Abstract
Purpose
Due to the limited specificity of prostate-specific antigen (PSA) for prostate cancer (CaP) screening, there is an ongoing search for adjunctive biomarkers. Retrospective studies have suggested that an isoform of proenzyme PSA called [−2] proPSA (p2PSA) may enhance the specificity of PSA-based screening. The objective of our study was to examine the utility of p2PSA in a prospective CaP screening study.
Materials and Methods
From a population of 2034 men undergoing CaP screening, we examined the relationship between p2PSA and CaP detection. Specifically, we compared the utility of total PSA, the ratio of free PSA (fPSA) to total PSA (%fPSA), the ratio of p2PSA to fPSA (%p2PSA) and a formula combining PSA, fPSA and p2PSA (called Beckman Coulter prostate health index or phi®) to predict CaP among men from the study undergoing prostate biopsy with PSA levels of 2.5–10 ng/ml and non-suspicious digital rectal examination (DRE).
Results
Despite similar total PSA levels (p=0.88), both %fPSA (p=0.02) and %p2PSA (p=0.0006) distinguished between positive and negative biopsy results. On receiver operating characteristic (ROC) analysis, %p2PSA (AUC 0.76) outperformed both PSA (AUC 0.50) and %fPSA (AUC 0.68) for differentiating between CaP and benign disease. Setting the sensitivity at 88.5%, p2PSA led to a substantial improvement in specificity, positive and negative predictive values. The Beckman Coulter phi® (AUC 0.77) had the best overall performance characteristics.
Conclusions
This is the first prospective study to demonstrate that p2PSA provides improved discrimination between CaP and benign disease in screened men with PSA levels from 2.5 to 10 ng/ml and negative DRE.
Keywords: prostate-specific antigen, PSA, free PSA, PSA isoforms, proPSA
INTRODUCTION
Prostate-specific antigen (PSA) is the most widely used serum marker for the early detection of prostate cancer, and its introduction in clinical practice has revolutionized contemporary management of this disease.1 However, since the serum PSA concentration is often increased in benign conditions, such as benign prostatic hyperplasia (BPH) and prostatitis, the test lacks specificity, especially at lower concentrations.2 Accordingly, only 20% to 30% of men with serum PSA levels from 2 to 4 ng/ml and 30% to 45% with serum PSA levels from 4 to 10 ng/ml have prostate cancer diagnosed on prostate needle biopsy.3–5 To address these limitations of PSA, adjunctive measurements, including the ratio of free to total PSA, called percent free PSA (%fPSA), have been investigated and shown to significantly improve cancer detection rates in the 4 to 10 ng/ml range.6–9
More recently, distinct molecular forms of free PSA (fPSA) have been characterized and found to be differentially associated with BPH or prostate cancer.10, 11 These precursor forms of PSA are enzymatically inactive and include: (1) proPSA, which is elevated in cancer tissue 12 and serum,10, 13, 14 as well as (2) benign PSA (BPSA), and (3) intact PSA (iPSA), which are associated with BPH.15, 16 Several isoforms of proPSA exist and are named based on the length of the pro-leader peptide. The 7 amino acid pro-leader peptide form, [−7] proPSA, is cleaved by human kallikrein 2 and trypsin to yield active PSA. Truncated forms containing leader sequences of 5, 4 or 2 amino acids are also present and can be measured in serum using immunoassays. These highly specific immunoassays have been tested clinically.13–15, 17, 18 The [−2] proPSA (p2PSA) has emerged as a promising marker for prostate cancer detection, as it is preferentially concentrated in cancerous tissue on histochemical staining19, and, compared to other isoforms, demonstrates superior accuracy in the detection of prostate cancer (the analyte is referred to as “[−2] proPSA” and the assay “p2PSA;” for simplicity, we use “p2PSA” throughout the remainder of this report). 20
Among men with PSA levels between 2 and 10 ng/ml, we have previously demonstrated in large retrospective studies that the ratios of total proPSA and p2PSA to fPSA (%proPSA and %p2PSA, respectively) were more cancer-specific than combinations of total PSA and fPSA.20, 21 Other research groups have similarly reported improved accuracy for cancer detection using p2PSA compared to free and total PSA.15, 17, 18 Moreover, subsequent studies have demonstrated a correlation between p2PSA levels with clinically significant cancer, including more advanced pathologic stage, higher tumor volume, and higher tumor grade.21 Also, p2PSA has been shown to improve discrimination in specific patient populations with particular diagnostic uncertainty, such as those with %fPSA > 25 and total PSA levels of 2 to 4 ng/ml. 13
However, all of these studies were retrospectively performed using archived serum samples and are limited by selection bias. In addition, more recently Beckman Coulter, Incorporated has also developed a mathematical formula combining total PSA, fPSA and p2PSA called the Beckman Coulter prostate health index, or phi®, that showed encouraging results in preclinical studies but requires clinical validation. Our objective in this study was, therefore, to perform the first prospective study of p2PSA and Beckman Coulter phi® for prostate cancer screening in men undergoing prostate biopsy for total PSA levels from 2.5 to 10 ng/ml with benign findings on digital rectal examination (DRE).
