Abstract
Current practices to maintain human pluripotent stem cells (hPSCs), which include induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs), in an undifferentiated state typically depend on the support of feeder cells such as mouse embryonic fibroblasts (MEFs), or an extracellular matrix such as Matrigel™. Culture conditions that depend on these undefined support systems limit our ability to interpret mechanistic studies aimed at resolving how hPSCs interact with their extracellular environment to remain in a unique undifferentiated state and to make fate-changing lineage decisions. Likewise, the xenogeneic components of MEFs and Matrigel™ ultimately hinder our ability to use pluripotent stem cells to treat debilitating human diseases. Many of these obstacles have been overcome by the development of synthetic coatings and bioreactors that support hPSC expansion and self-renewal within defined culture conditions that are free from xenogeneic contamination. The establishment of defined culture conditions andsynthetic matrices will facilitate studies tomore precisely probe the molecular basis of pluripotent stem cell self-renewal and differentiation. When combined with 3D cultures in bioreactors, these systems will also enable large-scale expansion for future clinical applications.
Keywords: pluripotent stem cells, human embryonic stem cells, induced pluripotent stem cells, polymer coatings, self-renewal, differentiation
Introduction
Like other stem cells, human pluripotent stem cells (hPSC) have the capacity for self-renewal and differentiation into specialized cell types. However, pluripotent cells are unique in their ability to self-renew indefinitely. They also feature the capacity to differentiate into all of the approximately 200 specialized cell types of the body. These two fundamental characteristics make hPSCs a potential source of cells for regenerative medicine, drug discovery, disease modeling and studies aimed to better understand human development. Human embryonic stem cells (hESCs) are isolated from the inner cell mass of the blastocyst1, while human induced pluripotent stem cells (hiPSCs) are derived by the over expression of key transcription factors in somatic cells2, 3. Both of these PSCs share the expression of transcription factors (Oct4, Sox2 and Nanog), cell surface markers such as the stage-specific embryonic antigens (SSEA) -3 and -4, the keratan sulphate-related antigens (TRA-1-60 and TRA-1-81), high telomerase and alkaline phosphatase activity, as well as the capacity to grow indefinitely in vitro when cultured under permissive conditions.
To maximize the potential of PSCs in regenerative medicine and for future transplantation studies, in vitro derivation and continuous culture conditions need to be performed using good manufacturing practices (GMP). This objective was clear from the first derivation and prolonged culture of hESCs1 and subsequently arapid evolution in derivation and culture methods has been realized. The early culture conditions for hESCs were determined by effectively following the methods developed for mouse ESCs4. These early methods included co-culture of hESCs with irradiated mouse embryonic fibroblasts (MEF) in an enriched culture medium containing fetal bovine serum. It soon became evident, however, that hESCs and mouse ESCs requirements for self-renewal are distinct. The principle difference between the two species is that the growth of undifferentiated hESCs cannot be maintained in feeder-free conditions in the presences of leukemia inhibitory factor (LIF), as it is possible for mouse ESCs5.
Since the initial description of the successful derivation and culture of hESCs1, several hundred lines of human ESCs and iPSCs have been derived and investigation of their biologic characteristics has contributed to the identification of key molecular pathways and transcription factors that are involved in the self-renewal and lineage differentiation of PSCs. This in turn has been translated into knowledge to optimize the culture conditions of PSCs. In this concise review we summarize the evolution in hPSC culture and place an emphasis on the use of synthetic coatings as substrates to support the unlimited proliferation of hPSCs in vitro (Fig. 1 and Table I).
Fig. 1. Evolution of human pluripotent stem cell (hPSC) culture.
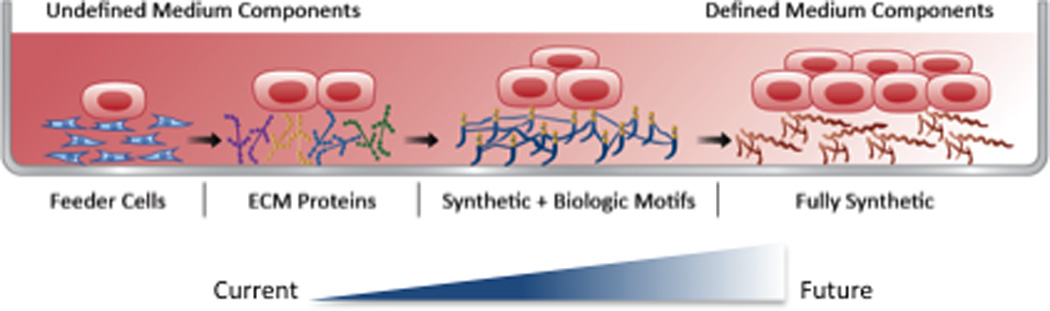
The in vitro culture of hPSCs has evolved to achieve xenogeneic-free and defined conditions. The illustration depicts this progression (left to right) from co-culture with feeder-cells and serum-containing medium, to feeder-independent cultures in chemical-defined medium. Feeder-free conditions have progressed from the use of complex combinations of extracellular matrix (ECM) proteins like Matrigel™ as a substrate, to individual ECM molecules such as laminin-511, vitronectin and fibronectin. The third generation substrates for hPSC culture is defined by the use of synthetic components in combination with biologic motifs. Advanced materials now provide a fully synthetic substrate that support clonal growth, derivation and long-term culture of genomically stable hPSCs. The gradient transition from dark to light red color (left to right) indicates the complexity of the culture medium, from undefined to defined components. hPSCs are illustrated as the rectangular cells with a prominent red nuclei, while fibroblasts are shown as elongated cells with blue nuclei.
