Abstract
Background aims
Mesenchymal stromal cells (MSC) have now been shown to reside in numerous tissues throughout the body, including the pancreas. Ex vivo culture-expanded MSC derived from many tissues display important interactions with different types of immune cells in vitro and potentially play a significant role in tissue homeostasis in vivo. In this study, we investigated the biologic and immunomodulatory properties of human pancreatic islet-derived MSC.
Methods
We culture-expanded MSC from cadaveric human pancreatic islets and characterized them using flow cytometry, differentiation assays and nuclear magnetic resonance-based metabolomics. We also investigated the immunologic properties of pancreatic islet-derived MSC compared with bone marrow (BM) MSC.
Results
Pancreatic islet and BM-derived MSC expressed the same cell-surface markers by flow cytometry, and both could differentiate into bone, fat and cartilage. Metabolomics analysis of MSC from BM and pancreatic islets also showed a similar set of metabolic markers but quantitative polymerase chain reactions showed that pancreatic islet MSC expressed more interleukin(IL)-1b, IL-6, STAT3 and FGF9 compared with BM MSC, and less IL-10. However, similar to BM MSC, pancreatic islet MSC were able to suppress proliferation of allogeneic T lymphocytes stimulated with anti-CD3 and anti-CD28 antibodies.
Conclusions
Our in vitro analysis shows pancreatic islet-derived MSC have phenotypic, biologic and immunomodulatory characteristics similar, but not identical, to BM-derived MSC. We propose that pancreatic islet-derived MSC could potentially play an important role in improving the outcome of pancreatic islet transplantation by promoting engraftment and creating a favorable immune environment for long-term survival of islet allografts.
Keywords: immunomodulation, mesenchymal stromal cells, pancreatic islets, pancreatic islet transplantation
Introduction
Type I diabetes mellitus, an autoimmune disorder leading to the destruction of insulin-producing pancreatic β-cells, represents an immense economic and social burden considering approximately 1 million people are currently afflicted with this disease in the USA (1). Although exogenous insulin administration is effective at preventing long-term complications of the disease, poor patient compliance and the inherent side-effects of using insulin, including hypoglycemic episodes, have prompted the search for curative measures (2). Pancreas transplantation provides a potentially curative approach; however, the procedure requires major surgery and life-long immunosuppressive drugs (3). Alternatively, pancreatic islet transplantation represents another possible curative option, and it is a much less invasive procedure. Pancreatic islets are collected from cadaveric donors and injected into the hepatic portal vein of the patient. In 2000, scientists from the University of Alberta (Edmonton, Canada) published a series of highly successful islet transplantations (4) by utilizing a novel protocol (the Edmonton protocol), which has revitalized the field. Despite this success, many challenges need to be resolved before islet transplantation becomes a practical therapeutic option. The issues of this procedure that must be addressed include the limited supply of available islets, technical difficulties inherent in isolation, culture, preservation and characterization of functional islets, and the need for two to three cadaveric donors for each recipient (5). As a longer follow-up of patients treated based on the Edmonton protocol revealed that many patients lose their originally achieved insulin independence (6), it is important to devise novel in vivo strategies to prolong engraftment and survival of the transplanted islets.
Bone marrow (BM)-derived MSC comprise an adherent, fibroblast-like cell population that are characterized by a combination of cell-surface markers and their potential to differentiate into bone, fat and cartilage (7,8). Over the last decade, enthusiasm about these cells has been fueled by a flurry of studies suggesting that BM-derived MSC not only differentiate into cells of mesodermal lineages, but also into cells of ectodermal and endodermal lineages, including pancreatic islets (9–13). This transdifferentiation potential originally provided the rationale for investigating the potential therapeutic effect of MSC in a variety of disorders, including pre-clinical models of type I diabetes mellitus (14). However, the degree of contribution of BM MSC to different tissues through transdifferentiation is now strongly debated (15–17). Nevertheless, BM MSC have become a very promising modality in human clinical cell therapies, and they are being actively investigated in many different clinical scenarios as new functional mechanisms for them are discovered. Specifically, exploitation of BM MSC in different clinical settings has been greatly facilitated by their unique immunomodulatory properties (18–23). Indeed, one of the most intriguing properties of ex vivo-expanded MSC is their ability to affect the immune response in vitro and in vivo through interaction with a broad range of immune cells, including T lymphocytes, B lymphocytes, natural killer (NK) cells, dendritic cells (DC), monocytes and macrophages (24–27). This study sought to isolate MSC from pancreatic islets and characterize their phenotypic, biologic, metabolomic and immunologic characteristics. Although derivation of MSC from pancreatic islets (28–30) has been reported previously, to our knowledge their immunomodulatory properties have not yet been investigated.
Methods
Isolation and maintenance of pancreatic islet MSC
Human pancreatic islets were obtained through the Integrated Islet Distribution Program (IIDP). In brief, following acquisition of written informed consent by the recovering Organ Procurement Organization (OPO), human pancreata were obtained from cadaveric organ donors sent to the IIDP islet isolation facility at the University of Wisconsin (UW; Madison, WI, USA) for processing. Our protocol was approved by the UW-Madison (Madison, WI, USA) Institutional Review Board (IRB). Islets from a total of nine donated pancreata, not suitable for clinical transplantation, were used. Approximately 9000–20,000 islet cell equivalents (IEQ) suspended in T175 flasks using Miami medium #1A were provided by the UW IIDP center and used for MSC derivation within 24 h of isolation. Islet cells were maintained in human MSC media composed of Minimum Essential Medium (αMEM) with 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA), 2 mM L-alanine-L-glutamine (Mediatech, Manassas, VA, USA) and 1% non-essential amino acids (Hyclone). Cells were cultured in a humidified incubator at 37°C and 5% CO2 and culture media were replaced every 2 days. When a flask reached confluency, adherent cells were harvested using TrypLE (Invitrogen, Carlsbad, CA, USA). Briefly, cells were washed with 5 mL phosphate-buffered saline (PBS) and then incubated with 2 mL 0.05% TrypLE for 5 min. An equivalent volume of MSC culture media was then added to neutralize TrypLE and cells were collected and underwent centrifugation at 1200 r.p.m. for 5 min to generate a cell pellet. Harvested cells were replated in a new flask at a density of 2000 cells/cm2.