MATERIALS AND METHODS
To evaluate the performance characteristics of p2PSA under “real world” conditions, we conducted a prospective screening van study of 2034 men in Chicago, IL. Over a period of one week in April 2007, men of all ages with no prior history of prostate cancer were offered screening. Each visit began with a focused medical history, after which serum samples were obtained by venipuncture for PSA, fPSA, and p2PSA testing. Lastly, a digital rectal examination (DRE) was performed by a urology resident or faculty urologist. Because of the “grass-roots” nature of the screening study, our population was heterogeneous, in that a variable proportion of the population had prior PSA values available for comparison or had undergone a previous prostate biopsy. Informed consent was obtained under institutional board review-approved and Health Insurance Portability and Accountability Act (HIPAA)-compliant protocols.
Our study protocol recommended biopsy for serum PSA levels greater than or equal to 2.5 ng/ml or any abnormality suspicious for cancer (i.e., induration, nodule, or irregularity) on DRE. For the purposes of this analysis, we focused on men with PSA levels between 2.5 and 10 ng/ml and a non-suspicious DRE. Although biopsies were performed at the institution selected by the patient, all participants were contacted for up to 2 years after a biopsy recommendation to follow-up on the results of biopsy and any subsequent treatment received.
Specimens were analyzed in a blinded fashion for PSA, fPSA, and p2PSA concentrations on a Beckman Coulter ACCESS 2 Immunoassay System (Beckman Coulter, Incorporated, Brea, California), which involve dual monoclonal immunoenzymatic, sandwich, paramagnetic particle, chemiluninescent automated Hybritech® assays. Beckman Coulter phi® was calculated for each patient as: phi = (p2PSA/fPSA) × sqrt(PSA). We performed receiver operating characteristic (ROC) analysis and compared the sensitivity, specificity, positive and negative predictive value of total PSA, %fPSA, %p2PSA, and phi® for cancer detection. All statistical analyses were performed using SAS® (Cary, NC).
RESULTS
From 2007 to 2008, 2034 men participated in the screening study. The demographic features of the population are listed in Table 1. The median age was 57 years with a median PSA concentration of 1.05 ng/ml and most men were Caucasian. Of the men screened, 322 were recommended to undergo a biopsy for PSA ≥ 2.5ng/ml or abnormality on DRE. Those recommended to undergo biopsy were older (median age 67 vs. 55 years) with higher total PSA (median 4.95 vs. 0.89 ng/ml) and lower %fPSA (median 18.70% vs. 26.55%) compared to healthy controls.
Table 1.
