1. Introduction
Individuals with Alzheimer’s disease (AD) experience cognitive loss and emotional and behavioral changes over the time course of the disease. Currently, it is tentatively estimated that at least 25% of people with early AD show impaired awareness of disease-related changes that are occurring in themselves (Derouesne et al., 1999; Feher, 1991; Kaszniak & Zak, 1996; Mograbi, Brown, & Morris, 2009; Salmon et al., 2008; Starkstein, Jorge, Mizrahi, Adrian, & Robinson, 2007). Because self-appraisals guide much human behavior, incorrect self-appraisals regarding one’s present-day abilities shown by many AD patients (alternately referred to as anosognosia or impaired self-awareness in this manuscript) can yield significant deleterious consequences – including important safety risks for patients and those around them (Cotrell & Wild, 1999; Starkstein, et al., 2007) and distress in families and caregivers (Clare, Whitaker, et al., 2011; Rymer et al., 2002; Seltzer, Vasterling, Yoder, & Thompson, 1997). Furthermore, impaired awareness of one’s own decline may delay medical consultation regarding incipient dementia and can interfere with treatment compliance (Arlt, Lindner, Rosler, & Von Renteln-Kruse, 2008; Griffith, Dymek, Atchison, Harrell, & Marson, 2005; Karlawish, Casarett, James, Xie, & Kim, 2005; Koltai, Welsh-Bohmer, & Schmechel, 2001). Thus, carefully characterizing the behavioral presentation of this symptom early in the AD time course and disseminating this information to health care providers is one important inroad to decreasing the prevalence of missed and delayed AD diagnosis and improving patient care (Bradford, Kunik, Schulz, Williams, & Singh, 2009). Investigating changes in brain activity that covary with the intactness of self-appraisal ability will improve our ability to identify and understand the causes of anosognosia and may point to methods for its treatment.
In the search for brain changes that contribute to anosognosia such as those shown in AD, several lines of research point to altered function of the medial prefrontal cortex (MPFC). Alexander Luria – a seminal voice in the study of how human brain function effects and is affected by a sense of self and one’s social context – highlighted the (MPFC) as a key part of a functional neural system underlying accurate self-appraisal (Luria, 1972, 1973). Contemporary lesion and functional neuroimaging studies that measure self-appraisal by asking people to make ratings regarding personal characteristics, preferences, and values corroborate this notion (Amodio & Frith, 2006; Johnson et al., 2002; Johnson & Ries, 2010; Johnson et al., 2006; Pronin, 2008; Schmitz & Johnson, 2007). Normative fMRI studies of this sort of self-appraisal yield reliable and robust activity in medial prefrontal and posterior cingulate regions (for reviews please see Johnson & Ries, 2010; Schmitz & Johnson, 2007). Observation of behavior changes in people with altered medial prefrontal structure and function secondary to brain injury (Prigatano, 2010; Schroeter, Ettrich, Menz, & Zysset, 2010), frontotemporal dementia (Bastin et al., 2011; Grossman et al., 2010; Neary et al., 1998; Orfei et al., 2010; Rankin, Baldwin, Pace-Savitsky, Kramer, & Miller, 2005; Schroeter, Raczka, Neumann, & von Cramon, 2008), and a variety of other neurodegenerative conditions (Rosen et al., 2010) underscores the MPFC’s central role in generating accurate self-appraisals as well as for guiding social decision-making and theory of mind or empathy for others. Furthermore, in people with amnestic mild cognitive impairment (MCI), medial prefrontal and posterior cingulate blood-oxygen-level-dependent (BOLD) response to self-appraisal corresponds directly to the accuracy of cognitive self-appraisal (Ries et al., 2007).
With this foundation of evidence regarding MPFC involvement in self-appraisal, research has turned to the MPFC’s involvement in networks of brain activity. The MPFC is a heteromodal region that possesses anatomic connections with a large number of other heteromodal and limbic regions (Price, 1999; Price & Drevets, 2010; Saleem, Kondo, & Price, 2008) responsible for drive and reward (e.g., anterior cingulate, nucleus accumbens, ventral tegmental area), mood (e.g., amygdala), and episodic, semantic, and autobiographical memory (e.g., posterior cingulate, lateral parietal lobe, hippocampus). Given that it is anatomically well-situated to integrate inputs important for self-appraisal,Schmitz et al. (2006) examined MPFC condition-dependent connectivity in the context of an fMRI self-appraisal task. This study found that BOLD response in dorsolateral prefrontal cortex (DLPFC) and bilateral hippocampal regions showed condition-dependent co-activation with dorsal MPFC that was modulated by self-appraisal (Schmitz & Johnson, 2006). The amygdala, nucleus accumbens, and insula also showed increased condition-dependent connectivity with ventral MPFC during a self-appraisal task relative to a semantic task.
The goal of the current project was to investigate changes in MPFC functional connectivity that correspond to impaired self-appraisal accuracy – specifically appraisal of current memory ability. Participants providing data for this project included older adults, some of whom had documented memory impairment and a diagnosis of MCI or early AD and some of whom had healthy memories. In this manuscript we conceptualize MCI and early AD as lying on a continuum of disease progression (Dubois et al., 2007) and report their descriptive data (Table 1) as one group. Research suggests that awareness is domain specific (i.e., a person may be aware of deficits in one cognitive or affect domain and not another; Clare, Whitaker, et al., 2011), and in the present study of anosognosia early in the AD course, we chose to focus on awareness of memory decline because it is the earliest and most consistent symptom at this stage of the disease. Memory self-appraisal was operationally defined as each participant’s explicitly expressed evaluation of his/her current memory ability (i.e., the participant’s rating of how well he was presently generally able to perform memory tasks in daily life). To measure accuracy of these self-appraisals, we used the Memory Function Scale of the Memory Awareness Rating Scale (MARS) – an index psychometrically validated for this purpose in people with AD (Clare, Whitaker, & Nelis, 2010; Clare, Wilson, Carter, Roth, & Hodgesm, 2002). This scale involves administering two questionnaires asking about the participant’s ability to perform memory tasks during everyday activities – a self-appraisal questionnaire is given to the participant and a parallel questionnaire is given to a person who knows the participant well. Memory appraisal accuracy is indexed via a discrepancy score between the two parallel forms of questionnaires – with the study partner’s report considered to be the “truth” or gold standard. Because study partner reports can be influenced by perceived caregiver burden (Quinn, Clare, & Woods, 2009), we assessed this with the Zarit Burden Interview (Zarit, Reever, & Bach-Peterson, 1980), and we also verified study partner reports against a neuropsychological memory test (Hopkins Verbal Learning Test – Revised; HVLT-R). We expected our healthy control participants to show close correspondence with their study partners in their memory appraisals – thus showing intact memory self-appraisal ability. Based on prior findings, we expected individuals with memory impairment due to MCI and early AD to show a range of accuracy in their memory self-appraisals (Ries, et al., 2007; Vogel et al., 2004). Using regression analysis, we tested the hypothesis that altered MPFC functional connectivity – particularly with other cortical midline structures – explains a significant portion of this variation in memory self-appraisal accuracy as measured by the Memory Function Scale. Furthermore, we expected that such a relationship exists independent of the severity of memory impairment. Therefore we included an index of learning (i.e., total number of items learned across trials on the HVLT-R), as a covariate in the regression analysis. In order to constrain our regression analyses to those regions that work in conjunction with the MPFC to evoke self-appraisals, we derived an explicit mask – generated from fMRI self-appraisal task data in a separate, large group of cognitively-healthy individuals. In the analysis yielding this explicit mask, psychophysiologic interaction analysis was used to identify regions showing condition-dependent connectivity with the MPFC that was modulated by a self-appraisal context.
Table 1.
