Abstract
Wnt-modulator in surface ectoderm (WISE) is a secreted modulator of Wnt signaling expressed in the adult kidney. Activation of Wnt signaling has been observed in renal transplants developing interstitial fibrosis and tubular atrophy; however, whether WISE contributes to chronic changes is not well understood. Here, we found moderate to high expression of WISE mRNA in a rat model of renal transplantation and in kidneys from normal rats. Treatment with a neutralizing antibody against WISE improved proteinuria and graft function, which correlated with higher levels of β-catenin protein in kidney allografts. In addition, treatment with the anti-WISE antibody reduced infiltration of CD68+ macrophages and CD8+ T cells, attenuated glomerular and interstitial injury, and decreased biomarkers of renal injury. This treatment reduced expression of genes involved in immune responses and in fibrogenic pathways. In summary, WISE contributes to renal dysfunction by promoting tubular atrophy and interstitial fibrosis.
The development of interstitial fibrosis and tubular atrophy with ongoing inflammation is a major risk for progressive graft dysfunction eventually leading to the failure of the majority of renal transplants. Although multiple approaches are available for preventing or at least blunting immune responses, there is currently no effective treatment for the development and progression of interstitial fibrosis and tubular atrophy.1 The formation of matrix proteins in parallel to a progressive loss of graft function is a hallmark of chronic allograft dysfunction (CAD). Several pathways, including those involving TGF-β and angiotensin receptor, have been found to promote fibrosis and thus significant efforts have been made to evaluate the potential utility of these two pathway inhibitors for CAD. TGF-β has been shown to play an important role in epithelial-mesenchymal transition (EMT), which may, in turn, promote deteriorating structural changes characteristic for CAD.2 However, inhibiting TGF-β may, because of its immunomodulatory effects, carry the risk of augmenting inflammation.3 Angiotensin II (AngII) is a growth factor that activates the Smad pathway during EMT involving TGF-β.4 AngII receptor antagonists were shown to reduce BP, proteinuria, and fibrosis in some studies.5,6 However, the application of AngII receptor antagonists is known to be associated with intimal hyperplasia and deteriorating renal function, thus making its application in CAD challenging.7
Wnt signaling is tightly regulated during kidney development and plays an important role in the formation of various structures of the developing kidney.8–11 In normal adult kidneys, Wnt signaling is progressively downregulated once the developmental phase is completed.12 Activation of Wnt signaling has been reported in a variety of human disease processes, including interstitial pulmonary fibrosis,13 and in transplanted kidneys undergoing interstitial fibrosis and tubular atrophy.14 To understand the contribution of Wnt-modulator in surface ectoderm (WISE) on tubular atrophy and interstitial fibrosis, we generated a potent rat inhibitory antibody to rat WISE, allowing long-term treatment while minimizing immune responses toward the injected antibody. Prophylactic treatment with a rat anti-WISE antibody, referred to hereafter as anti-WISE, reduced inflammatory infiltration, improved renal function, and reduced structural graft deterioration over a 6-month observation period. Serum biomarker and changes in gene expression suggested improvements in tubular epithelial integrity as well as decreases in profibrotic and inflammatory pathways, respectively. The improvement in graft function in our studies was associated with increased β-catenin levels. Moreover, WISE protein modulated Wnt signaling in vitro in a context-dependent manner, and directly affected E-cadherin expression and α-smooth muscle actin (α-SMA) expression in renal epithelial cells and interstitial fibroblasts.
Results
WISE is Expressed in Rat Renal Transplants
In initial experiments, we tested whether WISE was expressed in rat kidneys demonstrating interstitial fibrosis and tubular atrophy. WISE was moderately to highly expressed in distal tubules of the renal cortex and outer medulla and likely in collecting ducts in kidneys from rats at all stages after renal transplantation (Figure 1A) and at similar locations and levels in kidneys from normal rats (data not shown).
Figure 1.
WISE is expressed in renal transplants and modulates Wnt signaling in vitro. (A) WISE expression in transplanted kidney and in vitro activity on Wnt signaling. WISE expression in rat renal allograft with CAD. WISE was moderately to highly expressed in transplanted kidneys and was found in the distal tubules in renal cortex and outer medulla and in tubules thought to be collecting ducts as assessed by in situ hybridization. (B) WISE inhibited Wnt signaling (RLU) in MC3T3-E1/STF reporter cell. (C) WISE potentiates Wnt3a signaling and inhibits Wnt1 and Wnt10b. (D) Rat anti-WISE neutralized WISE inhibition in MC3T3-E1/STF reporter cell.
WISE Modulates Wnt Signaling In Vitro
Purified recombinant rat WISE protein was produced and used to determine the effect on canonical Wnt signaling in a Wnt responsive osteoblast cell line (MC3T3-E1) engineered with a Wnt-reporter construct. WISE protein inhibited endogenously produced, Wnt-induced, β-catenin-dependent transcriptional activity in a dose-dependent fashion (Figure 1B). In concordance with previously published data demonstrating that WISE exhibits varying activities dependent upon the type of Wnt,15,16 WISE inhibited Wnt-1 and Wnt10a induced SuperTOPFlash luciferase activity while exhibiting modestly agonistic activity toward Wnt3a in the 293 cell assay (Figure 1C). WISE exhibited no activity against bone morphogenetic proteins-4 (BMP4)-induced BRE-luciferase assay in MC3T3-E1 cells nor did it inhibit BMP7 activity in NRK49F cells (Supplemental Material). These data confirm and extend previously published data indicating that WISE is a modulator of Wnt signaling and that its inhibitory activity may be restricted to a specific subset of Wnt proteins.
Generation of Rat Anti-Rat WISE Antibody
The high level expression of WISE mRNA in the transplanted kidney suggested a link to the pathology of allograft dysfunction. To directly address this question, a potent rat anti-WISE neutralizing mAb was generated for use in a rat CAD model. Rat anti-WISE potently reversed the inhibition of WISE on β-catenin-dependent transcriptional activity in MC3T3-E1 cells (Figure 1D) and demonstrated an affinity toward rat WISE protein of approximately 130 pM (data not shown). Thus, this antibody was used in a kidney transplant model over a 6-month observation period.
