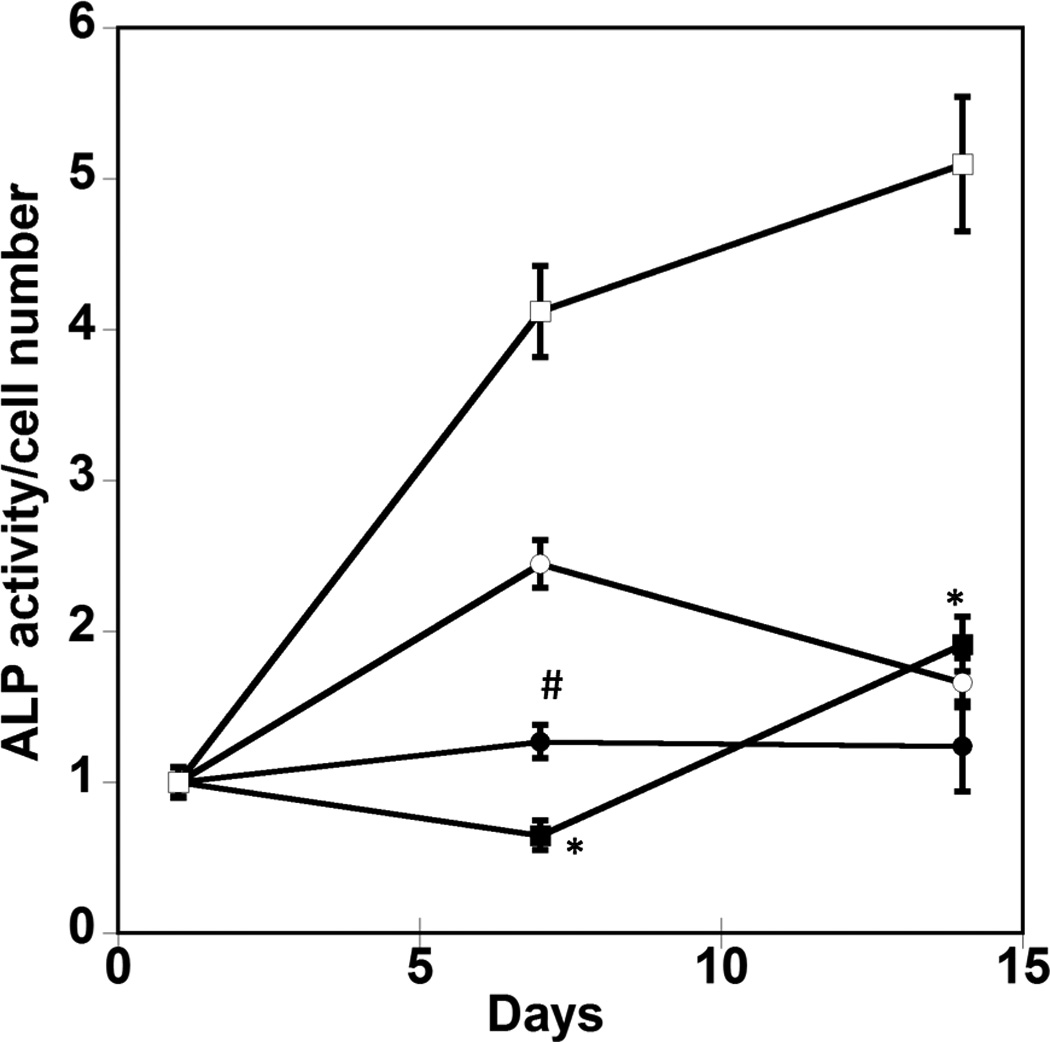
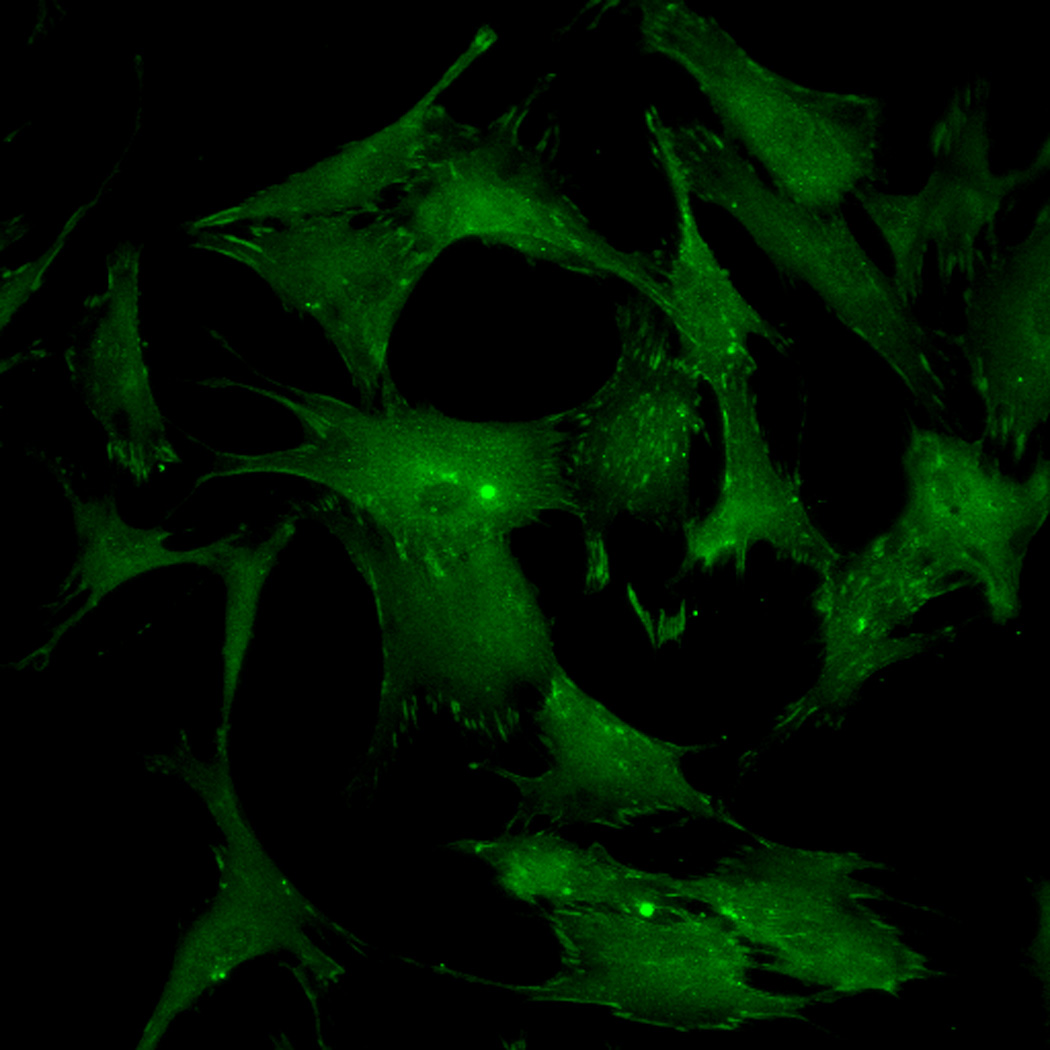
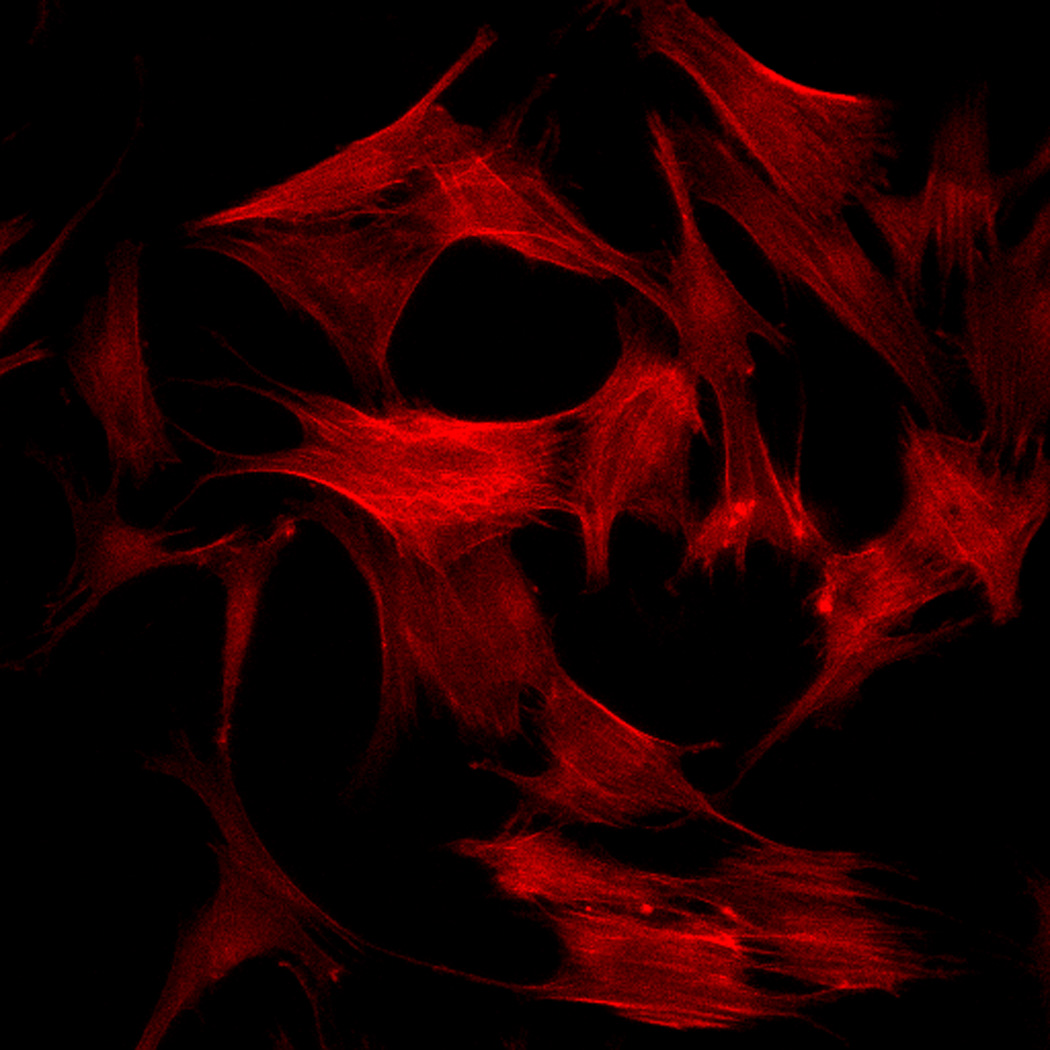
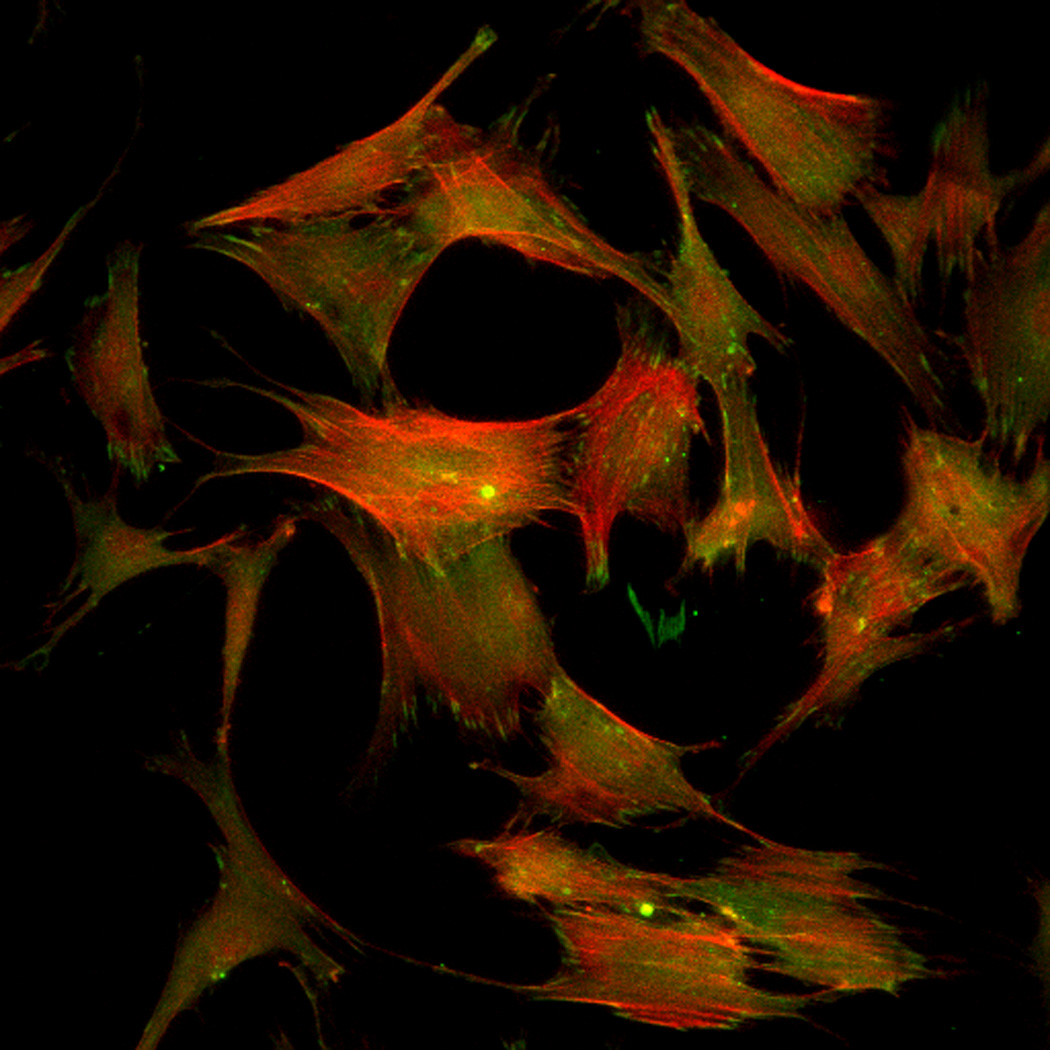
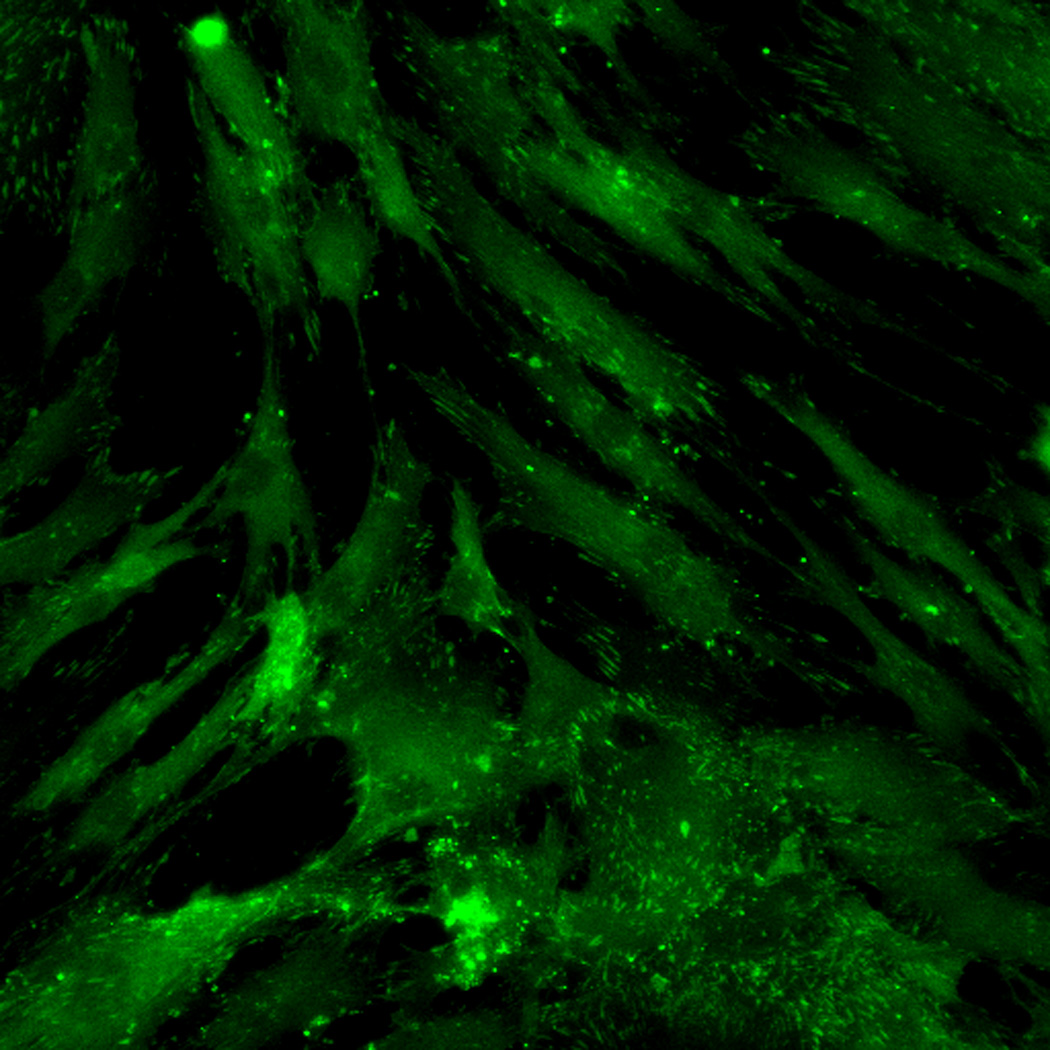
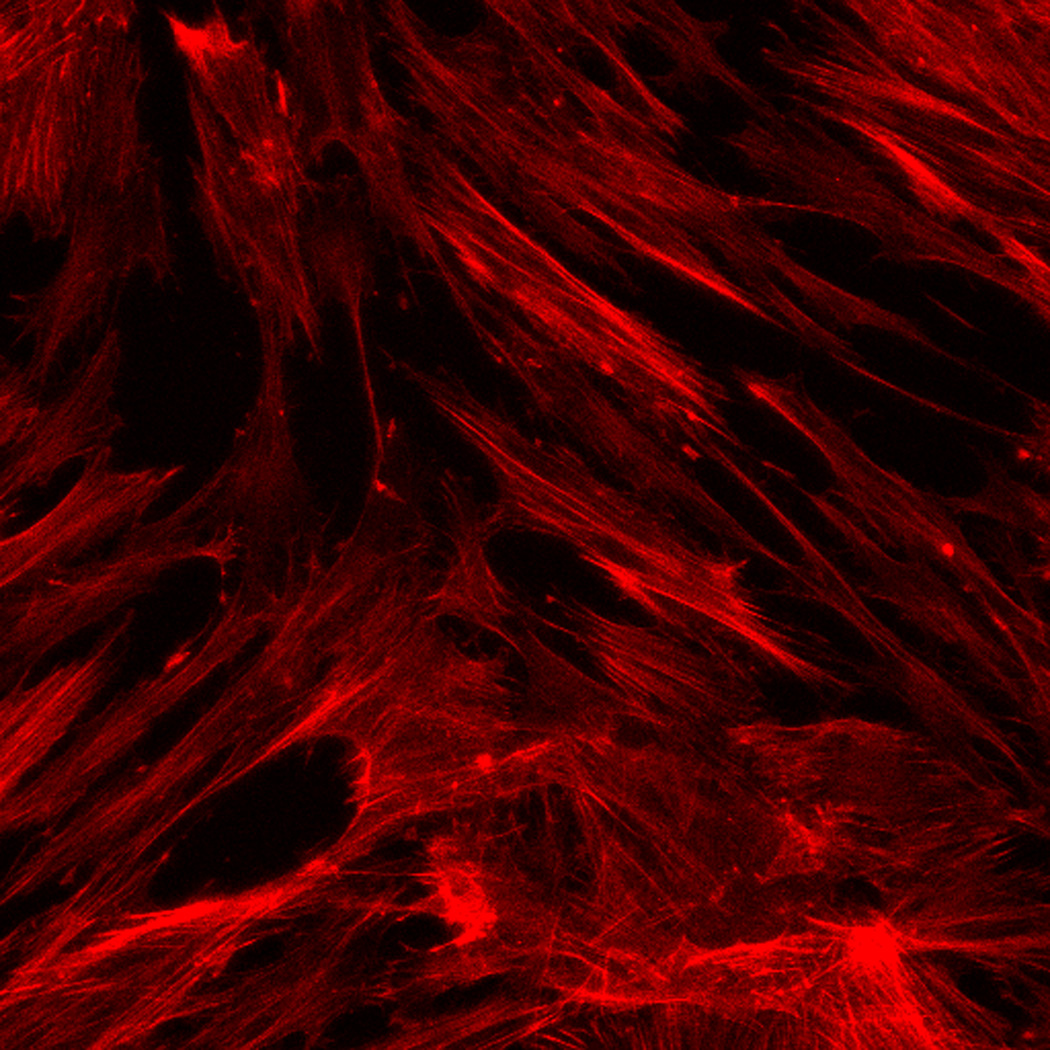
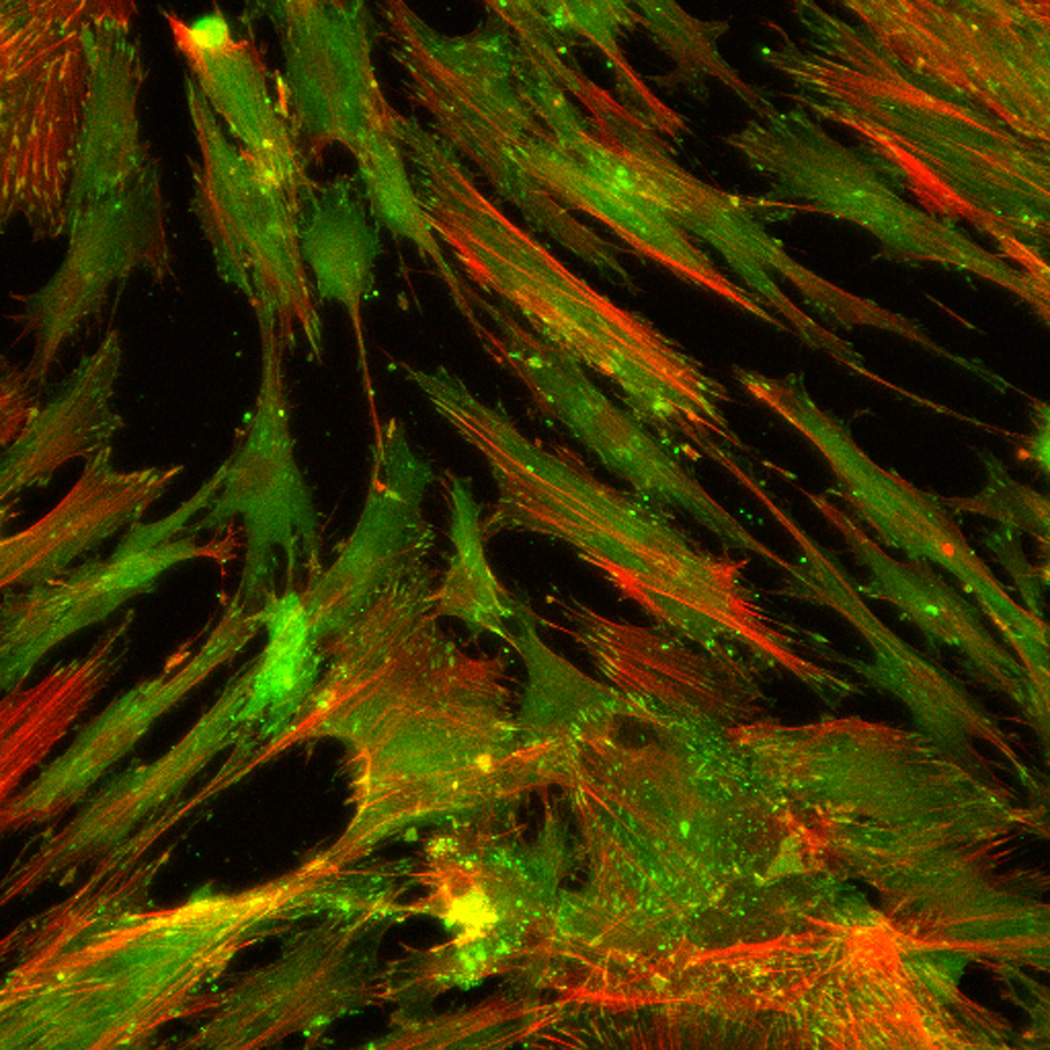
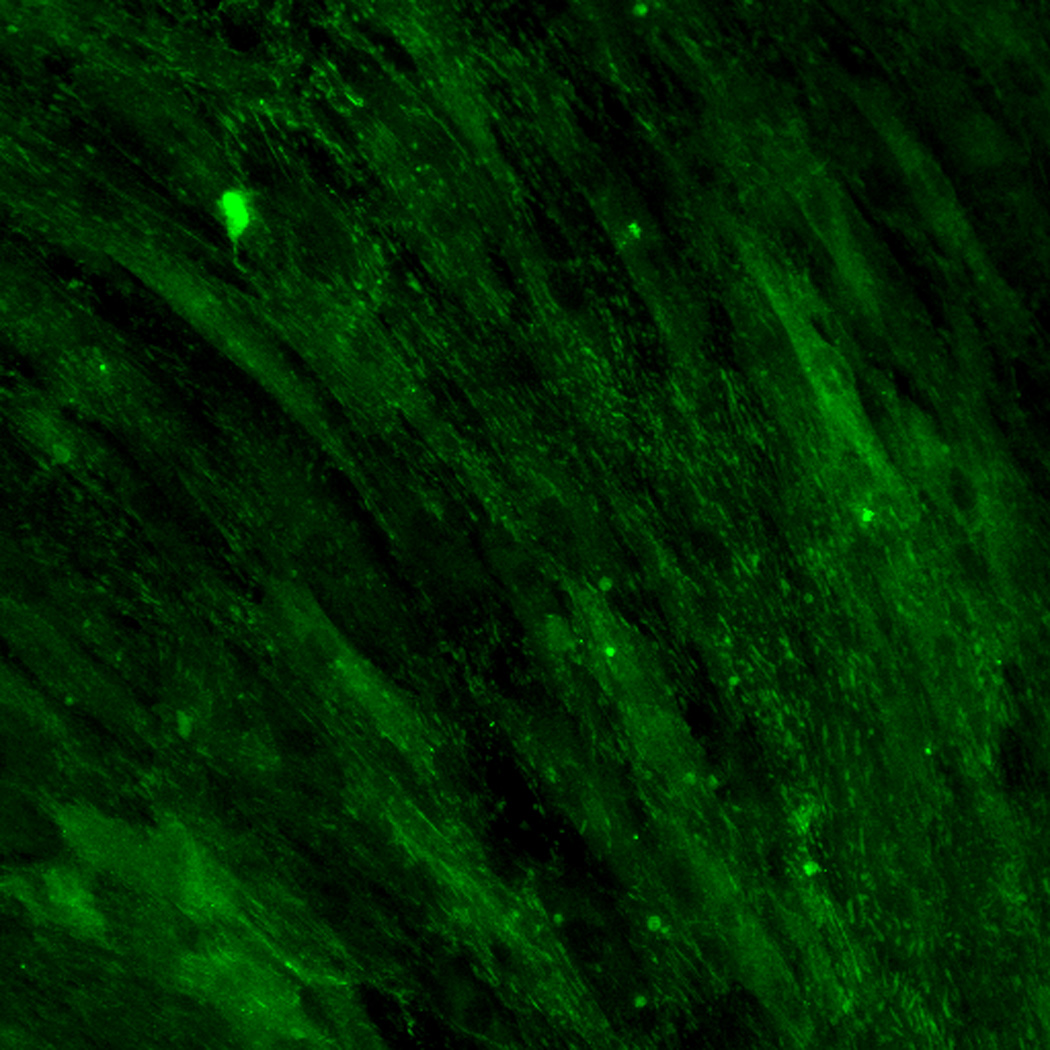
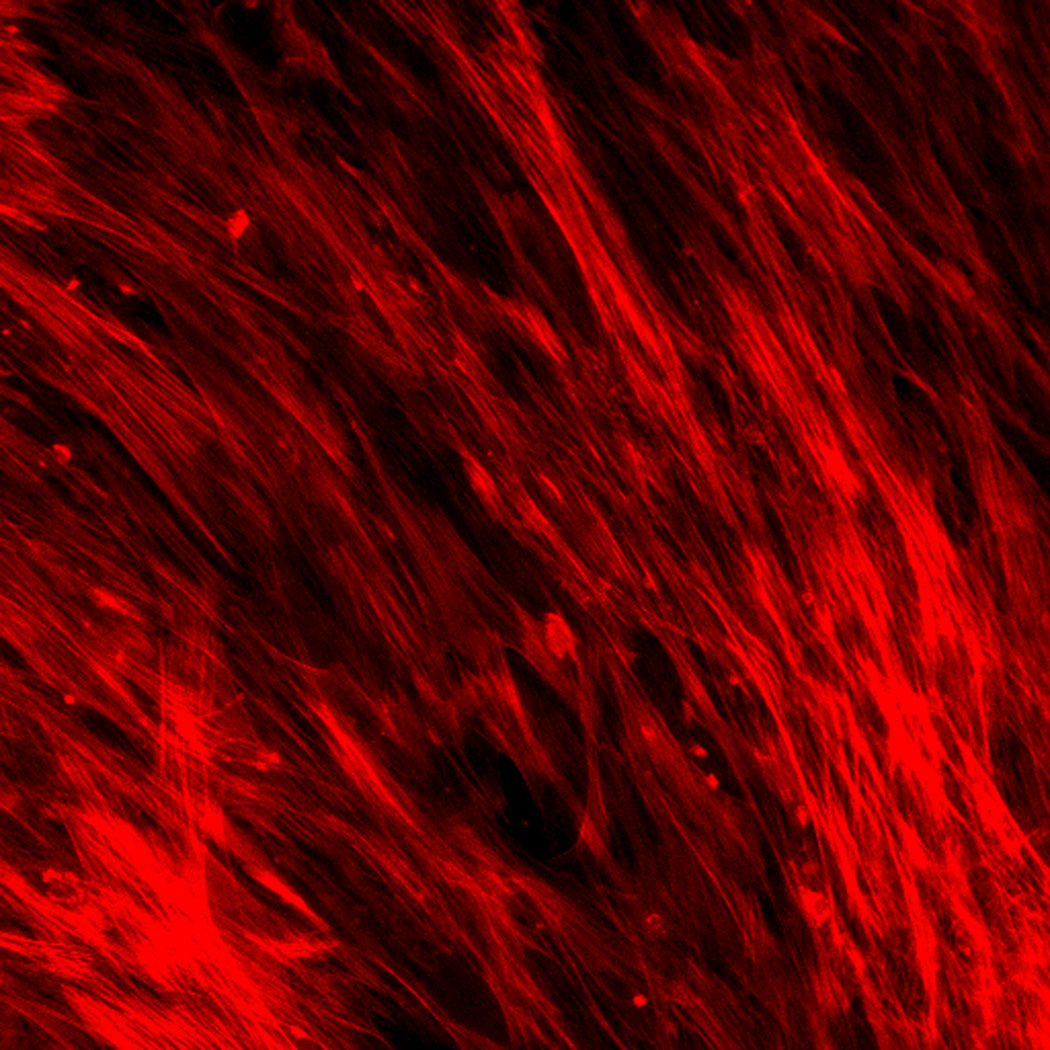
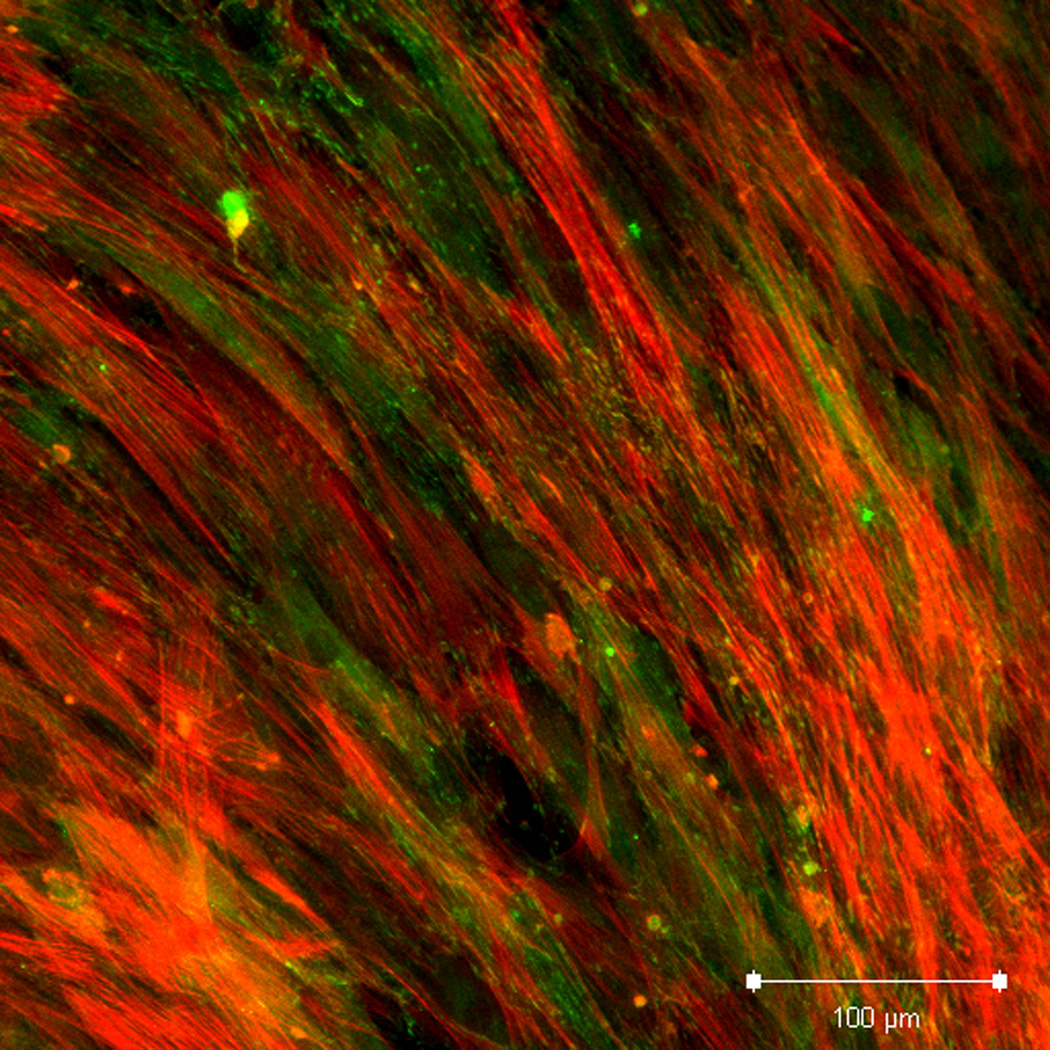