Demographic and neuropsychological data
| Controls (n = 12) |
MCI/AD MCI n = 7; AD n = 5) |
||||
|---|---|---|---|---|---|
| median | range | median | range | ||
| Age | 68 | 61–77 | 78 | 58–86 | * |
| Education | 18 | 16–20 | 16 | 12–22 | |
| Sex | 5m / 7f | 9m / 3f | * | ||
| Global Clinical Dementia Rating | 0 | 0 | 0.5 | 0.5–1 | * |
| Mini Mental Status Exam | 30 | 29–30 | 25 | 17–30 | * |
| HVLT-Ra Total Trials 1–3 | 29 | 19–34 | 16 | 5–24 | * |
| HVLT-Ra Delayed Recall | 11 | 4–12 | 2 | 0–8 | * |
| BVMT-Rb Total Trials 1–3 | 22.5 | 15–29 | 9 | 0–22 | * |
| BVMT-Rb Delayed Recall | 9.5 | 7–12 | 2 | 0–8 | * |
| Trail Making Test – A (seconds) | 25.5 | 22–43 | 52 | 26–197 | * |
| Trail Making Test–B (seconds) | 63.1 | 41–81 | 157.2 | 62–300 | * |
| Animal Naming | 20.5 | 17–30 | 14 | 5–20 | * |
| Memory Awareness Rating Scale: Memory Functioning Scale | |||||
| Self Rating | 41.5 | 24–48 | 36.5 | 32–45 | * |
| Study Partner Rating | 44 | 36–49 | 26.5 | 20–43 | * |
| Memory Function Discrepancy | −0.03 | −.4–.24 | 0.30 | −.15–.58 | * |
Significant group differences found employing Mann-Whitney U statistic or chi square, Alpha is p < .05;
Hopkins Verbal Learning Test, Revised,
Brief Visual Spatial Memory Test, Revised
2. Materials and Methods
2.1 Overall Study Design
The primary analysis examined how MPFC connectivity changes as a function of the accuracy of one’s memory self-appraisals as measured by the memory function discrepancy (MFD) score (described in Section 2.3.2). Participants, behavioral methods, and neuroimaging methods that yielded data for these analyses are detailed in sections 2.2, 2.3, and 2.4.2 respectively.
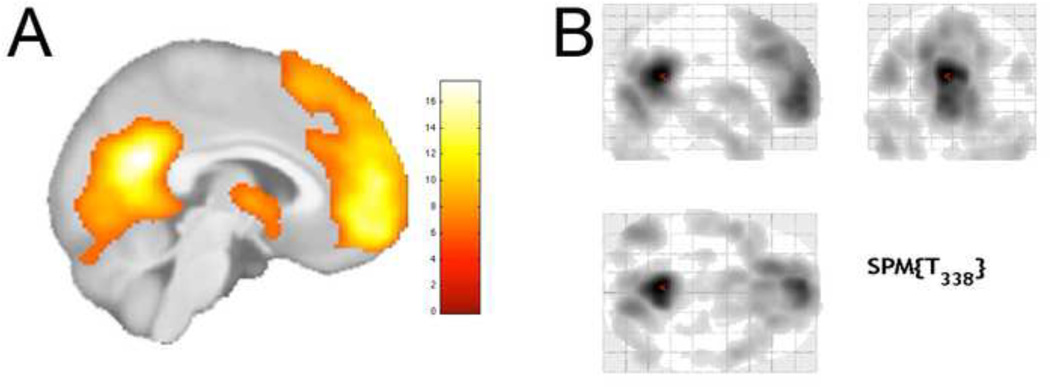
To reduce the risk of false positive (Type 1) statistical errors in these analyses, we chose to restrict (or explicitly mask) the above-described regression analysis to behaviorally-relevant brain regions. To do this, we employed fMRI self-appraisal task data from a separate, large group (N = 90) of cognitively-healthy adults (mean age: 58.7 ± 2.9), and we examined those brain regions that showed condition-dependent connectivity with the MPFC that was modulated by a self-appraisal context. Figure 1 shows the explicit mask derived from this analysis. Section 2.4.1 details the fMRI task design and psychophysiological interaction analysis methods used to derive this mask.
Figure 1.
Main effect (Self > Semantic) of the self-appraisal fMRI task administered to 90 cognitively-healthy adults (pFWE < .001). Renderings are on A) a mid-sagittal view of the statistical parametric map and B) a glass brain showing the extent of activation at this threshold in 3 dimensions.
2.2 Participants
The sample of participants included in our primary analysis consisted of twenty-four older adults – 12 cognitively healthy and 12 people with memory impairment due to MCI or early AD – who were recruited from the Madison community and from the University of Wisconsin memory clinics. To be included in the study, participants had to be age 60 or older. Control participants had a prior neuropsychological work-up (using a battery that did not overlap with that used in this study) that indicated that they were cognitively-healthy. MCI and AD diagnoses were determined through the Wisconsin Comprehensive Memory Program’s diagnosis consensus conference – a bi-monthly meeting attended by geriatricians, neurologists, and neuropsychologists. These diagnoses were made in accordance with published practice parameters (McKhann et al., 1984; Petersen et al., 2001). Of the participants with MCI, four had single-domain amnestic MCI and three had multi-domain amnestic MCI. Descriptive statistics regarding the sample’s demographics and neuropsychological performance are presented in Table 1.
Because the Memory Function Scale of the MARS requires a study partner report, we requested that each participant bring a study partner – someone who was a close friend or family member who knew the participant for over 5 years and had daily contact with the participant – to his/her study visit. Although we enrolled 27 participants in this study, only 24 were accompanied to study visits by an eligible study partner. Study partners for the 24 participants described in this paper were either spouses (83% of the study partners) or an adult child (17%). The 3 individuals without study partners had an MCI diagnosis.
Exclusion criteria included chronic major medical conditions (e.g., neurologic disease, cardiovascular disease, cancer), prior neurosurgical procedures, head trauma, diagnosis of a major psychiatric disorder, history of alcohol/substance abuse, learning disability, or vision/hearing impairment. All participants had a structural T1 weighted three-dimensional, spoiled gradient-recalled at steady-state (SPGR) scan that was collected subsequent to the functional EPI scans, and these were reviewed by a neuroradiologist for possible abnormalities. All participants provided written informed consent prior to engaging in study procedures and were treated in accordance with U.S. federal regulations and the ethical standards of the American Psychological Association; this study was also approved by the University of Wisconsin Health Sciences Institutional Review Board.
2.3 Behavioral Procedures
2.3.1 Neuropsychological Assessment
Participants received a neuropsychological test battery assessing general mental status (Mini Mental Status Examination; Folstein, Folstein, & McHugh, 1975) dementia staging (Clinical Dementia Rating), visual and verbal memory (Brief Visuospatial Memory Test - Revised, BVMT-R; Benedict, 1997 and Hopkins Verbal Learning Test - Revised, HVLTR; Brandt & Benedict, 2001), simple and shifting attention (Trails A & B), and semantic fluency (Animal Naming). Depressive symptoms were measured with the Geriatric Depression Scale (GDS).
2.3.2 Memory Awareness Measurement
Participants completed the Memory Awareness Rating Scale (MARS). The Memory Function Scale (MFS) of the MARS indexes memory awareness as a discrepancy between a study partner’s report and the participant’s self-report on parallel forms. The participant and study partner forms contain 13 items regarding everyday situations where a person would need to use his/her memory; one rates the frequency with which the person could effectively handle the situation on a scale of 0 (“never”) to 4 (“always”). Each participant and his study partner make subjective ratings of the participant’s memory function in relation to specified aspects of everyday memory (participant form: MFS-P; study partner form = MFS-SP). The discrepancy scores for this subscale (i.e., MFD scores) were calculated via the formula ((MFS-P – MFS-SP)/((MFS-P+ MFS-SP)/2) as recommended by the authors of this scale (Clare, et al., 2010). MFD scores close to zero indicate similar ratings between participants and study partners; positive scores indicate that participants rated themselves more favorably than their study partners. We also measured caregiver strain in the study partners via the Zarit Burden Interview – Revised (Zarit, 2008) to assess the relationship between perceived burden experienced by the study partners and their appraisals of the participants’ current memory ability.