Table I.
Summary of substrates and cell culture media used for feeder-free culture of human pluripotent stem cells
| Substrate coating |
Cell culture medium (chemically- defined)* |
Cell lines tested (passages)i |
Cell character- rizationJ |
Substrate | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| Sterilization capability |
Relative Cost |
Source of derivation |
Stability at RTK |
Preparation | |||||
| Matrigel™, laminin |
MEF-CM+FGF2B | H1, H7, H9, H14 (25) |
Full | No | Expensive | Xenogeneic | No | Absorption | Xu et al., 200113 |
| Laminin | X-VIVO10+GF*C | H1 (40) | Full | No | Expensive | Human protein | No | Absorption | Li et al., 200523 |
| Matrigel™, fibronectin |
HESCO*D | H9, BG01 (23) | Full | No | Expensive | Xenogeneic | No | Absorption | Lu et al., 200624 |
| Matrigel™, | DMEM/F12, N2, B12…*E |
H1, HSF6 (22) | Full | No | Expensive | Xenogeneic | No | Absorption | Yao et al., 200625 |
| Matrigel™, 4-ECM*A |
mTeSR™*F | H1, H7, H9, H14 (25) |
Full | No | Expensive | Xenogeneic | No | Absorption | Ludwig et al., 200626 |
| Matrigel™ | StemPro® *G | H1, BG01, BG02, BG03, CyT49 (25) |
Full | No | Expensive | Xenogeneic | No | Absorption | Wang et al., 200727 |
| Laminin- derived peptides |
MEF-CM+FGF2 | H1, H9 (Short- term: 7–10 days) |
Partial | No | Expensive | Protein | No | Peptide synthesis |
Derda et al., 200735 |
| RH laminin- 511 |
MEF-CM+FGF2 | KhES-1, KhES- 2, KhES-3 (10) |
Partial | No | Expensive | Recombinant Human protein |
No | Absorption | Miyazaki et al., 200815 |
| RH vitronectin | mTeSR* E-8*H |
HUES1, HES2, NL3 (5) H1, H9, hiPSCs (20) |
Partial Full |
No No |
Expensive Expensive |
Recombinant Human protein Recombinant Human protein |
No No |
Absorption Absorption |
Braam et al., 200819 Chen et al., 201128 |
| RH E- cadherin |
MEF-CM+FGF2, mTeSR* |
H1,H9, hiPSCs (37) |
Full | No | Expensive | Recombinant Human protein |
No | Absorption | Nagaoka et al., 201022 |
| PMVE-alt-MA | StemPro®* | HUES1, HUES9, hiPSCs (5) |
Partial | Yes | Inexpensive | Synthetic coating |
Yes | Free radical polymerization |
Brafman et al., 201029 |
| Heparin- binding GKKQ RFRHRNRKG |
mTeSR* | H1, H7, H9, H13, H14, IMR- 90-1 iPSCs, DF19-97T iPSCs (17) |
Partial | No | Expensive | Synthetic coating |
No | Chemisorption | Klim et al., 201030 |
| Hit 9 | mTeSR™* | BG01, WIBR3 (10) |
Full | Yes | Inexpensive | Synthetic coating |
Yes | Photo- polymerization |
Mei et al., 201031 |
| Synthemax™ | X-VIVO10+GF*, mTeSR™* |
H1 and H7 (10) | Full | Yes | Expensive | Synthetic coating |
Yes | Photo- polymerization & chemical conjugation of peptide |
Melkoumian et al., 201032 |
| APMAAm | mTeSR™* | H1, H9 (20) | Partial | Yes | Inexpensive | Synthetic coating |
Yes | Photo- polymerization |
Irwin et al., 201134 |
| PMEDSAH | MEF-CM+FGF2, hCCM+FGF2, StemPro®* |
H1, H7, H9,CHB-8, CHB-10, hiPSCs (20) |
Full | Yes | Inexpensive | Synthetic coating |
Yes | Surface initiated polymerization |
Villa-Diaz et al., 201033,36,37 |
4-ECM: combination of collagen IV, fibronectin, laminin and vitronectin.