Clinical characteristics of the screening population.
| Screened | No Biopsy Recommended |
Biopsy Recommended |
No Biopsy obtained |
Biopsy Obtained |
|
|---|---|---|---|---|---|
| No. of pts | 2034 | 1712 | 322 | 236* | 74** |
| Median age (years) | 57 | 55 | 67 | 67 | 65 |
| % Caucasian | 56% | 56% | 59% | 60% | 54% |
| % African American | 21% | 21% | 20% | 21% | 15% |
| Median total PSA(ng/ml) | 1.05 | 0.89 | 4.95 | 4.64 | 5.87 |
| Median %fPSA | 25.39 | 26.55 | 18.70 | 19.11 | 17.62 |
| Median %p2PSA | 2.59 | 2.75 | 1.74 | 1.73 | 1.72 |
| Median phi® | 26.97 | 25.34 | 39.93 | 39.27 | 41.48 |
12 patients were excluded for previous diagnosis of prostate cancer
63 patients were analyzed as part of the study
To date, 74 men (23%) complied with the recommendation for biopsy, of which 63 had total PSA levels from 2.5 to 10 ng/ml and non-suspicious DRE. Men who followed the biopsy recommendation were significantly younger (p=0.04) and had higher total PSA concentrations (p=0.04) than those who did not comply with biopsy. However, %fPSA and %p2PSA were similar between the groups.
Overall, 41% of men were diagnosed with prostate cancer on biopsy (i.e., had a positive biopsy), and all had a Gleason score of 6 (62%) or 7 (38%). Comparing men with negative versus positive biopsies, there was no statistically significant difference in total PSA (median 5.37 vs. 5.14 ng/ml, p=0.88); however, men diagnosed with prostate cancer had a significantly lower median %fPSA (14.3 vs. 20.2; p=0.02), and a significantly higher median %p2PSA (2.10 vs. 1.42, p=0.0006), compared to men with a negative biopsy. With increasing %p2PSA tertiles, the percent of positive biopsies continuously increased (p=0.002). Specifically, at %p2PSA <1.36, 1.36 to 2.15, and >2.15, prostate biopsy was positive in 12%, 38%, and 50%, respectively. Of note, %p2PSA levels in the general screening population (median total PSA concentration of 1.05 ng/ml) were relatively high (2.59), and were even higher (2.75) for those not recommended biopsy (median PSA of 0.89 ng/ml).
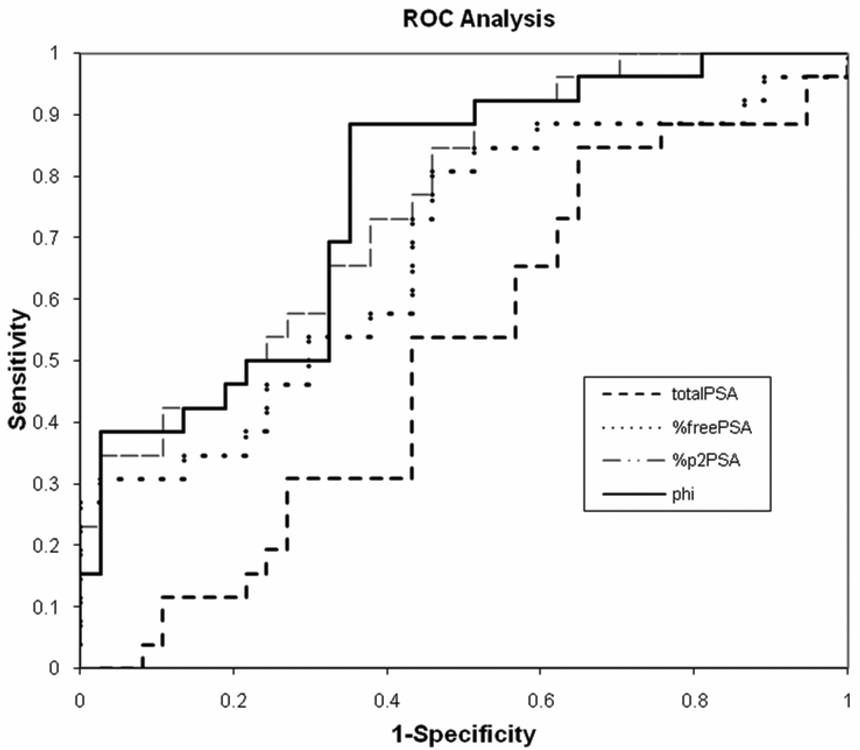
Figure 1 shows the results of ROC analysis. Total PSA alone lacked sensitivity and specificity in the 2.5–10 ng/ml range (AUC=0.50); whereas, %fPSA (AUC=0.68), %p2PSA (AUC=0.76), and phi® (AUC=0.77) had more favorable performance characteristics. Setting the level of sensitivity at 88.5%, %p2PSA outperformed %fPSA or total PSA (Table 2). Additionally, the phi® “score” further improved diagnostic performance to a greater extent than the other parameters examined (Table 2).