2.4 Neuroimaging Procedures
2.4.1 Behaviorally-Relevant Explicit Brain Mask
We developed an explicit mask in order to spatially-constrain our primary analyses – and all procedures within this section (2.4.1) refer to methods for deriving this mask. The data used to generate this explicit mask come from a self-appraisal fMRI task administered to a separate, large group (N = 90) of cognitively-healthy adults (mean age: 58.7 ± 2.9). We used psychophysiologic interaction (PPI) analysis to identify brain regions showing positive connectivity with the MPFC that was driven by a self-appraisal psychological context (Friston et al., 1997; Gitelman, Penny, Ashburner, & Friston, 2003). The psychological vector used in the PPI analysis was derived from the task described in the next paragraph and had two conditions: a self appraisal task and a semantic decision-making task.
The design of the self-appraisal task was a 2 × 2 factorial with 2 levels of self reference (self-appraisal vs semantic decision) and 2 levels of prior exposure (exposure to words prior to the scan vs no prior exposure) – with equal numbers of items in each cell. For the purposes of devising the explicit mask, we looked only at the main effect of selfreference. The fMRI task consisted of two conditions: In the self-appraisal (SA) condition, participants viewed trait adjectives and made yes/no button press responses to the question: “Does the word describe me?” In the semantic decision (SEM) comparison condition, participants viewed trait adjectives and made button press responses to the question: “Is the word positive?” Trait adjectives presented were obtained from the Affective Norms for English Words (ANEW) set (Bradley & Lang, 1999). The SA and SEM conditions were each composed of 48 words; words in each condition were chosen to be similar with respect to affective valence (SA mean = 4.9, SD = 2.1; SEM mean = 4.9, SD = 2.1), affective arousal (SA mean = 5.1, SD = 1.0; SEM mean = 5.1, SD = 1.1) and word frequency (SA mean = 18.8, SD = 19.8; SEM mean = 18.5, SD = 19.9).
The fMRI self-appraisal task was acquired over two runs during the same scanning session using alternate forms of the task composed of different words. Presentation order of the sessions was counterbalanced across participants. Within each session, the two self-reference task conditions (SA and SEM) were presented in blocks consisting of four words; the presentation order of the conditions was pseudorandom and 6 blocks of each condition were presented within a session. Stimuli were presented for 4000 ms. The task duration for each session was 5 minutes 34 seconds. Yes/no responses to each word were made with a two-button response device held in the right hand. Presentation order of SA and SEM conditions was counterbalanced across participants.
The imaging protocol during the self-appraisal fMRI task was as follows: Participants were situated on the scanner bed and provided with protective earplugs, a hand-held response device, and they were fitted with high-resolution goggles (Resonance Technologies, Northridge, CA). All MR images were acquired on a GE 3.0 Tesla Signa whole body long-bore MRI scanner (General Electric, Milwaukee, WI) with a standard quadrature head coil. BOLD measurements were achieved through a gradient-echo EPI pulse sequence with the following parameters: echo time = 30 ms; repetition time (TR) = 2000 ms; flip angle = 90°; acquisition matrix = 64 × 64; field of view (FOV) = 240 mm. Thirty 4-mm-thick sagittal slices with a 1 mm gap were acquired within each TR. The resulting voxel size was 3.75 × 3.75 × 5 mm. 167 temporal volume images were collected during each run; the initial 3 image volumes of each scan were discarded.
EPI data were first slice-time corrected using the Analysis of Functional NeuroImages (AFNI) software and then motion-corrected to the mean of the images after a preliminary realignment (after the first 3 TR’s were discarded) using Statistical Parametric Mapping software (SPM5). Next, the data were spatially normalized to the Montreal Neurological Institute (MNI) EPI template image, resampled to 2 mm isotropic voxels, and smoothed with a Gaussian kernel (8 mm FWHM) using SPM5.
SPM5 was used for statistical analysis. Analyses of the time series data were performed on individual participants using a boxcar model convolved with the canonical hemodynamic response function. The general linear model also included regressors for motion correction parameters, motion correction parameter derivatives, and a mean term each for session. Additionally, the model included a 128 s high-pass filter and an AR1 temporal autocorrelation estimation. Linear contrasts (e.g. SA > SEM) of the parameter estimates for each participant, obtained from the general linear model, were entered into a second-level random effects analysis to generate statistical parametric maps.
Condition-dependent connectivity was assessed using (PPI) analysis. We defined our seed region in the MPFC by drawing a 6 mm radius sphere around the peak voxel of activation in our task (-10, 58, 6). A PPI regressor was calculated as the product of the deconvolved time series data within the seed region and the psychological variable of interest (Gitelman, et al., 2003). In our PPI analysis, we used a psychological vector for each self-reference condition instead of using a single combined psychological contrast vector. For each participant, new statistical parametric maps were computed for the physiological variable (MPFC BOLD time series data), the psychological variables, and their interactions. Positive and negative relationships between the PPI interaction terms and activation in other voxels of the whole brain were tested. Single participant results were then entered into random effects analyses testing an interaction between condition and connectivity at the group level. A significant effect for PPI indicated that slope of the linear covariance between the seed origin and a coupled region during SA differed significantly from that during SEM.
All regions showing significance at a statistical threshold of puncorrected= .05, k = 500 (see section 3.2.1) were included in a binary mask that was used to constrain our primary analysis regarding the relationship between memory awareness and MPFC functional connectivity.
2.4.2 Primary Data Analysis Methods: Preprocessing and Statistical Analysis of Functional Connectivity Data
2.4.2.1 Resting BOLD Image Acquisition
Participants were outfitted with protective earplugs and underwent two 5.5 minute long resting BOLD sessions where they were instructed to lie still and remain awake within the scanner with their eyes closed. MR images were acquired on a GE 3.0T Signa whole body MRI scanner (General Electric, Milwaukee, WI) with an 8-channel head coil. A gradient recalled echo type echo-planar imaging (GRE-EPI) pulse sequence was used with higher order shimming applied to the static magnetic field (B0). The EPI parameters were as follows: TE = 30 ms; repetition time (TR) = 2000 ms; flip angle = 90°; acquisition matrix = 64×64; field of view (FOV) = 240 mm. Thirty-six continuous 4 mm thick sagittal slices were acquired within each TR. The resulting voxel size was 3.75 × 3.75 × 4 mm. 165 temporal volume images were collected during each run; the initial 3 image volumes of each run were discarded to allow for magnetization equilibrium.
2.4.2.2 Resting BOLD Data Processing
The following processing steps were completed to obtain normally-distributed voxel-wise correlations: All fMRI EPI data were first slice-time corrected using the Analysis of Functional NeuroImages (AFNI) software and then motion-corrected to the mean of the images after a preliminary realignment (after the first 3 TR’s were discarded) using Statistical Parametric Mapping software (SPM5). Next, the data were spatially normalized to the Montreal Neurological Institute (MNI) EPI template image, resampled to 2 mm isotropic voxels, and smoothed with a Gaussian kernel (8 mm FWHM) using SPM5.
2.4.3. Single Subject Functional Connectivity Analysis
Data preparation for the connectivity analysis consisted of the following steps: (1) high and low frequency components of the BOLD data were removed using a band-pass filter (0.009-0.08 Hz) in AFNI; and (2) variance due to motion parameters, ventricle signal, white matter signal, and the temporal derivatives of each of these variables was removed with regression to control for signal fluctuations unlikely to reflect neural activity. Our a priori region of interest (i.e., seed region) in the medial prefrontal cortex (-10, 58, 6) was the same one used in the PPI analysis. We computed the Pearson’s product-moment correlation coefficient between the regional mean of the MPFC seed region’s time course and the time course for each voxel in the whole brain. These correlation maps were converted to z’ values using Fisher’s r-to-z’ transformation, and these z’ values were entered into random-effects analyses.
2.4.4 Group Statistical Analyses
We did a voxel-wise regression of MFD scores on MPFC functional connectivity. Additional covariates included in the random effects model were age, sex, GDS scores, and performance on the total learning score for trials 1 through 3 on the HVLT-R. The HVLT-R was included because we wanted to evaluate the relationship between MPFC functional connectivity maps and memory awareness, while statistically controlling for participants’ level of memory impairment due to AD-like changes. We chose HVLT total learning given the hippocampally-based impairments in episodic memory encoding that occur in MCI and AD (Johnson et al., 2004). These analyses were spatially constrained to regions included in the binary mask described in 2.4.1 and 3.2.1. Using this mask reduced the number of voxel-wise comparisons from 257,143 (# of voxels in the whole brain) to 24,321 (# of voxels in the behaviorally-relevant explicit mask). Our alpha level was p < .001.
