Abstract
Vitamin A deficiency has been correlated with increased severity of human immunodeficiency virus type 1 (HIV-1)-associated disease. Moreover, vitamin A supplementation can reduce AIDS-associated morbidity and mortality. Our group and others have shown that retinoids, the bioactive metabolites of vitamin A, repress HIV-1 replication in monocytic cell lines and primary macrophages by blocking long-terminal-repeat (LTR)-directed transcription. Based on these studies, we hypothesize that retinoids are natural repressors of HIV-1 in vivo. We show here that all-trans-retinoic acid (RA)-mediated repression of HIV-1 activation requires pretreatment for at least 12 h and is blocked by the protein synthesis inhibitors cycloheximide and puromycin. Studies of the kinetics of RA-mediated repression in U1 cells and primary monocyte-derived macrophages (MDMs) reveal that the repressive effects of RA on HIV-1 expression are long-lasting but reversible. We demonstrate that HIV-1 expression is activated when U1 cells or MDMs are cultured in retinoid-free synthetic medium and show that physiological concentrations of RA repress this activation. In addition, the synthetic pan-retinoic acid receptor antagonist BMS-204 493 activates HIV-1 replication in U1 cells in a dose-dependent manner, suggesting that RA-induced transactivation of cellular gene expression is required for HIV-1 repression. Together, these data support the hypothesis that retinoids present in tissue culture media in vitro and serum in vivo maintain HIV-1 in a transcriptionally repressed state in monocytes/macrophages.
Retinoids, the bioactive metabolites of vitamin A, are likely candidates for natural repressors of human immunodeficiency virus type 1 (HIV-1) in vivo. These metabolites are required for a variety of normal biological processes, including development, growth, vision, and immunity (36, 54). Retinoids are found in serum and many other tissues (13, 52), including the lung, where they exert their effects as potent modulators of gene expression (36, 58). Their activity is mediated through their association with nuclear receptor transcription factors (NRs) that recognize and bind to specific DNA elements known as retinoic acid response elements (RAREs) (36, 58). To date, two families of these NRs have been identified: the retinoic acid receptors (RARs) and the retinoid X receptors (RXRs). These receptors bind to RAREs as either homodimers (RXR/RXR) or heterodimers (RAR/RXR) to either activate or repress gene transcription (64).
There is a wealth of epidemiological and molecular evidence suggesting that retinoids repress HIV-1 expression. Several properties of HIV-1-induced disease, including morbidity, mortality, the rate of heterosexual transmission, and the rate of mother-to-child transmission, have been inversely correlated with serum vitamin A levels (28, 39, 55-57, 59). Significantly, in clinical trials conducted in Africa, vitamin A supplementation has been shown to reduce HIV-1-associated disease and to slow the progression toward AIDS (15, 18, 19). Molecular studies conducted by our laboratory and others support the hypothesis that metabolites of vitamin A can repress HIV-1 transcription in monocytes/macrophages (8, 38, 47, 61, 65). In agreement with others, we found that physiological concentrations of vitamin A, in the form of all-trans-retinoic acid (RA), repressed HIV-1 long-terminal-repeat (LTR)-directed expression in THP-1 monocytes (38). In addition, Poli et al. found that RA repressed phorbol 12-myristate 13-acetate-, interleukin-6 (IL-6)-, and granulocyte/monocyte-colony stimulating factor-mediated activation of HIV-1 in latently infected U1 monocytes (47). We also found that RA pretreatment repressed HIV-1 replication in primary monocyte-derived macrophages (MDMs) (8, 38). Similarly, Yamaguchi et al. found that RA repressed HIV-1 replication in alveolar macrophages (65). Recently, we showed that RA consistently repressed HIV-1 replication in MDMs cultured in the presence of the proinflammatory cytokines IL-1β and IL-6 at concentrations expected in local sites of infection where HIV-1-infected macrophages reside in vivo (8).
HIV-1 infection initially leads to an effective immune response in the host, including the generation of virus-specific cytotoxic T lymphocytes (CTLs) believed to resolve the high levels of viremia that characterize the acute phase of infection (5). HIV-1 has evolved several mechanisms to avoid host immune surveillance (14), including the establishment of latent infections. Latently infected T cells and macrophages may avoid recognition by virus-specific CTLs and act as potential reservoirs for the dissemination of HIV-1 at later stages of disease. Vitamin A regulation is often impaired concomitant with progression toward AIDS, resulting in vitamin A deficiency (2, 9, 29, 44, 59). This deficiency may result in the loss of RA-mediated repression of HIV-1 and may contribute to viral expansion and progression toward AIDS.
We propose that RA is a natural repressor of HIV-1. Not only has vitamin A deficiency been inversely correlated with virus transmission and disease progression, but bioactive metabolites of vitamin A have been shown to repress HIV-1 replication in vitro. Here we show that RA represses HIV-1 expression in both latently infected U1 cells and productively infected MDMs and that this repression requires new cellular protein synthesis. We also demonstrate that HIV-1 expression is repressed when cells are grown in standard growth medium containing bioactive concentrations of retinoids but is activated when cells are grown in synthetic retinoid-free medium. Significantly, this activation is reversed by the addition of a physiological concentration of RA (10−9 M). Also, treatment of U1 cells with the synthetic pan-RAR antagonist BMS-204 493 activates HIV-1 expression in a dose-dependent manner, suggesting that RA-induced transactivation of cellular gene expression is required for HIV-1 repression. These data are consistent with the hypothesis that physiological concentrations of RA in serum actively repress HIV-1 in macrophages in vivo and that RA may play a role in maintaining latency in HIV-1-infected monocytes/macrophages. Further studies to more precisely determine the mechanism of RA-mediated repression of HIV-1 will be important for evaluating the therapeutic potential of vitamin A in underdeveloped nations, where malnourishment is endemic.
MATERIALS AND METHODS
Cell culture and reagents.
U1 cells were obtained from the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program courtesy of Tom Folks. U1 cells were cultured in RPMI 1640 medium supplemented with 100 U of penicillin/ml, 100 μg of streptomycin/ml, 0.29 mg of l-glutamine/ml, and 10% fetal bovine serum (FBS) (HyClone, Logan, Utah). To prepare monocyte-derived macrophages, CD14+ monocytes, purified from peripheral blood mononuclear cells of normal human donors by using anti-CD14-coated magnetic beads and the auto-MACS protocol (Miltenyi Biotech, Auburn, Calif.) (25), were allowed to differentiate in culture in the presence of 10% FBS and 10% normal human serum (Atlanta Biologicals, Norcross, Ga.). For low-serum experiments, U1 cells or MDMs were cultured in synthetic retinoid-free AIM V medium (Gibco/Invitrogen, Carlsbad, Calif.) supplemented with either 0.5 or 1% FBS, respectively.
RA, bacterial lipopolysaccharide (LPS) (Escherichia coli serotype O55:B5), trichostatin A (TSA), cycloheximide, and butylated hydroxytoluene were purchased from Sigma (St. Louis, Mo.). Synthetic retinoids, including a pan-RAR antagonist (BMS-204 493) and a pan-RAR agonist (BMS-348 997), were generously provided by Bristol Myers/Squibb. BMS-348 997 was originally developed by Ligand Pharmaceuticals as ALRT1550. Stock solutions of RA, retinol, and the synthetic retinoids (each at 5 × 10−2 M) were prepared in dimethyl sulfoxide with 10−4 M butylated hydroxytoluene to prevent oxidation. Stock solutions were stored under argon in glass vials at −80°C. Human IL-1β and IL-6 were purchased from R&D Systems (Minneapolis, Minn.). All reagents and tissue culture media were free of contaminating endotoxin.
HIV-1 p24 antigen ELISA.
HIV-1 expression was measured by p24 antigen capture enzyme-linked immunosorbent assay (ELISA; Beckman Coulter, Brea, Calif.) according to the manufacturer's instructions. The results with U1 cells were normalized for cell number and are expressed as picograms per 105 cells. The results with MDMs are expressed as nanograms per milliliter.
Semiquantitative RT-PCR assay.