Isolation and maintenance of BM MSC
Human BM MSC were isolated using a previously described protocol (25) from BM filters that were discarded at the end of clinical BM harvest; the protocol was approved by UW-Madison IRB. Briefly, filters were flushed with PBS (Hyclone) and mononuclear cells were isolated using Ficoll–Hypaque 1.073 (GE Lifesciences, Piscataway, NJ, USA). Red blood cells were lysed using ACK lysis buffer and the remaining cells were plated using human MSC media as described above.
Cell differentiation and characterization
Cell differentiation into osteogenic, adipogenic and chondrogenic lineages and subsequent detection was performed using established methodologies (31). Osteogenic, adipogenic and chondrogenic differentiation media were purchased from Miltenyi Biotech (Auburn, CA, USA) and used as per the recommendations of the manufacturer. Mineralization in osteogenic differentiation was detected by von Kossa staining (Sigma Inc., St Louis, MO, USA), whereas lipid accumulation in adipogenic differentiation was detected by Oil O Red stain (Sigma Inc.) Formalin-fixed paraffin-embedded (FFPE) chondrogenic pellets were stained with hematoxylin and eosin and Alcian Blue (Sigma Inc.) to detect glycosaminoglycans.
Flow cytometry analysis
Flow cytometry analysis was performed on pancreatic islet-derived MSC at passage (P) 4. Cell staining was done using the antibodies anti-CD14–fluorescein isothiocyanate (FITC), anti-CD29–phycoerythrin (PE), anti-CD34–allophyocyanin (APC), anti-CD44–PE, anti-CD73–PE, anti-CD90–APC, anti-CD105–APC, anti-HLA-DR–FITC (all BD Biosciences, San Diego, CA, USA), anti-CD31–APC, anti-CD45–PE, anti-HLA-ABC–FITC (all eBioscience, San Diego, CA, USA), and anti-CD54–FITC (Invitrogen). Cells were stained in cold PBS, 0.5 mM Ethylene-diaminetetraacetic acid (EDTA), 1% bovine serum albumin (BSA) and 0.05% NaN3 for 30 min at 4°C and then washed once before flow cytometry analysis. A total of 10,000–50,000 events was acquired using an Accuri C6 flow cytometer (Accuri, Ann Arbor, MI, USA) and data were analyzed with Flow Jo 7.0 (Tree Star Inc., Ashland, OR, USA).
Sample preparation for nuclear magnetic resonance
Cells were harvested using TrypLE and washed twice with PBS to remove trypsin. After a final centrifugation, all the liquid was aspirated and the cell pellets frozen. When all the samples were ready, cells were thawed on ice and immediately sonicated twice for 30–60 s with a small tip sonicator, followed by a centrifuge at 4000 r.p.m. for 10 min at 4°C. The supernatant was then dried in a speed-vacuum centrifuge overnight and stored at −80°C until time for nuclear magnetic resonance (NMR). The dried samples were resuspended with 99% Deuterium oxide (D2O) containing 0.1 mM NaF, 1 mM formate and 1 mM 4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS), with a final pH adjusted to 7.4. Both agents were used for referencing NMR chemical shifts and as internal standards for relative quantification of metabolite concentrations.
NMR data collection and analysis
All one-dimensional (1-D) 1H NMR spectra were collected, with a total of 512 transients for each, on a Varian NMR system, 600 MHz, equipped with a Cryoprobe at 25°C. Water suppression was achieved for all 1-D spectra by applying a weak saturation pulse (0.1 kHz field strength) of 2 s during the repetition delay, and an 80-ms spinlock pulse of 3.5 kHz was used prior to acquisition to suppress fast-relaxing signals from large molecules, such as proteins, leaving only peaks from slow-relaxing small metabolites. The software package vnmrJ 3 (Agilent Technologies, Colorado Springs, CO, USA) was used to process NMR data from five pancreatic islet MSC and six BM MSC. Spectra were analyzed both manually, and compared with high throughput binning of spectral data and with the Chenomx NMR Suite 5.1 software package (“http://www.chenomx.com” accessed Julu 2011) (Chenomx Inc, Edmonton, Canada) to identify and quantify metabolites (32). We used the online database MMCD (http://mmcd.nmfam.wisc.edu) to verify the identification of compounds.
Quantitative polymerase chain reaction assay
RNA was isolated from six pancreatic islet MSC and seven BM MSC using an RNeasy micro kit (Qiagen, Valencia, CA, USA), and the quality of isolated RNA was checked using Nanodrop 1000 (Fisher Scientific, Pittsburgh, PA, USA). RNA was converted to cDNA using a Quantitect reverse transcription kit (Qiagen). A quantitative polymerase chain reaction (PCR) assay was performed using Power SYBR green master mix (Applied Biosystems, Foster City, CA, USA) on a StepOne Plus instrument (Applied Biosystems) using a standard protocol. Verified primers were purchased from Qiagen. The threshold cycle (Ct) value for each gene was normalized with the average Ct number of three housekeeping genes (18S rRNA, GAPDH and b-Actin).
Interferon-γ challenge of pancreatic islet MSC
Pancreatic islet-derived MSC from seven different donors and BM-derived MSC from three different donors were plated at a density of 200 000/well in six-well plates. One well from each MSC group was treated with 200 IU interferon (IFN)-γ at day 0 and another well at day 2. Cells were then harvested at day 4 by trypsinization and stained with HLA-ABC–FITC, HLA-DR–PE, CD80–FITC, and CD86–PE (all eBioscience) using the protocol described above for the flow cytometry analysis. Cells without IFN-γ treatment were used as a control.