MEF-CM+FGF2: Conditioned medium from mouse embryonic fibroblasts (MEF) supplemented with recombinant human (RH) fibroblast growth factor (FGF)-2.
X-VIVO10+GF: X-VIVO10 medium, non-essential aminoacids (NEAA), L-glutamine (L-glu), β-mercapethanol (β-Mer), RH FGF2, RH stem cell factor (SCF), RH Flt3 ligand, RH leukemia inhibitory factor (LIF).
HESCO:DMEM/F12 medium, knock-out serum replacer (KSR), NEAA, L-glu, β-Mer, bovine serum albumin (BSA), Insulin (Ins), transferrin (Transf), FGF2, B-cell activating factor (BAFF), Wnt3a, cholesterol.
DMEM/F12, N2, B12: DMEM/F12 medium, N2 medium, B12 medium, NEAA, L-glu, β-Mer, BSA, RH FGF2.
mTeSR™ DMEM/F12 medium, NEAA, L-glu, β-Mer, BSA, Ins, Transf, HR FGF2, transforming growth factor (TGF) β1, pipecolic acid, lithium chloride, γ-aminobutyric acid (GABA).
StemPro®: DMEM/F12 medium, BSA, Trans, RH FGF2, IGF-I, activinA, heregulin 1β
E-8: DMEM/F12 medium, NaHCO3, Selenium, Ascorbic Acid, Ins, Transf, HR FGF2, TGFβ1.
Passages: Indicated the number of passages that cells were cultured at time of karyotype analysis
Cell characterization: Partial includes pluripotent stem cell marker expression (Oct4 [also known as Pou5f1], Sox2, Tra-1-60, Tra-1-81, SSEA3, SSEA4), in vitro differentiation in to derivatives of three germ layers, and karyotype analysis. Full characterization complements the partial characterization with in vivo differentiation through teratoma assay.
RT: room temperature.
Risks associated with feeder cells and xenogeneic components, and their impediment to mechanistic studies
Feeder-cells such as MEFs support the self-renewal of hPSCs by the secretion of essential growth factors, cytokines and extracellular matrices (ECM) such TGFβ, activin A, laminin-511 and vitronectin6. However, inconsistencies in expression and secretion of these factors by different feeder-cells6, 7 make it difficult to determine which components are indispensible for the support of hPSCs in an undifferentiated state. Moreover, the γ-irradiation of feeder-cells not only impedes their proliferation, but also induces apoptosis and subsequently alters the secretion of soluble factors and deposition of an ECM. All these factors may negatively affect the self-renewal and consistent culture of hPSCs8. Thus, the dynamic and undefined microenvironment that feeder-cells create, limits our ability to interpret mechanistic studies designed to understand the biology of hPSCs.
Feeder-cells and their products can also be a source of pathogens for hPSCs. For example, in the co-culture of hESCs and MEFs with animal-derived serum replacements, the detection of an immunogenic sialic acid (Neu5Gc) has been reported9. This is of particular concern because the presence of non-human sialic acid may induce an immune response upon transplantation of hPSC derivatives. Xenogeneic feeder-cells and serum are also a common source of mycoplasma contamination. Because mycoplasmas compete with host cells for essential nutrients, mycoplasma contamination of cultured cells may compromise diverse aspects of cell physiology such as cell growth, phenotype, karyotype and induction of cytokine expression. These infections often go undetected and consequently could alter the interpretation of key experimental observations (see reference10 for literature review). In an effort to prevent xenogeneic contaminations, human feeder-cells and serum have been proposed for the culture of hPSCs (see reference11 for literature review). However, the risk of contamination by viral and non-viral infectious agents also exists when using human feeder cells (see reference12 for literature review).
Feeder-free culture systems and their benefits in understanding the biology of hPSCs
One of the first examples of alternatives to feeder-dependent PSC cultures was the demonstration of long-term culture of hESCs on tissue culture plates coated with Matrigel™ in combination with MEF-conditioned medium13. Matrigel™ is composed mainly of laminin, collagen IV, heparin sulfate proteoglycans, entactin and growth factors14. However, Matrigel™ is derived from Engelbreth-Holm-Swarm mouse sarcomas14, exhibits lot-to-lot variability and can introduce unwanted xenogeneic contaminants. Therefore, Matrigel™ is not an ideal substrate for feeder-free culture of hPSCs if the primary objective is to culture these cells for eventual human therapies. Nevertheless, Matrigel™ remains one of the most commonly used substrates and has served as an important starting point to define the requirements for hPSCs growth and differentiation.