Total cytoplasmic RNA was isolated from U1 cells by using the RNeasy Minikit (Qiagen, Valencia, Calif.) according to the manufacturer's instructions. RNA (100 ng) was analyzed by reverse transcription-PCR (RT-PCR) by using the OneStep RT-PCR kit (Qiagen). RNA was reverse transcribed and amplified in a total volume of 50 μl containing 2.5 mM MgCl2, 400 μM concentrations of each deoxynucleoside triphosphate, 10 U of RNasin RNase inhibitor (Promega, Madison, Wis.), 5 μCi of [α-32P]dATP, and either 0.6 μM HIV-1 specific primers, 0.6 μM TGFβ1 specific primers, 0.6 μM RARβ specific primers, or 0.15 μM α-tubulin specific primers. RNA samples were reverse transcribed for 30 min at 50°C. After an initial denaturing step at 95°C for 15 min, cDNA products were amplified for 25 cycles (30 cycles for RARβ), each consisting of a 30-s denaturing step at 94°C, a 45-s annealing step at 65°C (62°C for RARβ), and a 1-min extension step at 72°C. The amplification concluded with a 10-min extension step at 72°C. Samples were resolved on 8% nondenaturing polyacrylamide gels and quantified in a Molecular Dynamics PhosphorImager SE by using ImageQuant software (Sunnyvale, Calif.). HIV-1 primers were specific for the R and U5 regions of the LTR: sense primer (nucleotides 41 to 62; 5′-GGCTAACTAGGGAACCCACTGC-3′) and antisense primer (nucleotides 158 to 181; 5′-CTGCTAGAGATTTTCCACACTGAC-3′). Other primers were as follows: TGFβ1 sense primer, 5′-TGGCGATACCTCAGCAACC-3′; TGFβ1 antisense primer, 5′-CTCGTGGATCCACTTCCAG-3′; RARβ sense primer, 5′-ACCAGCTCTGAGGAACTCGTCCCA-3′; RARβ antisense primer, 5′-AGGCGGCCTTCAGCAGGGTAATTT-3′; α-tubulin sense primer 5′-CACCCGTCTTCAGGGCTTCTTGGTTT-3′; and α-tubulin antisense primer, 5′-CATTTCACCATCTGGTTGGCTGGCTC-3′. RNA standards corresponding to 500, 50, and 5 ng of RNA from activated U1 cells were included in each experiment to ensure that all amplifications were within the linear range of the assay. The RNA standards represent serial dilutions of RNA from TSA-treated and/or RA-treated U1 cells.
HIV-1Ba-L infection of MDMs.
MDMs were plated at a density of 3 × 105 cells per well in 24-well plates and infected with HIV-1Ba-L (1 ng of p24/105 cells) for 2 h at 37°C. Infected MDMs were maintained in RPMI 1640 supplemented with 100 U of penicillin/ml, 100 μg of streptomycin/ml, 0.29 mg of l-glutamine/ml, 20% FBS, and 10 ng each of IL-1β and IL-6/ml. Virus replication was quantified by measuring p24 antigen release in cell-free culture supernatants every 3 days.
RESULTS
Retinoic acid represses HIV-1 activation in U1 cells.
We used chronically infected U1 cells as a model system to study the mechanism by which retinoids repress HIV-1 replication. U1 cells are derivatives of U937 promonocytes that contain two integrated copies of HIV-1 proviral DNA (21). Although these cells express very low levels of HIV-1 due to a defect in Tat (17), virus expression can be activated by a variety of stimuli, including phorbol esters (22), cytokines (45, 46), microbial components (48), and histone deacetylase inhibitors (63). Consequently, U1 cells are frequently used as a model for HIV-1 latency and virus expression (49).
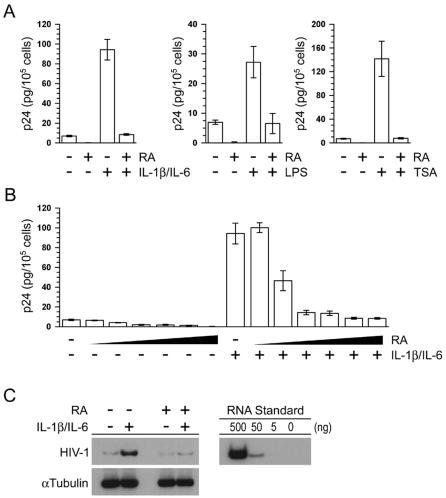
We first determined whether RA treatment could prevent IL-1β/IL-6 activation of HIV-1 expression in U1 cells. In agreement with earlier work (46), we found that cotreatment of the cells with IL-1β/IL-6 activated HIV-1 expression 13.5-fold as measured by p24 antigen release (Fig. 1A, left panel). RA pretreatment fully inhibited this activation. RA pretreatment also inhibited HIV-1 activation by LPS (Fig. 1A, center panel) and by the histone deacetylase inhibitor TSA (Fig. 1A, right panel). A physiological concentration of RA (10−9) repressed both basal and IL-1β/IL-6-activated HIV-1 expression in U1 cells (Fig. 1B). Similar results were obtained when HIV-1 expression in U1 cells was stimulated with either TSA or LPS (data not shown). These results are consistent with our earlier observations demonstrating that physiological concentrations of RA repressed HIV-1 replication in MDMs (38).
FIG. 1.
Retinoic acid represses HIV-1 activation in U1 cells. (A) U1 cells were cultured in the presence or absence of RA (10−6 M) for 48 h and treated with either IL-1β/IL-6 (2 ng/ml each) for an additional 48 h (left panel), LPS (1 μg/ml) for an additional 24 h (center panel), or TSA (150 nM) for an additional 18 h (right panel). Virus expression was measured by using a p24 antigen capture ELISA. The data are the averages (± the standard errors) of at least three independent experiments. (B) U1 cells were cultured in the absence or presence of various molar concentrations of RA (log increases from 10−11 to 10−6 M) for 48 h and then were treated with IL-1β/IL-6 (2 ng/ml each) for an additional 48 h. HIV-1 expression was measured by p24 antigen capture ELISA. The data are the averages (± the standard errors) of three independent experiments. (C) U1 cells were cultured in the presence or absence of RA (10−6 M) for 48 h and then treated with IL-1β/IL-6 (2 ng/ml each) for an additional 48 h. Total cytoplasmic RNA was prepared from the treated cultures and analyzed by semiquantitative RT-PCR for the expression of both HIV-1 (top panel) and α-tubulin (bottom panel) RNA. To ensure that all semiquantitative RT-PCR amplifications were within the linear range of the assay, 10-fold serial dilutions of total cytoplasmic RNA prepared from the IL-1β/IL-6-treated U1 cells shown in the top panel were included as a standard (right panel).
Our earlier studies showed that RA repressed both LTR-directed expression in THP-1 monocytes and HIV-1 transcription in infected MDMs (8, 38). To determine whether RA inhibited HIV-1 RNA expression in U1 cells, we used a semiquantitative RT-PCR assay. Total cytoplasmic RNA was prepared from untreated and RA-treated cells, and HIV-1 expression was measured by using primers corresponding to the R and U5 regions of the HIV-1 LTR. As expected, IL-1β/IL-6 treatment activated HIV-1 RNA expression, and RA repressed this activation (Fig. 1C). Similar results were obtained when U1 cells were activated with LPS and TSA (data not shown). RA treatment had no effect on the accumulation of α-tubulin RNA.
Retinoic acid repression of HIV-1 in U1 cells requires pretreatment and is dependent on new cellular protein synthesis.
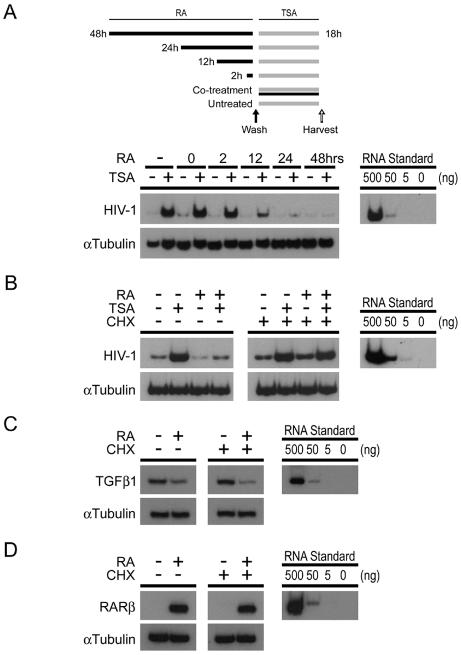
Retinoids can repress cellular gene expression in two general ways. The first occurs rapidly after retinoid treatment and is independent of new cellular gene expression (30, 32). The second requires new cellular gene expression. As a first step in distinguishing between these two possibilities, we examined the kinetics of RA-mediated repression of HIV-1 expression. U1 cells were treated with 10−6 M RA for various lengths of time prior to treatment with 150 nM TSA. Total cytoplasmic RNA was prepared from cells 18 h after TSA treatment, and HIV-1 expression was measured by semiquantitative RT-PCR (Fig. 2A). We found that RA had little to no effect when added at the same time as or 2 h prior to TSA treatment. In cells treated with RA 12 h prior to TSA stimulation, HIV-1 expression was inhibited ∼2-fold. Maximal repression required a 24-h pretreatment with RA (Fig. 2A). These results suggest that RA-mediated repression requires new cellular gene expression. To test this idea, we determined whether the cellular protein synthesis inhibitor cycloheximide blocked RA-mediated repression (Fig. 2B). U1 cells were treated for 24 h with RA and then stimulated with TSA (left panel). Some cells were also treated with cycloheximide at the same time as RA (right panel). Total cytoplasmic RNA was prepared from the cells after the 18-h treatment with TSA, and HIV-1 expression was measured by semiquantitative RT-PCR. We found that cycloheximide completely inhibited RA-mediated repression. Interestingly, if cycloheximide was added concurrently with TSA, after the 24-h pretreatment with RA, the repressive effect of RA, although slightly diminished, was still evident (data not shown). In addition, we looked at the expression of two genes, TGFβ1 and RARβ, known be to regulated by retinoids in a manner independent of new cellular protein synthesis. Retinoid-induced transrepression of TGFβ1, which occurs as a result of ligand-dependent RAR/RXR-mediated sequestration of the coactivator p300/CBP from the transcription factor AP-1 (30, 51), is unaffected by cycloheximide treatment (Fig. 2C). RARβ expression is induced in an acute fashion by retinoids (23) and is also unaffected by cycloheximide treatment (Fig. 2D). To control for any nonspecific effects of cycloheximide, we determined whether puromycin, another protein synthesis inhibitor that acts through a different mechanism, also blocked RA-mediated repression. As with cycloheximide, puromycin blocked RA-mediated repression in U1 cells (data not shown). Together, these data strongly suggest that RA does not repress HIV-1 gene expression by utilizing preexisting cellular components. It is therefore more likely that RA inhibits HIV-1 either by modulating cellular signaling pathways or by altering the expression of cellular factors that work directly on the LTR.