Effect of inhibition of FAK phosphorylation on ALP activity of hMSCs. (A) Total cell number of hMCSs on PO4-PEG gels ([PO4] = 50mM) in growth media with (45° striped bar) and without (white bar) pFAK inhibitor. Total cell number of hMSCs on TCPS in OS media with (135° striped bar) and without (black bar) pFAK inhibitor. (B) ALP activity profiles of hMSCs cultured on PO4-PEG gels ([PO4] = 50mM) in growth media with (filled circles) or without (open circles) PF-573228 normalized to cell number. Positive control cultures of hMSCs on TCPS with osteogenic supplements (OS) and with (filled squares) or without (open squares) PF-573228 are also shown. # indicates significant difference compared to hMSC cultures on PO4-PEG gel without pFAK inhibitor, (*) indicates significant difference compared to hMSC cultures on TCPS with OS. Data represent averages of normalized ALP activity of N=3 different experiments with n=3 replicates (C–K). Addition of pFAK inhibitor, PF-573228, doesn’t affect the formation of focal adhesion plaques. Immunostained images for vinculin (green, C,F,I), actin (red, D,G,J), overlay (E,H,K) of hMSCs at day1 (C–E), day 7 (F–H), day 14 (I–K) cultured on PO4-PEG constructs in the presence of FAK inhibitor. Vinculin staining shows the presence of well-developed focal adhesion plaques upon inhibiting phosphorylation of FAK. (Scale bar= 100µm)
Figure 6.