Lymphocyte proliferation suppression assay
Peripheral blood mononuclear cells were stained with carboxyfluorescein diacetate succinimidyl ester (CFSE; Sigma-Aldrich, St Louis, MO, USA) to track proliferation (33,34). Briefly, CFSE was added to cell suspensions at a concentration of 2 μM and incubated for 15 min at 37°C. Cells were washed twice with fresh R10 media (RPMI-1640 basal media supplemented with 10% FBS) to wash out excessive CFSE and then cultured with BM- or pancreatic islet-derived MSC at various concentrations in R10 media. Forty-eight-well plates were used and 100,000 lymphocytes were added per well. Lymphocytes were stimulated with a combination of anti-CD3 and anti-CD28 antibodies (RnD Systems, Minneapolis, MN, USA) at a concentration of 500 μg/mL for 4 days. Floating cells were harvested and stained with CD4–PE and CD8–APC (BD Biosciences) and then analyzed with the Accuri C6 using the CFlow plus program (Accuri cytometers). Proliferation assay analysis was performed using ModFit 3.2 (Verity software, Topsham, ME, USA). Briefly, a model histogram was generated to fit all the actual results with minimal deviation. The proliferation index (PI), which reflects the proliferative capacity of responding cells, is the average number of cell divisions that the responding cells have undergone. The PI was calculated by dividing the total number of cell divisions with the number of cells that had undergone division (33,34). For each experimental condition, the PI was normalized by dividing it with the PI of a control (stimulated lymphocytes without added MSC).
Statistical analysis
A t-test was performed using a GraphPad prism 5 software package (GraphPad Software, La Jolla, CA, USA). Statistical analysis of the metabolites was performed using two-tailed t-tests assuming type I errors. Differences were considered significant with P < .05 unless otherwise stated. The normality of metabolite data was confirmed by fitting 95% of the data within the range of the mean ± two standard deviations (SD).
Results
Derivation of MSC from pancreatic islets
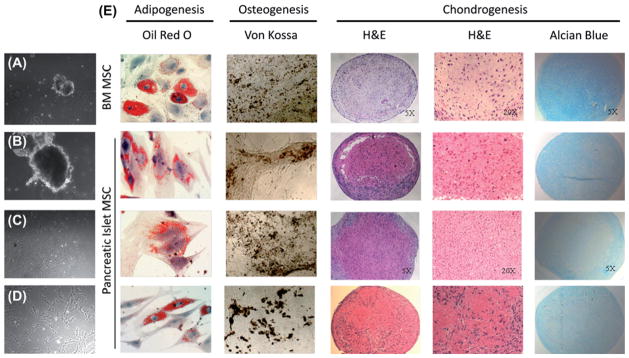
Human pancreatic islet cell equivalents cultured in the presence of MSC media (Figure 1A, B) started to generate spindle-shaped cells (Figure 1C) from the periphery of plated islet equivalents about 1 week after initial plating. Upon passaging cells, pancreatic islet MSC assumed a homogenous spindle-shaped morphology similar to that of other well-established MSC such as BM MSC (Figure 1D) and maintained MSC-like morphology up to P10 (data not shown). Pancreatic islet-derived MSC were able to differentiate into osteogenic, adipogenic and chondrogenic lineages when cultured in respective differentiation media (Figure 1E), satisfying the differentiation potential criteria of MSC. From 9000 to 20,000 IEQ, and between 1 × 106 and 2 × 106 cells, were harvested at P0. At a plating density of 2000 cells/cm2, up to 2 × 109 cells could be generated by P4.
Figure 1.
Derivation of pancreatic islet MSC (A–D). Pancreatic islets are plated in MSC media (A–B). Spindle shaped cells are visible by day 7 (C) and upon repeated passaging they become a homogenous population of MSC (D). Differentiation assays of BM MSC and three lines of pancreatic islet MSC (E). Adipogenic differentiated cells were stained with Oil O Red staining for detection of lipid deposition (orange/red). Osteogenic differentiation was detected by brown mineralization using von Kossa staining. Chondrogenic differentiated pellets were stained with hematoxylin and eosin for evaluation of chondrocyte morphology at both 5 × and 20 × magnification, and Alcian blue staining for detection of glycosaminoglycans (blue).
Flow cytometry analysis
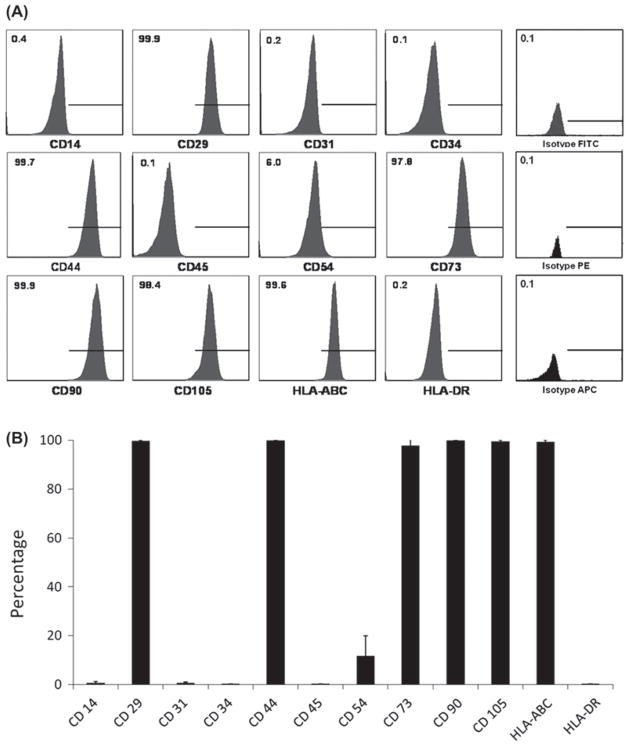
Flow cytometric analysis of nine MSC lines derived from human pancreatic islets at P4 showed that they did not express CD14 (0.69 ± 0.52%), CD31 (0.61 ± 0.49%), CD34 (0.13 ± 0.09%), CD45 (0.13 ± 0.09%) and HLA DR (0.17 ± 0.05%) markers. CD54 was generally negative but was expressed at a low level in some of the pancreatic islet MSC lines (11.80 ± 8.19%). Pancreatic islet MSC were all positive for CD29 (99.62 ± 0.48%), CD44 (99.89 ± 0.12%), CD73 (97.82 ± 3.34%), CD90 (99.81 ± 0.11%), CD105 (99.57 ± 0.45%) and HLA ABC (99.36 ± 0.69%) (Figure 2A). This cell-surface expression pattern corresponded well with results from more well-established MSC such as BM MSC (31). Flow cytometric analysis was performed for all nine pancreatic islet-derived MSC lines, and the results are summarized in Figure 2B.