FIG. 2.
Retinoic acid-mediated repression of HIV-1 requires pretreatment and is blocked by cycloheximide. (A) At the top of the panel is shown the treatment of U1 cells for the time course experiments. Black bars indicate the length of culture in the presence of RA (10−6 M). Gray bars indicate the length of culture in the presence of TSA. The solid arrow indicates a wash step to remove RA. The open arrow indicates the harvest of supernatant for ELISA. At the bottom of panel A, U1 cells were grown in the presence or absence of RA (10−6 M) for the indicated times prior to activation with TSA (150 nM) for an additional 18 h, as illustrated above. Total cytoplasmic RNA was prepared from the treated cells and analyzed for the expression of both HIV-1 and α-tubulin by semiquantitative RT-PCR. Lanes 3 and 4 (labeled “0”) indicate cultures that were treated with RA at the same time as TSA. The data are representative of two independent experiments. 10-fold serial dilutions of total cytoplasmic RNA prepared from TSA-treated U1 cells were included as a standard (right panel). (B to D) U1 cells were grown in the presence or absence of RA (10−6 M) for 24 h and then treated with TSA (150 nM) for an additional 18 h. Some cultures (right panels) were treated with cycloheximide (CHX; 10 μg/ml) at the time of RA treatment. Semiquantitative RT-PCR was used to measure the levels of HIV-1 (top panels, B), TGFβ1 (top panels, C), RARβ (top panels, D), and α-tubulin (bottom panels, B to D) RNA accumulation. The data are representative of four independent experiments. Tenfold serial dilutions of total cytoplasmic RNA prepared from TSA-treated U1 cells (B) or RA-treated U1 cells (C and D) were included as standards (right panels).
The repressive effects of RA on U1 cells and MDMs are not a general property of differentiation and are reversible.
Retinoids induce differentiation in a number of cell types, including monocytes. We were therefore interested in determining whether repression was a consequence of differentiation. Our previous studies (8, 38) indicated that repression is not a general property of differentiation. Our laboratory and others found that both phorbol 12-myristate 13-acetate and vitamin D3, which induce the terminal differentiation of monocytes, activate rather than repress HIV-1 expression in U1 cells (22, 24; data not shown). In addition, RA inhibits HIV-1 expression in U1 cells without affecting gross cell morphology or cellular proliferation (data not shown).
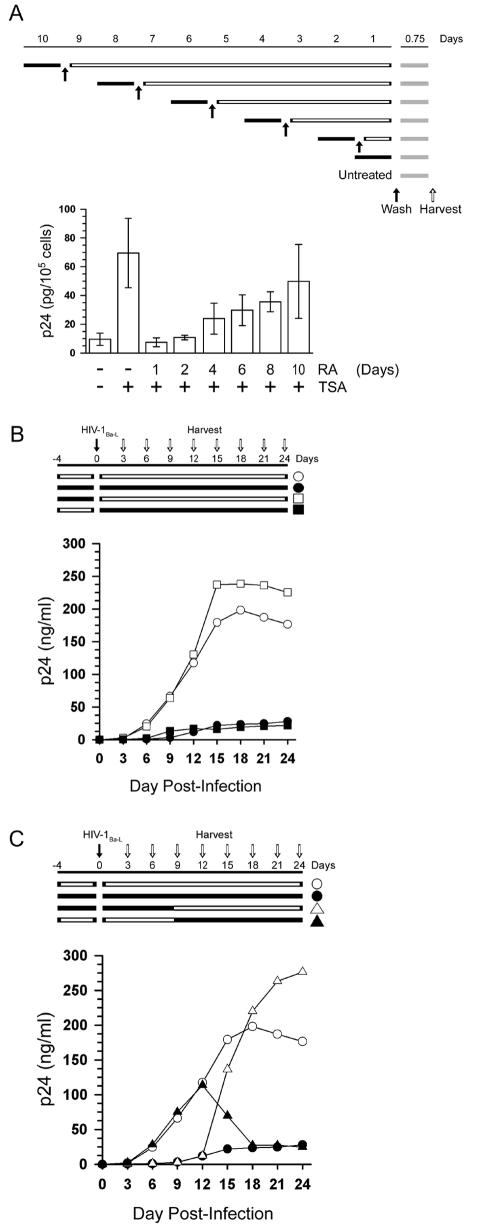
We also tested whether RA permanently repressed HIV-1 expression in U1 cells. Cells were treated with a single dose of RA and then stimulated with TSA at various times after RA treatment. We found that RA repressed TSA-stimulated expression of HIV-1 in a time-dependent manner. When added 1 to 2 days before TSA, RA repressed HIV-1 expression 6.5- to 9.3-fold. This suggested that a single dose of RA had a long-lasting repressive effect. However, this effect was reversible. When added 10 days prior to TSA, RA repressed HIV-1 expression only 1.4-fold (Fig. 3A). It is unlikely that rescue from the repressive phenotype is due to the outgrowth of retinoid-resistant clones, since the growth kinetics of RA-treated U1 cells do not differ significantly from those of untreated cells. In addition, when these cells (10-day single-dose pretreatment) were treated with a second dose of RA 1 day prior to activation, RA completely repressed HIV-1 expression (data not shown). In agreement with previous work (8), we found that RA represses HIV-1 replication in infected MDMs cultured in the presence of the proinflammatory cytokines IL-1β and IL-6 (Fig. 3B). Interestingly, we found that treatment of MDMs with RA prior to infection with HIV-1Ba-L did not prevent robust virus replication in cells that were cultured in the absence of exogenous retinoids after infection, suggesting that retinoids do not induce a permanent repressive phenotype in primary MDMs. As with U1 cells, the effects of RA on HIV-1 expression in MDMs are reversible. Infected MDMs that are removed from retinoid treatment 9 days after infection revert from the repressive phenotype and exhibit robust virus replication (Fig. 3C). We observed a delay of greater than 3 days between the removal of exogenous retinoids and the increase in virus replication, suggesting that the effects of retinoids in MDMs are long-lasting but reversible. In addition, as with U1 cells, the repressive affect of retinoids in MDMs requires a pretreatment. HIV-1 replication in infected MDMs is not diminished until at least 3 days after retinoids are first added (Fig. 3C). This lag in repression is consistent with the need to pretreat U1 cells with RA and suggests that RA-mediated repression requires new cellular gene expression. Together, our results support the conclusion that RA-mediated repression of HIV-1 expression is not a general consequence of cellular differentiation but is instead due to retinoid-specific changes in the cells.
FIG. 3.
The repressive effects of RA are long lasting but reversible. (A) The top part of panel A shows the treatment of U1 cells for the time course experiments. Solid bars indicate the length of culture in the presence of RA (10−6 M). Open bars indicate the length of culture after the removal of RA. Shaded bars indicate the length of culture in the presence of TSA. Solid arrows indicate wash steps to remove RA. The open arrow indicates the harvest of supernatant for ELISA. At thebottom of panel A, U1 cells were treated with a single dose of RA (10−6 M) at various times, as illustrated, prior to treatment with TSA (150 nM) for an additional 18 h. Virus expression was measured by p24 antigen capture ELISA. The data are the averages (± standard errors) of four independent experiments. (B and C) Treatments are as indicated in panel A except that solid arrows indicate the time of HIV-1Ba-L infection. MDMs were cultured in the presence of IL-1β/IL-6 and in the presence or absence of RA (10−7 M) as illustrated. Virus expression was measured by p24 antigen capture ELISA of cell-free culture supernatants. The averages of three independent infections from a single donor are shown. Similar results were obtained with MDMs from two additional donors.