Figure 1.
ROC curve comparing PSA, %fPSA, %p2PSA, and (Beckman Coulter phi®) for prostate cancer detection on prostate biopsy at total PSA levels of 2.5 to 10 ng/ml.
Table 2.
Performance characteristics of total PSA, %fPSA, %p2PSA and Beckman Coulter phi® at a set sensitivity of 88.5%.
| AUC | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|
| Total PSA | 0.50 | 88.5 | 24.3 | 45.1 | 75 |
| %fPSA | 0.68 | 88.5 | 40.5 | 51.1 | 83.3 |
| %p2PSA | 0.76 | 88.5 | 48.6 | 54.8 | 85.7 |
| phi® | 0.77 | 88.5 | 64.9 | 53.9 | 88.9 |
DISCUSSION
p2PSA, one of the recently identified forms of fPSA, is a promising candidate in the quest for better prostate cancer markers.11, 18, 21–23 In a retrospective series of 1091 men from two academic institutions, we previously reported that p2PSA was the marker with the highest specificity for prostate cancer detection.21 Moreover, the percentage of men with prostate cancer and with Gleason scores ≥7 increased with increasing levels of p2PSA and %p2PSA, suggesting that p2PSA may aid in the detection of clinically significant cancers.21 Recently, Sokoll and colleagues conducted a multi-institutional National Cancer Institute-sponsored validation study that evaluated PSA, fPSA, p2PSA, BPSA, and testosterone using stored serum samples from 123 men. They similarly demonstrated that %p2PSA was the most accurate predictor of prostate cancer, particularly in men with PSA levels in the 2–10 ng/ml range.18
The present study adds new information on the clinical utility of p2PSA as a prostate cancer marker, since it is the first prospective evaluation of its usefulness in a cross-sectional screening population and provides side-by-side comparisons with total and fPSA. Although previous studies from archived samples showed the potential clinical utility of p2PSA, so far no study has evaluated p2PSA in a “real world” setting in which it might be used in clinical practice. Furthermore, prospective studies are advantageous in the assessment of tumor markers by minimizing biases from data collection, sample storage, and interpretation.24, 25
With regard to the generalizability of our results, the study design, PSA distribution, and biopsy findings are all consistent with other contemporary screening populations. The overall prostate cancer detection rate of 41% in our population with total PSA of 2.5–10 ng/ml is consistent with the literature using contemporary prostate biopsy schemes.3, 5, 20 The 38% proportion of cancers with Gleason score 7 is also comparable to previous reports from multiple institutions.18, 21, 26 Thus, we believe that our population is representative of contemporary men undergoing prostate cancer screening.
Our results confirm that fPSA and p2PSA significantly improve overall diagnostic accuracy in men with total PSA concentrations from 2.5 to 10 ng/ml.13, 18, 21, 22 Indeed, despite similar total PSA levels between the groups, use of p2PSA was able to discriminate between men with and without prostate cancer diagnosed by biopsy. On ROC analysis, the AUC was greater for %p2PSA compared to total PSA and %fPSA, suggesting that it may be useful to reduce the morbidity and cost associated with unnecessary prostate biopsies. Regression analysis from our data indicated that the combination of %fPSA and %p2PSA could provide more discrimination between benign and cancer cases than either parameter alone [data not shown], but we could not use this information in the format of a test for the individual patient. In this regard, we found that the Beckman Coulter prostate health index (phi®) could be used to more accurately assess the risk for a positive biopsy. This mathematical index combining PSA, fPSA and p2PSA yielded even higher diagnostic accuracy than our regression model, with the advantages of being suitable for testing the individual patient.