Anti-WISE Treatment Preserved Renal Function
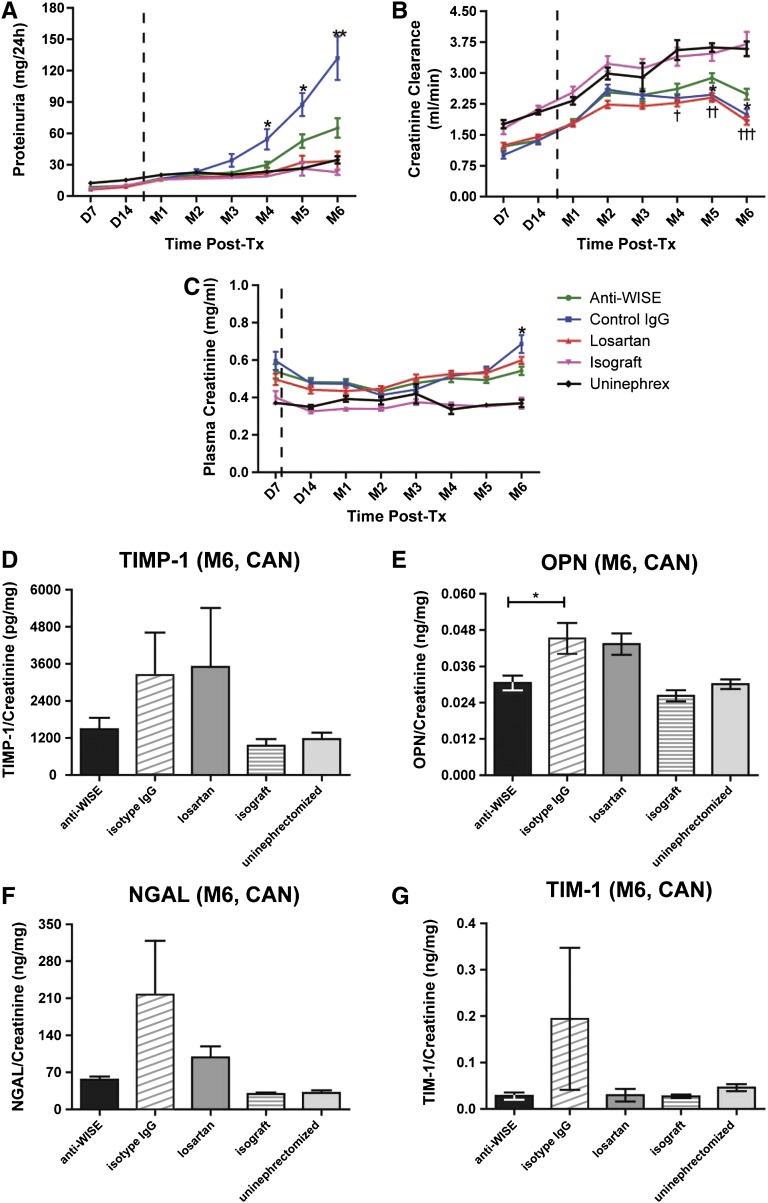
Renal function as measured by proteinuria, serum creatinine, and creatinine clearance was significantly improved in recipients of allografts receiving anti-WISE treatment relative to that of isotype control IgG (Figure 2, A–C). Because renal function was comparable between groups C-a and C-b (Losartan high and low dose), data from both groups were combined for statistical analysis. Creatinine clearance was significantly better preserved in anti-WISE-treated animals compared with both IgG and Losartan controls at the end of the observation period (P<0.05 and P<0.001 compared with IgG- and losartan-treated animals, respectively; Figure 2B). Proteinuria in our model increased progressively by 3 months in IgG-treated animals and was consistently and significantly lower in anti-WISE-treated allografts (P<0.05 on months 4 and 5; P<0.01 on month 6; Figure 2A). Likewise, we observed reduced serum creatinine levels in animals treated with anti-WISE compared with IgG controls (P<0.05 by month 6; Figure 2C).
Figure 2.
Anti-WISE treatment preserved renal function and reduced markers of urinary tubular injury. Renal function measured by (A) proteinuria, (B) plasma creatinine, and (C) creatinine clearance. Anti-WISE treatment animals showed a preserved renal function compared with IgG controls. Data are mean ± SEM. Statistical analysis was done by two-tailed t test between groups. *P<0.05, **P<0.01 (anti-WISE versus IgG); †P<0.05, ††P<0.01, †††P<0.001 (anti-WISE versus Losartan). Urinary tubular injury markers, such as (D) TIMP-1, (E) OPN, (F) NGAL, and (G) TIM-1, can be reduced by anti-WISE treatment. Data are mean ± SEM. Statistical analysis was done by ANOVA with the Tukey multiple comparison test. Tx, transplant; Uninephrex, uninephrectomized controls.
Urinary Tubular Injury Markers Were Reduced by Anti-WISE Treatment
Urinary markers of tubular injury were reduced in anti-WISE-treated animals. Urinary osteopontin (OPN) excretion was significantly reduced by anti-WISE treatment compared with IgG controls by 6 months (P<0.05). Urinary neutrophil gelatinase-associated lipocalin (NGAL), T cell-Ig-mucin (TIM-1), and TIMP metallopeptidase inhibitor 1 (TIMP-1) excretion were also reduced in anti-WISE-treated animals. Of note, excreted urinary markers of tubular injury in anti-WISE-treated animals were comparable with those observed in renal allograft and isograft controls at the end of the observation period, and were also comparable with those observed in Losartan-treated animals (Figure 2, D–G).
Anti-WISE Treatment Ameliorated Tubular Atrophy and Interstitial Fibrosis
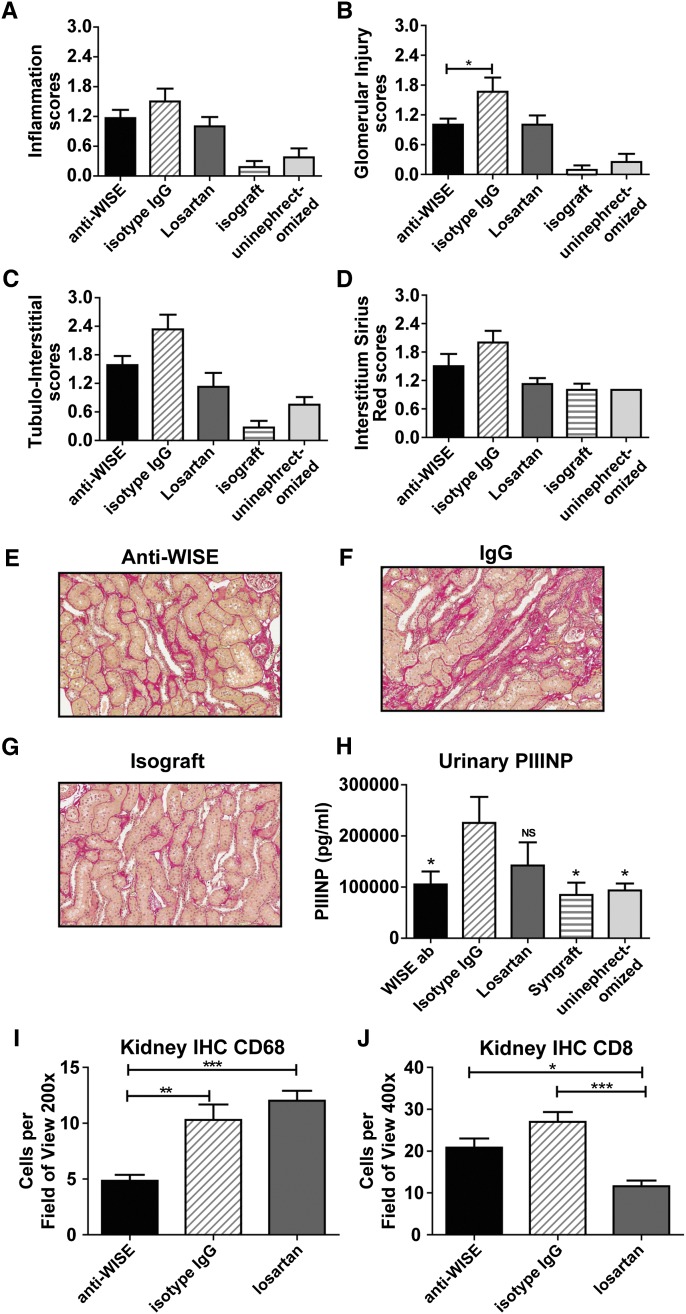
After the 6-month observation period, IgG-treated control animals demonstrated characteristic signs of CAD with prominent cellular infiltrates and advanced glomerular and tubulointerstitial injury (Figure 3, A–C). Anti-WISE-treated kidney allografts demonstrated reduced cellular infiltrates, and reduced glomerular and tubulointerstitial injuries (Figure 3, A–C). Sirius red staining confirmed a reduced expression of collagen in anti-WISE-treated animals relative to that in IgG-treated animals. (Figure 3, D–G). Furthermore, WISE antibody treatment resulted in significantly reduced levels of urinary marker of fibrosis such as N-terminal propeptide of collagen type III (PIIINP) compared with IgG-treated control animals (Figure 3H).