2.4.5. Structural Scans and Volumetric Analysis
Subsequent to acquiring the resting BOLD functional scans, we also acquired high-resolution T1- and T2-weighted images, and these image volumes were used for voxel-based morphometry (VBM) to determine whether there were volumetric brain alterations that might account for our fMRI findings. T1-weighted images were acquired with a 3D sagittal magnetization prepared rapid gradient echo (MP-RAGE) sequence. T1 imaging parameters were: TR = 6616 ms, TE = 2800 ms, inversion time = 900 ms, flip angle = 8°; acquisition matrix = 256 × 256; FOV = 240 mm; slice thickness = 1.2 mm (166 slices). T2-weighted images were acquired with a 3D sagittal extended echo train acquisition (XETA) sequence. T2 imaging parameters were: TR = 6200 ms, TE = 123.9 ms, inversion time = 1871, flip angle = 90°; acquisition matrix = 256 × 256; FOV = 240 mm; slice thickness (248 slices).
For our VBM analysis, we used the “new segment” tool in SPM8’s VBM8 toolbox – a function that segments, bias corrects and spatially normalizes within the same model (Ashburner & Friston, 2005). Native-space gray matter, white matter, and cerebrospinal fluid (CSF) segments were derived from T1- and T2-weighted images. The segments were then used to create a custom template using Diffeomorphic Anatomical Registration using Exponentiated Lie Algebra (DARTEL; Ashburner, 2007) to improve alignment of our volumetric images. The segments were transformed to MNI space using the "Normalise to MNI" function of the DARTEL toolbox and preserving tissue amount (with modulation). Prior to performing group statistics, the segments were smoothed with an 8 mm Gaussian filter.
To examine regions that show volumetric gray matter changes as a function of memory awareness, we performed a regression of MFD scores on participants’ modulated gray matter images. Age, sex, and HVLT total scores were included in the model as covariates of non-interest. The statistical threshold was p < .001 in 20 contiguous voxels.
3. RESULTS
3.1 Neuropsychological characterization and memory awareness
We present descriptive statistics in Table 1 – to most intelligibly characterize our sample of participants, we divided the sample into those with and without a diagnosed memory disorder. Because Shapiro-Wilk tests of normality indicated that the distributions of some of the following variables were not normal, we used Mann-Whitney U tests to compare participants with a diagnosed memory disorder with their counterparts. Individuals with a memory disorder were significantly older than their counterparts, U = 31.5, p = .02. Additionally, there were significant group differences across a number of neuropsychological domains. Participants diagnosed with a memory disorder performed significantly worse than those without such a disorder on tests of general cognitive status (MMSE), U = 15.0, p = .001, dementia staging (CDR), U = 3.5, p < .001, verbal learning (HVLT-R total 1-3), U = 9.0, p < .001, verbal delayed recall (HVLT-DR), U = 4.5, p < .001, visual learning (BVMT-R total 1-3), U = 6.5, p = .001, visual delayed recall (BVMT-R DR), U = 2.0, p < .001, simple attention (Trail Making Test, part A), U = 11.5, p < .001, shifting attention (Trail Making Test, part B), U = 7.0, p < .001, and semantic fluency (Animal naming), U = 15.5, p = .001. Individuals with a memory disorder did show between-subject heterogeneity in their cognitive scores. Mann-Whiney U tests revealed that the five people with an early AD diagnosis performed worse as a group than their counterparts with MCI on all measures, with the exception of verbal delayed recall, p < .05. For tests that yielded age-corrected standardized scores, we ran the same statistical tests using these scores. Each of the differences reported above remained significant. Using the Spearman rho statistic, we examined the correspondence of control participant memory self-appraisal ratings with their study partners’ ratings and found that they were significantly positively correlated (rs = -.60, p = .04). In contrast, self-appraisals of memory from participants with diagnosed memory disorders did not correlate with their study partners’ ratings (p > .05). The discrepancy scores in these participants ranged from -0.15 to 0.58 (median = 0.30); comparing this to published normative data from healthy control participants (Clare, et al., 2010), these scores correspond to above the 75th percentile and below the 1st percentile respectively – where low percentiles represent overestimation by the participant in relation to the study partner rating. In order to validate the veracity of study partner reports, we also tested the correspondence between each participant’s neuropsychological memory test performance and the study partner’s rating of the participant’s memory. Analysis of data from participants with aMCI and AD using the Spearman rho statistic shows a significant positive correlation between study partner reports and HVLT-R learning performance (rs = 0.68, p < .01). In contrast, no significant correlation existed between HVLT-R learning performance and the memory impaired participants’ self-appraisal of memory (p > .05). We also examined the relationship between aMCI and AD study partner reports and study partner reports of perceived strain on the Zarit Burden Interview, and we did not find a significant relationship (p > .05). Analysis of the relationship between MFD scores and other tests of executive function showed that MFD scores correlated with Animal Naming (rs = .58, p > .05) and the Trail Making Test, part B performances (rs = -.50, p > .05).
MFD scores – our primary independent variable in this study – were not normally distributed in our participants. Therefore, we used log transformed MFD scores in our neuroimaging regression analyses.
3.2 Neuroimaging
3.2.1 Explicit Mask
The explicit mask was derived from self-appraisal fMRI task data, and a test of the main effect of task (SELF > SEM) yields a similar pattern of activation in MPFC and PCC as found in our previous publications. Regions showing this task effect are displayed in Figure 1.
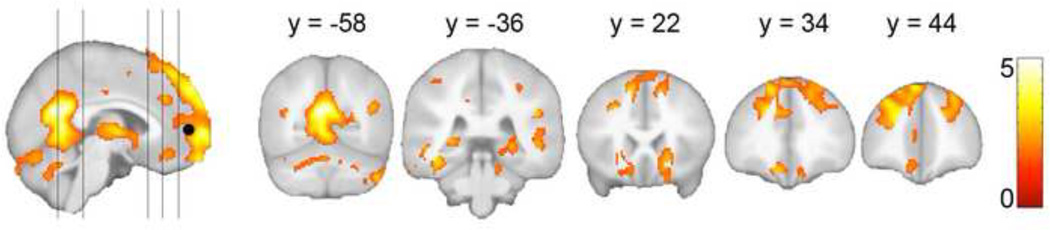
Figure 2 shows results of the psychophysiological interaction analysis; significant results were found only for the positive contrast (i.e., seed and coupled region exhibited coactive increases in signal). At a stringent, family-wise error corrected threshold (p FWE = .05), the MPFC showed condition-dependent coupling as a function of self-appraisal only in the posterior cingulate cortex. So as to not be too restrictive of regions included in our primary regression analyses, we applied a liberal threshold of puncorrected = .05, k = 500 for inclusion of regions in the explicit mask. Regions of significance at this threshold included ventral posterior cingulate extending into the cerebellar vermis, bilateral anterior and posterior hippocampi, bilateral middle and inferior temporal gyri, bilateral insular cortices, thalamus, anterior medial prefrontal cortex which extended into bilateral DLPFC, and anterior cingulate. The number of voxels that composed the explicit mask was 24,321.
Figure 2.
Psychophysiologic interaction analysis results from 90 cognitively-healthy adults who completed a self-appraisal fMRI task. Regions of significance show more positive correlation in BOLD response with the MPFC seed during self-appraisal than during judgment of affective valence. Results were used to create a binary explicit mask for the results of the memory awareness regression analysis in Figure 3.