HIV-1 expression is activated when U1 cells or MDMs are grown in retinoid-free synthetic medium, and RA represses this activation.
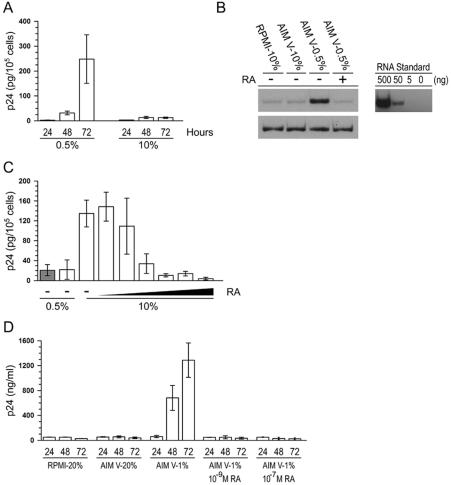
As a first test of our hypothesis that monocytes/macrophages are not permissive hosts for HIV-1 replication unless grown in subphysiological retinoid concentrations, we grew U1 cells in the synthetic medium AIM V. AIM V medium is retinoid-free and supports the normal proliferation of U1 cells when supplemented with 0.5% FBS (data not shown). We found that HIV-1 expression was activated in a time-dependent manner when U1 cells were grown under these conditions (Fig. 4A). The lag in activation we observed is consistent with the results shown in Fig. 3, which demonstrate that RA-mediated repression is long-lasting but reversible. HIV-1 transcription is activated in U1 cells grown in AIM V medium with 0.5% FBS, as demonstrated by semiquantitative RT-PCR (Fig. 4B). In addition, the comparison of HIV-1 expression in U1 cells cultured in RPMI supplemented with 10% serum and in AIM V supplemented with 10% serum demonstrates that AIM V does not contain components that activate virus expression. Importantly, culture of U1 cells in AIM V medium does not activate HIV-1 expression when supplemented with physiological concentrations of RA or 10% FBS, which typically results in a final RA concentration of ∼10−10 M (Fig. 4C). Although subphysiological, RA at these concentrations is nonetheless bioactive, as evidenced by the partial repression of HIV-1 expression seen in U1 cells cultured under these conditions (Fig. 1B and 4C). Perhaps this explains why activators such as IL-1β and IL-6 augment HIV-1 expression in U1 cells grown in standard tissue culture medium. When HIV-1-infected cells are supplemented with physiological concentrations of RA, virus expression is strongly repressed, even in the presence of potent viral activators (Fig. 1B and 3B). In accordance with results obtained in U1 cells, we found that HIV-1 replication increased greatly (∼48-fold) when infected MDMs were transferred to retinoid-free synthetic medium at day 10 postinfection and grown under retinoid-free conditions for an additional 3 days (Fig. 4D). As we found in U1 cells, HIV-1 expression in MDMs was activated in a time-dependent manner, demonstrating that RA-mediated repression is long-lasting but reversible. Moreover, a physiological concentration of RA (10−9 M) repressed HIV-1 expression in MDMs cultured in AIM V medium (Fig. 4D). These results support the hypothesis that HIV-1 expression is activated in monocytes/macrophages grown in subphysiological retinoid concentrations and that the retinoid component of serum represses HIV-1 expression in unstimulated cells.
FIG. 4.
HIV-1 expression is activated when U1 cells are cultured in retinoid-free synthetic medium. (A) U1 cells were grown for the indicated times in AIM V medium supplemented with either 0.5% serum or 10% serum. The levels of p24 antigen expressed over the final 24 h of cell culture were measured by ELISA. The data are the averages (± the standard errors) of three independent experiments. (B) U1 cells were treated as described in panel A except that total cytoplasmic RNA was prepared from the cultures and analyzed by semiquantitative RT-PCR for the expression of both HIV-1 (top panel) and α-tubulin (bottom panel). The data are representative of four independent experiments. Tenfold serial dilutions of total cytoplasmic RNA prepared from U1 cells grown in AIM V medium supplemented with 0.5% FBS were included as a standard (right panel). (C) U1 cells were grown in either RPMI 1640 (shaded bars) or AIM V (open bars) medium supplemented with the indicated amount of serum for 72 h. Some cultures were also treated with RA at various concentrations (log increases from 10−11 to 10−6 M). The levels of HIV-1 expression over the final 24 h were measured by p24 antigen capture ELISA. The data are the averages (± the standard errors) of four independent experiments. (D) HIV-1Ba-L-infected MDMs were transferred to the indicated culture media at day 10 postinfection. The levels of p24 expressed over the final 24 h of culture were measured by ELISA. The data are the averages (± the standard errors) of three independent experiments with MDMs from one donor. Similar results were obtained by using MDMs from an additional donor.
Treatment of U1 cells with the pan-RAR agonist BMS-348 997 represses HIV-1 expression.
In recent years, a number of novel synthetic retinoids have been developed that have proven to be very useful tools for understanding retinoid biochemistry (27, 34). We used two different synthetic retinoids in an attempt to elucidate the mechanism of RA-mediated repression: a pan-RAR agonist that selectively transactivates RARE-dependent gene expression and a pan-RAR antagonist that selectively blocks RARE-dependent transactivation by RA.
We initially determined the effect of the selective agonist BMS-348 997 on untreated and IL-1β/IL-6-treated U1 cells. Treatment of U1 cells with BMS-348 997 repressed both basal and IL-1β/IL-6-activated HIV-1 expression in U1 monocytes in a manner similar to RA as measured by p24 release (Fig. 5A). BMS-348 997 also repressed both LPS- and TSA-activated HIV-1 expression (data not shown). Moreover, as is the case with RA, this repression occurs at the level of HIV-1 transcription as measured by semiquantitative RT-PCR (Fig. 5B). These results suggest that both RA and the pan-RAR agonist BMS-348 997 likely repress HIV-1 expression in U1 cells by modifying the expression of a cellular factor(s) that influences HIV-1 transcription.
FIG. 5.
The pan-RAR agonist BMS-348 997 represses HIV-1 expression in U1 cells. (A) U1 cells were grown in the absence or presence of 10−7 M BMS-348 997 for 72 h prior to activation with IL-1β/IL-6 (2 ng/ml each) for an additional 48 h. HIV-1 expression was measured by p24 antigen capture ELISA. The data are the averages (± the standard errors) of three independent experiments. (B) U1 cells were treated as described in panel A except that total cytoplasmic RNA was prepared from the cultures and analyzed for the expression of both HIV-1 (top panel) and α-tubulin (bottom panel) RNA by semiquantitative RT-PCR. The data are representative of three independent experiments. Tenfold serial dilutions of total cytoplasmic RNA prepared from the IL-1β/IL-6-treated U1 cells were included as a standard (right panel).
Ligand-bound NRs, including RAR/RXR and glucocorticoid receptor (GR), can repress the transcription of cellular genes whose expression is dependent upon the transcription factor AP-1 (27, 30). This type of transrepression results from the competition between ligand-bound NRs and AP-1 for limiting amounts of p300/CBP coactivator. Because the HIV-1 LTR contains three AP-1 sites that influence virus transcription (16, 62), we sought to determine whether transrepression might play a role in RA-mediated repression. However, we found that dexamethasone, a ligand for GR, activated both basal and LPS-stimulated HIV-1 expression (data not shown). Dexamethasone has also been shown to activate HIV-1 expression in U1 cells synergistically with tumor necrosis factor alpha (6) and IL-6 (31). These results, together with our finding that RA-mediated transrepression of TGFβ1 is not inhibited by cycloheximide, whereas repression of HIV-1 is (Fig. 2B), suggest that RA does not repress HIV-1 through the prototypic AP-1 transrepression pathway.
The synthetic pan-RAR antagonist BMS-204 493 activates HIV-1 expression in U1 cells cultured in complete medium.