Due to the small sample size of men undergoing prostate biopsy, these promising findings will ultimately require validation in larger populations. If confirmed, our results suggest that p2PSA either alone or in combination with total PSA and fPSA may help reduce unnecessary biopsies, since only 12% of biopsies were positive at %p2PSA less than 1.36%.
Although our study confirms many of the findings from previous retrospective studies, there was insufficient statistical power to address the correlation with tumor features, such as Gleason grade. To date, only 23% of the men with an indication for prostate biopsy have complied with our biopsy recommendation. Nevertheless, our biopsy compliance rate is comparable to that of other screening studies with a similar design. For example, Crawford et al. 27 reported a 19.4% biopsy compliance rate in men with a PSA >4 ng/ml and normal DRE in the Prostate Cancer Awareness Week study, and Pinsky 28 reported a 38% rate of biopsy compliance within 1 year of an abnormal PSA test in the Prostate, Lung, Colorectal and Ovarian Cancer screening trial. Low biopsy compliance rates, therefore, are common in screening studies where biopsy is not mandated as part of the protocol, and may reflect the “real-world” conditions in which serum markers are currently used.
It is noteworthy that men who did not undergo biopsy in our study were older and had lower PSA levels than those who did have a biopsy. Since this was a grass-roots study using a relatively low PSA threshold for biopsy (2.5 ng/ml), some patients may have been advised by their physician that immediate biopsy was unnecessary. It is possible that these providers use higher cutoffs for recommending biopsy or had access to the patient’s previous PSA and biopsy history suggesting that the current value did not represent a considerable departure from their baseline. That notwithstanding, the resultant selection bias associated with poor biopsy compliance may have affected the results of our study.
Another important feature of our study design is that the ROC analysis was based upon men with total PSA levels of 2.5 to 10 ng/ml, such that these results may not be generalizable to other PSA ranges. Indeed, a finding of unknown clinical significance is that the median %p2PSA was relatively high (2.75) for the men in whom biopsy was not recommended because of “normal” screening results (median total PSA for this population was 0.89 ng/ml). Of course, samples with low total PSA concentrations also have low absolute levels of free PSA and p2PSA. The %p2PSA value observed in clinical practice is generally 1% to 3%; thus, in a patient with a total PSA of 2 ng/ml, %fPSA of 25%, and %p2PSA of 2.5%, the absolute quantity of p2PSA would be 0.013 ng/ml. The limit of detection for the p2PSA assay is 0.692 pg/ml (0.000692 ng/ml), and the limit of quantitation (20% coefficient of variation) is 3.266 pg/ml. In some men with low total PSA, concentrations of p2PSA may be lower than can be accurately measured by currently available assays (Weinzierl CF, personal communication).
Further studies are necessary to validate the use of p2PSA and any associated indices as screening tools and to establish clinical cut points. To this end, a pivotal trial is currently underway to obtain United States Food and Drug Administration approval of p2PSA as an aid to the early detection of prostate cancer. As we await further evidence, the current study adds to the growing literature suggesting a potential role for p2PSA in the prostate cancer screening algorithm.
CONCLUSIONS
Use of p2PSA, either incorporated in %p2PSA or as a mathematical index (Beckman Coulter phi®) provides superior discrimination between prostate cancer and benign disease in men with total PSA levels of 2.5 to 10 ng/ml and negative DRE. Confirmatory validation studies are needed to determine the optimal incorporation of this marker into clinical practice.