3.2.2 MPFC Functional Connectivity – Memory Awareness Regression
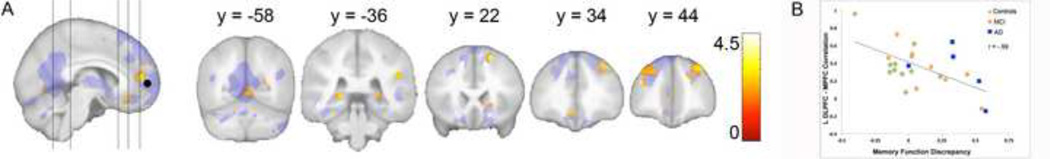
Figure 3a shows the results of the MFD regression analysis; regions of significance are those showing decreased functional connectivity with the MPFC corresponding to increased MFD scores (i.e., lower accuracy of self-appraisal). Table 2 contains the coordinates and correlation values for the significant clusters. For illustrative purposes, we graphically depict (figure 3b) the significant negative relation between MPFC functional connectivity and MFD scores from one cluster of statistical significance (containing the global maxima of this analysis) from the left DLPFC. MFD scores reported on the x-axis have been back transformed from log to raw scores to permit easy interpretation of the graph. Significant regions as shown in Figure 3a include medial prefrontal and anterior cingulate cortex, bilateral DLPFC, bilateral posterior hippocampus, right caudate nucleus, and cerebellar vermis. HVLT-R learning performances were included as a covariate of no interest in these analyses; thus, the association between awareness and BOLD signal in these regions does not appear to relate to the participants’ level of memory impairment. GDS scores were also included as covariates of no interest.
Figure 3.
Results of the regression of Memory Function Discrepancy scores on MPFC functional connectivity. Age, sex and scores on the Hopkins Verbal Learning Test Total 1–3 and GDS were included in the statistical model. A) Significant results (p < 0.001) are superimposed on the explicit mask generated from results shown in Figure 2. B) Graphical depiction (for illustrative purposes) of the negative relationship between MPFC functional connectivity with the left DLPFC cluster (which contained the global maxima at -38, 42, 22) and MFD scores. MFD scores reported on the x-axis have been back transformed from log to raw scores. Data points are color-coded to indicate whether the participant was a control or had a diagnosis of mild cognitive impairment or Alzheimer’s disease.
Table 2.
Regions showing negative correlation between MPFC functional connectivity and memory function discrepancy scores.
| Brain region | Cluster size |
r | x, y, z (MNI) | ||
|---|---|---|---|---|---|
| L DLPFC | 308 | −0.59 | −38 | 42 | 22 |
| −20 | 60 | 26 | |||
| −24 | 52 | 38 | |||
| R DLPFC | 43 | −0.59 | 20 | 20 | 56 |
| 339 | −0.52 | 30 | 38 | 44 | |
| 32 | 50 | 32 | |||
| 40 | 38 | 40 | |||
| Anterior MPFC | 150 | −0.53 | 2 | 60 | 8 |
| −6 | 52 | 12 | |||
| 10 | 62 | 10 | |||
| R TPO | 68 | −0.56 | 50 | −40 | 26 |
| −6 | 52 | 12 | |||
| −6 | 42 | 6 | |||
| Anterior Cingulate | 71 | −0.49 | −10 | 36 | −12 |
| −8 | 28 | −16 | |||
| L Post. Hippocampus | 56 | −0.39 | −20 | −34 | 2 |
| R Caudate | 29 | −0.42 | 18 | 22 | 2 |
| 12 | 24 | −8 | |||
| R Post. Hippocampus | 20 | −0.34 | 24 | −34 | 0 |
| Cerebellar Vermis | 132 | −0.35 | 4 | −60 | 0 |
Alpha for all correlations p < .001; MPFC = medial prefrontal cortex, DLPFC = dorsolateral prefrontal cortex, TPO = temporoparieto-occipital junction
3.2.3 Gray Matter Volume – Memory Awareness Regression
The regression of MFD scores on participants’ modulated gray matter images revealed only one small (k = 77) region of significance in the left posterior inferior temporal gyrus. This region did not overlap with any areas of significance in our regression of MFD scores on MPFC functional connectivity.
4. Discussion
4.1. Overview of findings
Older adults exhibiting behavioral changes associated with Alzheimer’s pathology show awareness of their own decline that ranges from full awareness to no demonstrated awareness (Galeone, Pappalardo, Chieffi, Iavarone, & Carlomagno, 2011; Vogel, Hasselbalch, Gade, Ziebell, & Waldemar, 2005), and our results reinforce the notion that self-awareness changes exist even in early stages of the disease (Clare, et al., 2010; Mimura & Yano, 2006; Vogel, et al., 2004; Vogel, Waldorff, & Waldemar, 2010). Consistent with our expectations and our prior published report, (Ries, et al., 2007) control participants accurately assessed their own memory ability; however, individuals with MCI and early AD showed variable accuracy in their memory self-appraisals. Our participants were older adults with a wide range of memory ability – including people with normal memory and those with impaired memory due to mild cognitive impairment and early Alzheimer’s disease. Results of our regression analysis were consistent with the hypothesis that altered MPFC functional connectivity – particularly with other cortical midline structures and DLPFC – explains variance in memory self-appraisal accuracy in these older adults. Greater MFS discrepancy scores corresponded to attenuated MPFC functional connectivity with subgenual anterior cingulate cortex, MPFC regions posterior and dorsal to the MPFC seed region, bilateral DLPFC, bilateral posterior hippocampus and right caudate nucleus. Contrary to expectations, MPFC functional connectivity with the posterior cingulate was not significantly related to MFD scores. The changes in MPFC functional connectivity that relate to self-appraisal accuracy noted here occur in the absence of gray matter loss as measured by VBM methods.
4.2 Measurement of Memory Awareness
The Memory Function Scale of the MARS was our index of memory self-appraisal accuracy. Because the MFS uses the study partner report as the gold standard index of the AD-affected person’s memory, we performed checks on the accuracy of these reports. Study partner reports correlated significantly with participants’ memory test performances – supporting the accuracy of these reports. In contrast, MCI and AD participant reports on the MFS questionnaire showed no significant relation to their memory test performance. As an additional check on the possible confounds to the accuracy of study partners’ MFS reports, we assessed their perceived strain as caregivers. Although some reports suggest that perceived strain as a result of providing care sometimes leads loved ones to underestimate the care receiver’s actual level of function (Quinn, et al., 2009; Quinn, Clare, & Woods, 2010), we did not find this in our sample.
4.3 Interpretation of results with regard to brain-behavior relationships
In this study, we examined the covariance between MPFC functional connectivity and a well-defined measure of awareness in a patient group showing heterogeneity in self-appraisal ability. Additionally, we examined MPFC functional connectivity within the spatial constraints of an explicit mask defined by regions that show task-dependent connectivity that is modulated by a self-appraisal context. Thus, our primary analysis was limited to regions determined a priori to be of behavioral significance.
The data presented here indicate that memory self-appraisal ability corresponds to the integrity of MPFC functional connectivity with several other of anteriorly-located cortical regions. This makes sense given the MPFC’s extensive intraregional anatomical connectivity and connections with the anterior cingulate cortex and the DLPFC. This finding is also consistent with fMRI studies of self-appraisal and social decision-making in adults with healthy brains and people with awareness deficits secondary to brain injury or degeneration that implicate each of these regions in this function (Fehr & Camerer, 2007; Ries, et al., 2007; Schmitz & Johnson, 2006, 2007).
The regression results in the present analysis mirror similar decrements in DLPFC perfusion (Reed, Jagust, & Coulter, 1993) and ventrolateral cerebral blood flow (Vogel, et al., 2005) that are observed in AD patients with anosognosia. These DLPFC findings coupled with the correlation between MFD scores and executive function tests show the executive contribution to our participants’ self-appraisal decrements. Our finding is also consist with transcranial magnetic stimulation studies showing that disruption of DLPFC activity interrupts moral social decision making – although this was only found with right-sided stimulation (Knoch & Fehr, 2007; Knoch, Pascual-Leone, Meyer, Treyer, & Fehr, 2006). Specifically, right DLPFC stimulation results in difficulty resisting temptation in an immediately rewarding fashion, even when there may be negative long-term social consequences (i.e., poor reputation). Thus, the MPFC-DLPFC physiologic circuit appears to play a key role in directing volitional social behavior, and its disruption causes changes in awareness and social decision-making.
Another area of significance in our regression analysis was in right caudate. This finding is consistent with research showing that dorsal striatum, like its ventral counterpart, plays a role in reward processing – a function that relates to the salience of information processed in reference to one’s self (Kelley & Berridge, 2002). Our data suggest that degradation of functional connectivity between MPFC and caudate contributes to deficits in memory self-appraisal.
