Abstract
Atherosclerosis is a peculiar form of inflammation triggered by cholesterol-rich lipoproteins and other noxious factors such as cigarette smoke, diabetes mellitus, and hypertension. Genetics also play an important role in the disease, accounting for about 40% of the risk. Of surprise in recent years of post-human genome sequencing, atherosclerosis-relevant genes discovered by non-biased techniques (ie, genome-wide association studies), did not rehash previously suspected pathways of lipid metabolism, diabetes, or hypertension. Instead these studies highlighted genes relevant to mechanisms of inflammation and stem cell biology. Only a minority of implicated genes were linked to lipid and other cardiac risk factor genes. Although such findings do not contradict the fact that atherosclerosis is triggered and exacerbated by elevated lipids, atherosclerosis “new genes” suggest that the mechanism responsible for the development of arterial lesions is more complex than a simple response to injury, where injury is necessary, but perhaps not sufficient, for disease progression.
Keywords: atherosclerosis, inflammation, genetics, stem cells
Introduction: Concept of Arterial Injury versus Repair
Mendelian traits such as familial hypercholesterolemia point toward injury as an important aspect of the mechanism of arterial lesion formation [1]. However, the timing and extent of lesion formation for situations of rather extreme cholesterol level seem to vary from individual to individual. The latter observation is interesting as it suggests that effectiveness of the response to injury may be as important as, if not more important than, the injury itself [2•, 3]. It is also known that individuals with rather benign recognizable risk factors may also be prone to atherosclerosis [4]. Taken together, this information suggests that although we have clearly identified factors that contribute to arterial lesion formation, the molecular and cellular activities at play at the level of the arterial wall exposed to such factors are just as important as, if not more important than, the injury itself.
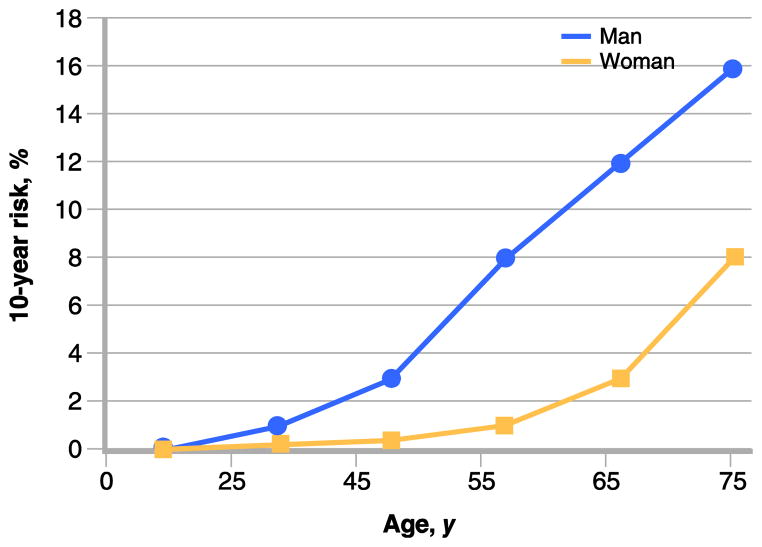
Established algorithms like the Framingham risk calculator [5] allow for an accurate measurement of the probability of an individual to develop a cardiac event over a period of time. This calculation takes into account established risk factors, both modifiable risks like smoking or cholesterol level, and non-modifiable risks like gender. An example of such risk calculation versus age is shown in (Fig. 1). One variable is included in the risk calculation that weighs more heavily than any other modifier, and that is the age of the individual. Keeping all variables constant, one can derive a risk that varies by more than two orders of magnitude by just changing age from 20 to 80 years. Yet the mechanism that accounts for the dominant effect of age on susceptibility for cardiac events had remained unknown. Although it is known that age can impact and worsen some of the variables that define risk, epidemiologic studies have provided evidence that even if there is no change in other risk factors, age itself can confer a marked increase in risk [6•].
Figure 1.
Effect of age on 10-year risk for coronary heart disease event. Ten-year risk as a function of age (in years) was calculated for an individual (man: blue; woman: red) who is a non-smoker, with no family history, no prior cardiac event, no diabetes mellitus, a fasting blood sugar of less than 100 mg/dL, a height of 5’ 8’’, a weight of 160 pounds, a waist circumference of less than 40 inches (man) or less than 35 inches (woman), a blood pressure of 120/70 mm Hg, and a cholesterol of 170 mg/dL (LDL 80 mg/dL, HDL 45 mg/dL, and triglycerides < 150 mg/dL). The risk increases by one to two orders of magnitude from 25 to 75 years of age in this individual with a relatively benign risk profile that was kept strictly unchanged over time. (Calculations made using the Heart Attack Risk Calculator from the American Heart Association. Available at https://www.heart.org/gglRisk/locale/en_US/index.html?gtype=health.)
There are other complexities in the field of atherosclerosis that challenge us in our ability to understand the disease process. The susceptibility for developing lesions for a given individual may vary markedly across the arterial tree, but will never occur in the venous or capillary systems, except in artificial conditions of post-transplant vasculopathy and of arterialized venous conduits (post-bypass surgery or arterio-venous shunt for dialysis). While for some, lesions are distributed consistently across the arterial system, for others, they may be markedly more pronounced within coronaries, or cerebral arteries, or peripheral arterial conduits. Such variability could be explained, at least in part, by the dominance of risk factors that are predominantly impactful for certain arterial thromboembolic complications, such as hypertension and physical inactivity for stroke or cholesterol elevation for myocardial infarction. However, the entire arterial system is exposed to these injuries when present, and the selectivity of their effect on certain arterial conduits remains poorly understood.