Figure 3.
Anti-WISE improved structural changes of CAD. (A–C) Semiquantitative evaluation of H&E and polarized Sirius red staining showed that anti-WISE treatment (A) reduced cellular infiltrates, (B) while improving glomerular changes, and (C) tubulointerstitial injury. (D) Semiquantitative staining of Sirius red staining results showed reduced collagen in anti-WISE-treated animals. (E) Sirius red staining in anti-WISE-treated allograft. (F) IgG-treated allografts with Sirius red staining. (G) Isografts stained for Sirius red. (H) WISE antibody-treated animals have reduced urinary PIIINP level, a marker of fibrosis, relative to those treated with control IgG. (I and J) Anti-WISE treatment downregulated CD68 (I) and CD8 (J). Quantified kidney immunohistology showed that CD68 was significantly reduced by anti-WISE treatment. CD8 was also reduced, though the difference did not reach significance. Data are mean ± SEM. Statistical analysis was done by ANOVA with the Tukey multiple comparison test. *P<0.05, **P<0.01, ***P<0.001.
Anti-WISE Treatment Decreased Macrophage (CD68+) and T Cell (CD8+) Infiltration
Macrophages and T cells play a critical role in the progression of CAD in the F-344→Lewis (LEW) model.17 Expression of the macrophage specific marker CD68 was significantly reduced in anti-WISE-treated allografts compared with both IgG and losartan controls (P<0.01 and 0.001, respectively, by 6 months; Figure 3I). Similarly, we observed reduced numbers of CD-8+ T cell infiltrates in anti-WISE-treated animals compared with IgG controls (Figure 3J).
Anti-WISE-Induced Total β-Catenin Correlated with Improved Graft Function
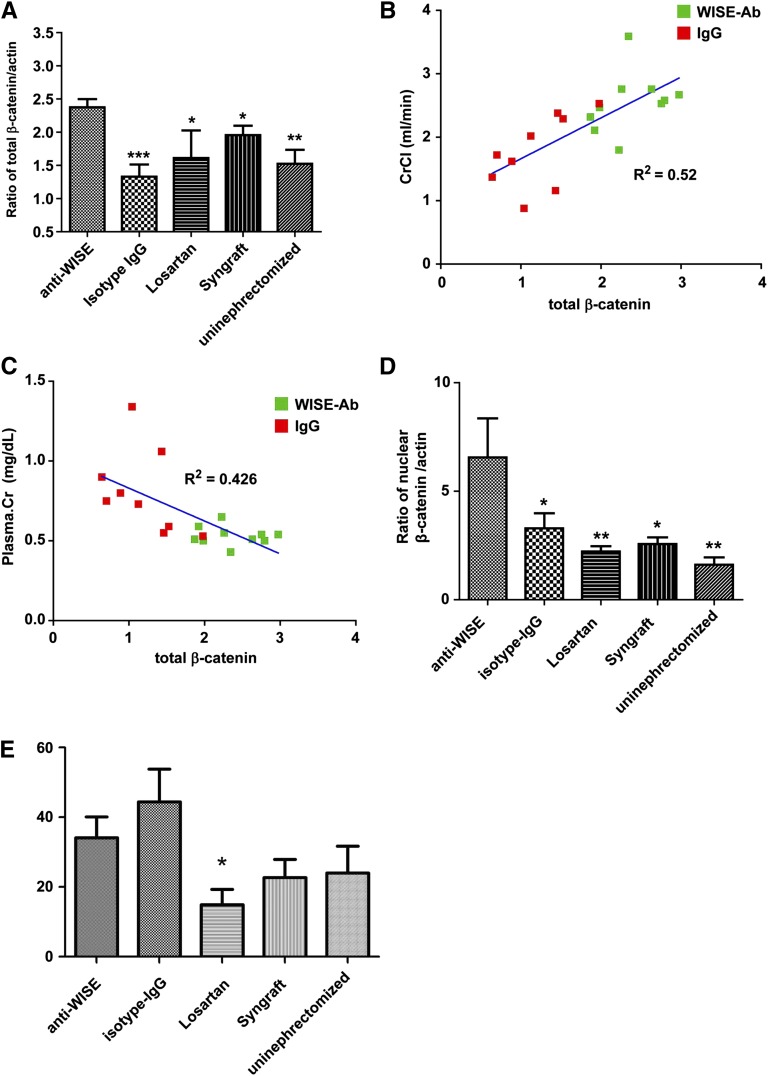
Total β-catenin in allografts treated with IgG control antibody was significantly lower. At the same time, total β-catenin protein was increased in anti-WISE-treated allografts (Figure 4A). Total β-catenin correlated with graft function measured by creatinine clearance and serum creatinine (R2=0.52 and R2=0.43, respectively) in anti-WISE- and IgG-treated animals, indicating that total β-catenin and likely the degree of Wnt signaling activation correlates with graft function (Figure 4, B and C). To study Wnt induced activation of β-catenin in more detail, we generated nuclear extracts from the same material. Nuclear β-catenin was significantly increased in anti-WISE-treated animals (Figure 4D), although we were not able to demonstrate the same robust correlation between this parameter and improved renal function. We did, however, observe that anti-WISE-treated animals with the greatest increase in nuclear β-catenin were among those with the best maintained renal function (data not shown). In contrast to effects on total β-catenin, we did not observe significant alterations in TGF-β1 levels in allograft from anti-WISE-treated animals compared with IgG controls, whereas Losartan treatment reduced TGF-β1 level by >50% (Figure 4E).