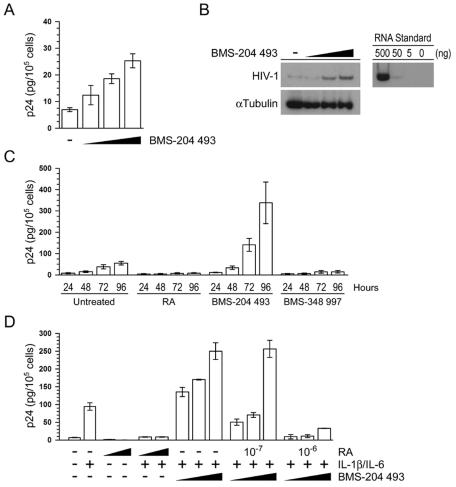
We have shown that HIV-1 expression is activated when U1 cells are grown in retinoid-free synthetic medium and that RA represses this activation. We have also shown that RA-mediated repression requires new cellular protein synthesis and that a pan-RAR agonist that selectively transactivates RARE-dependent gene expression represses HIV-1 expression. These results suggest that retinoid-dependent cellular gene expression is necessary for repression. To confirm that retinoids modulate the expression of a gene that affects HIV-1 transcription, we selectively blocked RARE-dependent transactivation in U1 cells cultured in complete medium by using the synthetic pan-RAR antagonist BMS-204 493. The pan-RAR antagonist activated HIV-1 expression in a dose-dependent manner. A 72-h treatment with 10−5 M BMS-204 493 resulted in a 3.6-fold increase in HIV-1 expression, as measured by p24 levels in cell-free culture supernatants (Fig. 6A). Treatment of U1 cells with BMS-204 493 increased the accumulation of HIV-1 RNA, as measured by semiquantitative RT-PCR (Fig. 6B), a finding consistent with our earlier findings that the primary target of RA-mediated repression of HIV-1 is viral transcription (8, 38). Under these culture conditions, BMS-204 493 was not toxic to the cells (data not shown). These results suggest that retinoids and not another component of serum are responsible for the repression of HIV-1 that we report here. We also found that HIV-1 expression increased in a time-dependent manner when U1 cells were cultured in the presence of the pan-RAR antagonist. A 72-h treatment with BMS-204 493 resulted in a 3.7-fold increase in HIV-1 expression, whereas a 96-h treatment resulted in a 6.1-fold increase (Fig. 6C). This lag in activation is consistent with the results shown in Fig. 3 and 4, which demonstrate that the repressive effect of RA is long-lasting but reversible.
FIG. 6.
HIV-1 expression is activated when U1 cells are cultured in the presence of a pan-RAR antagonist. (A) U1 cells were grown for 72 h in the absence or presence of various concentrations (10−7, 10−6, or 10−5 M) of the pan-RAR antagonist, BMS-204 493. Levels of p24 expression over the last 24 h of cell culture were measured by p24 antigen capture ELISA. The data are the averages (± the standard errors) of three independent experiments. (B) U1 cells were treated as described in panel A except that total cytoplasmic RNA was prepared from the cultures and analyzed for the expression of both HIV-1 (top panel) and α-tubulin (bottom panel) RNA by semiquantitative RT-PCR. The data are representative of three independent experiments. Tenfold serial dilutions of total cytoplasmic RNA prepared from the BMS-204 493-treated U1 cells were included as a standard (right panel). (C) U1 cells were grown for the indicated times in the absence or presence of either RA (10−6 M), the pan-RAR antagonist BMS-204 493 (10−5 M), or the pan-RAR agonist BMS-348 997 (10−5 M). Accumulation of p24 in the cell culture was measured by p24 antigen capture ELISA. The data are the averages (± the standard errors) of five independent experiments. (D) U1 cells were cultured in the presence or absence of various molar concentrations of RA (either 10−7 or 10−6 M) and various molar concentrations of BMS-204 493 (10−7, 10−6, or 10−5 M) for 72 h prior to activation with IL-1β/IL-6 for an additional 48 h. HIV-1 expression was measured by p24 antigen capture ELISA. The data are the means (± the standard errors) of three independent experiments.
We also determined the effect of the selective pan-RAR antagonist on IL-1β/IL-6-activated HIV-1 expression in U1 cells. Interestingly, BMS-204 493 and IL-1β/IL-6 synergistically activated HIV-1 expression in U1 cells (Fig. 6D), suggesting that endogenous retinoids present in complete culture medium repress HIV-1 expression. Similar results were obtained with LPS and TSA (data not shown). In order to test whether RA-mediated transactivation of a cellular gene is required for HIV-1 repression, we cotreated U1 cells with RA and increasing concentrations of BMS-204 493. Importantly, BMS-204 493 reversed the RA-mediated repression of IL-1β/IL-6-, LPS-, and TSA-induced HIV-1 expression in a dose-dependent fashion (Fig. 6D and data not shown), suggesting that RA-mediated transactivation of a cellular gene is required for HIV-1 repression.
DISCUSSION
We have been studying the role of retinoids, the bioactive metabolites of vitamin A, as natural repressors of HIV-1 transcription in monocytes/macrophages. The data presented here are consistent with the hypothesis that physiological concentrations of retinoids in serum potently repress HIV-1 in macrophages in vivo and play a role in maintaining latency in HIV-1-infected monocytes/macrophages.
Recent epidemiological findings suggest that vitamin A is a natural repressor of HIV-1 expression in vivo. Several properties of HIV-1-induced disease, including morbidity, mortality, and the rates of viral transmission, have been inversely correlated with vitamin A levels in serum (28, 39, 55-57, 59). Moreover, vitamin A supplementation has been shown to reduce HIV-1-associated disease (15, 18, 19). Similarly, we have observed a strong inverse relationship between the levels of retinoids present in tissue culture medium and the ability of HIV-1 to replicate in monocytes/macrophages. Here we show that HIV-1 expression is repressed in U1 cells and primary MDMs cultured in growth medium containing 10 to 20% FBS (10−10 M RA, final concentration) compared to cells cultured in retinoid-free synthetic medium (AIM V with low serum; 10−11 M RA, final concentration) (Fig. 4). Significantly, supplementation of AIM V culture medium with a physiological concentration of RA (10−9 M) decreases virus replication 30- to 65-fold (Fig. 4). We also previously reported that HIV-1 replication in infected MDMs cultured in complete medium (20% FBS) can be repressed by the addition of 10−9 M RA (41). Our findings may also help to explain why vitamin A supplementation therapy has been demonstrated to decrease the severity of HIV-1-associated disease and to reduce the rates of mother-to-child transmission in individuals with severe deficiencies in vitamin A levels in serum. This is also consistent with our hypothesis that unstimulated monocytes/macrophages are normally inefficient hosts for HIV-1 replication but become more efficient hosts when the cells are grown in subphysiological retinoid concentrations. This may help to explain why HIV-1 expression in macrophages increases at later stages of disease (35). In vivo, HIV-1 infection often leads to vitamin A deficiency at times when the levels of virus production increase (2, 9, 29, 44, 59). The loss of retinoid-mediated repression may account, in part, for the increased levels of HIV-1 replication seen at later stages of disease.
Our data suggest that monocytes/macrophages cultured in the presence of physiological concentrations of either retinol, RA, and 9-cis-retinoic acid (Fig. 1B and 3B) (8, 38) are innately refractory to HIV-1 replication. Retinol is present in serum at micromolar concentrations (ca. 10−6 M), whereas retinoic acid is typically present in nanomolar concentrations (ca. 10−9 to 10−8 M). However, both retinoids can be found in much higher concentrations in localized tissues, including the liver and the lung (13, 52). RA has a relatively short half-life of less than 3 h in culture (4), so it is likely that other retinoids also contribute to the repressive effect that we observe. Another major metabolite of vitamin A, 4-oxoretinol, has a much longer half-life than RA (>15 h) and is bioactive at concentrations as low as 10−8 M (1). Experiments with RA and the pan-RAR agonist BMS-348 997, both specific ligands for RAR, suggest that the repressive effect of retinoids is mediated by RAR/RXR heterodimers. Much like RA and BMS-348 997, 4-oxoretinol selectively activates RAR (3), suggesting that it may play a role in the repression of HIV-1 in monocytes/macrophages that we observe.
Treatment of U1 cells with the synthetic pan-RAR antagonist BMS-204 493 activates HIV-1 expression in a dose-dependent manner, suggesting that RA-induced transactivation of cellular gene expression is required for HIV-1 repression. It is important to note that physiological concentrations of RA repress HIV-1 expression in response to stimulation with concentrations of IL-1β/IL-6 expected at local sites where HIV-1-infected macrophages reside in vivo (Fig. 1B and 3B) (8). In addition to demonstrating that HIV-1 expression is activated in the absence of retinoids, we also show that the synthetic pan-RAR antagonist BMS-204 493 activates HIV-1 expression in U1 cells cultured in complete medium (Fig. 6A). Interestingly, the antagonist and IL-1β/IL-6 synergistically activate HIV-1 expression (compare Fig. 6A and D), suggesting that retinoids present in standard culture medium repress IL-1β/IL-6 stimulation of HIV-1 expression. These results imply that retinoids present in culture medium in vitro and in serum in vivo induce the expression of a potent inhibitor of HIV-1 expression in monocytes/macrophages.