The individual components of Matrigel™ exhibit varying degrees of support for PSCs. Laminin-coated surfaces support the growth of hESCs13. In contrast, when cultured on fibronectin and collagen IV, the self-renewal of hESCs is compromised13. It has also been reported that the specific laminin isoforms, -111, -332, and -511 support the adhesion and proliferation of undifferentiated hESCs, while isoforms -211 and 411 do not15. In addition, it has been shown that supportive feeder cells6 and hESCs13, 15 produce laminin isoforms -511/-521 and express the integrin α6β1 receptor, the primary receptor for these laminin isoforms16. The laminin isoform -511 is abundant in the embryonic basement membrane17 and is also supportive of mouse ESC cultures18. Therefore, these findings suggest that mechanisms responsible for self-renewal of hPSCs in vitro may be conserved between species.
Because integrins are the principal cell-surface receptors that mediate cell-ECM interactions, it is likely that the identification of integrins in hPSCs signal the use of other supporting ECM proteins for self-renewal of these pluripotent stem cells. Following this hypothesis, vitronectin has been shown to support hESC self-renewal via integrin αVβ519. Similarly, E-cadherin, which mediates cell-cell interactions and has been involved in hESC colony formation20 and self-renewal21, has been used as a substrate for long-term culture of hPSCs22. The use of recombinant human (rh) laminin -51115, rh vitronectin19 and rh E-cadherin22 represent significant milestones in the culture of hPSCs because they were the first examples of defined and xenogeneic-free substrates.
From non-defined to defined conditions: culture medium and supportive substrates
While this review places an emphasis on the cell-culture substrates, one must acknowledge that the culture medium plays an essential role in achieving a defined culture system for hPSCs. As mentioned above, the secretion of soluble factors by feeder cells influences the fate of PSCs in self-renewal and differentiation. Consequently, conditioned medium from feeder cells has been commonly used to culture hPSCs in feeder-free conditions. However, due to its undefined characteristics, variability and the risks associated with contaminants, more rigorous practices include the use of chemically-defined culture medium. While several culture media formulations23–28 have been developed to support the culture of hPSCs in feeder-free conditions, it should be noted that all these media formulations were developed using Matrigel™ as a substrate. Additionally, some have also been shown to be effective when used with individual ECM proteins like fibronectin24, laminin23 or vitronectin28 and with synthetic substrates29–34 that support undifferentiated hPSCs proliferation.
The combination of chemically-defined medium and xenogeneic-free biological substrates represents significant progress in the generation of clinically compliant culture systems for hPSCs. Nevertheless, for large-scale expansion of hPSCs in chemically-defined and clinically compliant conditions, biological substrates have drawbacks that must be overcome. These barriers include factors such as batch-to-batch variability, limited scalability, difficultly in isolation, expense to manufacture and the need to ensure pathogen-free conditions. As important alternatives, synthetic substrates that support the proliferation of undifferentiated hPSCs have been developed29–33. These synthetic environments will likely prove to be superior because they exhibit little batch-to-batch variation, are defined, reproducible, stable, are amenable to standard sterilization techniques and can be readily tuned to meet the culture requirements for different hPSC lines.
Due to the anchorage-dependent nature of hPSCs, synthetic substrates must allow cell adhesion, spreading, self-renewal and subsequent colony formation of undifferentiated hPSCs. Furthermore, hPSCs cultured on synthetic substrates must retain the unique nature of pluripotency, and must not develop chromosomal and genomic abnormalities. In addition, synthetic coatings should demonstrate efficacy in the long-term culture of multiple stem cell lines/types, be compatible with common biomedical sterilization methods, be cost effective and have the potential to be scaled-up for commercial purposes.
To create defined synthetic substrates that allow the long-term propagation of hPSCs, several materials and material combinations have been developed and tested. For example, peptide or protein-based systems, polymers, and polymers in conjunction with biomolecules have been developed. Many of these systems exploit biologic moieties to achieve appropriate culture conditions. The success of this approach was realized when the heparin-binding peptide GKKQRFRHRNRKG was conjugated to an alkanethiol self assembled monolayer for the long-term culture of hESCs30. Similarly, arrays of laminin-derived peptides have also been shown to support hESCs35.
Among the polymer-based substrates is the zwitterionic hydrogel, poly[2-(methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide] (PMEDSAH), that has been developed using a surface initiated graft polymerization technique. The surface coatings composed of PMEDSAH are fully synthetic and are extremely effective in sustaining the long-term expansion of hESCs and hiPSCs33, 36, 37. Other polymers coatings for hPSCs include polymer Hit931 and the aminopropylmethacrylamide (APMAAm)34, a methacrylamide containing polymer. Both of these polymers are fabricated by photopolymerization. Poly(methyl vinyl ether-alt-maleic anhydride) (PMVE-alt-MA), an anhydride containing polymer coating generated by radical polymerization29, is another synthetic material that supports hPSCs. Other research groups have taken a hybrid approach, using polymers as the base substrate and then modifying the surface with biomolecules. For example, Synthemax™ (Corning™), an acrylate polymer modified with amino-containing peptides, has been shown to be effective in the prolonged culture of multiple hESC lines32.