A unique artery for its resistance to the development of atherosclerotic lesions is the internal mammary artery (IMA). Very rarely is the mammary artery found to be burdened by atherosclerosis. This fact led to its successful use for coronary artery bypass. In a study that started in 1972, investigators have followed the impact of the IMA on survival of patients after coronary artery bypass grafting surgery (CABG). They observed the impact of the use of IMA on improved survival [7]. This observation was confirmed by other large surgical series with long follow-up [8]. Indeed, Kurlansky et al. [9] also reported that the use of bilateral IMAs was better than one IMA, independent of the coronary to which the second IMA was anastomosed [10]. Thus, whereas the left IMA is practically always anastomosed to the left anterior descending coronary, the second IMA could be grafted to the right coronary or circumflex coronary branches with no difference in outcome. Post-CABG patients with two IMAs seem to have life expectancy that matches that of the general population [9]. The remarkable ability of the grafted IMA to remain patent, more so than any other know human vascular graft, including vein grafts (and perhaps even more than other grafted arterial conduits such as radial artery) remains unexplained.
The dominant impact of aging on the process of atherosclerosis led to a series of experiments performed in genetically derived mouse models of atherosclerosis. The basic hypothesis advanced at the time was a new concept that the arterial tree is capable of self-repair, and that such repair becomes impaired with aging [11]. Because of the discovery of precursors for endothelial cells in the circulation, it was hypothesized that such cells could contribute to the self-repair process for arteries. Actually, we were able to show that in mice genetically engineered to be prone to atherosclerosis (by marked elevation of circulating lipoproteins, normally not a condition that affects rodents), the atherosclerosis lesions become detectable not as soon as the cholesterol level becomes elevated, but instead only once the self-repair of arteries becomes deficient [3].
With aging, and in the presence of noxious factors (such as cigarette smoke or cholesterol), endothelial cells and perhaps other cells of the arterial wall become senescent and dysfunctional [12], rendering the arterial wall more susceptible to atherosclerosis [12]. Replacement of these senescent cells by progenitors may lead to repair and restoration of function, through cellular and/or paracrine molecular effects that may be occurring locally at the level of the arterial wall. One source for endothelial progenitor cells is the bone marrow, so we decided to inject lineage-negative bone marrow cells (Lin-BMC) into the circulation of mice that otherwise would develop atherosclerosis as a consequence of high lipid load (apolipoprotein E-deficient [ApoE−/−] mice on a western diet with high fat and cholesterol, resulting in blood cholesterol levels of ~10 g/L) [13]. Repeated injections of such cells led to less lesion formation in the aorta of recipient mice. Cholesterol level (the injury) was unaffected by the injected cells.
The latter observation was replicated when Lin-BMC originated from wild-type mice, and even from young ApoE−/− mice. Of interest was the fact that the same cells extracted from the bone marrow of older ApoE−/− mice were no longer capable of suppressing the development of lesions of atherosclerosis, suggesting that with aging, and in the presence of such elevated lipid burden, Lin-BMC capable of arterial repair may become exhausted [11,13]. We also observed that the injected cells do bind to the aorta of ApoE−/− mice (not to the aorta of wild type mice) [13]. The cells lining the recipient's aorta wall were less senescent according to the detected length of their chromosome telomeres, relative to sham-treated ApoE−/ − mice [13]. And again, in no instance was the cholesterol level (~10 g/L) affected by the repeated injections of Lin-BMC [13]. Recently, we were able to show that the impact of injected Lin-BMC could be enhanced and made more reproducible by a culture step for these cells in the presence of interleukins (3 and 6) and growth factor (stem cell factor) [14•]. With this enhanced culture step, atherosclerotic lesions were reduced by 50% to 80%, without altering the cholesterol level.
Hence, many of the complexities of atherosclerosis remain challenging and poorly understood, including the biological consequences of aging that expose most of us to the disease process. While intriguing, the possibility of endogenous arterial repair and its deterioration with advancing age in the presence of risk factors will also require further studies to become an established scientific fact. Instructively, both aging and arterial repair, or loss thereof, have been linked to inflammation [16, 17].
Inflammation: The Dominant Process of Atherosclerosis
Inflammation is a stepwise process that is fundamentally designed to enhance resistance of the organism to infections, and to lead to tissue repair after various forms of injury [17]. The process typically involves the initial formation of a blood clot with platelet activation and aggregation and fibrin polymer formation, the recruitment of inflammatory cells, including dendritic cells, neutrophils, lymphocytes, and monocytes/macrophages (all cells that may induce some degree of tissue destruction), and then angiogenesis, cellular growth, and fibrosis contribute to restoration of tissue integrity. When triggered acutely (eg, skin cut), inflammation is a short-lived process that occurs and is self-limited (constructive inflammation) [16]. However, when triggered chronically by a persistent noxious stimulus such as a concealed tuberculosis bacillus or elevated cholesterol, chronic inflammation ensues that can in itself become the process of tissue dysfunction and destruction (destructive inflammation) [16].