Figure 2.
Cell-surface marker expression pattern of pancreatic islet MSC. (A) Typical cell-surface marker expression of pancreatic islet MSC. (B) Expression pattern of all nine pancreatic islet MSC.
NMR metabolic analysis of pancreatic islet and BM MSC
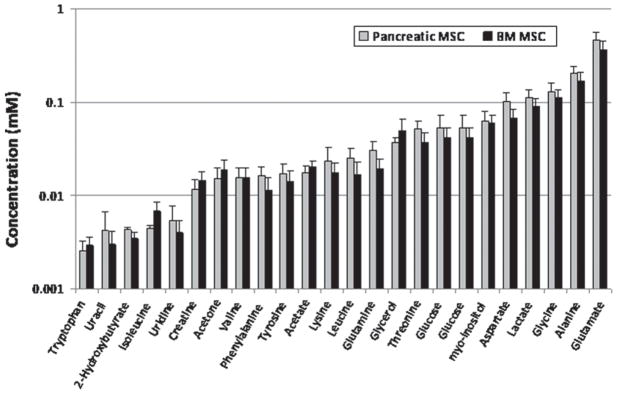
A set of metabolites was identified and quantified in cells prepared from pancreatic islet and BM MSC by NMR spectral analysis. These metabolites comprised a set of amino acids (such as alanine, glutamate, glutamine, glycine, tyrosine and phenylalanine), nucleotides (uracil and uridine), organic acids and ketogenic intermediates (2-hydroxybutyrate, lactate, acetate and acetone). The relative concentrations of the metabolites to the internal reference standards (DSS and formate) were measured and are shown in bar graphs in Figure 3. Although glutamate and alanine were increased in pancreatic islet-derived MSC compared with BM MSC, these differences did not reach statistical significance (P-values of 0.53 and 0.48, respectively).
Figure 3.
Metabolomic profiles of pancreatic islet and BM MSC. The x-axis indicates metabolites and the y-axis shows concentrations of metabolites relative to the internal references (formate and DSS) at 0.1 mM. Standard error bars are shown for all metabolites as calculated using two-tailed t-tests.
Quantitative PCR analysis
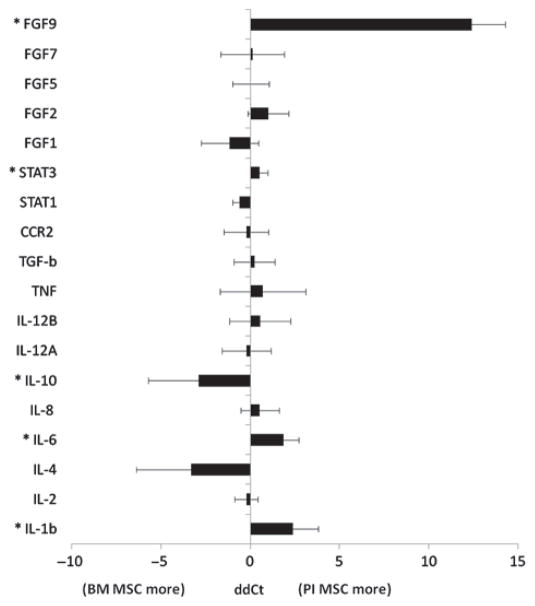
A total of six human BM MSC lines and seven pancreatic islet MSC lines was used for quantitative polymerase chain reaction (qPCR) analysis. Three housekeeping genes (18S rRNA, GAPDH and b-actin) were averaged and then used for normalization of the 17 genes of interest [interleukin (IL)-1b, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12A, IL-12B, Tumor necrosis factor (TNF), Chemokine (C-C motif) receptor (CCR2), Signal transducer and activator of transcription (STAT1), STAT3, Fibroblast growth factor (FGF1), FGF2, FGF5, FGF7 and FGF9]. These genes were chosen because of their known role in either MSC or pancreatic islet biology. The comparison of pancreatic islet MSC and BM MSC yielded five genes that were differentially regulated between human BM MSC and human pancreatic islet MSC in a statistically significant way (P < 0.05). IL-1b (P < 0.0042), IL-6 (P < 0.0097), STAT3 (P < 0.0123) and FGF9 (P < 0.0001) were more abundant in pancreatic islet MSC, while IL-10 (P < 0.0197) was more abundant in BM MSC (Figure 4).
Figure 4.
Comparison of BM MSC and pancreatic MSC using quantitative PCR. *P < 0.05.
IFN-γ challenge of pancreatic islet MSC
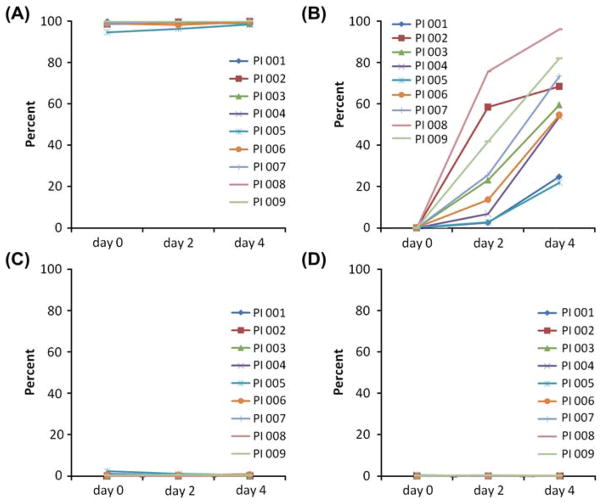
The expression level of HLA-ABC was high in all the MSC lines tested, and IFN-γ challenge did not generate noticeable changes (day 0, 98.5 ± 1.8%; day 2, 99.2 ± 1.3%; day 4, 99.5 ± 0.7%; Figure 5A). After IFN-γ challenge, pancreatic islet MSC expressed higher levels of HLA-DR compared with non-challenged cells at day 2 (32.9 ± 27.5%), which increased further by day 4 (60.9 ± 28.0%; Figure 5B). CD80 and CD86 were not expressed on pancreatic islet MSC at baseline, and their expression level did not change after 2 or 4 days of IFN-γ treatment (Figure 5C, D). These results were comparable with reported effects of IFN-γ challenge on BM MSC (35).