Anatomic and functional connections exist between MPFC with several posterior cortical regions, one of the most notable being the posterior cingulate cortex. Therefore, we were surprised that poorer memory self-appraisal ability was not associated with degraded MPFC-PCC functional connectivity given their consistent co-activation during fMRI self-appraisal tasks (Johnson & Ries, 2010) and the finding of attenuated MPFC and PCC BOLD activity elicited by a self-appraisal task in people with self-appraisal deficits (Ries, et al., 2007). One possible explanation for our finding is that small-world networks of connectivity may show disease-related decrements before more far-reaching connections break down (Supekar, Menon, Rubin, Musen, & Greicius, 2008), although evidence for this is not unequivocal (Sanz-Arigita et al., 2010). Also, the posterior cingulate and precuneus are regions of high pathologic burden in AD. Our null result in this brain region may relate to alterations in neurovascular coupling secondary to perfusion and metabolic changes in MCI and AD (Schroeter, Stein, Maslowski, & Neumann, 2009).
Our analysis did reveal a direct relationship between accuracy of memory self-appraisals and MPFC functional connectivity with the left posterior hippocampus. This finding fits with theoretical accounts that emphasize the relationship between self and memory (Mograbi, et al., 2009) and empirical results showing that posterior hippocampal activity is modulated by the personal salience of retrieved autobiographical memories (Addis, Moscovitch, Crawley, & McAndrews, 2004). However, it is important to note that self-appraisal ability and episodic memory ability – even autobiographical episodic memory – are related, but dissociable psychological constructs (Libby & Eibach, 2007). In the present study, the self-appraisal impairments we observed in a subset of our participants were not fully explained by a deficit of memory – our analysis of the brain correlates of memory self-appraisal accuracy statistically controlled for standardized memory performance.
A large literature focuses on MPFC functional connectivity – particularly as a central hub in a functional connectivity network often called the default mode network (Buckner et al., 2009). Many researchers have noted the striking neuroanatomic overlap between brain activity during fMRI self-appraisal tasks (see Figure 1) and the default mode network – with common regions including MFPC, posterior cingulate, lateral parietal regions, and hippocampus. Regions of the default mode network are more active during “resting” baseline conditions (e.g., fixation cross) than during fMRI task conditions requiring attention and response to an external stimulus – and this observation has guided reasoning that these regions are involved in mentalizing about one’s self and one’s internal milieu (Gusnard, 2005). However, well-controlled studies supplying behavioral empirical data required for making strong inference regarding brain-behavior relationships have been sparse. Our results suggest that regions often specified as default mode network do show degraded MPFC functional connectivity in people with poorer self-appraisal ability – most notably the anterior cingulate and other medial prefrontal regions. However, some areas of significance in our regression analysis fall outside of regions typically denoted as the default mode network – most notably bilateral DLPFC. Our results suggest that MPFC functional connectivity with regions both within and without the default mode network are important for supporting self-appraisal.
4.4. Study Limitations
Some study limitations are of note. We chose to focus on one aspect of self-awareness: explicitly expressed appraisals of current everyday episodic memory ability. However, anosognosia is a multidimensional construct – with behavioral measures of awareness of various cognitive, affective, and functional showing only modest or no correlation (Clare, Whitaker, et al., 2011; Derouesne, et al., 1999; Okwonko, Spitznagel, Alosco, & Tremont, 2010). Thus, our data definitively shed light on the behavioral presentation and brain substrates of only a small sector of a vast clinical phenomenon.
A second potential limitation is the clinical heterogeneity of our participants – our sample includes healthy older adults as well as individuals with memory impairment diagnosed with MCI and early AD. Our participants were variable in their performance on tests of executive function and these scores were correlated with MFD scores – a finding that was expected given our focus on frontally-medially awareness deficits. Having a range in cognitive in brain function is better suited to associative studies such as this one. However, our small sample size limited our ability to make inference specific to a particular stage of early AD (i.e., MCI or AD). Given results of a recent meta-analysis that found medial frontal reduction in glucose utilization in AD – but not MCI – when compared to controls, investigating group differences (i.e., MCI versus AD) in symptom-related changes in medial frontal physiology would be of interest. As an additional caveat, our small sample size may have limited our power to detect regions of significant correlation between MPFC functional connectivity and self-appraisal accuracy.
A final limitation relates to our use of a BOLD measure (functional connectivity) as an index of neural health in our sample. This measure assumes that neurovascular coupling is constant across participants (Pike, 2011). However, two factors that varied in our participants – presence of AD and acetylcholine-esterase inhibitor (AChEI) treatment – have been linked to changes in neurovascular coupling (Rosengarten, Paulsen, Burr, & Kaps, 2009). AD is associated with greater cerebrovascular stiffness and a slower perfusion response to neural activity. However, there is evidence that AChEI treatment may mitigate some impairment in cerebral vasoregulation in response to neural activity. These vascular alterations may have introduced error into our index of neurally-based changes associated with self-appraisal deficits – particularly in regions of high disease burden such as the posterior cingulate cortex.
4.5. Future Directions
Findings reported here indicate that impaired awareness of memory decline is present in a subset of people in the earliest stages of AD. Although the causes of this behavioral phenomenon are likely multiple, with environmental and cultural factors mediating the manifestation of altered self-appraisal ability (Clare, Nelis, et al., 2011), our data join other published findings indicating that memory awareness deficits in this population have a physiologic brain substrate. Because people who are unaware of AD-related changes in themselves are unlikely to present with complaints in clinic, anosognosia may contribute to missed diagnosis of MCI and AD, especially in primary care clinics (Bradford, et al., 2009) and would lead to a selection bias in research. This is a serious concern – since delay in diagnosis due to anosognosia denies the AD-affected individual timely pharmacologic treatment and (in the United States) also blocks caregivers from receiving Medicare-funded support, resources, and education that can potentially mitigate their burden (Weimer & Sager, 2009). Furthermore, delay in diagnosis also results in greater costs to the health care system as a whole (Weimer & Sager, 2009). Work to more fully characterize the prevalence of anosognosia in early AD, the behavioral presentation and phenomenology of this condition, and its neural substrates is critical during this time of international research focus on early disease detection.
HIGHLIGHTS.
People with early Alzheimer’s symptoms vary in memory self-appraisal accuracy.
We indexed memory self-appraisal via a psychometrically-validated measure.
Our a priori neuroimaging focus was medial prefrontal functional connectivity.