Two lines of evidence suggest that RARE-dependent transactivation of gene expression is required for RA-mediated repression of HIV-1. First, the requirement for prolonged retinoid treatment (at least 12 h) and new cellular protein synthesis implies that retinoids induce the expression of a cellular factor(s) that inhibits HIV-1 transcription. Second, studies with synthetic retinoids that selectively target RARE-dependent transactivation of gene expression (Fig. 5 and 6) indicate that the induction of cellular gene expression is necessary for retinoids to repress HIV-1. Together, these data provide support for the hypothesis that RA-mediated repression requires new cellular gene transcription. Although RAR and RXR do not bind to the core promoter region of the HIV-1 LTR, RA treatment does induce the binding of four as-yet-unidentified protein complexes to this region in THP-1 monocytes (38). This is consistent with the hypothesis that retinoids induce the expression of a negative regulatory factor(s) that binds to the viral core promoter. Several candidate proteins have been described that bind to this segment of the LTR and alter transcription (reviewed in reference 43). Further studies are necessary both to identify these RA-induced complexes and to determine their effects on viral transcription.
It is unlikely that either transrepression or ligand-dependent active repression through DNA-bound NRs is involved in retinoid-mediated repression of HIV-1. Both are acute responses to ligand treatment that utilize preexisting cellular complexes. Here, we show that retinoid-mediated repression of HIV-1 requires at least 12 h of retinoid treatment (Fig. 2A) and requires new cellular protein synthesis (Fig. 2B), indicating that neither pathway is involved in retinoid repression of HIV-1. Furthermore, ligand-bound RAR/RXR, like GR, can transrepress AP-1 activity in a number of ways, including competition with AP-1 for limiting amounts of coactivator p300/CBP (30). However, dexamethasone, a ligand for GR, activates HIV-1 expression in U1 cells (data not shown). Furthermore, the RA-mediated transrepression of TGFβ1 expression is not inhibited by cycloheximide, whereas repression of HIV-1 is inhibited by cycloheximide (Fig. 2C), suggesting that the canonical AP-1 transrepression pathway is not involved in the RA-mediated repression we observe in latently infected U1 cells. An alternative possibility is that retinoid-bound RAR/RXR can actively repress transcription by binding to RAREs or CCAAT boxes within the promoters of certain genes (32). In addition to three CCAAT boxes, the HIV-1 LTR also contains an atypical RARE that can be bound by RAR/RXR and therefore might play a role in transcription (41). However, the cis-acting sequences necessary for RA-mediated repression have been mapped to the core promoter region of the HIV-1 LTR (−51 to +12, where +1 is the start site of transcription) (38), which contains neither CCAAT boxes nor sequences similar to known RAREs. Moreover, neither RARs nor RXRs bind to this region in vitro (38). In addition, retinoids have been shown to activate HIV-1 transcription through this atypical RARE (33). These data suggest that it is unlikely repression results from ligand-associated RARs binding to the HIV-1 core promoter. Another possibility is that RA-mediated repression is coupled with cellular differentiation. However, this is unlikely since HIV-1 replication in MDMs can be inhibited by RA even when it is added after the cells have terminally differentiated, and since the RA effect is reversible (Fig. 3).
Three properties of retinoid-mediated repression of HIV-1 implicate the general transcription machinery as a possible target. First, the cis-acting sequences required for repression are located in the viral core promoter (38), which includes binding sites for components of the general transcription machinery in addition to binding sites for several transcription factors (reviewed in reference 43). Second, retinoids repress HIV-1 expression stimulated by multiple independent factors (Fig. 1A). RA represses activation by LPS, which induces the recruitment of NF-κB (48), NF-IL-6 (26), and PU.1 (37) to the LTR; by IL-1β/IL-6, which stimulate HIV-1 expression through NF-κB (42), NF-IL-6 (60), and undefined transcriptional and posttranscriptional mechanisms (46); and by TSA, which induces histone hyperacetylation and chromatin remodeling (63). These data imply that retinoids target a component common to all three activation pathways, possibly a component of the general transcription machinery. Finally, the magnitude of RA repression is consistent with retinoids interfering with the basal transcription machinery. It is intriguing that RA inhibits activation of HIV-1 transcription by TSA. Given that TSA has been shown to induce the remodeling of a regulatory nucleosome, nuc-1, at the HIV-1 promoter (63), it will be important to determine the affects of RA on the HIV-1 chromatin structure. Interestingly, we recently found that treatment of U1 cells with the pan-RAR antagonist BMS-204 493 not only activates HIV-1 expression (Fig. 6) but also promotes the remodeling of nuc-1 (T. M. Hanley and G. A. Viglianti, unpublished data).
In the present study we have shown that retinoids contribute to the maintenance of HIV-1 transcriptional latency in monocytes/macrophages. Reservoirs of latently infected cells pose a challenge to current HIV/AIDS therapies. Recent studies have demonstrated a rapid emergence of plasma viremia after the cessation of highly active antiretroviral therapy (HAART) (10, 20); this emergence of plasma viremia is likely generated by persistent reservoirs of latently infected cells. Adjuvant therapies designed to eliminate the pool of latently infected CD4+ T cells in patients on HAART have not been able to fully eradicate viral reservoirs (12). Interestingly, the rebounding virus is genetically distinct from the replication-competent virus associated with the pool of latently infected T cells, suggesting that other latent reservoirs of HIV-1 exist (11). Other potential cellular reservoirs for HIV-1 include circulating CD14+ monocytes (66) and myeloid cells in the lung and the central nervous system (7, 53). It is conceivable that latently infected monocytes and tissue macrophages, such as alveolar macrophages (40, 50), contribute to the rapid emergence of plasma viremia seen with the cessation of HAART. Perhaps potent myeloid-selective activators of HIV-1, such as retinoid antagonists, will be useful in specifically reactivating virus expression in latently infected macrophages, contributing to a decrease in the size of this important viral reservoir.
Acknowledgments
We thank Lynn Denekamp, Jianming Hu, Lia Luus, Vikram Suri, and David Talmage for their helpful comments regarding the manuscript and Rahm Gummuluru for providing CD14+ monocytes. We also thank Thomas Folks and the NIH AIDS Research and Reference Reagent Program for providing the U1 cell line.
This study was supported by grant HL57882 from the National Heart, Lung, and Blood Institute and grant AI49098 from the National Institute of Allergy and Infectious Diseases to G.A.V.