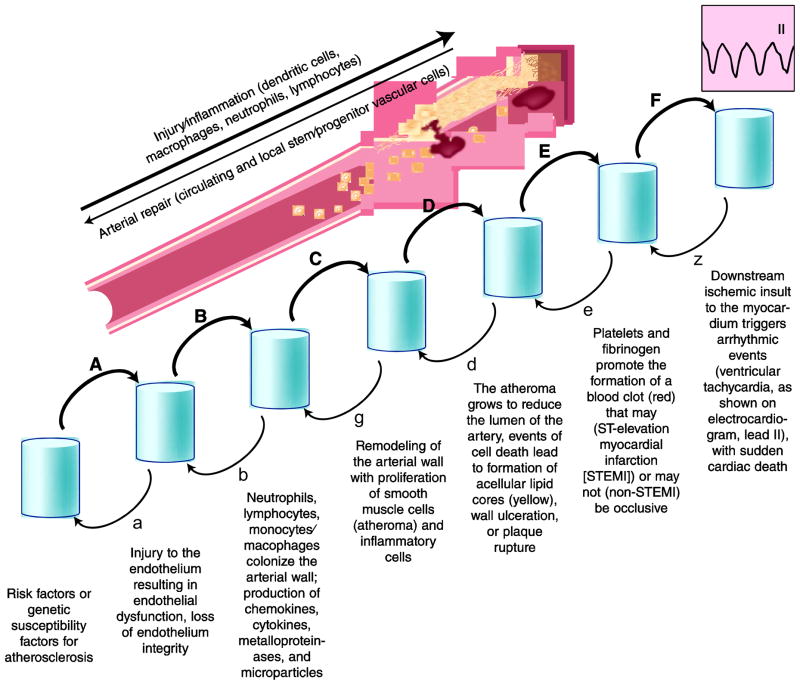
For end-organ damage to occur as a result of arterial thromboembolic events, the following steps need to occur (Fig. 2) [17]: 1) the presence of factors, established risk factors or genetic susceptibility factors that trigger and sustain atherosclerosis and its thromboembolic complications; 2) injury to the endothelium resulting in endothelial dysfunction, loss of endothelium integrity, and open access to sub-endothelial layers to noxious molecules and cells, with platelet adhesion, aggregation, fibrin polymer formation, and adhesion of nucleated cells; 3) propagation of the process with colonization of the arterial wall by neutrophils, lymphocytes and monocytes/macrophages, production of chemokines, cytokines, metalloproteinases, micro-particles (from platelets and other cells) with transfer of microRNA molecules and extracellular matrix damage and apoptosis, occasionally necrosis, of inflammatory cells and cells belonging to the arterial wall (endothelial and smooth muscle cells mainly); 4) remodeling of the arterial wall ensues, with a well described proliferation of smooth muscle cells that have switched from a non-proliferating, contractile phenotype to a proliferating, migrating, and non-contractile phenotype, within the neointima layer (atheroma); 5) the atheroma grows to reduce the lumen of the artery, such growth is not an equilibrium state, and events of cellular death (apoptosis and necrosis), often triggered by activated macrophages, can lead to destabilization of the atheroma, with ulceration or “plaque rupture” that leads to exposure of prothrombotic material (collagen, lipid particles, etc.) to the circulating blood; 6) platelets and fibrinogen can then promote the formation of a blood clot that may (ST-elevation myocardial infarction [STEMI]), or may not (acute coronary syndrome, non-STEMI) be occlusive; 7) on occasion, the downstream ischemic insult to the myocardium may trigger an arrhythmic event (ventricular tachycardia or fibrillation, less frequently asystole) that may, in turn, trigger sudden cardiac death [18].
Figure 2.
Progression of atherosclerosis inflammation. Atherosclerosis as a complex disease process that can be deconstructed into a series of discrete events whose individual probability (A/α, B/β, C/γ, etc.) and timing vary with risk factors and intrinsic ability of self-repair for the artery. In general, the disease progression is presented as irreversible and one-directional as a function of time. However, for each step a reverse probability, even if rather small, does exist. Indeed, some individuals are genetically provided with a substantial ability for self-repair of their arteries, and consequently, even in the presence of potent risk factors, they remain sheltered from the consequences of atherosclerosis and related thromboembolic events.
Hence, inflammation is the dominant process of atherosclerosis lesion formation and is particular for the accumulation of foam cells, macrophages loaded with phagocytozed lipids, and the formation of acellular lipid cores (high cholesterol esters content) that weaken the arterial wall and make atheroma prone to rupture. The proliferation and accumulation of smooth muscle cells, proliferation that has been shown on occasion to be clonal, is also typical and probably secondary to the destabilization and destruction of the media layer of arterial wall. Not fully recognized until recently is the role of the adventitia layer. It was recently shown to contribute precursor cells that could contribute to arterial remodeling with smooth muscle cell migration and proliferation, but also endothelial progenitor cells with abnormal angiogenesis and destabilization of the arterial wall [19]. However, adventitial precursor cells could also be responsible for a rather different role in atherosclerosis or more specifically its prevention [20].
Limitations of the Repair Capacity of the Arterial Wall
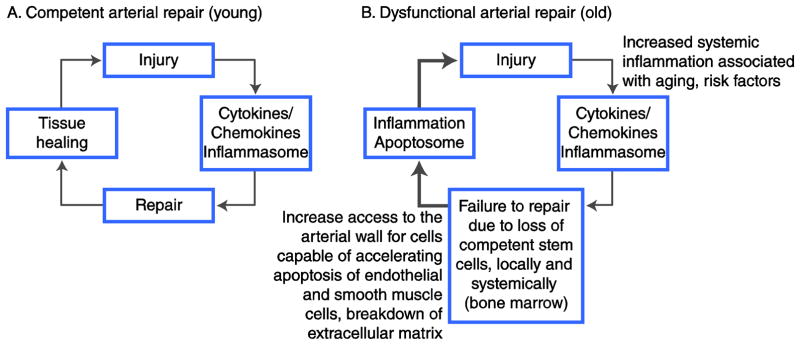
Progenitor cells that can differentiate in endothelial cells (endothelial progenitor cells [EPCs]) are known to be present in the circulation [21]. Indeed, an inverse relationship was shown to exist between the level of circulating EPCs and clinically relevant conditions from endothelial dysfunction to atheroma destabilization and ensuing myocardial infarction [22]. It is likely that this population of circulating precursor cells is in equilibrium with stem cell reservoirs like the bone marrow, and also with local sources of progenitors such as the adventitia of arteries [20]. Should this equilibrium be disturbed by events such as the excessive destruction of circulating progenitor cells (autoimmune diseases) [23], the failure of the marrow to produce such progenitor cells (aging) [11], or the excessive consumption of progenitor cells at the arterial wall level because of exposure to excessive injury, effective repair of the arterial wall would cease, and arterial inflammation would accelerate, with consequent acceleration of atherosclerosis, where atheroma destabilization may occur (Fig. 3).