Medial prefrontal functional connectivity covaries with self-appraisal accuracy.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the study participants who generously gave their time and effort to make this project possible. We also thank Caitlin Cleary, Robbie Kattappuram, Amy Hawley, Kelli Hellenbrand, Sara Pladziewicz, and Jennifer Oh for their dedicated work in participant recruitment and execution of the study procedures. This work was supported by grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources (NCRR), National Institutes of Health (NIH). Michele Ries’ contributions to this paper were supported through the CTSA grant – both as a Clinical Scholar and through a pilot grant that funded acquisition of data used in the primary analysis. Acquisition of data used in the explicit mask was made possible by support through NIH grants R01AG21155 (PI: Sterling Johnson) and R01AG027161 (PI: Mark Sager). We also acknowledge facilities and resources provided through the Wisconsin Alzheimer’s Disease Research Center (NIH grant P50AG033514; PI: Sanjay Asthana) and the Geriatric Research Education and Clinical Center (GRECC) of the William S. Middleton Memorial Veterans Hospital (GRECC manuscript number 2011-10).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Addis D, Moscovitch M, Crawley A, McAndrews M. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004;14(6):752–762. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- 2.Amodio D, Frith C. Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 3.Arlt S, Lindner R, Rosler A, Von Renteln-Kruse W. Adherence to medication in patients with dementia: Predictors and strategies for improvement. Drugs & Aging. 2008;25:1033–1047. doi: 10.2165/0002512-200825120-00005. [DOI] [PubMed] [Google Scholar]
- 4.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Bastin C, Feyers D, Souchay C, Guillaume B, Pepin J, Lemaire C, Salmon E. Frontal and posterior cingulate metabolic impairment in the behavioral variant of frontotemporal dementia with impaired autonoetic consciousness. Human Brain Mapping, on-line version published before inclusion in an issue. 2011 doi: 10.1002/hbm.21282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benedict R. Brief Visuospatial Memory Test-Revised: Psychological Assessment Resources Inc. 1997 [Google Scholar]
- 7.Bradford A, Kunik M, Schulz P, Williams S, Singh H. Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer Disease and Associated Disorders. 2009;23:306–314. doi: 10.1097/WAD.0b013e3181a6bebc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley M, Lang P. Affective Norms for English Words (ANEW): Instruction Manual and Affective Ratings. 1999 [Google Scholar]
- 9.Buckner R, Sepulcre J, Talukdar T, Krienen F, Liu H, Hedden T, Johnson K. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer's disease. The Journal of neuroscience: The official journal of the Society for Neuroscience. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clare L, Nelis S, Martyr A, Roberts J, Whitaker C, Markova I, Morris R. The influence of psychological, social and contextual factors on the expression and measurement of awareness in early-stage dementia: Testing a biopsychosocial model. International Journal of Geriatric Psychiatry, on-line version published before inclusion in an issue. 2011 doi: 10.1002/gps.2705. [DOI] [PubMed] [Google Scholar]
- 11.Clare L, Whitaker C, Nelis S. Appraisal of memory functioning and memory performance in healthy ageing and early-stage Alzheimer's disease. Neuropsychology, Development, and Cognition. Section B Aging, Neuropsychology and Cognition. 2010;17:462–491. doi: 10.1080/13825580903581558. [DOI] [PubMed] [Google Scholar]
- 12.Clare L, Whitaker C, Nelis S, Martyr A, Markova I, Roth I, Morris R. Multidimensional assessment of awareness in early-stage dementia: A cluster analytic approach. Dementia and Geriatric Cognitive Disorders. 2011;31:317–327. doi: 10.1159/000327356. [DOI] [PubMed] [Google Scholar]
- 13.Clare L, Wilson B, Carter G, Roth I, Hodgesm J. Assessing awareness in early-stage Alzheimer's disease: Development and piloting of the Memory Awareness Rating Scale. Neuropsychological Rehabilitation. 2002;12:341–362. [Google Scholar]
- 14.Cotrell V, Wild K. Longitudinal study of self-imposed driving restrictions and deficit awareness in patients with Alzheimer disease. Alzheimer Disease and Associated Disorders. 1999;13:151–156. doi: 10.1097/00002093-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Derouesne C, Thibault S, Lagha-Pierucci S, Baudouin-Madec V, Ancri D, Lacomblez L. Decreased awareness of cognitive deficits in patients with mild dementia of the Alzheimer type. International Journal of Geriatric Psychiatry. 1999;14:1019–1030. [PubMed] [Google Scholar]
- 16.Dubois B, Feldman H, Jacova C, Dekosky S, Barberger-Gateau P, Cummings J, Scheltens P. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet neurology. 2007;6(8):734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 17.Feher E. Anosognosia in Alzheimer's disease. Neuropsychiatry, Neuropsychology, & Behavioral Neurology. 1991;4:136–146. [Google Scholar]
- 18.Fehr E, Camerer C. Social neuroeconomics: The neural circuitry of social preferences. Trends in Cognitive Sciences. 2007;11:419–427. doi: 10.1016/j.tics.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 21.Galeone F, Pappalardo S, Chieffi S, Iavarone A, Carlomagno S. Anosognosia for memory deficit in amnestic mild cognitive impairment and Alzheimer's disease. International Journal of Geriatric Psychiatry. 2011;26:695–701. doi: 10.1002/gps.2583. [DOI] [PubMed] [Google Scholar]
- 22.Gitelman D, Penny W, Ashburner J, Friston K. Modeling regional and psychophysiologic interactions in fMRI: The importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- 23.Griffith H, Dymek M, Atchison P, Harrell L, Marson D. Medical decisionmaking in neurodegenerative disease: Mild AD and PD with cognitive impairment. Neurology. 2005;65:483–485. doi: 10.1212/01.wnl.0000171346.02965.80. [DOI] [PubMed] [Google Scholar]
- 24.Grossman M, Eslinger P, Troiani V, Anderson C, Avants B, Gee J, Antani S. The role of ventral medial prefrontal cortex in social decisions: Converging evidence from fMRI and frontotemporal lobar degeneration. Neuropsychologia. 2010;48:3505–3512. doi: 10.1016/j.neuropsychologia.2010.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gusnard D. Being a self: Considerations from functional imaging. Consciousness and Cognition. 2005;14(4):679–697. doi: 10.1016/j.concog.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Johnson S, Baxter L, Susskind-Wilder L, Connor D, Sabbagh M, Caselli R. Hippocampal adaptation to face repetition in healthy elderly and mild cognitive impairment. Neuropsychologia. 2004;42(7):980–989. doi: 10.1016/j.neuropsychologia.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Johnson S, Baxter L, Wilder L, Pipe J, Heiserman J, Prigatano G. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- 28.Johnson S, Ries M. Functional imaging of self-appraisal. In: Prigatano G, editor. The Study of Anosognosia. New York: Oxford University Press; 2010. [Google Scholar]
- 29.Johnson S, Schmitz T, Moritz C, Meyerand M, Rowley H, Alexander A, Alexander G. Activation of brain regions vulnerable to Alzheimer's disease: The effect of mild cognitive impairment. Neurobiology of Aging. 2006;27(11):1604–1612. doi: 10.1016/j.neurobiolaging.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karlawish J, Casarett D, James B, Xie S, Kim S. The ability of persons with Alzheimer disease to make a decision about taking an AD treatment. Neurology. 2005;64:1514–1519. doi: 10.1212/01.WNL.0000160000.01742.9D. [DOI] [PubMed] [Google Scholar]
- 31.Kaszniak A, Zak M. On the neuropsychology of metamemory: Contributions from the study of amnesia and dementia. Learning and Individual Differences. 1996;8:355–381. [Google Scholar]
- 32.Kelley A, Berridge K. The neuroscience of natural rewards: Relevance to addictive drugs. The Journal of neuroscience: The official journal of the Society for Neuroscience. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knoch D, Fehr E. Resisting the power of temptations: The right prefrontal cortex and self-control. Annals of the New York Academy of Sciences. 2007;1104:123–134. doi: 10.1196/annals.1390.004. [DOI] [PubMed] [Google Scholar]
- 34.Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science. 2006;314:829–832. doi: 10.1126/science.1129156. [DOI] [PubMed] [Google Scholar]
- 35.Koltai D, Welsh-Bohmer K, Schmechel D. Influence of anosognosia on treatment outcome among dementia patients. Neuropsychological Rehabilitation. 2001;11:455–475. [Google Scholar]
- 36.Libby L, Eibach R. How the self affects and reflects the content and subjective experience of autobiographical memory. In: Sedikides C, Spencer S, editors. The Self. New York: Psychology Press; 2007. [Google Scholar]