Figure 5.
Expression of cell-surface markers on pancreatic islet MSC before and after IFN-γ challenge. HLA-ABC (A), HLA-DR (B), CD80 (C) and CD86 (D).
Lymphocyte proliferation suppression assay
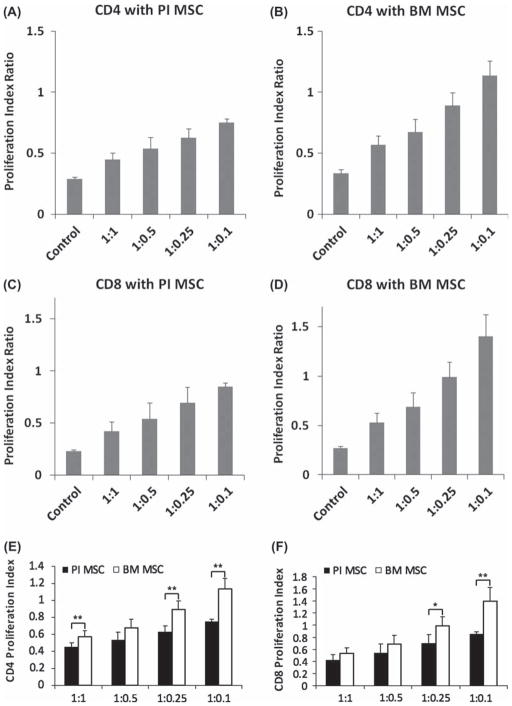
To determine the proliferation of T lymphocytes in the presence of pancreatic islet-derived MSC, lymphocytes were stained with CFSE and cultured for 4 days with various ratios of MSC. Five pancreatic islet MSC lines and three BM MSC lines were used for these experiments. After harvesting floating cells, they were stained with CD4 and CD8 to gate respective lymphocyte groups. The number of lymphocytes that remained attached to MSC was minimal after washing by gentle pipetting. Representative raw flow data are depicted in Supplementary Figure 1 to be found online at http://www.informahealthcare.com/cyt/doi/10.3109/14653249.2012.684376. Briefly, lymphocytes were gated for CD4-positive and CD8-positive cells and then CFSE signal was analyzed using software as described in the Methods (see Supplementary Figure 1A). Upon each cell division the signal intensity of CFSE decreases as each daughter cell retains approximately half the amount of CFSE compared with the parent population. Co-culture of stimulated lymphocytes with pancreatic islet MSC at 200,000/ well increased the CFSE signal intensity compared with stimulated lymphocyte-only controls, and similar results were observed with BM MSC (see Supplementary Figure 1C). Both BM MSC and pancreatic islet MSC exhibited suppression of lymphocyte proliferation at a high MSC to lymphocyte ratio, but this suppressive effect was decreased at lower concentrations of MSC. The effect of MSC on lymphocyte suppression was observed for both CD4-positive helper T cells (Figure 6A, B) and CD8-positive cytotoxic T cells (Figure 6C, D). For CD4 lymphocytes, the PI of each group divided by the control (no MSC) group was 0.45 ± 0.05(lymphocyte:MSCata1:1ratio),0.54± 0.09 (1:0.5), 0.63 ± 0.07 (1:0.25) and 0.75 ± 0.03 (1:0.1) for pancreatic islet MSC, and 0.57 ± 0.07 (1:1), 0.68 ± 0.10 (1:0.5), 0.89 ± 0.11 (1:0.25) and 1.13 ± 0.12 (1:0.1) for BM MSC. CD4 lymphocytes without stimulation had a PI of 0.31 ± 0.03. In the case of CD8 lymphocytes, the PI were 0.42 ± 0.09 (lymphocyte:MSC at a ratio of 1:1), 0.54 ± 0.15 (1:0.5), 0.69 ± 0.15 (1:0.25) and 0.85 ± 0.03 (1:0.1) for pancreatic islet MSC, and 0.53 ± 0.10 (1:1), 0.69 ± 0.14 (1:0.5), 0.99 ± 0.15 (1:0.25) and 1.40 ± 0.22 (1:0.1) for BM MSC. The no stimulation control for CD8 lymphocytes had a relative PI of 0.25 ± 0.02. Interestingly, pancreatic islet MSC exhibited suppression of lymphocyte proliferation even at low MSC:lymphocyte ratios, while BM MSC failed to demonstrate comparable inhibitory effects on lymphocyte proliferation at these low ratios (Figure 6E, F).
Figure 6.
Lymphocyte proliferation suppression assay. Stimulated lymphocytes without any added MSC were used as a control (PI 1.0). (A, B) CD4-positive lymphocyte proliferation in co-culture with pancreatic islet MSC (A) and BM MSC (B). (C, D) CD8-positive lymphocyte proliferation in co-culture with pancreatic islet MSC (C) and BM MSC (D). (E, F) Comparison of pancreatic islet MSC and BM MSC in terms of lymphocyte proliferation suppression. (E) CD4 lymphocytes. (F) CD8 lymphocytes. *P < 0.05, **P < 0.01.