Figure 4.
Correlation of total β-catenin with graft function. (A) Total β-catenin was significantly increased after anti-WISE treatment. *P<0.05, **P<0.01, ***P<0.001 compared with anti-WISE. (B and C) Total β-catenin correlated with graft function measured by creatinine clearance and plasma creatinine (R2=0.52 and 0.43, respectively). Calculations by linear regression analysis using Prism GraphPad software. (D) Nuclear β-catenin was significantly increased by anti-WISE. *P<0.05, **P<0.01 compared with anti-WISE. One-way ANOVA with Dunnett’s post test. (E) Total TGF-β levels were not altered by anti-WISE treatment.
The QuantiGene Plex 2.0 assay was utilized to explore the expression of Wnt-pathway genes and the potential effect of anti-WISE (Table 1). The study was conducted in two parts: first, we collected mRNA from all anti-WISE- or IgG-treated allografts (Table 1), and second, RNA was isolated from the three animals showing the greatest improvement in renal function in the anti-WISE group and compared with the three animals from the IgG-treated group with the worst renal function (Table 1). Expression of Wnt1, 3, 6, and 10b was not detected in either group. Expression of most pathway members was not altered; however, Wnt8a and Wnt16 mRNAs were increased, and the downstream signaling components Lef1 and Tcf7 were decreased. Overall, these data indicated that Wnt-pathway components are present in this model and although some are changed globally, they do not inform on the local activation or distribution of these molecules in the damaged or repairing kidney.
Table 1.
Expression of Wnt-pathway genes in anti-WISE-treated renal allografts normalized to IgG-treated allografts
| All Animals | Selected Animals | ||
|---|---|---|---|
| WISE-Ab | WISE-Ab | ||
| Wnt1 | ND | Wnt1 | ND |
| Wnt2 | 1.06 | Wnt2 | 0.86 |
| Wnt2b | 1.11 | Wnt2b | 1.20 |
| Wnt3 | ND | Wnt3 | ND |
| Wnt3a | 1.85 | Wnt3a | 1.52 |
| Wnt4 | 0.81 | Wnt4 | 0.75 |
| Wnt5a | 0.99 | Wnt5a | 1.22 |
| Wnt5b | 0.89 | Wnt5b | 0.77 |
| Wnt6 | ND | Wnt6 | ND |
| Wnt7a | 1.07 | Wnt7a | 0.71 |
| Wnt7b | 0.88 | Wnt7b | 0.82 |
| Wnt8a | 2.12 | Wnt8a | 2.76 |
| Wnt8b | 1.70 | Wnt8b | 1.56 |
| Wnt9a | 1.15 | Wnt9a | 1.13 |
| Wnt9b | 1.56 | Wnt9b | 1.27 |
| Wnt10a | 1.32 | Wnt10a | 1.22 |
| Wnt10b | ND | Wnt10b | ND |
| Wnt11 | 1.52 | Wnt11 | 1.34 |
| Wnt16 | 2.07 | Wnt16 | 1.59 |
| Lrp4 | 0.96 | Lrp4 | 0.96 |
| Lrp5 | 0.90 | Lrp5 | 0.86 |
| Lrp6 | 0.95 | Lrp6 | 0.88 |
| APC | 1.00 | APC | 1.03 |
| GSK3β | 0.97 | GSK3β | 0.95 |
| Axin1 | 0.90 | Axin1 | 0.94 |
| Axin2 | 0.81 | Axin2 | 0.53 |
| Lef1 | 0.56 | Lef1 | 0.31 |
| Tcf7 | 0.58 | Tcf7 | 0.41 |
ND, not detected.
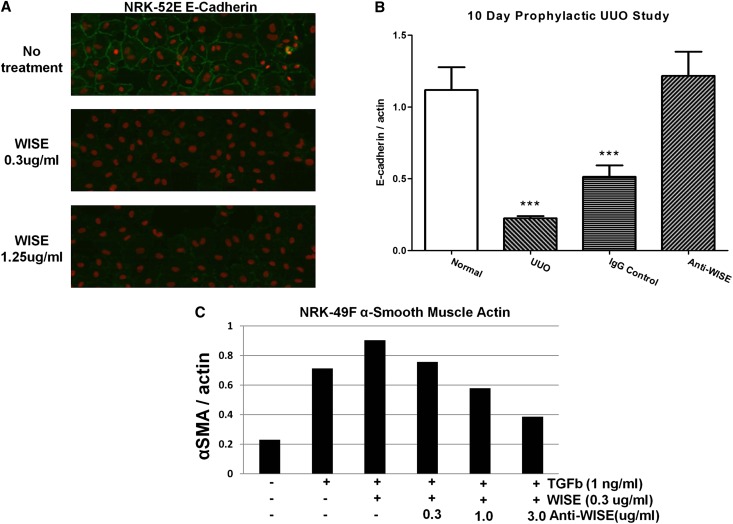
WISE Promotes E-Cadherin Degradation in Renal Epithelial Cells and α-SMA Expression in Renal Fibroblasts
The addition of WISE protein to the rat renal proximal tubular cell line NRK-52E led to the disappearance of E-cadherin staining after 48 hours (Figure 5A). Furthermore, as mentioned previously, in the unilateral ureteral obstruction (UUO) model of kidney injury, WISE deletion exerted a tubular protective effect in the damaged kidney as evidenced by E-cadherin stabilization.18,19 We confirmed that anti-WISE treatment prevented E-cadherin loss in the UUO model (Figure 5B). In addition, WISE deletion in the UUO model resulted in an ameliorated fibrotic response. To see if WISE could alter TGF-β-induced fibrogenesis directly, normal rat kidney fibroblast NRK-49F cells were treated with TGF-β and WISE. WISE addition increased the ratio of α-SMA/actin; this effect was reversed in a dose-responsive manner by the addition of anti-WISE (Figure 5C). These data suggest that WISE is capable of having a direct effect on both the tubular epithelium and on fibrosis in the kidney.
Figure 5.
WISE compromises epithelial integrity and promotes fibrosis. (A) Rat proximal tubular NRK-52E cells treated with WISE and stained for E-cadherin. (B) Anti-WISE prevents E-cadherin loss in UUO kidneys. ***P<0.001 compared with anti-WISE (one-way ANOVA with Dunnett’s post test). n=10 per group. (C) WISE potentiated TGF-β-induced increases in α-SMA in rat kidney fibroblast NRK-49F cells and anti-WISE dose-dependently inhibited this response.