Figure 3.
Impact of effective arterial repair, or lack thereof, on arterial inflammation. A, In young individuals, the repair capacity of the arterial wall is almost always intact, with local and systemic reservoirs of stem/progenitor cells that are competent to repair an injured artery. As a consequence, an arterial injury triggers a limited inflammatory response, which is mediated by chemokines, cytokines, interleukins, and growth factors (such as vascular endothelial growth factor). The cellular inflammation response (cytoplasmic inflammasome) serves as a signal for the recruitment of inflammatory cells, but also stem/progenitor cells capable of repair, to the area of damaged arteries. Consequently, the artery is successfully repaired and the inflammation ceases (ie, a negative feedback loop). B, In older individuals, the repair capacity may become progressively exhausted. Once the bone marrow and other local reservoirs for repair-competent stem/progenitor cells are depleted, injury to the arterial wall triggers an inflammatory reaction that does not result in successful repair and, consequently, in the absence of negative feedback loop, inflammation continues unregulated (systemic elevation of C-reactive protein and other markers of inflammation). An unregulated inflammatory reaction at the level of the arterial wall contributes to larger atheroma, and unstable lesions ensue when the cellular inflammation response leads to rapid cell death (cytoplasmic apoptosome). In this situation, a positive feedback loop occurs, and unstable clinical events can occur as a consequence of plaque ulceration/rupture and thrombotic complications.
Clinical relevance of the process of self-repair, or lack thereof, is illustrated by the fact that blood inflammatory markers are efficient at signaling patients that are at particular risk for arterial thromboembolic events [2•, 4]. It is well documented that markers such as C-reactive protein (CRP) help identify individuals at increased risk for myocardial infarction, even if their cholesterol is not particularly elevated (most patients with coronary events) [4]. Elevation of markers of inflammation may actually reflect a deficient arterial repair (Fig. 3), and thus a propensity for arterial wall destabilization, atheroma rupture, and thromboembolic events [16]. Of interest is the fact that repeated injections of Lin-BMC in a mouse model of atherosclerosis not only retard the development of arterial lesions, but also modify inflammation [13, 14•]. Thus, Lin-BMC reduces pro-atherosclerosis inflammation markers and elevates anti-atherosclerosis inflammation markers. While the mechanism(s) that explain(s) such regulation of inflammation by Lin-BMC still needs to be demonstrated, it may be linked to the preservation of efficient arterial repair by injected cells [13, 14•].
Hence, there is more to atherosclerosis than the simple response to lipid injury and the development of a classic inflammatory response. There is remodeling and repair process that progressively degrades. The inflammation reaction becomes increasingly overwhelming to the local arterial tissue. It is instructive to note that except for acetylsalicylic acid, no other classic anti-inflammatory drug has been shown to prevent or improve atherosclerotic inflammation (actually most worsen atherosclerosis), probably because anti-inflammatory drugs do not resolve the causative problem of atherosclerosis (ie, injury and a listing repair process). In the new framework of atherosclerosis, with its intricate cellular and molecular activities linked to injury, inflammation, and tissue repair or lack thereof, it is interesting to consider novel discoveries that were provided by the application of non-biased studies of atherosclerosis genetics in large human cohorts (in genome-wide association studies) [6•, 24•].
Recent Genetic and Epigenetic Information on Atherosclerosis Supports a New Model
Several excellent reviews have been published on the topic [2•, 6•, 24•, 25, 26•, 27, 28), and thus this report focuses especially on a few dominant genetic findings from 2011. The year 2011 has been a productive one for the field of atherosclerosis genetics. The number of identified loci by genome-wide association study (GWAS) with significant association (P < 5×10e−8, the currently accepted threshold for genome-wide significance) has more than doubled during 2011 [6•]. Information was also provided on loci that may be more specific for particular ethnic groups.
In the United States, African Americans are at greatest risk of dying of coronary heart disease (CHD). Single nucleotide polymorphism (SNP) rs1859023 on chromosome 7q21 near the PFTK1 gene was significantly associated with atherosclerosis in the Atherosclerosis Risk in Communities (ARIC) study and replicated in the Women’s Health Initiative (WHI) study [29]. PFTK1 encodes a serine/threonine protein kinase (PFTAIRE-protein kinase 1), a cyclin-dependent kinase that regulates cell cycle progression and proliferation. PFTAIRE-PK1 has been shown to promote a motile and metastatic gestalt for cancer cells, and to be present in dendritic cells. It is possible that PFTAIRE-PK1 and variants thereof contribute to atherosclerosis by affecting the biology of arterial dendritic cells or other vascular cells and thus promote arterial inflammation or repair.
In the Chinese Han population, rs6903956 on chromosome 6p24.1 was significantly associated with CHD [30]. The minor allele of rs6903956 decreases the expression of C6orf105 mRNA, which codes for a protein that has been shown to affect the biology of endothelial cells. It is too early to speculate how C6orf105 regulates the biology of the endothelium or other aspects of arterial biology, but it is interesting to note that the Chinese population has a lower normalized rate of coronary artery disease death than Americans (~3 times less) [30, 31]. This difference remains true even after correction for life expectancy, when Hong Kong Chinese are compared to Americans. While it is assumed that such differences in death are due to differences in diet, blood lipid level, and other environmental risk factors, or to prevention of CHD, it is tempting to speculate that genetic susceptibility could be at play. Some genes linked to CHD have been shown to present alleles whose frequency varies substantially among cohorts of European versus Asian ancestry [32–34].