Discussion
Despite tremendous progress in the field of pancreatic islet transplantation since the 1970s, there still remains a need for novel strategies to prevent the destruction of islet allografts post-transplantation. In general, graft failure after transplantation arises from a multitude of effects, including inadequate revascularization, autoimmune-mediated destruction, inflammatory reactions and allogeneic immunologic rejection. Unfortunately, the diversity of the reactions leading to islet allograft rejection is beyond the scope of systemic immunosuppression alone. Therefore, research efforts must focus on new therapeutic avenues that have more global effects and capabilities to improve islet survival, and we believe MSC could represent the key to improving pancreatic islet transplantation. Substantial in vitro and in vivo data, including recent promising results in several clinical trials, suggest that MSC derived from BM or other tissues promote regeneration of many different tissues through a variety of mechanisms, such as secretion of angiogenic factors, trophic cytokines and growth factors, as well as modulation of inflammatory reactions and immunologic responses (36–40). BM-derived MSC are being investigated in an ever increasing multitude of clinical trials because of their ease of isolation and expansion, lack of infusional toxicity, and the fact that these cells can be administered safely without the need for HLA matching (41). Thus it is conceivable that BM MSC could become a therapeutic modality for use in pancreatic islet transplantation (42–44). However, we hypothesize that pancreatic islet MSC retain theoretical advantages in pancreatic islet transplantation compared with MSC from BM or other tissues. For example, pancreatic islet-derived MSC could potentially provide a more suitable feeder layer for pancreatic islet co-culture ex vivo compared with other cells that have been used for the same purpose, such as small intestinal submucosa (45,46).
Not surprisingly, pancreatic islet MSC showed cell-surface marker characteristics and differentiation potential similar to BM MSC. Additionally, we used an NMR metabolomics approach to study their cell metabolism at the molecular level. Metabolomics is a fast, sensitive and economical approach to scanning quickly for metabolic processes in cells or animals (47,48). The metabolome comparison between the two types of MSC indicated that the two cell derivatives had a very similar metabolism and their differences between the two types of cells did not reach statistical significance. Surprisingly, glutamine, one of the most abundant amino acids in cells, was found to be at significantly lower concentrations than its toxic counterpart glutamate in both pancreatic and BM MSC, possibly indicating a larger degradation of glutamine to glutamate via a shift toward glutamate–alanine pathways in both types of MSC. At the level of gene expression, distinct differences between pancreatic islet MSC and BM MSC included increased expression of IL-1b, IL-6 and STAT3 and decreased expression of IL-10. However, we cannot speculate about the impact of these biologic differences on the intended immunomodulatory effects of these cells. One interesting finding is that FGF9 is expressed at very high levels in pancreatic islet MSC compared with BM MSC. FGF9 have been reported to interact with FGFR3 and regulate proliferation of pancreatic epithelial cells, and the expression level of FGF9 increases during pancreatic regeneration (49–51). Higher levels of FGF9 expression in pancreatic islet MSC could make them advantageous compared with BM MSC for the purpose of pancreatic islet regeneration or protection.
The main purpose of our study was to investigate the immunomodulatory properties of MSC derived from cadaveric pancreatic islets. Our results show that pancreatic islet MSC exhibit all the characteristics of BM MSC, including an increase in the level of HLA-DR upon stimulation with IFN-γ and the ability to suppress proliferation of stimulated lymphocytes (52). Indeed, in our study, pancreatic islet MSC suppressed proliferation of lymphocytes even when the ratios of MSC to lymphocytes were lower, while BM MSC were less inhibitory or had no inhibitory effect at these ratios; this is in line with previous reports in which low ratios of BM MSC are not as suppressive (53–55). The immunomodulatory properties of BM-derived MSC and their ability to suppress proliferation of activated lymphocytes is well known, and the fact that pancreatic islet MSC exhibit similar traits is very encouraging.
Currently, use of BM-derived MSC in the context of islet transplantation is being investigated in a limited number of clinical trials. Based on our results, we propose that in the future it will be conceivable to generate banks of MSC from a few high-quality pancreatic islet sources and use them as universal donor cells for immunomodulation and improvement of engraftment after pancreatic islet transplantation. The number of islet equivalents harvested from a single donor varies between 300,000 to 600,000, and we could consistently derive MSC from 9000–20,000 islet equivalents. By deriving pancreatic islet MSC from a small portion of islets intended for transplantation, it is also possible to exploit these autogenic MSC later for boosting engraftment of transplanted islets or delaying their loss. Our results suggest that pancreatic islet MSC could be a useful alternative to BM MSC as a cell-based therapy targeted toward pancreatic islet transplantation, or even treatment of diabetes mellitus, because of their immunosuppressive properties and by providing a microenvironment more suitable for pancreatic islet cell survival. Our report could pave the way for further investigation of the potential therapeutic applicability of pancreatic islet MSC.
Supplementary Material
Acknowledgments
We would like to thank the Integrated Islet Distribution Program (IIDP) and University of Wisconsin IIDP Center, especially Dr. Luis Fernandez, for generously providing pancreatic islet samples. We would also like to acknowledge the Experimental Histology Core Facility at the University of Wisconsin Carbone Cancer Center for processing and staining of chondrogenic pellets.
This work was supported by NHLBI-NIH K08 HL081076 grant to PH, NIH R01 DC009018 and RC4EY021357 to FPA, and P41 RR 02301 from the NIH Center for Research Resources.
Footnotes
Author contribution summary: Jaehyup Kim, conception and design, performing experiments, collection of data, data analysis, manuscript writing; Melissa J. Breunig, performing experiments, collection of data; Leah E. Escalante, performing experiments; Neehar Bhatia, performing experiments, collection of data, data analysis; Ryan A. Denu, performing experiments, Bridget A. Dollar, performing experiments; Andrew P. Stein, performing experiments; Summer E. Hanson, performing experiments, collection of data; Nadia Naderi, data analysis; James Radek, NMR data collection; Dermot Haughy, NMR data processing and analysis; Debra D. Bloom, data analysis; Fariba M. Porter-Assadi, NMR data interpretation, manuscript writing; Peiman Hematti, conception and design, provision of study material, data analysis and interpretation, manuscript writing.
Disclosure of potential conflicts of interest: The authors have declared that no potential conflicts of interests exist.