Gene Expression is Skewed toward an Anti-Inflammatory and Antifibrotic Profile in Anti-WISE-Treated Kidneys
Gene expression profiles based on an analysis of progressive injuries showed significant differences within control IgG-treated animals when samples with advanced CAD (additive hematoxylin and eosin [H&E] inflammation, tubulointerstitial and glomerular injury scores >9) were compared with those with minor changes (score of <5). We identified 793 downregulated (≥2 fold changes) and 263 upregulated (≤2 fold changes) genes. Comparing gene expression between IgG-treated allograft recipients with advanced structural changes and animals treated with anti-WISE, we identified 907 downregulated and 285 upregulated genes. Among the downregulated genes, fibrosis-related genes such as collagen types Ia1 and IIIa1, Fsp1/S100a4, Fibronectin-1, matrix metalloproteinases, Serpine1/plasminogen activator inhibitor-1, TGF-β1, TIMP-1, and Vimentin, as well as inflammatory transcripts such as IL-17 receptor B, IL-6 receptor α, IL-1 receptor-associated kinase 3, integrin (α 2,10; β 2,4), were identified, suggesting that anti-WISE treatment inhibits the progression of renal injury in this model by interfering with fibrosis and inflammation (Table 2).
Table 2.
Summary of gene expression related to inflammation and fibrosis by microarray analysis
| Sequence Name(s) | IgG Kidney >9 H&E versus WISE-Ab Kidney | IgG Kidney >9 H&E versus IgG Kidney <5 H&E |
|---|---|---|
| Fold changes | Fold Changes | |
| Fibrosis-related genes | ||
| Col Ia1 | −2.4 | −2.4 |
| Col IIIa1 | −2.5 | −2.6 |
| Fsp1/S100a4 | −2.2 | −2.1 |
| Fibronectin-1 | −2.2 | −2.1 |
| MMP2 | −2.5 | −2.6 |
| MMP12 | −2.5 | −2.7 |
| MMP16 | −3.4 | −2.8 |
| Serpine1/PAI-1 | −3.3 | −2.8 |
| SPP1 (OPN) | −4.0 | −2.9 |
| TIMP-1 | −3.5 | −2.9 |
| Vim | −2.8 | −2.8 |
| OSMR | −2.2 | −2.1 |
| Inflammatory transcripts | ||
| IL-17 receptor β | −2.6 | −2.6 |
| Allograft inflammatory factor 1 | −2.3 | −2.2 |
| CD19 | −2.8 | −2.9 |
| CCR4 | −3.7 | −3.6 |
| CCL19 | −2.8 | −2.9 |
| CD4 | −2.1 | −2.1 |
| IL-7r | −3.1 | −2.5 |
| IL-16 | −2.3 | −2.2 |
| IL-6 receptor ɑ | −2.4 | −2.2 |
| IL-1 receptor-associated kinase 3 | −2.1 | −2.1 |
| Integrin ɑ2 | −4.0 | −3.2 |
| Integrin ɑ10 | −3.0 | −2.8 |
| Integrin β2 | −2.0 | −2.0 |
| Integrin β4 | −2.9 | −2.4 |
Discussion
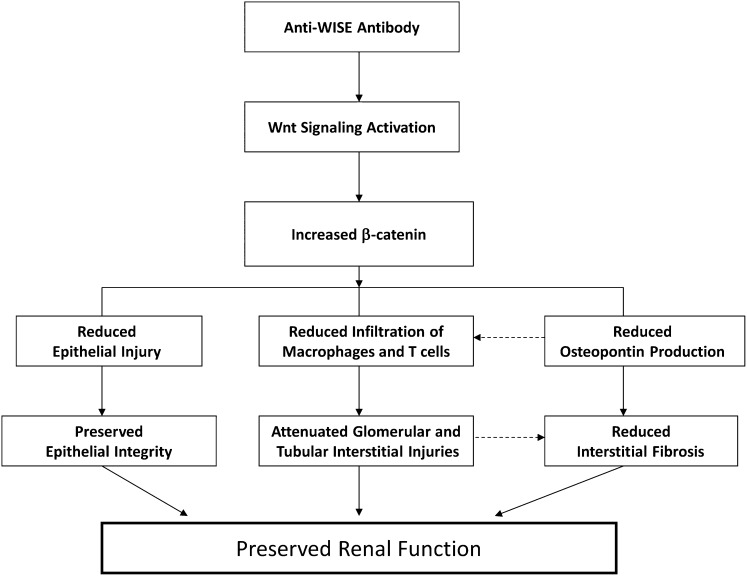
Our data have shown that WISE mRNA is abundantly expressed in kidneys with progressive tubular atrophy and interstitial fibrosis. The application of anti-WISE treatment preserved renal function and structure, and reduced inflammation in this rodent kidney transplant model. At the same time, total β-catenin was increased in parallel to the prevention of CAD. In addition, we demonstrated that Wnt signaling components were present in the renal allograft and that anti-WISE treatment increased nuclear β-catenin, suggesting that modifications of the Wnt signaling pathway contributed to the improvement of CAD (see Figure 6 for the proposed mechanism of action). Microarray analysis revealed that genes of both inflammatory response and fibrotic pathways were significantly downregulated through WISE antibody treatment emphasizing the interference with profibrotic programs initiated with CAD.
Figure 6.
Proposed mechanisms of action of anti-WISE to preserve renal function. Anti-WISE inhibits the inhibitory activity of WISE on Wnt signaling in kidney cells, leads to an increase in total β-catenin, which in turn reduces epithelial damage, infiltration of inflammatory cells such as macrophage and T cells, and the production of OPN. These effects result in preserved epithelial integrity, attenuated glomerular and tubulointerstitial injuries and interstitial fibrosis, and finally, in preserved renal graft function.
We used a rat renal transplant model in a F-344→LEW donor/recipient combination. Although this model has been used by some to simulate the situation of clinical chronic allograft deterioration,20 it does not reflect all characteristic clinical functional and structural hallmarks of this process.20,21 The F-344→LEW model demonstrates, however, characteristic features of chronic kidney injury, including interstitial fibrosis, tubular atrophy, and FSGS, all of which are of critical clinical significance in a variety of renal disease processes.