With a meta-analysis (CARDIoGRAM consortium) that surveyed 14 GWAS of 22,233 coronary artery disease (CAD) patients and 64,762 controls, all of European descent, and further genotyping of 56,682 additional individuals to confirm top association signals, 13 new loci were discovered (P < 5 × 10e−8) and 10 of 12 previously reported loci were further established [35•]. The risk alleles varied from infrequent (0.13) to frequent (0.91), and increased the risk for CHD from 6% to 17% per allele. In another study by the Coronary Artery Disease Genetics Consortium, of Europeans and South Asians (from Pakistan and India), 5 new loci were significantly associated with CHD: LIPA on 10q23, PDGFD on 11q22, ADAMTS7-MORF4L1 on 15q25, a gene-rich locus on 7q22, and KIAA1462 on 10Q11; all loci were novel except for the 15q25 locus [36]. So far chromosomes 1, 6, 9, 10, and 17 display more CHD loci than the other human chromosomes [6•, 35•].
Most of the new genes associated with atherosclerosis susceptibility using non-biased techniques (GWAS) have no established connection with lipoprotein biology (> 80%) or any of the classic risk factors for CHD (> 50%). They belong to general cell biology and have been identified as tumor suppressors for a variety of cancers (from head and neck, lung, breast cancers, etc., to liquid tumors and lymphomas, to melanoma), as regulators of inflammation, stem cells, endothelial cells, extracellular matrix, cellular signaling, motility and growth, and as part of canonical biochemical pathways that mediate cell metabolism in a variety of ways (Table 1). It is tempting to speculate that many contribute to atherosclerosis inflammation and to the arterial repair process [24•].
Table 1.
Loci identified genome-wide as significantly (P < 5 × 10e−8) associated with coronary heart disease
| # | SNPa | Band | Gene(s) |
|---|---|---|---|
| 1 | rs11206510 | 1p32.3 | PCSK9 |
| 2 | rs17114036 | 1p32.2 | PPAP2B |
| 3 | rs599839 | 1p13.3 | SORT1 |
| 4 | rs17465637 | 1q41 | MIA3 |
| 5 | rs6725887 | 2q33.1 | WDR12 |
| 6 | rs2306374 | 3q22.3 | MRAS |
| 7 | rs12526453 | 6p24.1 | PHACTR1 |
| 8 | rs6903956 | 6p24.1 | C6orf105 |
| 9 | rs17609940 | 6p21.31 | ANKS1A |
| 10 | rs12190287 | 6q23.2 | TCF21 |
| 11 | rs3798220 | 6q25.3 | LPA |
| 12 | rs10953541 | 7q22.3 | DUS4L, BCAP29 |
| 13 | rs11556924 | 7q32.2 | ZC3HC1 |
| 14 | rs4977574 | 9p21.3 | CDKN2A, CDKN2B |
| 15 | rs7025486 | 9q33 | DAB2IP |
| 16 | rs579459 | 9q34.2 | ABO |
| 17 | rs2505083 | 10p11.23 | KIAA1462 |
| 18 | rs1746048 | 10q11.21 | CXCL12 |
| 19 | rs1412444 | 10q23.31 | LIPA |
| 20 | rs12413409 | 10q24.32 | CYP17A1, CNNM2, NT5C2 |
| 21 | rs974819 | 11q22.3 | PDGFD |
| 22 | rs964184 | 11q23.3 | ZNF259, APOA5-A4-C3-A1 |
| 23 | rs3184504 | 12q24.12 | SH2B3 |
| 24 | rs4773144 | 13q34 | COL4A1, COL4A2 |
| 25 | rs2895811 | 14q32.2 | HHIPL1 |
| 26 | rs3825807 | 15q25.1 | ADAMTS7 |
| 27 | rs216172 | 17p13.3 | SMG6, SRR |
| 28 | rs12936587 | 17p11.2 | RSAD1, SMCR3, PEMT |
| 29 | rs46522 | 17q21.32 | ATP5G1, UBE2Z, GIP, SNF8 |
| 30 | rs1122608 | 19p13.2 | LDLR |
| 31 | rs9982601 | 21q22.11 | MRPS6 |
OR for individual SNP are < 1.10 (about 50%), < 1.20 (about 50%), only two SNP have been attributed reproducible OR > 1.20 (rs3798220 on band 6q25.3 [OR about 1.50] and rs4977574 on band 9p21.3 [OR about 1.30]).
OR–odds ratio; SNP–single nucleotide polymorphism.
Because CHD is a major obstacle to human longevity, the study of centenarians may be relevant to the identification of genes and their variants that reduce susceptibility for CHD and other common lethal diseases. Iannitti et al. [37] have reviewed genetic information obtained from centenarians whose health remains in good condition and who are able to perform their daily routine. Their key findings relate to gene variants in genes that code for pro- or anti-inflammatory cytokines [37]. While worsening inflammation has been associated with aging in the general population, successful aging seems to be related to the robust function of the immune system even at the limit of human life span.
One locus that is clearly relevant to both inflammation and arterial repair is the 10q11.21 with variant rs1746048 near the gene that codes for chemokine CXCL12 [6•, 38]. Chemokines are small secreted proteins with two subfamilies: CC chemokines and CXC chemokines, where the two conserved cysteine residues present in all chemokines are separated by an intervening amino acid [24•]. The seven transmembrane domain, G-protein coupled chemokine receptors (cell surface GPCRs), are designated CXCR and CCR based on their specific preference for certain chemokines. CXCL12 (also called SDF1α) is a ligand for CXCR4 and contributes to arterial inflammation. CXCL12 expression in arterial inflammation is triggered by the binding and membrane fusion of apoptotic bodies released by senescent endothelial cells to the plasma membrane of other arterial-wall cells [39]. Such fusion results in the paracrine transfer of a microRNA (miRNA), miR-126, abundant in endothelial cell-derived apoptotic bodies, to surrounding endothelial and other vascular cells. Once transferred, miR-126 activates CXCR4, triggering a feedback loop that increases the production of CXCL12 [39]. Consequently, CXCR2 enhances homing of EPC (putative arterial repair-competent cells) to sites of arterial injury, and may reduce atherosclerosis.