Supplementary material available online
References
- 1.Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997;14(Suppl 5):S1–85. [PubMed] [Google Scholar]
- 2.Bloomgarden ZT. Diabetes complications. Diabetes Care. 2004;27:1506–14. doi: 10.2337/diacare.27.6.1506. [DOI] [PubMed] [Google Scholar]
- 3.Robertson RP, Davis C, Larsen J, Stratta R, Sutherland DE. Pancreas and islet transplantation for patients with diabetes. Diabetes Care. 2000;23:112–6. doi: 10.2337/diacare.23.1.112. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immuno-suppressive regimen. New Engl J Med. 2000;343:230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 5.Neidlinger NA, Odorico JS, Sollinger HW, Fernandez LA. Can ‘extreme’ pancreas donors expand the donor pool? Curr Opinion Organ Transplant. 2008;13:67–71. doi: 10.1097/MOT.0b013e3282f44a51. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, et al. International trial of the Edmonton protocol for islet transplantation. New Engl J Med. 2006;355:1318–30. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 7.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–47. [PubMed] [Google Scholar]
- 8.Battiwalla M, Hematti P. Mesenchymal stem cells in hematopoietic stem cell transplantation. Cytotherapy. 2009;11:503–15. doi: 10.1080/14653240903193806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moriscot C, de Fraipont F, Richard MJ, Marchand M, Savatier P, Bosco D, et al. Human bone marrow mesenchymal stem cells can express insulin and key transcription factors of the endocrine pancreas developmental pathway upon genetic and/or microenvironmental manipulation in vitro. Stem Cells. 2005;23:594–603. doi: 10.1634/stemcells.2004-0123. [DOI] [PubMed] [Google Scholar]
- 10.Kadam SS, Sudhakar M, Nair PD, Bhonde RR. Reversal of experimental diabetes in mice by transplantation of neo-islets generated from human amnion-derived mesenchymal stromal cells using immuno-isolatory macrocapsules. Cytotherapy. 2010;12:982–91. doi: 10.3109/14653249.2010.509546. [DOI] [PubMed] [Google Scholar]
- 11.Phadnis SM, Joglekar MV, Dalvi MP, Muthyala S, Nair PD, Ghaskadbi SM, et al. Human bone marrow-derived mesenchymal cells differentiate and mature into endocrine pancreatic lineage in vivo. Cytotherapy. 2011;13:279–93. doi: 10.3109/14653249.2010.523108. [DOI] [PubMed] [Google Scholar]
- 12.Chang C, Niu D, Zhou H, Zhang Y, Li F, Gong F. Mesenchymal stromal cells improve hyperglycemia and insulin deficiency in the diabetic porcine pancreatic microenvironment. Cytotherapy. 2008;10:796–805. doi: 10.1080/14653240802461924. [DOI] [PubMed] [Google Scholar]
- 13.Limbert C, Path G, Ebert R, Rothhammer V, Kassem M, Jakob F, et al. PDX1- and NGN3-mediated in vitro reprogramming of human bone marrow-derived mesenchymal stromal cells into pancreatic endocrine lineages. Cytotherapy. 2011;13:802–13. doi: 10.3109/14653249.2011.571248. [DOI] [PubMed] [Google Scholar]
- 14.Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA. 2006;103:17438–43. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phinney DG, Prockop DJ. Concise review. Mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair. Current views. Stem Cells. 2007;25:2896–902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 16.Horwitz EM, Dominici M. How do mesenchymal stromal cells exert their therapeutic benefit? Cytotherapy. 2008;10:771–4. doi: 10.1080/14653240802618085. [DOI] [PubMed] [Google Scholar]
- 17.Tolar J, Le Blanc K, Keating A, Blazar BR. Concise review. Hitting the right spot with mesenchymal stromal cells. Stem Cells. 2010;28:1446–55. doi: 10.1002/stem.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Blanc K, Ringden O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:321–34. doi: 10.1016/j.bbmt.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Prockop DJ, Olson SD. Clinical trials with adult stem/progenitor cells for tissue repair: let’s not overlook some essential precautions. Blood. 2007;109:3147–51. doi: 10.1182/blood-2006-03-013433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stagg J, Galipeau J. Immune plasticity of bone marrow-derived mesenchymal stromal cells. Handbk Exp Pharmacol. 2007;180:45–66. doi: 10.1007/978-3-540-68976-8_3. [DOI] [PubMed] [Google Scholar]
- 21.Kode JA, Mukherjee S, Joglekar MV, Hardikar AA. Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy. 2009;11:377–91. doi: 10.1080/14653240903080367. [DOI] [PubMed] [Google Scholar]
- 22.Le Blanc K. Mesenchymal stromal cells: tissue repair and immune modulation. Cytotherapy. 2006;8:559–61. doi: 10.1080/14653240601045399. [DOI] [PubMed] [Google Scholar]
- 23.Le Blanc K, Pittenger M. Mesenchymal stem cells: progress toward promise. Cytotherapy. 2005;7:36–45. doi: 10.1080/14653240510018118. [DOI] [PubMed] [Google Scholar]
- 24.Sundin M, D’Arcy P, Johansson CC, Barrett AJ, Lonnies H, Sundberg B, et al. Multipotent mesenchymal stromal cells express FoxP3: a marker for the immunosuppressive capacity? J Immunother. 2011;34:336–42. doi: 10.1097/CJI.0b013e318217007c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445–53. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 27.Keating A. How do mesenchymal stromal cells suppress T cells? Cell Stem Cell. 2008;2:106–8. doi: 10.1016/j.stem.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Carlotti F, Zaldumbide A, Loomans CJ, van Rossenberg E, Engelse M, de Koning EJ, et al. Isolated human islets contain a distinct population of mesenchymal stem cells. Islets. 2010;2:164–73. doi: 10.4161/isl.2.3.11449. [DOI] [PubMed] [Google Scholar]