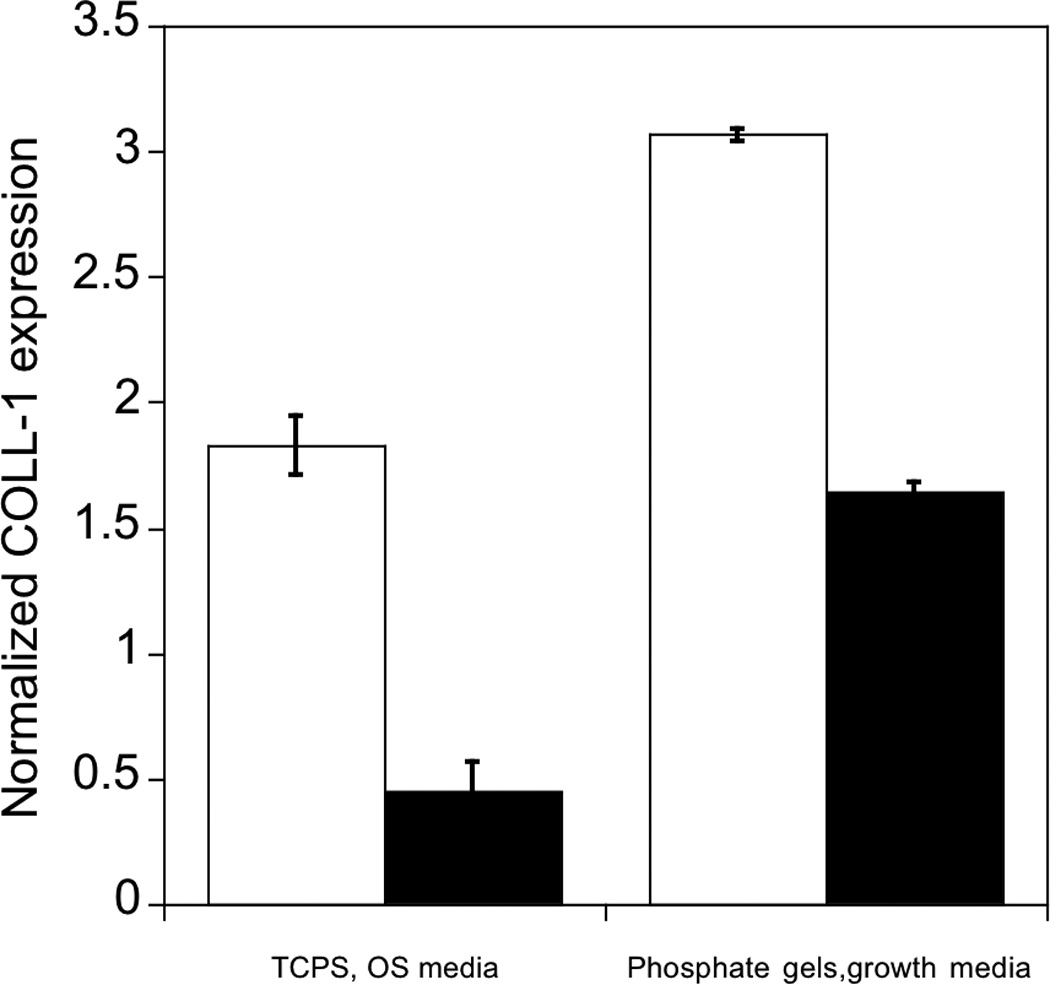
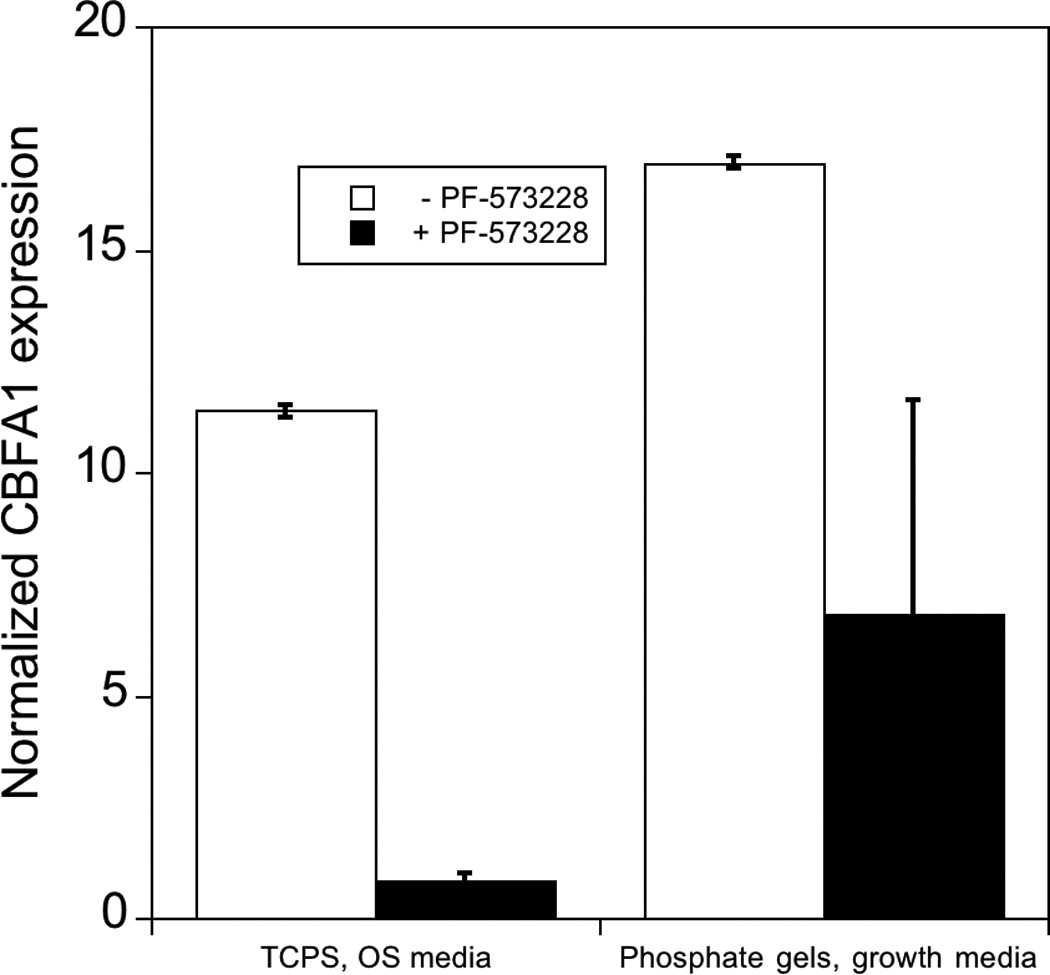
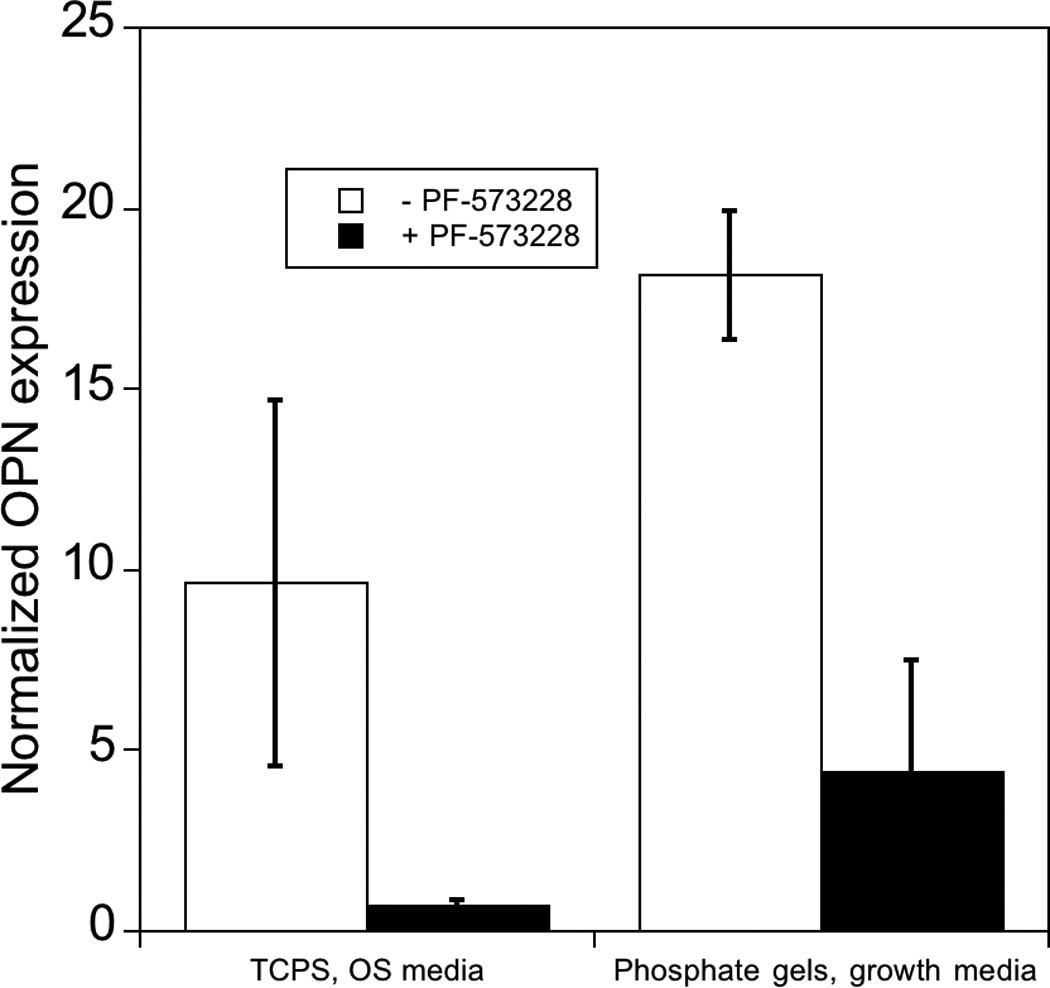
Effect of inhibition of phosphorylation of FAK on expression of osteogenic related genes in hMSCs. hMSCs cultured on PO4-PEG gels ([PO4] = 50mM) in growth media on TCPS in OS media in the presence (black bars) or absence (white bars) of FAK inhibitor (PF-573228). All data are normalized to GAPDH gene and further normalized to a corresponding TCPS, growth media control. Data are shown for OS genes, a) Collagen-1 (COLL-1), b) CBFA1, c) Osteopontin (OPN). # indicates significant difference compared to cultures that did not receive pFAK inhibitor p<=0.05. Data represent fold increase averaged over N=3 biological replicates with n=2 technical replicates of each.