REFERENCES
- 1.Achkar, C. C., F. Derguini, B. Blumberg, A. Langston, A. A. Levin, J. Speck, R. M. Evans, J. Bolado, Jr., K. Nakanishi, J. Buck, et al. 1996. 4-Oxoretinol, a new natural ligand and transactivator of the retinoic acid receptors. Proc. Natl. Acad. Sci. USA 93:4879-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baum, M. K., G. Shor-Posner, G. Zhang, H. Lai, J. A. Quesada, A. Campa, M. Jose-Burbano, M. A. Fletcher, H. Sauberlich, and J. B. Page. 1997. HIV-1 infection in women is associated with severe nutritional deficiencies. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 16:272-278. [DOI] [PubMed] [Google Scholar]
- 3.Blumberg, B., J. Bolado, Jr., F. Derguini, A. G. Craig, T. A. Moreno, D. Chakravarti, R. A. Heyman, J. Buck, and R. M. Evans. 1996. Novel retinoic acid receptor ligands in Xenopus embryos. Proc. Natl. Acad. Sci. USA 93:4873-4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boylan, J. F., and L. J. Gudas. 1992. The level of CRABP-I expression influences the amounts and types of all-trans-retinoic acid metabolites in F9 teratocarcinoma stem cells. J. Biol. Chem. 267:21486-21491. [PubMed] [Google Scholar]
- 5.Brander, C., and B. D. Walker. 1999. T lymphocyte responses in HIV-1 infection: implications for vaccine development. Curr. Opin. Immunol. 11:451-459. [DOI] [PubMed] [Google Scholar]
- 6.Bressler, P., G. Poli, J. S. Justement, P. Biswas, and A. S. Fauci. 1993. Glucocorticoids synergize with tumor necrosis factor alpha in the induction of HIV expression from a chronically infected promonocytic cell line. AIDS Res. Hum. Retrovir. 9:547-551. [DOI] [PubMed] [Google Scholar]
- 7.Brodie, S. J. 2000. Nonlymphoid reservoirs of HIV replication in children with chronic-progressive disease. J. Leukoc. Biol. 68:351-359. [PubMed] [Google Scholar]
- 8.Brown, X. Q., T. M. Hanley, and G. A. Viglianti. 2002. Interleukin 1β and interleukin 6 potentiate retinoic acid-mediated repression of human immunodeficiency virus type 1 replication in macrophages. AIDS Res. Hum. Retrovir. 18:649-656. [DOI] [PubMed] [Google Scholar]
- 9.Camp, W. L., S. Allen, J. O. Alvarez, P. E. Jolly, H. L. Weiss, J. F. Phillips, E. Karita, A. Serufilira, and S. H. Vermund. 1998. Serum retinol and HIV-1 RNA viral load in rapid and slow progressors. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 18:401-406. [DOI] [PubMed] [Google Scholar]
- 10.Chun, T. W., R. T. Davey, Jr., D. Engel, H. C. Lane, and A. S. Fauci. 1999. Re-emergence of HIV after stopping therapy. Nature 401:874-875. [DOI] [PubMed] [Google Scholar]
- 11.Chun, T. W., R. T. Davey, Jr., M. Ostrowski, J. Shawn Justement, D. Engel, J. I. Mullins, and A. S. Fauci. 2000. Relationship between preexisting viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat. Med. 6:757-761. [DOI] [PubMed] [Google Scholar]
- 12.Chun, T. W., D. Engel, S. B. Mizell, C. W. Hallahan, M. Fischette, S. Park, R. T. Davey, Jr., M. Dybul, J. A. Kovacs, J. A. Metcalf, J. M. Mican, M. M. Berrey, L. Corey, H. C. Lane, and A. S. Fauci. 1999. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat. Med. 5:651-655. [DOI] [PubMed] [Google Scholar]
- 13.Chytil, F. 1992. The lungs and vitamin A. Am. J. Physiol. 262:L517-L527. [DOI] [PubMed] [Google Scholar]
- 14.Collins, K. L., and D. Baltimore. 1999. HIV's evasion of the cellular immune response. Immunol. Rev. 168:65-74. [DOI] [PubMed] [Google Scholar]
- 15.Coutsoudis, A., R. A. Bobat, H. M. Coovadia, L. Kuhn, W. Y. Tsai, and Z. A. Stein. 1995. The effects of vitamin A supplementation on the morbidity of children born to HIV-infected women. Am. J. Public Health 85:1076-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.el Kharroubi, A., and M. A. Martin. 1996. cis-Acting sequences located downstream of the human immunodeficiency virus type 1 promoter affect its chromatin structure and transcriptional activity. Mol. Cell. Biol. 16:2958-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emiliani, S., W. Fischle, M. Ott, C. Van Lint, C. A. Amella, and E. Verdin. 1998. Mutations in the tat gene are responsible for human immunodeficiency virus type 1 postintegration latency in the U1 cell line. J. Virol. 72:1666-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fawzi, W. W., R. Mbise, D. Spiegelman, M. Fataki, E. Hertzmark, and G. Ndossi. 2000. Vitamin A supplements and diarrheal and respiratory tract infections among children in Dar es Salaam, Tanzania. J. Pediatr. 137:660-667. [DOI] [PubMed] [Google Scholar]
- 19.Fawzi, W. W., R. L. Mbise, E. Hertzmark, M. R. Fataki, M. G. Herrera, G. Ndossi, and D. Spiegelman. 1999. A randomized trial of vitamin A supplements in relation to mortality among human immunodeficiency virus-infected and uninfected children in Tanzania. Pediatr. Infect. Dis. J. 18:127-133. [DOI] [PubMed] [Google Scholar]
- 20.Finzi, D., M. Hermankova, T. Pierson, L. M. Carruth, C. Buck, R. E. Chaisson, T. C. Quinn, K. Chadwick, J. Margolick, R. Brookmeyer, J. Gallant, M. Markowitz, D. D. Ho, D. D. Richman, and R. F. Siliciano. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295-1300. [DOI] [PubMed] [Google Scholar]
- 21.Folks, T. M., J. Justement, A. Kinter, C. A. Dinarello, and A. S. Fauci. 1987. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science 238:800-802. [DOI] [PubMed] [Google Scholar]
- 22.Folks, T. M., J. Justement, A. Kinter, S. Schnittman, J. Orenstein, G. Poli, and A. S. Fauci. 1988. Characterization of a promonocyte clone chronically infected with HIV and inducible by 13-phorbol-12-myristate acetate. J. Immunol. 140:1117-1122. [PubMed] [Google Scholar]
- 23.Giannini, G., M. I. Dawson, X. Zhang, and C. J. Thiele. 1997. Activation of three distinct RXR/RAR heterodimers induces growth arrest and differentiation of neuroblastoma cells. J. Biol. Chem. 272:26693-26701. [DOI] [PubMed] [Google Scholar]
- 24.Goletti, D., A. L. Kinter, P. Biswas, S. M. Bende, G. Poli, and A. S. Fauci. 1995. Effect of cellular differentiation on cytokine-induced expression of human immunodeficiency virus in chronically infected promonocytic cells: dissociation of cellular differentiation and viral expression. J. Virol. 69:2540-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gummuluru, S., M. Rogel, and M. Emerman. 2003. Binding of human immunodeficiency virus type 1 to immature dendritic cells can occur independently of DC-SIGN and mannose binding C-type lectin receptors in a cholesterol-dependent manner. J. Virol. 12865-12874. 77: [DOI] [PMC free article] [PubMed]
- 26.Henderson, A. J., X. Zou, and K. L. Calame. 1995. C/EBP proteins activate transcription from the human immunodeficiency virus type 1 long terminal repeat in macrophages/monocytes. J. Virol. 69:5337-5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang, C., W. Y. Ma, M. I. Dawson, M. Rincon, R. A. Flavell, and Z. Dong. 1997. Blocking activator protein-1 activity, but not activating retinoic acid response element, is required for the antitumor promotion effect of retinoic acid. Proc. Natl. Acad. Sci. USA 94:5826-5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.John, G. C., R. W. Nduati, D. Mbori-Ngacha, J. Overbaugh, M. Welch, B. A. Richardson, J. Ndinya-Achola, J. Bwayo, J. Krieger, F. Onyango, and J. K. Kreiss. 1997. Genital shedding of human immunodeficiency virus type 1 DNA during pregnancy: association with immunosuppression, abnormal cervical or vaginal discharge, and severe vitamin A deficiency. J. Infect. Dis. 175:57-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kafwembe, E. M., P. Kelly, and P. Ngalande. 2001. Vitamin A levels in HIV/AIDS. East Afr. Med. J. 78:451-453. [DOI] [PubMed] [Google Scholar]
- 30.Kamei, Y., L. Xu, T. Heinzel, J. Torchia, R. Kurokawa, B. Gloss, S. C. Lin, R. A. Heyman, D. W. Rose, C. K. Glass, and M. G. Rosenfeld. 1996. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell 85:403-414. [DOI] [PubMed] [Google Scholar]
- 31.Kinter, A. L., P. Biswas, M. Alfano, J. S. Justement, B. Mantelli, C. Rizzi, A. R. Gatti, E. Vicenzi, P. Bressler, and G. Poli. 2001. Interleukin-6 and glucocorticoids synergistically induce human immunodeficiency virus type-1 expression in chronically infected U1 cells by a long terminal repeat independent posttranscriptional mechanism. Mol. Med. 7:668-678. [PMC free article] [PubMed] [Google Scholar]
- 32.Kirfel, J., M. Kelter, L. M. Cancela, P. A. Price, and R. Schule. 1997. Identification of a novel negative retinoic acid responsive element in the promoter of the human matrix Gla protein gene. Proc. Natl. Acad. Sci. USA 94:2227-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ladias, J. A. 1994. Convergence of multiple nuclear receptor signaling pathways onto the long terminal repeat of human immunodeficiency virus-1. J. Biol. Chem. 269:5944-5951. [PubMed] [Google Scholar]