The precise pathway by which WISE protein exerts its effect is under debate.18,19,22,23 WISE has been reported to be a dual modulator of BMP and Wnt signaling. WISE was also reported as a context-dependent activator and inhibitor of Wnt signaling.15 Moreover, WISE was recently found to be capable of modulating both BMP and Wnt signaling, even though it had been demonstrated that WISE binds preferentially to the Wnt coreceptor LPR6 over the BMP signaling molecule BMP4.24 Our study demonstrated a direct effect on Wnt signaling by WISE, yet we were unable to demonstrate an effect on BMP signaling in the cell lines utilized. Our data demonstrate for the first time that treatment with neutralizing anti-WISE resulted in increased total and nuclear β-catenin in the transplanted kidney. This is consistent with the observation of an increased nuclear β-catenin and Wnt signaling in tooth mesenchyma in WISE/ uterine sensitization-associated gene-1 knockout mice.16,25 Although we have not formally demonstrated the target cell type of increased nuclear β-catenin that correlated to the activation of Wnt signaling in the kidney, we believe that the tubular epithelium is the likely target. This assumption is based on the observation that anti-WISE treatment resulted in significant improvements in markers of tubular damage, showing that WISE is capable of directly inhibiting Wnt1-class signaling.
Increased Wnt/β-catenin signaling may exert multiple effects in the transplanted kidney. First, WISE antibody treatment-mediated Wnt signaling resulted in a significantly reduced infiltration of macrophages and T cells associated with attenuated glomerular and tubular interstitial injuries. In addition to the work by Lin et al.,26 Manicassamy et al.27 recently reported that β-catenin signaling can limit the inflammatory response while balancing tolerance and immunity through the control of dendritic cell function in the intestine. This suggests that reduced inflammation could be a direct effect of increased Wnt/β-catenin signaling in the injured allograft kidney.
Second, dampened inflammatory responses subsequent to anti-WISE treatment may result in reduced injury to kidney epithelial cells evidenced by reduced urinary NGAL levels, a parameter of renal injury. In addition, our microarray analysis demonstrated preserved levels of many genes coding for tubular solute carrier organic anion transporters in WISE antibody-treated animals. This is consistent with our observation that WISE negatively regulates E-cadherin expression, a protein critically involved in maintaining epithelial cell integrity18 in tubular epithelial cells.
Finally, reduced inflammatory infiltration may also, in turn, lead to a decreased production of macrophage- and T cell-derived profibrotic factors known to stimulate the proliferation and activation of quiescent resident myofibroblasts. This interference may result in the reduced expansion of activated myofibroblasts, a key effector cell type in matrix production and fibrosis progression.28 This notion is supported by our observation that the expression of genes coding for α-SMA and FSP-1 and other fibrosis signature genes such as plasminogen activator inhibitor-1 and fibronectin are significantly reduced in WISE antibody-treated grafts. Furthermore, WISE antibody treatment reduced fibrosis as evidenced by reduced interstitial Sirius Red staining, a hallmark of tissue fibrosis, and urinary PIIINP secretion, a product formed during collagen synthesis during tissue fibrosis.29
Interestingly, the urinary level of OPN, a target gene of Wnt/β-catenin signaling known to be expressed in macrophage and epithelial cells,30–33 was significantly reduced in WISE antibody-treated relative to IgG-treated and Losartan-treated allograft kidneys. OPN itself is a proinflammatory and profibrotic factor that has been shown to be upregulated in several models of fibrosis in rodents, and, clinically in idiopathic pulmonary fibrosis, systemic sclerosis, and chronic allograft injury.34 OPN knockout mice have reduced fibrosis relative to wild-type mice in mouse models of renal injury or bleomycin-induced lung injury.35,36 The antifibrotic effect of WISE antibody is likely secondary to its effects on reducing inflammation and preserving epithelial cell integrity, subsequently leading to a reduced number of activated myofibroblasts and matrix production. Importantly, the effects of WISE antibody appear independent of changes in TGF-β expression.
Several studies with long-term follow-up of patients with chronic allograft injuries demonstrated that ongoing inflammation in the presence of fibrosis is a key factor for graft loss.37 The ability of WISE inhibition to reduce inflammation and fibrosis and to limit structural changes makes it an attractive therapeutic approach. Thus, we propose that WISE contributes to the development of progressive allograft dysfunction through the modification of Wnt signaling.
We submit that WISE inhibition serves to protect and repair injured renal epithelial cells and propose that WISE inhibition may represent a novel therapeutic approach for renal disease processes resulting in interstitial fibrosis and tubular atrophy. Ongoing experimental studies are correlating early injury and immune responses after transplantation to dissect the dynamics of Wnt signaling and associated inflammatory and fibrosis programs. Additional studies will test whether anti-WISE treatment may not only improve chronic structural injuries when utilized immediately after transplantation, as in our study, but also when applied at a time point when early functional deterioration is becoming obvious.
Concise Methods
Antibody
Recombinant human WISE protein was used to immunize LEW and Brown Norway rats. Rats with high titers were used for hybridoma generation and antibody screening with recombinant rat WISE protein. A potent neutralizing antibody was selected, gene cloned and a stable cell line was generated for antibody production for this study.
Animals
Male, inbred Fischer-344 [(F-344, RT11v1)→LEW (RT11) and LEW→LEW (Charles River, Wilmington, MA) rats weighing 209.1±25.8 g (mean ± SD) were utilized as donor/recipients for allogeneic and syngeneic transplant experiments, respectively. LEW rats were used as native controls. The F-344→LEW strain combination differs at “weak histocompatibility loci,” including 2 MHC-I and various non-MHC genes and is not different for MHC-II loci. All animal procedures were performed according to local approved animal protocols (#04077).
Surgical Techniques
Donor kidneys were flushed with sterile physiologic saline after systemic application of heparin saline (100 U) and preserved at 4°C. Renal transplants were performed with an end-to-side anastomosis of the aortic stump of the donor kidney and recipient’s aorta, and between the recipient inferior vena cava and donor renal vein, respectively, using 10–0 running nylon sutures. The mean time for anastomosis averaged 31.8±3.3 (mean ± SD) minutes. Uretero-ureterostomy was performed with an end-to-end interrupted sutures technique using 11–0 nylon sutures. Both native kidneys were removed at the time of engraftment.
UUO Model
UUO was performed in 7- to 8-week-old CD1 mice (Charles River Laboratories). Starting on the day of UUO, animals were treated with 20 mg/kg of anti-WISE or IgG control antibody per day. Animals were sacrificed on day 10 and kidneys were isolated for analysis. E-cadherin levels were determined by Western blotting and normalized to β-actin levels. Normal mice and nontreated UUO mice served as control groups. Each group contained 10 animals.
Treatment and Procurement
Three groups of allogeneic transplants were studied: group A (n=14) was treated with anti-WISE (20 mg/kg intraperitoneally biweekly); group B (n=14) served as the negative control and was treated with isotype control rat IgG antibody (20 mg/kg intraperitoneally biweekly); and groups C-a (n=14) and C-b (n=8) served as positive controls and were treated with the AngII receptor blocker Losartan (C-a: 30 mg/kg per day; and C-b: 10 mg/kg per day). Group D comprised syngeneic LEW→LEW animals (n=14) that were treated with vehicle solution (20 mg/kg intraperitoneally biweekly). Group E was composed of uninephrectomized native animals without further treatment (n=8) that served as additional controls. Transplants were randomized to avoid potential biases due to improved surgical techniques during the course of the studies. Allogeneic transplant recipients received low dose cyclosporine A (1.5 mg/kg per day for 10 days) to prevent early acute rejection episodes. Treatment was initiated immediately after surgery.