As with ALP activity, FAK inhibited several genes associated with osteogenesis. Specifically, gene expression of OPN, Coll-1 and CBFA1 were increased by 18, 3, and 17 fold respectively on phosphate functionalized gels cultured in growth media and by 9, 1.7, 10 fold respectively on TCPS cultured with OS supplements by day 14, consistent with osteogenic differentiation. However, when treated with FAK inhibitor, expression was reduced significantly compared to controls for all the genes studied. Coll-1 expression was reduced to 1.5 fold, while CBFA1 and OPN expression reduced to 6 and 4 fold relative to control, respectively. Similarly, FAK inhibition reduced expression of each of these genes on TCPS in the presence of OS media. The effect of pFAK inhibition was not due to changes in shape or focal complex formation, as spreading and focal adhesion expression was similar with or without FAK inhibitor (Figure 5C–K). Therefore, we conclude that FAK signaling is required for osteogenic differentiation for hMSCs seeded on PO4-PEG hydrogels, and we again note that osteogenic supplements are not required to mediate this signaling.
4. DISCUSSION
Biomaterial strategies aimed at harnessing stem cells for regenerative medicine applications typically provide cells with biologically relevant signals to guide cell function, such as differentiation down tissue-specific lineages [38]. Discovery-based approaches have become increasingly prevalent for screening chemical and biological functionality for desired influences on cell function, often without any prior knowledge of potential outcomes [39–43]. While many strategies are aimed towards designing peptide mimics or attaching other biomolecules to mimic physiological signaling mechanisms [38, 44], approaches that minimize complexity have also been pursued [15, 23, 40, 41].
Recently, Benoit et al. demonstrated that small chemical functional groups induced differentiation of hMSCs down several lineages without the use of differentiation media [15]. Several functional groups were chosen to mimic chemical characteristics of microenvironments associated with common hMSC lineages and screened at different concentrations using a 2D array-based approach, and it was discovered that osteogenic differentiation was induced by PO4 groups while adipogenesis was induced by t-butyl groups. Results were then validated in 3D to demonstrate that the chemical functional groups, and not cell shape, were the guiding influence for differentiation. The 3D results were crucial for demonstrating the specific role of the functionalized hydrogels, since cell shape has been shown to be an important factor for directing hMSC differentiation, with osteogenic differentiation being preferential for spread morphologies while rounded morphologies often favors adipogenic differentiation, even when exposed to the same soluble components in the media [45, 46]. Benoit et al. [15] have shown that PO4-PEG gels promoted a spread morphology and t-butyl functional groups promoted a rounded morphology, consistent with the previous studies on the effects of cell shape on hMSC differentiation pathway. However, while it was definitively shown that the functionalized hydrogels specifically induced hMSC differentiation, it was not determined if the mechanism was due to direct interactions with the functional groups or proteins preferentially adsorbed or presented by the different chemical environments. Therefore, we aimed to gain a better understanding of how small chemical functional groups were able to direct specific differentiation of hMSCs down the osteogenic lineage in an effort to guide more rational choices for future biomaterials designs.
First, we aimed to determine whether attachment and spreading of hMSCs on PO4-PEG hydrogels was due to direct interactions with phosphate groups or due to adsorbed serum proteins. Our results indicated that hMSCs did not attach to PO4-PEG hydrogels when seeded in serum free medium. However, hMSC attachment and spreading on gels that were pre-incubated in serum containing media prior to seeding with cells in serum free media was similar to seeding in serum. As a control, very low hMSC attachment was observed on blank PEG gels (i.e., unfunctionalized) for serum-free conditions under all treatments. Since attachment occurred on PO4-PEG hydrogels incubated in serum media prior to seeding under serum-free conditions, soluble components were not required to mediate attachment. Thus, our combined results demonstrated that adsorbed serum components, and not phosphate groups alone, mediated hMSC attachment to PEG-PO4 hydrogels. Interestingly, the cell seeding efficiency on PO4-PEG gels (~600 cells/cm2 when seeded at 5000 cells/cm2) is lower compared to that usually observed on TCPS (90–95%). We note that this cell lower density is often observed when seeding cells on PEG hydrogels, and a potential complication is that one might enrich for a particular subpopulation of hMSCs that are more adherent to these gels. Since hMSCs are a heterogeneous population [47, 48], this subset might be more predisposed to undergo osteogenic differentiation in growth media. It is difficult to de-convolute the effects of preferential selection of an hMSC population from the effects of the phosphate groups on osteogenic differentiation, but this is a point worthy of further investigation.
Next, we have quantified the amount of serum proteins adsorbed onto PO4-PEG gels. Our results show that presence of PO4 functionalities promote protein adsorption significantly compared to the control PEG gel. Although the exact cause of this protein adsorption is not clear from this study, incorporation of charged PO4 functional groups could result in altered zeta potential around the PEG gel compared to the control gels resulting in strong protein adsorption. This hypothesis is supported by finding of a recent study by Meder et al. [49], in which alumina particles functionalized with PO4 functional groups resulted in negative zeta potential and preferentially adsorbed positively charged proteins. These results suggest that electrostatic forces play an important role in determining the protein adsorption to these particles. Interestingly, PO4 concentration did not have any effect on level of protein adsorption to PO4-PEG gels. This observation could be explained by considering the network structure of polymers formed by chain polymerization of PEGDM monomers. Here, PO4 functionalities are incorporated only in the kinetic chains and results in a network structure with dense regions of PO4 functionalities connected by long PEG chains [50]. This is also supported by our observation that the swelling ratio of the gels is also unchanged with increasing PO4 concentration (data not shown). The amount of protein adsorbed onto TCPS (which is used to expand hMSCs) is significantly higher than PO4-PEG gels. This suggests that the PO4-PEG gels could preferentially adsorb specific ECM proteins (compared to proteins adsorbed on TCPS) that signal hMSCs to differentiate down the osteogenic pathway.
Several studies have shown that the identity and structural orientation of adsorbed proteins are strongly dependent on the biophysical and biochemical aspects of the biomaterial [24, 28, 51–54], leading to different influences on cell behavior even when the same protein is present [28, 29, 51, 55]. For example, Keselowsky et al. [28, 29] showed that substrate chemistry controlled the presentation of adhesion sites present on adsorbed fibronectin and hence influenced the differentiation of osteoblasts. Other studies have shown that interaction of hMSCs with ECM proteins, Coll-1 and FN, influenced the matrix mineralization, expression of osteogenic genes and ALP activity of in vitro hMSC cultures [26, 27, 41, 45, 46]. For example, Salasznyk et al. [30] demonstrated that hMSCs cultured on Coll-1 up-regulated ALP activity and secretion of osteopontin without any need for osteogenic supplements. Since, Coll-1 and FN were each present on PO4-PEG hydrogels, suggesting a similar possible role for these ECM components here. Previously, published results on TCPS also show that coll-1 and FN can upregulate expression of osteogenic signal in hMSCs. To complement the results presented here, further studies exploring how osteogenic differentiation is affected by blocking adhesion sites of coll-1 and FN might provide insight as to the role of these proteins in osteogenic differentiation of hMSCs on PO4-PEG gels.