Abbreviations
- PSA
prostate-specific antigen
- fPSA
free PSA
- [−2] proPSA
the isoform of proenzyme PSA with 2 amino acid residues remaining on its 7 amino acid leader peptide and the analyte measured in Beckman Coulter Incorporated ACCESS 2 assay
- p2PSA
that measures [−2] proPSA concentration in serum
- %fPSA
ratio of free PSA to total PSA × 100
- %p2PSA
ratio of p2PSA to free PSA × 100
- DRE
digital rectal examination
- ROC
receiver operating curve
- AUC
area under receiver operating curve
- Beckman Coulter phi®
Beckman-Coulter, Incorporated name for the “prostate health index,” a mathematical combination of PSA, fPSA and [−2]proPSA
Footnotes
Supported by Beckman Coulter, Urological Research Foundation, Prostate SPORE grant (P50 CA90386-05S2), the Robert H. Lurie Comprehensive Cancer grant (P30 CA60553) and Zero, the Project to End Prostate Cancer, formerly called the National Prostate Cancer Coalition (Quentin “Skip” Lockwood, III, CEO)
Presented in part at the American Urological Association Annual Meeting, April 25–30, 2009, Chicago, IL
REFERENCES
- 1.Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156. doi: 10.1056/NEJM199104253241702. [DOI] [PubMed] [Google Scholar]
- 2.Tchetgen MB, Oesterling JE. The effect of prostatitis, urinary retention, ejaculation, and ambulation on the serum prostate-specific antigen concentration. Urol Clin North Am. 1997;24:283. doi: 10.1016/s0094-0143(05)70374-8. [DOI] [PubMed] [Google Scholar]
- 3.Emiliozzi P, Longhi S, Scarpone P, et al. The value of a single biopsy with 12 transperineal cores for detecting prostate cancer in patients with elevated prostate specific antigen. J Urol. 2001;166:845. [PubMed] [Google Scholar]
- 4.Okihara K, Fritsche HA, Ayala A, et al. Can complexed prostate specific antigen and prostatic volume enhance prostate cancer detection in men with total prostate specific antigen between 2.5 and 4.0 ng./ml. J Urol. 2001;165:1930. doi: 10.1097/00005392-200106000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Roehl KA, Antenor JA, Catalona WJ. Robustness of free prostate specific antigen measurements to reduce unnecessary biopsies in the 2.6 to 4.0 ng/ml range. J Urol. 2002;168:922. doi: 10.1016/S0022-5347(05)64543-0. [DOI] [PubMed] [Google Scholar]
- 6.Catalona WJ, Partin AW, Slawin KM, et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA. 1998;279:1542. doi: 10.1001/jama.279.19.1542. [DOI] [PubMed] [Google Scholar]
- 7.Lilja H. Significance of different molecular forms of serum PSA. The free, noncomplexed form of PSA versus that complexed to alpha 1-antichymotrypsin. Urol Clin North Am. 1993;20:681. [PubMed] [Google Scholar]
- 8.Stenman UH, Leinonen J, Alfthan H, et al. A complex between prostate-specific antigen and alpha 1-antichymotrypsin is the major form of prostate-specific antigen in serum of patients with prostatic cancer: assay of the complex improves clinical sensitivity for cancer. Cancer Res. 1991;51:222. [PubMed] [Google Scholar]
- 9.Woodrum DL, Brawer MK, Partin AW, et al. Interpretation of free prostate specific antigen clinical research studies for the detection of prostate cancer. J Urol. 1998;159:5. doi: 10.1016/s0022-5347(01)63996-x. [DOI] [PubMed] [Google Scholar]
- 10.Mikolajczyk SD, Grauer LS, Millar LS, et al. A precursor form of PSA (pPSA) is a component of the free PSA in prostate cancer serum. Urology. 1997;50:710. doi: 10.1016/S0090-4295(97)00449-4. [DOI] [PubMed] [Google Scholar]
- 11.Mikolajczyk SD, Marks LS, Partin AW, et al. Free prostate-specific antigen in serum is becoming more complex. Urology. 2002;59:797. doi: 10.1016/s0090-4295(01)01605-3. [DOI] [PubMed] [Google Scholar]
- 12.Mikolajczyk SD, Millar LS, Wang TJ, et al. A precursor form of prostate-specific antigen is more highly elevated in prostate cancer compared with benign transition zone prostate tissue. Cancer Res. 2000;60:756. [PubMed] [Google Scholar]