Animals with free access to water were housed in metabolic cages overnight (>12 h) to collect urine for functional analysis on days 7 and 14, and then at monthly intervals. Blood was drawn at similar time intervals.
Analyses of Renal Function
Creatinine levels were measured using the COBAS INTEGRA Creatinine Jaffe Gen.2 system (Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s instructions. Urine protein levels were measured using the COBAS INTEGRA Total Protein Urine/CSF Gen.3 system (Roche Diagnostics). Proteinuria was calculated by urine protein (mg/dl) × collecting urine volume (ml) × 24/(collecting time (h) × 100). Creatinine clearance was calculated as urine creatinine (mg/dl) × collecting urine volume (ml)/(plasma creatinine (mg/dl) × collecting time (min)).
Histology
Kidneys were fixed in 10% neutral buffered formalin, processed, and embedded in paraffin. Sections were stained with H&E and in addition with Picrosirius red and evaluated for the amount of interstitial, polarized Picrosirius red-stained collagen. Scores for inflammation, tubulointerstitial changes, and glomerular injury were determined using a semiquantitative scale (0 = not remarkably different from expected in control animals; 1 = minimal, 2 = mild, 3 = moderate, and 4 = marked increase in severity over controls) and evaluated in a blinded fashion by a board-certified veterinary pathologist (S.V.).
Immunohistology
Paraffin-embedded sections were rehydrated, blocked with peroxidase (Dako, Carpinteria, CA), and rinsed in Tris-buffered saline. After rinses, sections were blocked with biotin (Dako, Glostrup, Denmark), rinsed in Tris-buffered saline, and blocked with goat serum (MP Biomedicals, Solon, OH) before adding CD8 or CD68 mAbs (AbD Serotec, Oxford, UK). After antibody incubation, sections were exposed with biotinylated linked streptavidin-horseradish peroxidase, and DAB chromogen substrate (Dako).
RNA Labeling and Microarray Hybridization
Total RNA was profiled after the Agilent One-Color Microarray-Based Gene Expression Analysis Protocol (version 6.0) Cy3-labeled cRNA was fragmented and hybridized to a customized Agilent Rat Whole Genome V2 Microarray (Agilent Technologies, Santa Clara, CA) with every reporter replicated at least four times (AMIDID 026843). Arrays were scanned at 3 μm, 20-bit resolution, and data were extracted using Agilent Feature Extraction Software (version 10.7.3.1). Data were imported into Rosetta Resolver software (version 7.2.2).
Microarray Analyses
Ratios were built in Resolver using the Agilent/Intensity–Pairwise Ratio Builder pipeline. For IgG-treated control animals, ratios used data from 3 animals with H&E scores >9 versus 7 animals with scores <5. A second ratio was built using data from the same 3 animals with H&E scores >9 versus 10 anti-WISE-treated animals with a H&E score of <5. The reported genes had a Resolver-calculated P value of ≤1.0E-5 and an absolute fold change of at least 2.
Analyses of Wnt-Pathway Gene Expression
Wnt-pathway gene expression was determined using the Quantigene 2.0 Plex Assay following the manufacturer’s instructions (Affymetrix, Santa Clara, CA). All samples were analyzed in quadruplicate and normalized to the geometric mean of housekeeping controls (hypoxanthine-guanine phosphoribosyltransferase, Glyceraldehyde 3-phosphate dehydrogenase, and peptidyl-prolyl cis-trans isomerase B). The ratio of anti-WISE/IgG-treated Wnt-pathway genes was then determined and is presented in Table 1. One sample from IgG-treated animals was discounted due to low expression levels of housekeeping and target genes; renal function of this animal was near the mean for the group.
In Situ Hybridization
A 622 bp mouse WISE cDNA fragment corresponding to nucleotides 54–676 bp (Genbank BC021458.1) gene was used as a template to generate an antisense 33P-labeled RNA probe. A standard in situ hybridization protocol was performed as previously described.38
Statistical Analyses
Data are presented as mean ± SEM and SD, respectively. Statistical significance was analyzed by ANOVA or unpaired t test, as appropriate.
Disclosures
X.Q., S.V., J.L., L.Z., A.Y., V.D.F, A.W., E.G.V., J.P., J.L.S, B.T., A.G.W., K.G., D.M.B., M.D., H.S.-M., Y.G., C.P., W.S.S., and W.G.R. are or were Amgen employees and own Amgen stock and/or stock options.
Supplementary Material
Acknowledgments
The authors thank Holly Tomlin (employee and stockholder, Amgen Inc) for her editing and manuscript formatting assistance; Ursula Rush, on behalf of Amgen Inc, for manuscript styling; and Sharda Jha for technical assistance with the UUO model.
This research was funded by Amgen Inc.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Present address for Dr. Xiaodong Yuan, Department of Urology, Shandong Provincial Hospital, Shandong University, Jinan, Shandong, 250021 China.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012010012/-/DCSupplemental.