In addition to endothelial cell microparticles, platelet microparticles (PMPs) also boost the potential of EPC for restoring endothelial integrity after vascular injury [39–43]. Several mechanisms are involved in the ability of platelets and derived PMPs to promote arterial repair, including enhancement of EPC recruitment, migration and differentiation, and the release of pro-angiogenic factors. In particular, the angiogenic chemokines CCL2, CXCL1, CXCL7, CXCL12, as well as growth factors (eg, receptor tyrosine kinase agonists such as VEGF) and cytokines, contribute to arterial repair activity [30–34, 35•, 36–43]. However, most chemokines, cytokines, interleukins and growth factors involved in repair may also contribute to arterial inflammation, and thus will represent challenging targets for therapeutic intervention [43].
In the absence of competent arterial repair, as a result of aging and exposure to common arterial injuries such as cigarette smoke and excess circulating lipids, arterial inflammatory activity increases without regulation and results in the accumulation of inflammatory cells within the thickness of the arterial wall (Fig. 3). These inflammatory cells include dendritic cells, macrophages, neutrophils and activated NK cells that have the ability to destroy the extracellular matrix of the arterial wall and induce apoptosis of endothelial and smooth muscle cells, leading to arterial remodeling and thromboembolic complications (Fig. 2) [2•].
Differences in susceptibility to inflammation and ability to promote arterial repair may also explain the variable resistance of arteries to atherosclerosis, coronary versus internal mammary artery (IMA) for example. Archacki et al. [44] have used a microarray technique to compare gene expression between IMA and left anterior descending (LAD) coronary artery. They have found that 29 genes were significantly differently expressed between LAD and IMA, including TES (the gene that codes for the protein testin). Testin mRNA and protein concentration are higher in IMA than LAD. Both testin mRNA and protein were significantly (P < 0.0001) lower in CHD patients versus controls without CHD. Reducing testin expression, or overexpression, led to more versus less inflammation, respectively.
Conclusions
Genetics and epigenetics will continue to contribute to a better understanding of susceptibility for, and mechanism of, atherosclerosis, and its distribution across the arterial tree. As illustrated with this review, the traditional concept of damage to the arterial wall induced by lipid injury, leading to fatty streak, atheroma, lipid core, plaque rupture, and thromboembolic complications, no longer suffices to account for the more recent discoveries. More relevant is the new model of balance between arterial inflammation and repair, between genetic susceptibility and resistance to injury-induced arterial inflammation (Fig. 4). For years, the focus has been on reducing arterial injury with lifestyle modification (smoking avoidance, treatment of depression, diet, and physical activity), treating dyslipidemia (diet and statins), controlling blood pressure (diet, exercise, and drugs), and preventing and limiting thrombosis (acetylsalicylic acid, purinergic receptor antagonists). The fact that in animal models we have shown that atherosclerosis can be prevented significantly without modifying the culprit injury (high cholesterol), simply by preserving the animal repair capacity through injections of conditioned progenitor cells, suggests that the future will bring a new armamentarium of therapeutic opportunities. Furthermore, the atherosclerosis genetics revolution will increasingly allow us to personalize our approach to the individual patient, especially when applying preventive measures and treatments.
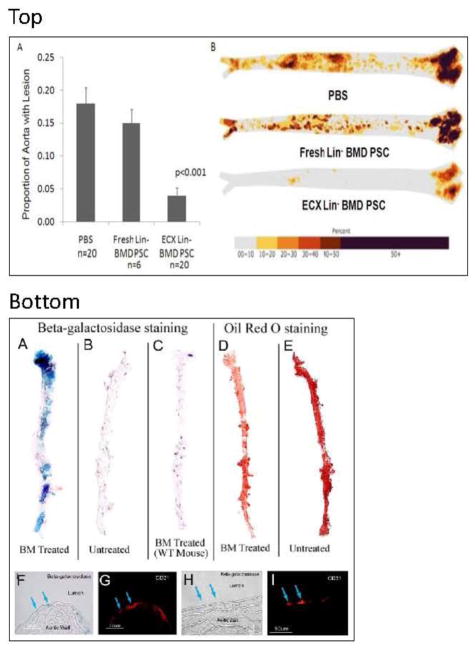
Figure 4.
Effective arterial repair supported by periodic injections of bone marrow cells. Top panel: Enhanced culture step and bone marrow cells (BMC) repair efficacy. A, Proportion of total aorta containing atherosclerosis following injections of PBS, freshly harvested Lin BMCs, or enhanced culture (ECX) cells. B, Composite images for all analyzed aortas in each group; color indicates probability for the region to contain atherosclerosis (see color bar). From Song et al.[14]; with permission. Bottom panel: Cell biology or arterial repair. A, Cultured bone marrow (BM) cells from ROSA26 mice (expressing β-galactosidase in their nucleus) were injected intravenously into apolipoprotein E (ApoE)-deficient (knockout) C57 black 6 mice fed a high-fat diet. Shown are the aortae of recipient animals, after staining for β-galactosidase (blue coloration of positive cells). Blue cells have anchored in areas of the recipient aorta that otherwise would develop atherosclerosis. B, ApoE-knockout mice that did not receive ROSA26 cells (medium alone). In this case, no blue cells could be detected in the recipient aorta. C, Wild-type (WT) C57 black 6 mice who received cultured BMCs from ROSA26 mice do not display blue cells, indicating that attachment and homing of progenitor cells require an injury to the aorta (for ApoE knockout animals, injury corresponds to high circulating lipid levels). D, Oil Red O staining of ApoE knockout mice. Aorta from a mouse that received repeated injections of cultured BMCs showing reduced atherosclerosis (Oil Red O-positive lesions) of treated animals. E, Same staining as in D, for a mock-treated ApoE knockout mouse. F, H Fate of injected cultured BMCs (blue cells) in recipient aorta. G, I, A majority of the cells with a blue nucleus double-stain positive for markers of endothelial cells, such as PECAM (CD 31), indicating that most blue cells become endothelial cells that are incorporated within the surface area of the artery. Reproduced from Raucher et al. [13]
Acknowledgments
I am indebted to Dr. Joshua M. Hare, Director of the Interdisciplinary Stem Cell Institute at the University of Miami for his invaluable review of the manuscript, and to Chris Morris and Irene Hung for outstanding editorial support.