Osteogenesis is influenced by the ECM due to attachment that is mediated by integrins, with β1- and β3-integrins having each been shown to be important regulating osteogenic differentiation of hMSCs [31]. Interaction of hMSCs with Coll-1 occurs via β1 integrins and has been shown to be critical for their osteogenic differentiation when cultured on Coll-1. Also, hMSC interaction with FN via α5β1 integrins has been shown to enhance osteogenic related genes CBFA1, bone sialoprotein and ALP. hMSC adhesion to FN functionalized surfaces mediated by β3 integrins has been shown to negatively influence growth and differentiation of hMSCs [35, 31]. We demonstrated that hMSC attachment was decreased on PO4-PEG hydrogels when either β1- or β3-integrins were blocked with functional antibodies, demonstrating that β1- and β3-integrins can each mediate attachment and spreading.
Outside-in signaling through integrin binding is due to the recruitment of focal complexes, which are required for phosphorylation of FAK [58]. Phosphorylated FAK then triggers downstream signaling through the MAP kinase pathway, which ultimately controls expression of osteogenic transcription factors that are crucial for guiding hMSC differentiation down the osteogenic lineage [30, 36]. Inhibition of FAK led to significant down-regulation of each of the osteogenic markers studied, which included alkaline phosphatase activity and gene expression for CBFA1, osteopontin, and Coll-1A. The influence of FAK inhibition was not due to changes in cell shape or focal adhesions, as hMSCs showed similar morphology on PO4-PEG gels and continued to express vinculin in a fibrillar organization similar to non-inhibited controls. Substrate elasticity has been previously shown to play an important role during osteogenic differentiation of hMSCs [17, 18, 59]. Previous studies have also shown that phosphorylation of FAK is dependent on the underlying substrate elasticity [60, 61]. For example, Khatiwala et al. [60] have shown that levels of phosphorylation (Tyr397) of FAK increased with increasing stiffness when pre-osteoblast cells are cultured on poly(acrylamide) substrates. Although not studied in this work, it is possible that substrate elasticity also affects the osteogenic differentiation of hMSCs on PO4-functionalized hydrogels. Thus, we conclude that the influence of PO4-PEG hydrogels on differentiation of hMSCs down the osteogenic lineage requires FAK signaling and that shape or cell attachment alone is not sufficient for inducing this effect.
Previously, Benoit et al. [15] showed (1) PO4-PEG hydrogels induced osteogenesis, while other functional groups with similar charges did not induce similar effects, (2) Incorporation of PO4 functionality was at a low enough concentration to prevent changes in gel hydrophobicity, (3) Shape was not responsible for differentiation, as similar results were obtained for rounded cells in 3D environments [15]. Here, we provided further insight into the mechanism by providing evidence that PO4-PEG hydrogels induce differentiation of hMSCs down the osteogenic lineage through specific outside-in signaling induced by interactions with serum proteins adsorbed onto the PO4-containing matrix. This mechanism is supported by several pieces of evidence that we presented here: (1) Adsorbed serum proteins are required for attachment and spreading, (2) Attachment is partially mediated by β1- and β3-integrins, (3) Cells seeded on PO4-PEG hydrogels form well-defined focal complexes, (4) FAK signaling is required for osteogenic differentiation independent of cell shape. Our results are particularly important for researchers pursuing strategies aimed at simplifying biomaterials design since the influence of small molecules may not be easily predicted. While combinatorial approaches to screen for small molecules are often predicated on little knowledge of how materials may lead to changes in cell function, our results suggest that the dominating influence is likely due to differences in how proteins adsorb to these materials. Therefore, an exciting potential direction for these combinatorial approaches may be to identify specific influences on cell function, and how these materials adsorb and present proteins that are important for these changes. Ultimately, a better understanding of how biomaterials interact with proteins in the soluble environment will be important for both guiding cell function and avoiding potential side effects associated with in vivo application.
5. CONCLUSIONS
In this work we investigated the role of hydrogel chemistry on osteogenic differentiation of hMSCs. We demonstrate that the presence of phosphate functional groups promoted adsorption of ECM proteins, including fibronectin and collagen-1, while hMSC attachment was partially mediated via β1 and β3 integrins. Adsorbed ECM proteins directed hMSCs down the osteogenic lineage despite the absence of osteogenic supplements, as demonstrated by elevated alkaline phosphatase production and up-regulation of classic osteogenic markers. Combined with our previous study [15], we demonstrated that attachment and cell shape alone were insufficient for promoting osteogenesis of hMSCs, but that FAK signaling was necessary for the influence of PO4-PEG hydrogels on differentiation. This study provides evidence that adsorbed serum proteins can play a crucial role for the influence of synthetic biomaterials in guiding cell function, including PEG hydrogels which are designed to prevent non-specific adsorption. While the specific result reported here (PO4-PEG hydrogel induction of osteogenic differentiation for hMSCs) is directly applicable to bone tissue engineering, our work is more generally important due to the broader implications associated with the mechanisms by which functionalized hydrogels guide cell function.
ACKNOWLEDGEMENTS
We wish to thank Dr. Sarah Anderson for helpful discussions in designing the experiments. The authors gratefully acknowledge financial support from National Institute of Health (R01, DE016523) and the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- 3.Friedman MS, Long MW, Hankenson KD. Osteogenic differentiation of human mesenchymal stem cells is regulated by bone morphogenetic protein-6. J Cell Biochem. 2006;98:538–554. doi: 10.1002/jcb.20719. [DOI] [PubMed] [Google Scholar]
- 4.Cheng SL, Yang JW, Rifas L, Zhang SF, Avioli LV. Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone. Endocrinology. 1994;134:277–286. doi: 10.1210/endo.134.1.8275945. [DOI] [PubMed] [Google Scholar]
- 5.Aubin JE. Advances in the osteoblast lineage. Biochemistry and cell biology = Biochimie et biologie cellulaire. 1998;76:899–910. [PubMed] [Google Scholar]
- 6.Dorozhkin SV. Bioceramics of calcium orthophosphates. Biomaterials. 2010;31(7):1465–1485. doi: 10.1016/j.biomaterials.2009.11.050. [DOI] [PubMed] [Google Scholar]
- 7.Oh S, Oh N, Appleford M, Ong JL. Bioceramics for tissue engineering applications – A review. Am. J. Biochem. & Biotechnol. 2006;2(2):49–56. [Google Scholar]
- 8.LeGeros RZ. Calcium Phosphate-based osteoinductive materials. Chem Rev. 2008;108:4742–4753. doi: 10.1021/cr800427g. [DOI] [PubMed] [Google Scholar]
- 9.Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. 2006;27(18):3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 10.Holland TA, Mikos AG. Review:Biodegradable polymeric scaffolds. Improvements in bone tissue engineering through controlled drug delivery. Adv Biochem Engin/Biotechnol. 2006;102:161–185. doi: 10.1007/b137205. [DOI] [PubMed] [Google Scholar]
- 11.Lin CC, Anseth KS. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. 2009;26(3):631–643. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31(17):4639–4656. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang F, Williams CG, Wang DA, Lee H, Manson PN, Elisseff J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials. 2005;26(30):5991–5981. doi: 10.1016/j.biomaterials.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 14.He X, Ma J, Jabbari E. Effect of grafting RGD and BMP-2 protein-derived peptides to a hydrogel substrate on osteogenic differentiation of marrow stromal cells. Langmuir. 2008;24:12508–12516. doi: 10.1021/la802447v. [DOI] [PubMed] [Google Scholar]
- 15.Benoit DS, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat Mater. 2008;7:816–823. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller T, et al. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21:513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 17.Khatiwala CB, Kim PD, Peyton SR, Putnam AJ. ECM compliance regulates osteogenesis by influencing MAPK signaling downstream of RhoA and ROCK. J Bone Miner Res. 2009;24(5):886–898. doi: 10.1359/JBMR.081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parekh SH, Chatterjee K, Lin-Gibson S, Moore NM, Cicerone MT, Young MF, Simon CG., Jr Modulus-driven differentiation of marrow stromal cells in 3D scaffolds that is independent of myosin-based cytoskeleton tension. Biomaterials. 2011;32(9):2256–2264. doi: 10.1016/j.biomaterials.2010.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curran JM, Chen R, Hunt JA. Controlling the phenotype and function of mesenchymal stem cells in vitro by adhesion to silane-modified clean glass surfaces. Biomaterials. 2005;26:7057–7067. doi: 10.1016/j.biomaterials.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Curran JM, Chen R, Hunt JA. The guidance of human mesenchymal stem cell differentiation in vitro by controlled modifications to the cell substrate. Biomaterials. 2006;27:4783–4793. doi: 10.1016/j.biomaterials.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Curran JM, Chen R, Hunt JA. Using silane modified surfaces to control the differentiation pathways of human mesenchymal stem cells. Tissue Engineering. 2006;12:1015. [Google Scholar]