Of all the synthetic surfaces detailed thus far, only PMEDSAH33, 36 and APMAAm34 have been reported to maintain hPSCs for greater than 20 passages in defined and xenogeneic-free culture medium. Because of the great number of potential applications in regenerative medicine and tissue engineering using hPSCs, it is desirable for culture substrates to maintain numerous cell lines and cell types in an undifferentiated state. To date, all of the substrates detailed in this review meet the criteria of having been utilized in the culture of multiple hESC and hiPSC lines.
Biomedical products such as culture plates to expand PSCs must be batch sterilized before use and thus it is important for synthetic substrates to be compatible with common sterilization techniques such as electron beam- and γ-radiation. However, substrates with biologic components such as proteins and peptides do not readily lend themselves to common sterilization methods because the sterilization treatment may denature or degrade the biologic moieties. At this time, only Synthemax™ and PMEDSAH have shown compatibility with common sterilization methods32, 33, 38. Furthermore, the inclusion of peptides and proteins in stem cell culture substrates may not allow the surfaces to be reused because biomolecules are known to undergo degradation from metalloproteinases secreted by cultured cells39. This observation should also be considered as a factor for future clinical adoption, as the inclusion of biomolecules leads to increased costs. Due to the large number of stem cells that will likely be needed for cell-based regenerative therapies40, it will be important to utilize processes to generate substrates that can be scaled-up from the bench top to high throughput manufacturing processes. Modifying surfaces with peptides and proteins can also hinder scale-up as it increases process complexity.
Biology of synthetic substrate-PSC interactions: maintenance of self-renewal and control of differentiation
Several strategies have been used to develop synthetic substrates for long-term hPSC culture. While the molecular mechanisms responsible for this support are not yet fully characterized, the influence of material properties on cells is currently an area of intense investigation. For substrates that present cell adhesion elements such as heparin-binding peptides30 and laminin-derived peptides35, the mechanism of support for hPSCs may be due to these biological-mimicking components. It is possible that substrates like PMVE-alt-MA29 and PMEDSAH33, which contain carboxyl and sulfonyl groups, mimic the functional characteristics of heparin, and in this way, support hPSCs. However, other material properties, including hydrophilicity/hydrophobicity, surface roughness, and stiffness of the culture substrate can influence hPSC behavior. For example, the wettability properties of polymer coatings affect the adhesion of hPSCs. In this case, hydrophobic materials are less permissive33. The roughness of the substrate also impacts the fate of hPSCs. In terms of adhesion, proliferation and self-renewal, surfaces categorized as smooth by a root-mean-square roughness (Rq) of 1 nm, provide superior support to hESCs over nanorough surfaces with an Rq = 75–150 nm41. Similarly, it has been shown that rigid substrates maintain self-renewal of hESCs, while on soft substrates stem cells are more prone to differentiate. These data demonstrate that hESCs are mechanosensitive and increase their cytoskeleton contractility with substrate rigidity42.
The chemical properties of the substrate also affect the structural conformation of proteins that interact with the synthetic coating. The source of the protein may be from factors that are used to coat the surface, factors that are added to the culture medium and those factors that are secreted as stem cells adhere to their physical environment in vitro31. For example, the network structure of Matrigel™ and its ability to support attachment and self-renewal of hESCs changes depending on the surface on which it is adsorbed43. In the case of supplementary proteins, it has been shown that APMAAm surfaces adsorb bovine serum albumin from the culture medium in an unfolded state, allowing the proliferation of undifferentiated hESCs34. Taken together, these examples illustrate the importance and the complexity of the role the microenvironment plays in self-renewal and differentiation of hPSCs and suggests that synthetic substrates that support hPSCs exhibit both chemical and mechanical properties that support hPSC proliferation.
Culture of pluripotent stem cells in 3D bioreactors
While the establishment of synthetic substrates and defined culture conditions for hPSCs allows us to more precisely probe the molecular basis of pluripotent stem cell self-renewal and differentiation, the large-scale expansion of these cells for future clinical use could be accelerated by the development of 3D cell suspension cultures40. It is accepted that hPSCs grow as adherent colonies and upon detachment from supportive substrates, stem cells randomly differentiate and the formation of embryoid bodies (EB) is enabled. However, with the proper culture medium, undifferentiated hPSCs can proliferatein suspension as spheroid clumps. Homogenous populations of small cells with large nuclei can survive and retain the expression of pluripotent stem cell markers and the capability to differentiate in vivo and in vitro44–47. Interestingly, when hPSC spheroid clumps are cultured in suspension with serum-containing medium, cystic EBs form and evidence of differentiation is observed44.