- 34.Lee, M. O., M. I. Dawson, N. Picard, P. D. Hobbs, and M. Pfahl. 1996. A novel class of retinoid antagonists and their mechanism of action. J. Biol. Chem. 271:11897-11903. [DOI] [PubMed] [Google Scholar]
- 35.Li, S., J. Juarez, M. Alali, D. Dwyer, R. Collman, A. Cunningham, and H. M. Naif. 1999. Persistent CCR5 utilization and enhanced macrophage tropism by primary blood human immunodeficiency virus type 1 isolates from advanced stages of disease and comparison to tissue-derived isolates. J. Virol. 73:9741-9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linney, E. 1992. Retinoic acid receptors: transcription factors modulating gene regulation, development, and differentiation. Curr. Top. Dev. Biol. 27:309-350. [DOI] [PubMed] [Google Scholar]
- 37.Lodie, T. A., M. Reiner, S. Coniglio, G. Viglianti, and M. J. Fenton. 1998. Both PU.1 and nuclear factor-κB mediate lipopolysaccharide-induced HIV-1 long terminal repeat transcription in macrophages. J. Immunol. 161:268-276. [PubMed] [Google Scholar]
- 38.Maciaszek, J. W., S. J. Coniglio, D. A. Talmage, and G. A. Viglianti. 1998. Retinoid-induced repression of human immunodeficiency virus type 1 core promoter activity inhibits virus replication. J. Virol. 72:5862-5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehendale, S. M., M. E. Shepherd, R. S. Brookmeyer, R. D. Semba, A. D. Divekar, R. R. Gangakhedkar, S. Joshi, A. R. Risbud, R. S. Paranjape, D. A. Gadkari, and R. C. Bollinger. 2001. Low carotenoid concentration and the risk of HIV seroconversion in Pune, India. J. Acquir. Immune Defic. Syndr. 26:352-359. [DOI] [PubMed] [Google Scholar]
- 40.Moreno, P., M. J. Rebollo, F. Pulido, R. Rubio, A. R. Noriega, and R. Delgado. 1996. Alveolar macrophages are not an important source of viral production in HIV-1-infected patients. AIDS 10:682-684. [DOI] [PubMed] [Google Scholar]
- 41.Orchard, K., G. Lang, J. Harris, M. Collins, and D. Latchman. 1993. A palindromic element in the human immunodeficiency virus long terminal repeat binds retinoic acid receptors and can confer retinoic acid responsiveness on a heterologous promoter. J. Acquir. Immune Defic. Syndr. 6:440-445. [PubMed] [Google Scholar]
- 42.Osborn, L., S. Kunkel, and G. J. Nabel. 1989. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor κB. Proc. Natl. Acad. Sci. USA 86:2336-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pereira, L. A., K. Bentley, A. Peeters, M. J. Churchill, and N. J. Deacon. 2000. A compilation of cellular transcription factor interactions with the HIV-1 LTR promoter. Nucleic Acids Res. 28:663-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Periquet, B. A., N. M. Jammes, W. E. Lambert, J. Tricoire, M. M. Moussa, J. Garcia, J. Ghisolfi, and J. Thouvenot. 1995. Micronutrient levels in HIV-1-infected children. AIDS 9:887-893. [DOI] [PubMed] [Google Scholar]
- 45.Poli, G., A. Kinter, J. S. Justement, J. H. Kehrl, P. Bressler, S. Stanley, and A. S. Fauci. 1990. Tumor necrosis factor alpha functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proc. Natl. Acad. Sci. USA 87:782-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poli, G., A. L. Kinter, and A. S. Fauci. 1994. Interleukin 1 induces expression of the human immunodeficiency virus alone and in synergy with interleukin 6 in chronically infected U1 cells: inhibition of inductive effects by the interleukin 1 receptor antagonist. Proc. Natl. Acad. Sci. USA 91:108-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poli, G., A. L. Kinter, J. S. Justement, P. Bressler, J. H. Kehrl, and A. S. Fauci. 1992. Retinoic acid mimics transforming growth factor beta in the regulation of human immunodeficiency virus expression in monocytic cells. Proc. Natl. Acad. Sci. USA 89:2689-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pomerantz, R. J., M. B. Feinberg, D. Trono, and D. Baltimore. 1990. Lipopolysaccharide is a potent monocyte/macrophage-specific stimulator of human immunodeficiency virus type 1 expression. J. Exp. Med. 172:253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pomerantz, R. J., D. Trono, M. B. Feinberg, and D. Baltimore. 1990. Cells nonproductively infected with HIV-1 exhibit an aberrant pattern of viral RNA expression: a molecular model for latency. Cell 61:1271-1276. [DOI] [PubMed] [Google Scholar]
- 50.Rose, R. M., A. Krivine, P. Pinkston, J. M. Gillis, A. Huang, and S. M. Hammer. 1991. Frequent identification of HIV-1 DNA in bronchoalveolar lavage cells obtained from individuals with the acquired immunodeficiency syndrome. Am. Rev. Respir. Dis. 143:850-854. [DOI] [PubMed] [Google Scholar]
- 51.Salbert, G., A. Fanjul, F. J. Piedrafita, X. P. Lu, S. J. Kim, P. Tran, and M. Pfahl. 1993. Retinoic acid receptors and retinoid X receptor-α down-regulate the transforming growth factor-β1 promoter by antagonizing AP-1 activity. Mol. Endocrinol. 7:1347-1356. [DOI] [PubMed] [Google Scholar]
- 52.Schmitz, H. H., C. L. Poor, R. B. Wellman, and J. W. Erdman, Jr. 1991. Concentrations of selected carotenoids and vitamin A in human liver, kidney and lung tissue. J. Nutr. 121:1613-1621. [DOI] [PubMed] [Google Scholar]
- 53.Schrager, L. K., and M. P. D'Souza. 1998. Cellular and anatomical reservoirs of HIV-1 in patients receiving potent antiretroviral combination therapy. JAMA 280:67-71. [DOI] [PubMed] [Google Scholar]
- 54.Semba, R. D. 1994. Vitamin A, immunity, and infection. Clin. Infect. Dis. 19:489-499. [DOI] [PubMed] [Google Scholar]
- 55.Semba, R. D., W. T. Caiaffa, N. M. Graham, S. Cohn, and D. Vlahov. 1995. Vitamin A deficiency and wasting as predictors of mortality in human immunodeficiency virus-infected injection drug users. J. Infect. Dis. 171:1196-1202. [DOI] [PubMed] [Google Scholar]
- 56.Semba, R. D., P. G. Miotti, J. D. Chiphangwi, G. Liomba, L. P. Yang, A. J. Saah, G. A. Dallabetta, and D. R. Hoover. 1995. Infant mortality and maternal vitamin A deficiency during human immunodeficiency virus infection. Clin. Infect. Dis. 21:966-972. [DOI] [PubMed] [Google Scholar]
- 57.Semba, R. D., P. G. Miotti, J. D. Chiphangwi, A. J. Saah, J. K. Canner, G. A. Dallabetta, and D. R. Hoover. 1994. Maternal vitamin A deficiency and mother-to-child transmission of HIV-1. Lancet 343:1593-1597. [DOI] [PubMed] [Google Scholar]
- 58.Stunnenberg, H. G. 1993. Mechanisms of transactivation by retinoic acid receptors. Bioessays 1 5:309-315. [DOI] [PubMed] [Google Scholar]
- 59.Tang, A. M., N. M. Graham, A. J. Kirby, L. D. McCall, W. C. Willett, and A. J. Saah. 1993. Dietary micronutrient intake and risk of progression to acquired immunodeficiency syndrome (AIDS) in human immunodeficiency virus type 1 (HIV-1)-infected homosexual men. Am. J. Epidemiol. 138:937-951. [DOI] [PubMed] [Google Scholar]
- 60.Tesmer, V. M., A. Rajadhyaksha, J. Babin, and M. Bina. 1993. NF-IL6-mediated transcriptional activation of the long terminal repeat of the human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 90:7298-7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Towers, G., J. Harris, G. Lang, M. K. Collins, and D. S. Latchman. 1995. Retinoic acid inhibits both the basal activity and phorbol ester-mediated activation of the HIV long terminal repeat promoter. AIDS 9:129-136. [PubMed] [Google Scholar]
- 62.Van Lint, C., A. Burny, and E. Verdin. 1991. The intragenic enhancer of human immunodeficiency virus type 1 contains functional AP-1 binding sites. J. Virol. 65:7066-7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Lint, C., S. Emiliani, M. Ott, and E. Verdin. 1996. Transcriptional activation and chromatin remodeling of the HIV-1 promoter in response to histone acetylation. EMBO J. 15:1112-1120. [PMC free article] [PubMed] [Google Scholar]
- 64.Xu, L., C. K. Glass, and M. G. Rosenfeld. 1999. Coactivator and corepressor complexes in nuclear receptor function. Curr. Opin. Genet. Dev. 9:140-147. [DOI] [PubMed] [Google Scholar]
- 65.Yamaguchi, K., J. E. Groopman, and R. A. Byrn. 1994. The regulation of HIV by retinoic acid correlates with cellular expression of the retinoic acid receptors. AIDS 8:1675-1682. [DOI] [PubMed] [Google Scholar]
- 66.Zhu, T., D. Muthui, S. Holte, D. Nickle, F. Feng, S. Brodie, Y. Hwangbo, J. I. Mullins, and L. Corey. 2002. Evidence for human immunodeficiency virus type 1 replication in vivo in CD14+ monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J. Virol. 76:707-716. [DOI] [PMC free article] [PubMed] [Google Scholar]