- 22.Phillips JE, Petrie TA, Creighton FP, Garcia AJ. Human mesenchymal stem cell differentiation on self-assembled monolayers presenting different surface chemistries. Acta Biomater. 2010;6:12–20. doi: 10.1016/j.actbio.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nuttelman CR, Tripodi MC, Anseth KS. Synthetic hydrogel niches that promote hMSC viability. Matrix Biology. 2005;24:208–218. doi: 10.1016/j.matbio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Allen LT, Tosetto M, Miller IS, O'Connor DP, Penney SC, Lynch I, et al. Surface-induced changes in protein adsorption and implications for cellular phenotypic responses to surface interaction. Biomaterials. 2006;27:3096–3108. doi: 10.1016/j.biomaterials.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Shen M, Horbett TA. The effects of surface chemistry and adsorbed proteins on monocyte/macrophage adhesion to chemically modified polystyrene surfaces. J Biomed Mater Res. 2001;57:336–345. doi: 10.1002/1097-4636(20011205)57:3<336::aid-jbm1176>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 26.Steele JG, Dalton BA, Johnson G, Underwood PA. Adsorption of fibronectin and vitronectin onto Primaria and tissue culture polystyrene and relationship to the mechanism of initial attachment of human vein endothelial cells and BHK-21 fibroblasts. Biomaterials. 1995;16:1057–1067. doi: 10.1016/0142-9612(95)98901-p. [DOI] [PubMed] [Google Scholar]
- 27.Iuliano DJ, Saavedra SS, Truskey GA. Effect of the conformation and orientation of adsorbed fibronectin on endothelial cell spreading and the strength of adhesion. J Biomed Mater Res. 1993;27:1103–1113. doi: 10.1002/jbm.820270816. [DOI] [PubMed] [Google Scholar]
- 28.Keselowsky BG, Collard DM, Garcia AJ. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J Biomed Mater Res A. 2003;66:247–259. doi: 10.1002/jbm.a.10537. [DOI] [PubMed] [Google Scholar]
- 29.Keselowsky BG, Collard DM, Garcia AJ. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc Natl Acad Sci U S A. 2005;102:5953–5957. doi: 10.1073/pnas.0407356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salasznyk RM, Klees RF, Williams WA, Boskey A, Plopper GE. Focal adhesion kinase signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells. Experimental Cell Research. 2007;313:22–37. doi: 10.1016/j.yexcr.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shekaran A, Garcia AJ. Extracellular matrix-mimetic adhesive biomaterials for bone repair. J Biomed Mater Res A. 2011;96:261–272. doi: 10.1002/jbm.a.32979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizuno M, Fujisawa R, Kuboki Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen- alpha 2 beta 1 integrin interaction. J Cell Physiol. 2000;184:207–213. doi: 10.1002/1097-4652(200008)184:2<207::AID-JCP8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 33.Mizuno M, Kuboki Y. Osteoblast-related gene expression of bone marrow cells during the osteoblastic differentiation induced by type I collagen. J Biochem. 2001;129:133–138. doi: 10.1093/oxfordjournals.jbchem.a002824. [DOI] [PubMed] [Google Scholar]
- 34.Moursi AM, Globus RK, Damsky CH. Interactions between integrin receptors and fibronectin are required for calvarial osteoblast differentiation in vitro. J Cell Sci. 1997;110(Pt 18):2187–2196. doi: 10.1242/jcs.110.18.2187. [DOI] [PubMed] [Google Scholar]
- 35.Martino MM, Mochizuki M, Rothenfluh DA, Rempel SA, Hubbell JA, Barker TH. Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials. 2009;30:1089–1097. doi: 10.1016/j.biomaterials.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salasznyk RM, Klees RF, Hughlock MK, Plopper GE. ERK signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells on collagen I and vitronectin. Cell Communication and Adhesion. 2004;11:137–153. doi: 10.1080/15419060500242836. [DOI] [PubMed] [Google Scholar]
- 37.Slack-Davis JK, Martin KH, Tilghman RW, Iwanicki M, Ung EJ, Autry C, et al. Cellular characterization of a novel focal adhesion kinase inhibitor. J Biol Chem. 2007;282:14845–14852. doi: 10.1074/jbc.M606695200. [DOI] [PubMed] [Google Scholar]
- 38.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature biotechnology. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 39.Davies MC, Alexander MR, Hook AL, Yang J, Mei Y, Taylor M, et al. High throughput surface characterization: A review of a new tool for screening prospective biomedical material arrays. Journal of drug targeting. 2010;18:741–751. doi: 10.3109/1061186X.2010.521941. [DOI] [PubMed] [Google Scholar]
- 40.Anderson DG, Levenberg S, Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat Biotech. 2004;22:863–866. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 41.Flaim CJ, Teng D, Chien S, Bhatia SN. Combinatorial signaling microenvironments for studying stem cell fate. Stem Cells Dev. 2008;17:29–39. doi: 10.1089/scd.2007.0085. [DOI] [PubMed] [Google Scholar]
- 42.Peters A, Brey DM, Burdick JA. High-throughput and combinatorial technologies for tissue engineering applications. Tissue engineering Part B, Reviews. 2009;15:225–239. doi: 10.1089/ten.TEB.2009.0049. [DOI] [PubMed] [Google Scholar]
- 43.Simon CG, Jr, Lin-Gibson S. Combinatorial and high-throughput screening of biomaterials. Adv Mater. 2011;23:369–387. doi: 10.1002/adma.201001763. [DOI] [PubMed] [Google Scholar]
- 44.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8:457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 45.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell Shape, Cytoskeletal Tension, and RhoA Regulate Stem Cell Lineage Commitment. Developmental Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 46.Treiser MD, Yang EH, Gordonov S, Cohen DM, Androulakis IP, Kohn J, et al. Cytoskeleton-based forecasting of stem cell lineage fates. Proceedings of the National Academy of Sciences. 2010;107:610–615. doi: 10.1073/pnas.0909597107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogel W, Grünebach F, Messam CA, Kanz L, Brugger W, Bühring HJ. Heterogeneity among human bone marrow-derived mesenchymal stem cells and neural progenitor cells. Haematologica. 2003;88(2):126–133. [PubMed] [Google Scholar]
- 48.Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32(5):414–425. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Meder F, Daberkow T, Treccani L, Wilhelm M, Schowalter M, Rosenauer A, Madler L, Rezwan K. Protein adsorption on colloidal alumina particles functionalized with amino, carboxyl, sufonate and phosphate groups. Acta Biomat. 2012;8(3):1221–1229. doi: 10.1016/j.actbio.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 50.Lin-Gibson S, Jones RL, Washburn NR, Horkay F. Structure-Property relationships of photopolymerizable poly(ethylene glycol) dimethacrylate hydrogels. Macromolecules. 2005;38:2897–2902. [Google Scholar]
- 51.Guerra NB, Gonzalez-Garcia C, Llopis V, Rodriguez-Hernandez JC, Moratal D, Rico P, et al. Subtle variations in polymer chemistry modulate substrate stiffness and fibronectin activity. Soft Matter. 2010:6. [Google Scholar]
- 52.Rowlands AS, George PA, Cooper-White JJ. Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation. Am J Physiol Cell Physiol. 2008;295:C1037–C1044. doi: 10.1152/ajpcell.67.2008. [DOI] [PubMed] [Google Scholar]
- 53.Elliott JT, Woodward JT, Umarji A, Mei Y, Tona A. The effect of surface chemistry on the formation of thin films of native fibrillar collagen. Biomaterials. 2007;28:576–585. doi: 10.1016/j.biomaterials.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 54.Sherratt MJ, Bax DV, Chaudhry SS, Hodson N, Lu JR, Saravanapavan P, et al. Substrate chemistry influences the morphology and biological function of adsorbed extracellular matrix assemblies. Biomaterials. 2005;26:7192–7206. doi: 10.1016/j.biomaterials.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 55.Keselowsky BG, Collard DM, Garcia AJ. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials. 2004;25:5947–5954. doi: 10.1016/j.biomaterials.2004.01.062. [DOI] [PubMed] [Google Scholar]
- 56.Popov C, Radic T, Haasters F, Prall WC, Aszodi A, Gullberg D, et al. Integrins [alpha]2[beta]1 and [alpha]11[beta]1 regulate the survival of mesenchymal stem cells on collagen I. Cell Death and Dis. 2011;2:e186. doi: 10.1038/cddis.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lund AW, Stegemann JP, Plopper GE. Inhibition of ERK promotes collagen gel compaction and fibrillogenesis to amplify the osteogenesis of human mesenchymal stem cells in three-dimensional collagen I culture. Stem Cells Dev. 2009;18:331–341. doi: 10.1089/scd.2008.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boudreau NJ, Jones PL. Extracellular matrix and integrin signalling: the shape of things to come. Biochem J. 1999;339:481–488. [PMC free article] [PubMed] [Google Scholar]
- 59.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 60.Khatiwala CB, Peyton SR, Putnam AJ. Intrinsic mechanical properties of the extracellular matrix affect the behavior of pre-osteoblastic MC3T3-E1 cells. Am J Physiol Cell Physiol. 2006;290:C1640–C1650. doi: 10.1152/ajpcell.00455.2005. [DOI] [PubMed] [Google Scholar]
- 61.Friedland JC, Lee MH, Boettinger D. Mechanically activated integrin switch controls a5b1 function. Science. 2009;323:642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]