Footnotes
Disclosure
Pascal J. Goldschmidt-Clermont serves on the boards of the companies MEDNAX and HMA; he receives compensation for these activities as well as share options. He also serves on the scientific board of SYNECOR, a non-compensated activity, and he is a share-owner (20,000 shares). Dr. David Seo and PJG-C also cofounded the company Atherorepairix, without related compensation (cash, stock, gift or any other form of compensation). For further details, please consult our UM public faculty disclosure web site: (http://med.miami.edu/about-miller/faculty-disclosures). C. Dong: none. O. Velazquez: none.
References
- 1.Brown MS, Goldstein JL. Koch's postulates for cholesterol. Cell. 1992;71(2):187–188. doi: 10.1016/0092-8674(92)90346-e. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–25. doi: 10.1038/nature10146. Review. • This is a thorough review of the most recent information on atherosclerosis inflammation and its cellular and molecular agents. Novel clinical trials with anti-inflammatory drugs are also surveyed. [DOI] [PubMed] [Google Scholar]
- 3.Karra R, Vemullapalli S, Dong C, et al. Molecular evidence for arterial repair in atherosclerosis. Proc Natl Acad Sci USA. 2005;102(46):16789–16794. doi: 10.1073/pnas.0507718102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 5.American Heart Association. [Accessed December 2011.];Heart Attack Risk Calculator. Available at: https://www.heart.org/gglRisk/locale/en_US/index.html?gtype=health.
- 6.Stylianou IM, Bauer RC, Reilly MP, Rader DJ. Genetic basis of atherosclerosis: insights from mice and humans. Circ Res. 2012 doi: 10.1161/CIRCRESAHA.110.230854. In press. • This is a well-written review of the genetic (epigenetic) discoveries relative to atherosclerosis in mice and humans. It is probably the most complete review to date on the topic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galbut DL, Traad EA, Dorman MJ, et al. Twelve-year experience with bilateral internal mammary artery grafts. Ann Thorac Surg. 1985;40(3):264–70. doi: 10.1016/s0003-4975(10)60039-2. [DOI] [PubMed] [Google Scholar]
- 8.Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314(1):1–6. doi: 10.1056/NEJM198601023140101. [DOI] [PubMed] [Google Scholar]
- 9.Kurlansky PA, Traad EA, Dorman MJ, et al. Thirty-year follow-up defines survival benefit for second internal mammary artery in propensity-matched groups. Ann Thorac Surg. 2010;90:101–8. doi: 10.1016/j.athoracsur.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Kurlansky PA, Traad EA, Dorman MJ, et al. Location of the second internal mammary artery graft does not influence outcome of coronary artery bypass grafting. Ann Thorac Surg. 2011;91(5):1378–83. doi: 10.1016/j.athoracsur.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 11.Goldschmidt-Clermont PJ. Loss of bone marrow-derived vascular progenitor cells leads to inflammation and atherosclerosis. Am Heart J. 2003;146(Suppl 4):S5–S12. doi: 10.1016/j.ahj.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Xiao Q, Kiechl S, Patel S, et al. Endothelial progenitor cells, cardiovascular risk factors, cytokine levels and atherosclerosis – Results from a large population-based study. PLoS One. 2007;2(10):e975. doi: 10.1371/journal.pone.0000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, et al. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108(4):457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 14.Song X, Ma Q, Liu X, et al. Will periodic intravenous injections of conditioned bone marrow cells effectively reduce atherosclerosis? Antioxid Redox Signal. 2012;16(1):85–91. doi: 10.1089/ars.2011.4139. • This is the first report on reduction of atherosclerosis by some 80% in ApoE−/− mice without altering the lipidic injury (6 g/L) and without additional genetic intervention, using repeated infusions of cultured lineage-negative bone marrow cells in enhanced conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106(5):506–12. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 16.Goldschmidt-Clermont PJ, Creager MA, Losordo DW, et al. Atherosclerosis 2005: Recent discoveries and novel hypotheses. Circulation. 2005;112(21):3348–3353. doi: 10.1161/CIRCULATIONAHA.105.577460. [DOI] [PubMed] [Google Scholar]
- 17.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 18.Gutstein DE, Fuster V. Pathophysiology and clinical significance of atherosclerotic plaque rupture. Cardiovasc Res. 1999;41(2):323–333. doi: 10.1016/s0008-6363(98)00322-8. [DOI] [PubMed] [Google Scholar]
- 19.Khurana R, Simons M, Martin JF, Zachary IC. Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation. 2005;112(12):1813–24. doi: 10.1161/CIRCULATIONAHA.105.535294. Review. [DOI] [PubMed] [Google Scholar]
- 20.Majesky MW, Dong XR, Hoglund V, Daum G, Mahoney WM., Jr The adventitia: a progenitor cell niche for the vessel wall. Cells Tissues Organs. 2012;195(1–2):73–81. doi: 10.1159/000331413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 22.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 23.Zhu S, Evans S, Yan B, et al. Transcriptional regulation of Bim by FOXO3a and Akt mediates scleroderma serum-induced apoptosis in endothelial progenitor cells. Circulation. 2008;118(21):2156–65. doi: 10.1161/CIRCULATIONAHA.108.787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldschmidt-Clermont PJ, Seo DM, Wang L, et al. Inflammation, stem cells and atherosclerosis genetics. Curr Opin Mol Ther. 2010;12(6):712–23. Review. • This is a prior review by our group addressing the genetic revolution triggered by serial GWAS that unveiled an unanticipated series of genomic loci relative to our understanding of atherosclerosis. [PubMed] [Google Scholar]