References
- 1.Vadivel N, Tullius SG, Chandraker A: Chronic allograft nephropathy. Semin Nephrol 27: 414–429, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Gaciong Z, Koziak K, Religa P, Lisiecka A, Morzycka-Michalik M, Rell K, Kozlowska-Boszko B, Lao M: Increased expression of growth factors during chronic rejection of human kidney allograft. Transplant Proc 27: 928–929, 1995 [PubMed] [Google Scholar]
- 3.Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick A, Sheppard D: Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature 422: 169–173, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Carvajal G, Rodríguez-Vita J, Rodrigues-Díez R, Sánchez-López E, Rupérez M, Cartier C, Esteban V, Ortiz A, Egido J, Mezzano SA, Ruiz-Ortega M: Angiotensin II activates the Smad pathway during epithelial mesenchymal transdifferentiation. Kidney Int 74: 585–595, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Campistol JM, Iñigo P, Jimenez W, Lario S, Clesca PH, Oppenheimer F, Rivera F: Losartan decreases plasma levels of TGF-beta1 in transplant patients with chronic allograft nephropathy. Kidney Int 56: 714–719, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Amuchastegui SC, Azzollini N, Mister M, Pezzotta A, Perico N, Remuzzi G: Chronic allograft nephropathy in the rat is improved by angiotensin II receptor blockade but not by calcium channel antagonism. J Am Soc Nephrol 9: 1948–1955, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Smit-van Oosten A, Stegeman CA, van Goor H: RAS blockade in experimental renal transplantation. Benefits and limitations. Curr Drug Targets Cardiovasc Haematol Disord 3: 73–79, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Stark K, Vainio S, Vassileva G, McMahon AP: Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372: 679–683, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Kispert A, Vainio S, McMahon AP: Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development 125: 4225–4234, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP: Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 9: 283–292, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Pulkkinen K, Murugan S, Vainio S: Wnt signaling in kidney development and disease. Organogenesis 4: 55–59, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iglesias DM, Hueber PA, Chu L, Campbell R, Patenaude AM, Dziarmaga AJ, Quinlan J, Mohamed O, Dufort D, Goodyer PR: Canonical WNT signaling during kidney development. Am J Physiol Renal Physiol 293: F494–F500, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Königshoff M, Balsara N, Pfaff E-M, Kramer M, Chrobak I, Seeger W, Eickelberg O: Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS ONE 3: e2142, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Toerne C, Schmidt C, Adams J, Kiss E, Bedke J, Porubsky S, Gretz N, Lindenmeyer MT, Cohen CD, Gröne HJ, Nelson PJ: Wnt pathway regulation in chronic renal allograft damage. Am J Transplant 9: 2223–2239, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Itasaki N, Jones CM, Mercurio S, Rowe A, Domingos PM, Smith JC, Krumlauf R: Wise, a context-dependent activator and inhibitor of Wnt signalling. Development 130: 4295–4305, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Ahn Y, Sanderson BW, Klein OD, Krumlauf R: Inhibition of Wnt signaling by Wise (Sostdc1) and negative feedback from Shh controls tooth number and patterning. Development 137: 3221–3231, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hancock WH, Whitley WD, Tullius SG, Heemann UW, Wasowska B, Baldwin WM, 3rd, Tilney NL: Cytokines, adhesion molecules, and the pathogenesis of chronic rejection of rat renal allografts. Transplantation 56: 643–650, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Yanagita M, Okuda T, Endo S, Tanaka M, Takahashi K, Sugiyama F, Kunita S, Takahashi S, Fukatsu A, Yanagisawa M, Kita T, Sakurai T: Uterine sensitization-associated gene-1 (USAG-1), a novel BMP antagonist expressed in the kidney, accelerates tubular injury. J Clin Invest 116: 70–79, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka M, Asada M, Higashi AY, Nakamura J, Oguchi A, Tomita M, Yamada S, Asada N, Takase M, Okuda T, Kawachi H, Economides AN, Robertson E, Takahashi S, Sakurai T, Goldschmeding R, Muso E, Fukatsu A, Kita T, Yanagita M: Loss of the BMP antagonist USAG-1 ameliorates disease in a mouse model of the progressive hereditary kidney disease Alport syndrome. J Clin Invest 120: 768–777, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melk A, Kittikowit W, Sandhu I, Halloran KM, Grimm P, Schmidt BM, Halloran PF: Cell senescence in rat kidneys in vivo increases with growth and age despite lack of telomere shortening. Kidney Int 63: 2134–2143, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Gourishankar S, Halloran PF: Late deterioration of organ transplants: A problem in injury and homeostasis. Curr Opin Immunol 14: 576–583, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Tanaka M, Endo S, Okuda T, Economides AN, Valenzuela DM, Murphy AJ, Robertson E, Sakurai T, Fukatsu A, Yancopoulos GD, Kita T, Yanagita M: Expression of BMP-7 and USAG-1 (a BMP antagonist) in kidney development and injury. Kidney Int 73: 181–191, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Yanagita M, Oka M, Watabe T, Iguchi H, Niida A, Takahashi S, Akiyama T, Miyazono K, Yanagisawa M, Sakurai T: USAG-1: A bone morphogenetic protein antagonist abundantly expressed in the kidney. Biochem Biophys Res Commun 316: 490–500, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Lintern KB, Guidato S, Rowe A, Saldanha JW, Itasaki N: Characterization of wise protein and its molecular mechanism to interact with both Wnt and BMP signals. J Biol Chem 284: 23159–23168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murashima-Suginami A, Takahashi K, Sakata T, Tsukamoto H, Sugai M, Yanagita M, Shimizu A, Sakurai T, Slavkin HC, Bessho K: Enhanced BMP signaling results in supernumerary tooth formation in USAG-1 deficient mouse. Biochem Biophys Res Commun 369: 1012–1016, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Lin S-L, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS: Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A 107: 4194–4199, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, Pulendran B: Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science 329: 849–853, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G: The myofibroblast: One function, multiple origins. Am J Pathol 170: 1807–1816, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soylemezoglu O, Wild G, Dalley AJ, MacNeil S, Milford-Ward A, Brown CB, el Nahas AM: Urinary and serum type III collagen: Markers of renal fibrosis. Nephrol Dial Transplant 12: 1883–1889, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Rohde F, Rimkus C, Friederichs J, Rosenberg R, Marthen C, Doll D, Holzmann B, Siewert JR, Janssen KP: Expression of osteopontin, a target gene of de-regulated Wnt signaling, predicts survival in colon cancer. Int J Cancer 121: 1717–1723, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Pardo A, Gibson K, Cisneros J, Richards TJ, Yang Y, Becerril C, Yousem S, Herrera I, Ruiz V, Selman M, Kaminski N: Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med 2: e251, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okada H, Moriwaki K, Kalluri R, Takenaka T, Imai H, Ban S, Takahama M, Suzuki H: Osteopontin expressed by renal tubular epithelium mediates interstitial monocyte infiltration in rats. Am J Physiol Renal Physiol 278: F110–F121, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Nicholas SB, Liu J, Kim J, Ren Y, Collins AR, Nguyen L, Hsueh WA: Critical role for osteopontin in diabetic nephropathy. Kidney Int 77: 588–600, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Rödder S, Scherer A, Raulf F, Berthier CC, Hertig A, Couzi L, Durrbach A, Rondeau E, Marti HP: Renal allografts with IF/TA display distinct expression profiles of metzincins and related genes. Am J Transplant 9: 517–526, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Persy VP, Verhulst A, Ysebaert DK, De Greef KE, De Broe ME: Reduced postischemic macrophage infiltration and interstitial fibrosis in osteopontin knockout mice. Kidney Int 63: 543–553, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Berman JS, Serlin D, Li X, Whitley G, Hayes J, Rishikof DC, Ricupero DA, Liaw L, Goetschkes M, O’Regan AW: Altered bleomycin-induced lung fibrosis in osteopontin-deficient mice. Am J Physiol Lung Cell Mol Physiol 286: L1311–L1318, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Matas AJ, Leduc R, Rush D, Cecka JM, Connett J, Fieberg A, Halloran P, Hunsicker L, Cosio F, Grande J, Mannon R, Gourishankar S, Gaston R, Kasiske B: Histopathologic clusters differentiate subgroups within the nonspecific diagnoses of CAN or CR: Preliminary data from the DeKAF study. Am J Transplant 10: 315–323, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Wilcox JN: Fundamental principles of in situ hybridization. J Histochem Cytochem 41: 1725–1733, 1993 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.