The long-term and large-scalepropagation of both hESCs and hiPSCs in bioreactors with serum-free medium and without microcarriers in both static and dynamic cultures has been reported44–47. Under these culture conditions hPSCs have been expanded for over 20 passages44 and the proliferation rates, although varying from study to study, have been reported to beas high as 20-fold in 6 days which is higher than that reported for feeder cell cultures (~5-fold) or feeder-free cultures (~9-fold) for the same period of time46. It has been calculated that an initial seeding density of 5×107hPSCs per spinner flask would result in a yield of at least 1.6×109 cells over 5 passages47.
Derivation and long-term culture of hPSCs on synthetic substrates and in cell-suspension cultures: phenotypic, genetic and epigenetic stability
As the field of regenerative medicine advances towards the development of in vitro disease models from hPSC lines, both directed genetic modification and derivation of new stem cell lines need to be performed. This implies the successful expansion of genetically modified single cells into an entire population of hPSCs. Therefore, synthetic substrates should support clonal growth of PSCs. To date, the proliferation of undifferentiated single hPSCs has been reported on hit-9 surfaces31, while the derivation of new hiPSC lines on defined substrates has been achieved on rh vitronectin28 and in suspension conditions with the addition of fibronectin, laminin and gelatin in the culture medium45.These newly derived populations of hPSCs were shown to be phenotypically similar to cells derived on feeder cells. They expressed the characteristic markers of PSCs, showed comparable cell-doubling rates and demonstrated the ability to differentiate into derivatives of the three germ layers in vitro and in vivo. However, equally important is the finding that derivation and long-term culture of hPSCs on synthetic substrates and in bioreactors may be accomplished without the introduction of genomic abnormalities that may generate a selective advantage such as a greater propensity for self-renewal48. Chromosomal abnormalities have been reported in hPSCs after prolonged culture as well as in early passages. These aberrations commonly involve nonrandom gains of chromosomes 12, 17, 20 and X, or fragments of these chromosomes49 as detected by standard G-banding metaphase karyotype analysis. High-resolution genome-wide analysis using array-based comparative genomic hybridization (aCGH) techniques have shown recurrent alterations in the same regions, as well as others not detected by standard karyotyping methods. One alteration frequently reported is an amplification in the 20q11.21 region that includes genes such as DNMT3B, ID1, HM13 and BCL2L149, 50, which have been shown to be involved in cell proliferation, inhibition of differentiation and apoptosis51–53 and may provide a strong selective advantage in culture compared to normal cells. To date, all studies investigating the expansion of hPSCs on synthetic substrates have reported normal karyotypes using the low-resolution G-banding technique. However, for more rigorous interpretation of biological studies and a greater safety profile for eventual cell therapy, high-resolution genome-wide studies should be performed.
The epigenetic stability of hPSCs is also important to consider when using or developing new culture systems. The epigenetic status of genes can change dynamically with culture time and has been shown to be highly variable among different hPSC lines and between sibling lines49. There have been no systematic reports of potential epigenetic changes when hPSC are cultured on synthetic substratesor in suspension. However, this will be an important consideration in light of recent studies that related derivation and culture conditions of female hiPSCs with erosion of DNA methylation and gene expression on the X chromosome inactivation. Such changes have the potential to affect disease modeling, differentiation potential and clinical applications (see reference54 for review).
Future directions
The current knowledge of synthetic substrates and their characteristics responsible for supporting the proliferation of undifferentiated hPSCs will likely continue to evolve. A new and deeper understanding of how chemical moieties support or direct biologic behavior will lead to improvement in, and development of, new synthetic substrates and will improve our understanding of the biology of pluripotent stem cells. Using the tuning capacity in chemical synthesis of polymer substrates, it will be possible to investigate the response of hPSCs to custom tailored chemical and mechanical signals to maintain self-renewal, or to perhaps induce cell lineage-specific differentiation. Initial examples of this potential have been demonstrated with mesenchymal stem cells, where matrix elasticity of the culture substrate contributed to lineage progression toward neurons, myoblasts and osteoblasts55. One can envision that in combination with high-throughput screenings, the use of small molecules, gene-transfection libraries and directed chemical manipulations, that synthetic substrates will facilitate the development of defined culture conditions for multiple cell-lineage commitment of hPSCs. In this regard, the impact that synthetic substrates and chemically-defined, xenogeneic-free medium for the culture of hPSCs is already high and research in this area will continue to play a prominent role in the development of strategies to use hPSCs to treat debilitating human diseases.