- 29.Davani B, Ikonomou L, Raaka BM, Geras-Raaka E, Morton RA, Marcus-Samuels B, et al. Human islet-derived precursor cells are mesenchymal stromal cells that differentiate and mature to hormone-expressing cells in vivo. Stem Cells. 2007;25:3215–22. doi: 10.1634/stemcells.2007-0323. [DOI] [PubMed] [Google Scholar]
- 30.Karaoz E, Ayhan S, Gacar G, Aksoy A, Duruksu G, Okcu A, et al. Isolation and characterization of stem cells from pancreatic islet: pluripotency, differentiation potential and ultrastructural characteristics. Cytotherapy. 2010;12:288–302. doi: 10.3109/14653240903580296. [DOI] [PubMed] [Google Scholar]
- 31.Hanson SE, Kim J, Johnson BH, Bradley B, Breunig MJ, Hematti P, et al. Characterization of mesenchymal stem cells from human vocal fold fibroblasts. Laryngoscope. 2010;120:546–51. doi: 10.1002/lary.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang D, Weljie A, Newton J. Leveraging latent information in NMR spectra for robust predictive models. Pac Symp Biocomput. 2007:115–26. [PubMed] [Google Scholar]
- 33.Hasbold J, Gett AV, Rush JS, Deenick E, Avery D, Jun J, et al. Quantitative analysis of lymphocyte differentiation and proliferation in vitro using carboxyfluorescein diacetate succinimidyl ester. Immunol Cell Biol. 1999;77:516–22. doi: 10.1046/j.1440-1711.1999.00874.x. [DOI] [PubMed] [Google Scholar]
- 34.Lyons AB, Hasbold J, Hodgkin PD. Flow cytometric analysis of cell division history using dilution of carboxyfluorescein diacetate succinimidyl ester, a stably integrated fluorescent probe. Methods Cell Biol. 2001;63:375–98. doi: 10.1016/s0091-679x(01)63021-8. [DOI] [PubMed] [Google Scholar]
- 35.Trivedi P, Hematti P. Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Exp Hematol. 2008;36:350–9. doi: 10.1016/j.exphem.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uccelli A, Pistoia V, Moretta L. Mesenchymal stem cells: a new strategy for immunosuppression? Trends Immunol. 2007;28:219–26. doi: 10.1016/j.it.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Keating A. Mesenchymal stromal cells. Curr Opin Hematol. 2006;13:419–25. doi: 10.1097/01.moh.0000245697.54887.6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prockop DJ. ‘Stemness’ does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs) Clinical Pharmacol Ther. 2007;82:241–3. doi: 10.1038/sj.clpt.6100313. [DOI] [PubMed] [Google Scholar]
- 39.Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28:875–84. doi: 10.1016/s0301-472x(00)00482-3. [DOI] [PubMed] [Google Scholar]
- 40.Dazzi F, Horwood NJ. Potential of mesenchymal stem cell therapy. Curr Opin Oncol. 2007;19:650–5. doi: 10.1097/CCO.0b013e3282f0e116. [DOI] [PubMed] [Google Scholar]
- 41.Tolar J, Villeneuve P, Keating A. Mesenchymal stromal cells for graft-versus-host disease. Human Gene Ther. 2011;22:257–62. doi: 10.1089/hum.2011.1104. [DOI] [PubMed] [Google Scholar]
- 42.Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes. 2008;57:1759–67. doi: 10.2337/db08-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berman DM, Willman MA, Han D, Kleiner G, Kenyon NM, Cabrera O, et al. Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes. 2010;59:2558–68. doi: 10.2337/db10-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hematti P. Role of mesenchymal stromal cells in solid organ transplantation. Transplant Rev. 2008;22:262–73. doi: 10.1016/j.trre.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woods EJ, Walsh CM, Sidner RA, Zieger MA, Lakey JR, Ricordi C, et al. Improved in vitro function of islets using small intestinal submucosa. Transplant Proc. 2004;36:1175–7. doi: 10.1016/j.transproceed.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 46.Jung EJ, Kim SC, Wee YM, Kim YH, Choi MY, Jeong SH, et al. Bone marrow-derived mesenchymal stromal cells support rat pancreatic islet survival and insulin secretory function in vitro. Cytotherapy. 2011;13:19–29. doi: 10.3109/14653249.2010.518608. [DOI] [PubMed] [Google Scholar]
- 47.Khoo SH, Al-Rubeai M. Metabolomics as a complementary tool in cell culture. Biotechnol App Biochem. 2007;47:71–84. doi: 10.1042/BA20060221. [DOI] [PubMed] [Google Scholar]
- 48.Cuperlovic-Culf M, Barnett DA, Culf AS, Chute I. Cell culture metabolomics: applications and future directions. Drug Discov Today. 2010;15:610–21. doi: 10.1016/j.drudis.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 49.Arnaud-Dabernat S, Kritzik M, Kayali AG, Zhang YQ, Liu G, Ungles C, et al. FGFR3 is a negative regulator of the expansion of pancreatic epithelial cells. Diabetes. 2007;56:96–106. doi: 10.2337/db05-1073. [DOI] [PubMed] [Google Scholar]
- 50.Dichmann DS, Miller CP, Jensen J, Scott Heller R, Serup P. Expression and misexpression of members of the FGF and TGFbeta families of growth factors in the developing mouse pancreas. Dev Dynamics. 2003;226:663–74. doi: 10.1002/dvdy.10270. [DOI] [PubMed] [Google Scholar]
- 51.Gittes GK. Developmental biology of the pancreas: a comprehensive review. Dev Biol. 2009;326:4–35. doi: 10.1016/j.ydbio.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 52.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–6. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 53.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 54.Gotherstrom C, Ringden O, Tammik C, Zetterberg E, Westgren M, Le Blanc K. Immunologic properties of human fetal mesenchymal stem cells. Am J Obstet Gynecol. 2004;190:239–45. doi: 10.1016/j.ajog.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 55.Najar M, Rouas R, Raicevic G, Boufker HI, Lewalle P, Meuleman N, et al. Mesenchymal stromal cells promote or suppress the proliferation of T lymphocytes from cord blood and peripheral blood: the importance of low cell ratio and role of interleukin-6. Cytotherapy. 2009;11:570–83. doi: 10.1080/14653240903079377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.