- 37.Luria A. The man with a shattered world: The history of a brain wound. New York: Basic Books; 1972. [Google Scholar]
- 38.Luria A. The working brain: An introduction to neuropsychology. New York: Peguin Books; 1973. [Google Scholar]
- 39.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA workgroup under the auspices of the Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 40.Mimura M, Yano M. Memory impairment and awareness of memory deficits in early-stage Alzheimer's disease. Reviews in the Neurosciences. 2006;17:253–266. doi: 10.1515/revneuro.2006.17.1-2.253. [DOI] [PubMed] [Google Scholar]
- 41.Mograbi D, Brown R, Morris R. Anosognosia in Alzheimer's disease--The petrified self. Consciousness and Cognition. 2009;18(4):989–1003. doi: 10.1016/j.concog.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Neary D, Snowden J, Gustafson L, Passant U, Stuss D, Black S, Benson D. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 43.Okwonko O, Spitznagel M, Alosco M, Tremont G. Associations among measures of awareness of cognitive deficits in dementia. Alzheimer's & Dementia: The Journal of the Alzheimer's Association. 2010;6:312–318. doi: 10.1016/j.jalz.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orfei M, Varsi M, Blundo C, Celia E, Casini A, Caltagirone C, Spalletta G. Anosognosia in mild cognitive impairment and mild Alzheimer's disease: Frequency and neuropsychological correlates. The American Journal of Geriatric Psychiatry. 2010;18:1133–1140. doi: 10.1097/JGP.0b013e3181dd1c50. [DOI] [PubMed] [Google Scholar]
- 45.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 46.Pike G. Quantitative functional MRI: Concepts, issues and future challenges. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 47.Price J. Prefrontal cortical networks related to visceral function and mood. Annals of the New York Academy of Sciences. 1999;877:383–396. doi: 10.1111/j.1749-6632.1999.tb09278.x. [DOI] [PubMed] [Google Scholar]
- 48.Price J, Drevets W. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prigatano G. Anosognosia after traumatic brain injury. In: Prigatano G, editor. The Study of Anosognosia. New York: Oxford University Press; 2010. [Google Scholar]
- 50.Pronin E. How we see ourselves and how we see others. Science. 2008;320:1177–1180. doi: 10.1126/science.1154199. [DOI] [PubMed] [Google Scholar]
- 51.Quinn C, Clare L, Woods B. The impact of the quality of relationship on the experiences and wellbeing of caregivers of people with dementia: A systematic review. Aging & Mental Health. 2009;13:143–154. doi: 10.1080/13607860802459799. [DOI] [PubMed] [Google Scholar]
- 52.Quinn C, Clare L, Woods R. The impact of motivations and meanings on the wellbeing of caregivers of people with dementia: A systematic review. International Psychogeriatrics. 2010;22:43–55. doi: 10.1017/S1041610209990810. [DOI] [PubMed] [Google Scholar]
- 53.Rankin K, Baldwin E, Pace-Savitsky C, Kramer J, Miller B. Self awareness and personality change in dementia. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76:632–639. doi: 10.1136/jnnp.2004.042879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reed B, Jagust W, Coulter L. Anosognosia in Alzheimer's disease: Relationships to depression, cognitive function, and cerebral perfusion. Journal of Clinical & Experimental Neuropsychology. 1993;15:231–244. doi: 10.1080/01688639308402560. [DOI] [PubMed] [Google Scholar]
- 55.Ries M, Jabbar B, Schmitz T, Trivedi M, Gleason C, Carlsson C, Johnson S. Anosognosia in mild cognitive impairment: Relationship to activation of cortical midline structures involved in self-appraisal. Journal of the International Neuropsychological Society. 2007;13:450–461. doi: 10.1017/S1355617707070488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosen H, Alcantar O, Rothlind J, Sturm V, Kramer J, Weiner M, Miller B. Neuroanatomical correlates of cognitive self-appraisal in neurodegenerative disease. Neuroimage. 2010;49:3358–3364. doi: 10.1016/j.neuroimage.2009.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosengarten B, Paulsen S, Burr O, Kaps M. Neurovascular coupling in Alzheimer patients: Effect of acetylcholine-esterase inhibitors. Neurobiology of Aging. 2009;30(12):1918–1923. doi: 10.1016/j.neurobiolaging.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 58.Rymer S, Salloway S, Norton L, Malloy P, Correia S, Monast D. Impaired awareness, behavior disturbance, and caregiver burden in Alzheimer disease. Alzheimer Disease and Associated Disorders. 2002;16(4):248–253. doi: 10.1097/00002093-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Saleem K, Kondo H, Price J. Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. The Journal of Comparative Neurology. 2008;506:659–693. doi: 10.1002/cne.21577. [DOI] [PubMed] [Google Scholar]
- 60.Salmon E, Perani D, Collette F, Feyers D, Kalbe E, Holthoff V, Herholz K. A comparison of unawareness in frontotemporal dementia and Alzheimer's disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2008;79:176–179. doi: 10.1136/jnnp.2007.122853. [DOI] [PubMed] [Google Scholar]
- 61.Sanz-Arigita E, Schoonheim M, Damoiseaux J, Rombouts S, Maris E, Barkhof F, Stam C. Loss of 'small-world' networks in Alzheimer's disease: Graph analysis of FMRI resting-state functional connectivity. PloS One. 2010;5(11):e13788. doi: 10.1371/journal.pone.0013788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmitz T, Johnson S. Self-appraisal decisions evoke dissociated dorsalventral aMPFC networks. Neuroimage. 2006;30:1050–1058. doi: 10.1016/j.neuroimage.2005.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmitz T, Johnson S. Relevance to self: A brief review and framework of neural systems underlying appraisal. Neuroscience & Biobehavioral Reviews. 2007;31:585–596. doi: 10.1016/j.neubiorev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schroeter M, Ettrich B, Menz M, Zysset S. Traumatic brain injury affects the frontomedian cortex--An event-related fMRI study on evaluative judgments. Neuropsychologia. 2010;48:185–193. doi: 10.1016/j.neuropsychologia.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 65.Schroeter M, Raczka K, Neumann J, von Cramon D. Neural networks in frontotemporal dementia--A meta-analysis. Neurobiology of Aging. 2008;29:418–426. doi: 10.1016/j.neurobiolaging.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 66.Schroeter M, Stein T, Maslowski N, Neumann J. Neural correlates of Alzheimer's disease and mild cognitive impairment: A systematic and quantitative meta-analysis involving 1351 patients. Neuroimage. 2009;47(4):1196–1206. doi: 10.1016/j.neuroimage.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seltzer B, Vasterling J, Yoder J, Thompson K. Awareness of deficit in Alzheimer's disease: Relation to caregiver burden. The Gerontologist. 1997;37:20–24. doi: 10.1093/geront/37.1.20. [DOI] [PubMed] [Google Scholar]
- 68.Starkstein SE, Jorge R, Mizrahi R, Adrian J, Robinson RG. Insight and danger in Alzheimer's disease. European Journal of Neurology. 2007;14(4):455–460. doi: 10.1111/j.1468-1331.2007.01745.x. [DOI] [PubMed] [Google Scholar]
- 69.Supekar K, Menon V, Rubin D, Musen M, Greicius M. Network analysis of intrinsic functional brain connectivity in Alzheimer's disease. PLoS Computational Biology. 2008;4:e1000100. doi: 10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vogel A, Hasselbalch S, Gade A, Ziebell M, Waldemar G. Cognitive and functional neuroimaging correlates for anosognosia in mild cognitive impairment and Alzheimer's disease. International Journal of Geriatric Psychiatry. 2005;20:238–246. doi: 10.1002/gps.1272. [DOI] [PubMed] [Google Scholar]
- 71.Vogel A, Stokholm J, Gade A, Andersen B, Hejl A, Waldemar G. Awareness of deficits in mild cognitive impairment and Alzheimer's disease: Do MCI patients have impaired insight? Dementia and Geriatric Cognitive Disorders. 2004;17(3):181–187. doi: 10.1159/000076354. [DOI] [PubMed] [Google Scholar]
- 72.Vogel A, Waldorff F, Waldemar G. Impaired awareness of deficits and neuropsychiatric symptoms in early Alzheimer's disease: the Danish Alzheimer Intervention Study (DAISY) The Journal of Neuropsychiatry and Clinical Neurosciences. 2010;22:93–99. doi: 10.1176/jnp.2010.22.1.93. [DOI] [PubMed] [Google Scholar]
- 73.Weimer D, Sager M. Early identification and treatment of Alzheimer's disease: Social and fiscal outcomes. Alzheimer's & Dementia: The Journal of the Alzheimer's Association. 2009;5:215–226. doi: 10.1016/j.jalz.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zarit S. Diagnosis and management of caregiver burden in dementia. In: Vinken P, Bruyn G, editors. Handbook of clinical neurology. Vol. 89. 2008. pp. 101–106. [DOI] [PubMed] [Google Scholar]
- 75.Zarit S, Reever K, Bach-Peterson J. Relatives of the impaired elderly: Correlates of feelings of burden. The Gerontologist. 1980;20:649–655. doi: 10.1093/geront/20.6.649. [DOI] [PubMed] [Google Scholar]