Acknowledgements
This work was supported by NIH grant DE016530.
Footnotes
Author contribution. All authors contributed in the preparation of this concise review.
REFERENCES
- 1.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 5.Williams RL, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 6.Hongisto H, et al. Laminin-511 expression is associated with the functionality of feeder cells in human embryonic stem cell culture. Stem Cell Res. 2012;8:97–108. doi: 10.1016/j.scr.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Eiselleova L, et al. Comparative study of mouse and human feeder cells for human embryonic stem cells. Int J Dev Biol. 2008;52:353–363. doi: 10.1387/ijdb.082590le. [DOI] [PubMed] [Google Scholar]
- 8.Villa-Diaz LG, et al. Analysis of the Factors That Limit the Ability of Feeder-Cells to Maintain the Undifferentiated State of Human Embryonic Stem Cells. Stem Cells Dev. 2008;18:641–651. doi: 10.1089/scd.2008.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228–232. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 10.Rottem S, Barile MF. Beware of mycoplasmas. Trends Biotechnol. 1993;11:143–151. doi: 10.1016/0167-7799(93)90089-R. [DOI] [PubMed] [Google Scholar]
- 11.Mallon BS, Park KY, Chen KG, Hamilton RS, McKay RD. Toward xeno-free culture of human embryonic stem cells. Int J Biochem Cell Biol. 2006;38:1063–1075. doi: 10.1016/j.biocel.2005.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stacey GN, et al. The development of 'feeder' cells for the preparation of clinical grade hES cell lines: challenges and solutions. J Biotechnol. 2006;125:583–588. doi: 10.1016/j.jbiotec.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Xu C, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 14.Kleinman HK, et al. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- 15.Miyazaki T, et al. Recombinant human laminin isoforms can support the undifferentiated growth of human embryonic stem cells. Biochem Biophys Res Commun. 2008;375:27–32. doi: 10.1016/j.bbrc.2008.07.111. [DOI] [PubMed] [Google Scholar]
- 16.Nishiuchi R, et al. Ligand-binding specificities of laminin-binding integrins: a comprehensive survey of laminin-integrin interactions using recombinant alpha3beta1, alpha6beta1, alpha7beta1 and alpha6beta4 integrins. Matrix Biol. 2006;25:189–197. doi: 10.1016/j.matbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Miner JH, Li C, Mudd JL, Go G, Sutherland AE. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development. 2004;131:2247–2256. doi: 10.1242/dev.01112. [DOI] [PubMed] [Google Scholar]
- 18.Domogatskaya A, Rodin S, Boutaud A, Tryggvason K. Laminin-511 but not -332, 111, or -411 enables mouse embryonic stem cell self-renewal in vitro. Stem Cells. 2008;26:2800–2809. doi: 10.1634/stemcells.2007-0389. [DOI] [PubMed] [Google Scholar]
- 19.Braam SR, et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells. 2008;26:2257–2265. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- 20.Li L, et al. A unique interplay between Rap1 and E-cadherin in the endocytic pathway regulates self-renewal of human embryonic stem cells. Stem Cells. 2010;28:247–257. doi: 10.1002/stem.289. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y, et al. Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc Natl Acad Sci U S A. 2010;107:8129–8134. doi: 10.1073/pnas.1002024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagaoka M, Si-Tayeb K, Akaike T, Duncan SA. Culture of human pluripotent stem cells using completely defined conditions on a recombinant E-cadherin substratum. BMC Dev Biol. 2010;10:60. doi: 10.1186/1471-213X-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Powell S, Brunette E, Lebkowski J, Mandalam R. Expansion of human embryonic stem cells in defined serum-free medium devoid of animal-derived products. Biotechnol Bioeng. 2005;91:688–698. doi: 10.1002/bit.20536. [DOI] [PubMed] [Google Scholar]
- 24.Lu J, Hou R, Booth CJ, Yang SH, Snyder M. Defined culture conditions of human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:5688–5693. doi: 10.1073/pnas.0601383103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao S, et al. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci U S A. 2006;103:6907–6912. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ludwig TE, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, et al. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood. 2007;110:4111–4119. doi: 10.1182/blood-2007-03-082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen G, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brafman DA, et al. Long-term human pluripotent stem cell self-renewal on synthetic polymer surfaces. Biomaterials. 2010;31:9135–9144. doi: 10.1016/j.biomaterials.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klim JR, Li L, Wrighton PJ, Piekarczyk MS, Kiessling LL. A defined glycosaminoglycan-binding substratum for human pluripotent stem cells. Nat Methods. 2010;7:989–994. doi: 10.1038/nmeth.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mei Y, et al. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat Mater. 2010;9:768–778. doi: 10.1038/nmat2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melkoumian Z, et al. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat Biotechnol. 2010;28:606–610. doi: 10.1038/nbt.1629. [DOI] [PubMed] [Google Scholar]