- 25.Sivapalaratnam S, Motazacker MM, Maiwald S, et al. Genome-wide association studies in atherosclerosis. Curr Atheroscler Rep. 2011;13(3):225–32. doi: 10.1007/s11883-011-0173-4. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lund G, Zaina S. Atherosclerosis: an epigenetic balancing act that goes wrong. Curr Atheroscler Rep. 2011;13(3):208–14. doi: 10.1007/s11883-011-0174-3. Review. • This is a review how dietary components could induce atherosclerotic atherosclerosis through epigenetic changes that shift the physiologic program of differential gene activation and repression. [DOI] [PubMed] [Google Scholar]
- 27.Manolio TA, Green ED. Genomics Reaches the Clinic: From Basic Discoveries to Clinical Impact. Cell. 2011;147:14–16. doi: 10.1016/j.cell.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Ginsburg D. Genetics and Genomics to the Clinic: A Long Road ahead. Cell. 2011;147:17–19. doi: 10.1016/j.cell.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Barbalic M, Reiner AP, Wu C, et al. Genome-wide association analysis of incident coronary heart disease (CHD) in African Americans: a short report. PLoS Genet. 2011;7(8):e1002199. doi: 10.1371/journal.pgen.1002199. Epub 2011 Aug 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang F, Xu CQ, He Q, et al. Genome-wide association identifies a susceptibility locus for coronary artery disease in the Chinese Han population. Nat Genet. 2011;43(4):345–9. doi: 10.1038/ng.783. [DOI] [PubMed] [Google Scholar]
- 31.Roger VL, Go AS, Lloyd-Jones DM, et al. on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics--2012 Update: A Report From the American Heart Association. Circulation. 2011 Dec 15; [Google Scholar]
- 32.Kim HO, Jin Y, Kickler TS, et al. Gene frequencies of the five major human platelet antigens in African American, white, and Korean populations. Transfusion. 1995;35(10):863–7. doi: 10.1046/j.1537-2995.1995.351096026369.x. [DOI] [PubMed] [Google Scholar]
- 33.Weiss EJ, Bray PF, Tayback M, et al. A polymorphism of a platelet glycoprotein receptor as an inherited risk factor for coronary thrombosis. N Engl J Med. 1996;334(17):1090–4. doi: 10.1056/NEJM199604253341703. [DOI] [PubMed] [Google Scholar]
- 34.Kucharska-Newton AM, Monda KL, Campbell S, et al. Association of the platelet GPIIb/IIIa polymorphism with atherosclerotic plaque morphology: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 2011;216(1):151–6. doi: 10.1016/j.atherosclerosis.2011.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schunkert H, König IR, Kathiresan S, et al. CARDIoGRAM Consortium, Samani NJ. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43(4):333–8. doi: 10.1038/ng.784. • The CARDIoGram Consortium is a comprehensive analysis (GWAS) of very large cohorts to identify SNPs that significantly associate with CHD. The study not only confirmed previously identified SNPs, but also pointed to 13 new, not previously recognized SNPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coronary Artery Disease (C4D) Genetics Consortium. A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat Genet. 2011;43(4):339–44. doi: 10.1038/ng.782. [DOI] [PubMed] [Google Scholar]
- 37.Iannitti T, Palmieri B. Inflammation and genetics: an insight in the centenarian model. Hum Biol. 2011;83(4):531–59. doi: 10.3378/027.083.0407. [DOI] [PubMed] [Google Scholar]
- 38.Samani NJ, Erdmann J, Hall AS, et al. WTCCC and the Cardiogenics Consortium. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357(5):443–53. doi: 10.1056/NEJMoa072366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zernecke A, Bidzhekov K, Noels H, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2(100):ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 40.Mause SF, Ritzel E, Liehn EA, et al. Platelet microparticles enhance the vasoregenerative potential of angiogenic early outgrowth cells after vascular injury. Circulation. 2010;122(5):495–506. doi: 10.1161/CIRCULATIONAHA.109.909473. [DOI] [PubMed] [Google Scholar]
- 41.Hristov M, Zernecke A, Weber C. Regulation of endothelial progenitor cell homing after arterial injury. Thromb Haemost. 2007;98(2):274–277. [PubMed] [Google Scholar]
- 42.Hristov M, Zernecke A, Bidzhekov K, et al. Importance of CXC chemokine receptor 2 in the homing of human peripheral blood endothelial progenitor cells to sites of arterial injury. Circ Res. 2007;100(4):590–597. doi: 10.1161/01.RES.0000259043.42571.68. [DOI] [PubMed] [Google Scholar]
- 43.Liehn EA, Tuchscheerer N, Kanzler I, et al. Double-Edged Role of the CXCL12/CXCR4 Axis in Experimental Myocardial Infarction. J Am Coll Cardiol. 2011;58(23):2415–23. doi: 10.1016/j.jacc.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 44.Archacki SR, Angheloiu G, Moravec CS, et al. Comparative gene expression analysis between coronary arteries and internal mammary arteries identifies a role for the TES gene in endothelial cell functions relevant to coronary artery disease. Hum Mol Genet. 2011 Dec 21; doi: 10.1093/hmg/ddr574. [DOI] [PubMed] [Google Scholar]