- 13.Mikolajczyk SD, Catalona WJ, Evans CL, et al. Proenzyme forms of prostate-specific antigen in serum improve the detection of prostate cancer. Clin Chem. 2004;50:1017. doi: 10.1373/clinchem.2003.026823. [DOI] [PubMed] [Google Scholar]
- 14.Mikolajczyk SD, Marker KM, Millar LS, et al. A truncated precursor form of prostate-specific antigen is a more specific serum marker of prostate cancer. Cancer Res. 2001;61:6958. [PubMed] [Google Scholar]
- 15.Khan MA, Sokoll LJ, Chan DW, et al. Clinical utility of proPSA and "benign" PSA when percent free PSA is less than 15% Urology. 2004;64:1160. doi: 10.1016/j.urology.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 16.Mikolajczyk SD, Millar LS, Wang TJ, et al. "BPSA," a specific molecular form of free prostate-specific antigen, is found predominantly in the transition zone of patients with nodular benign prostatic hyperplasia. Urology. 2000;55:41. doi: 10.1016/s0090-4295(99)00372-6. [DOI] [PubMed] [Google Scholar]
- 17.Sokoll LJ, Chan DW, Mikolajczyk SD, et al. Proenzyme PSA for the early detection of prostate cancer in the 2.5–4.0 ng/ml total PSA range: preliminary analysis. Urology. 2003;61:274. doi: 10.1016/s0090-4295(02)02398-1. [DOI] [PubMed] [Google Scholar]
- 18.Sokoll LJ, Wang Y, Feng Z, et al. [−2] proenzyme prostate specific antigen for prostate cancer detection: a national cancer institute early detection research network validation study. J Urol. 2008;180:539. doi: 10.1016/j.juro.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan TY, Mikolajczyk SD, Lecksell K, et al. Immunohistochemical staining of prostate cancer with monoclonal antibodies to the precursor of prostate-specific antigen. Urology. 2003;62:177. doi: 10.1016/s0090-4295(03)00138-9. [DOI] [PubMed] [Google Scholar]
- 20.Catalona WJ, Bartsch G, Rittenhouse HG, et al. Serum pro prostate specific antigen improves cancer detection compared to free and complexed prostate specific antigen in men with prostate specific antigen 2 to 4 ng/ml. J Urol. 2003;170:2181. doi: 10.1097/01.ju.0000095460.12999.43. [DOI] [PubMed] [Google Scholar]
- 21.Catalona WJ, Bartsch G, Rittenhouse HG, et al. Serum pro-prostate specific antigen preferentially detects aggressive prostate cancers in men with 2 to 4 ng/ml prostate specific antigen. J Urol. 2004;171:2239. doi: 10.1097/01.ju.0000127737.94221.3e. [DOI] [PubMed] [Google Scholar]
- 22.de Vries SH, Raaijmakers R, Blijenberg BG, et al. Additional use of [−2] precursor prostate-specific antigen and "benign" PSA at diagnosis in screen-detected prostate cancer. Urology. 2005;65:926. doi: 10.1016/j.urology.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 23.Naya Y, Fritsche HA, Bhadkamkar VA, et al. Evaluation of precursor prostate-specific antigen isoform ratios in the detection of prostate cancer. Urol Oncol. 2005;23:16. doi: 10.1016/j.urolonc.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 26.Billis A, Guimarães MS, Freitas LL, et al. The impact of the 2005 international society of urological pathology consensus conference on standard Gleason grading of prostatic carcinoma in needle biopsies. J Urol. 2008;180:548. doi: 10.1016/j.juro.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 27.Crawford ED, Leewansangtong S, Goktas S, et al. Efficiency of prostate-specific antigen and digital rectal examination in screening, using 4.0 ng/ml and age-specific reference range as a cutoff for abnormal values. Prostate. 1999;38:296. doi: 10.1002/(sici)1097-0045(19990301)38:4<296::aid-pros5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 28.Pinsky PF, Andriole GL, Kramer BS, et al. Prostate biopsy following a positive screen in the prostate, lung, colorectal and ovarian cancer screening trial. J Urol. 2005;173:746. doi: 10.1097/01.ju.0000152697.25708.71. [DOI] [PubMed] [Google Scholar]