- 33.Villa-Diaz LG, et al. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat Biotechnol. 2010;28:581–583. doi: 10.1038/nbt.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irwin EF, Gupta R, Dashti DC, Healy KE. Engineered polymer-media interfaces for the long-term self-renewal of human embryonic stem cells. Biomaterials. 2011;32:6912–6919. doi: 10.1016/j.biomaterials.2011.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Derda R, et al. Defined substrates for human embryonic stem cell growth identified from surface arrays. ACS Chem Biol. 2007;2:347–355. doi: 10.1021/cb700032u. [DOI] [PubMed] [Google Scholar]
- 36.Nandivada H, et al. Fabrication of synthetic polymer coatings and their use in feeder-free culture of human embryonic stem cells. Nat Protoc. 2011;6:1037–1043. doi: 10.1038/nprot.2011.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villa-Diaz LG, et al. Derivation of mesenchymal stem cells from human induced pluripotent stem cells cultured on synthetic substrates. Stem Cells. 2012;30:1174–1181. doi: 10.1002/stem.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross AMNH, Ryan AL, Lahann J. Synthetic substrates for long-term stem cell culture. Polymer. 2012;53:2533–2539. [Google Scholar]
- 39.Fonseca KB, Bidarra SJ, Oliveira MJ, Granja PL, Barrias CC. Molecularly designed alginate hydrogels susceptible to local proteolysis as three-dimensional cellular microenvironments. Acta Biomater. 2011;7:1674–1682. doi: 10.1016/j.actbio.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 40.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 41.Chen W, et al. Nanotopography influences adhesion, spreading, and self-renewal of human embryonic stem cells. ACS Nano. 2012;6:4094–4103. doi: 10.1021/nn3004923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Y, et al. Mechanics regulates fate decisions of human embryonic stem cells. PLoS One. 2012;7:e37178. doi: 10.1371/journal.pone.0037178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohen NT, Little LE, Healy KE. Characterization of Matrigel interfaces during defined human embryonic stem cell culture. Biointerphases. 2009;4:69–79. doi: 10.1116/1.3274061. [DOI] [PubMed] [Google Scholar]
- 44.Amit M, et al. Suspension culture of undifferentiated human embryonic and induced pluripotent stem cells. Stem Cell Rev. 2010;6:248–259. doi: 10.1007/s12015-010-9149-y. [DOI] [PubMed] [Google Scholar]
- 45.Steiner D, et al. Derivation, propagation and controlled differentiation of human embryonic stem cells in suspension. Nat Biotechnol. 2010;28:361–364. doi: 10.1038/nbt.1616. [DOI] [PubMed] [Google Scholar]
- 46.Olmer R, et al. Long term expansion of undifferentiated human iPS and ES cells in suspension culture using a defined medium. Stem Cell Res. 2010;5:51–64. doi: 10.1016/j.scr.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Singh H, Mok P, Balakrishnan T, Rahmat SN, Zweigerdt R. Up-scaling single cell-inoculated suspension culture of human embryonic stem cells. Stem Cell Res. 2010;4:165–179. doi: 10.1016/j.scr.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Maitra A, et al. Genomic alterations in cultured human embryonic stem cells. Nat Genet. 2005;37:1099–1103. doi: 10.1038/ng1631. [DOI] [PubMed] [Google Scholar]
- 49.Amps K, et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat Biotechnol. 2011;29:1132–1144. doi: 10.1038/nbt.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elliott AM, Elliott KA, Kammesheidt A. High resolution array-CGH characterization of human stem cells using a stem cell focused microarray. Mol Biotechnol. 2010;46:234–242. doi: 10.1007/s12033-010-9294-1. [DOI] [PubMed] [Google Scholar]
- 51.Beaulieu N, et al. An essential role for DNA methyltransferase DNMT3B in cancer cell survival. J Biol Chem. 2002;277:28176–28181. doi: 10.1074/jbc.M204734200. [DOI] [PubMed] [Google Scholar]
- 52.Bai H, et al. Bcl-xL enhances single-cell survival and expansion of human embryonic stem cells without affecting self-renewal. Stem Cell Res. 2012;8:26–37. doi: 10.1016/j.scr.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barone MV, Pepperkok R, Peverali FA, Philipson L. Id proteins control growth induction in mammalian cells. Proc Natl Acad Sci U S A. 1994;91:4985–4988. doi: 10.1073/pnas.91.11.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wutz A. Epigenetic alterations in human pluripotent stem cells: a tale of two cultures. Cell Stem Cell. 2012;11:9–15. doi: 10.1016/j.stem.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 55.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]


