Abstract
A missense mutation in mouse Nek8, which encodes a ciliary kinase, produces the juvenile cystic kidneys (jck) model of polycystic kidney disease, but the functions of Nek8 are incompletely understood. Here, we generated a Nek8-null allele and found that homozygous mutant mice die at birth and exhibit randomization of left-right asymmetry, cardiac anomalies, and glomerular kidney cysts. The requirement for Nek8 in left-right patterning is conserved, as knockdown of the zebrafish ortholog caused randomized heart looping. Ciliogenesis was intact in Nek8-deficient embryos and cells, but we observed misexpression of left-sided marker genes early in development, suggesting that nodal ciliary signaling was perturbed. We also generated jck/Nek8 compound heterozygotes; these mutants developed less severe cystic disease than jck homozygotes and provided genetic evidence that the jck allele may encode a gain-of-function protein. Notably, NEK8 and polycystin-2 (PC2) proteins interact, and we found that Nek8−/− and Pkd2−/− embryonic phenotypes are strikingly similar. Nek8-deficient embryos and cells did express PC2 normally, which localized properly to the cilia. However, similar to cells lacking PC2, NEK8-depleted inner medullary collecting duct cells exhibited a defective response to fluid shear, suggesting that NEK8 may play a role in mediating PC2-dependent signaling.
Cystic diseases of the kidney are prevalent in the human population and there is compelling interest in understanding the functions of the associated genes. The most common human cystic disease is autosomal dominant polycystic kidney disease (ADPKD) with an incidence of 1 in 750 individuals.1 ADPKD is caused by mutations in either PKD1 or PKD2, which encode the polycystin proteins PC1 and PC2, respectively. PC1 is a G-protein coupled receptor that partners with PC2, a transient receptor potential polycystic family ion channel that regulates both calcium entry into and intracellular calcium release within renal epithelial cells.2–7 The nephronophthisis (NPHP) types 1–11 and NPHPL1 comprise a less common group of autosomal recessive disorders, but they are collectively the leading genetic cause of ESRD within the first 3 decades of life.8–10
We previously reported that the mouse juvenile cystic kidneys (jck) model of recessive polycystic kidney disease (PKD) is due to a missense mutation in the Nek8 gene; jck animals develop cysts in distal nephron segments and collecting ducts within the first week of life and the disease rapidly progresses, causing renal failure and death by 6 months of age.11 Nek8 encodes a highly conserved member of the Nek family of serine/threonine kinases, characterized by an N-terminal kinase domain homologous to that of the Aspergillus nidulans NIMA protein that controls cell cycle entry during mitosis.12 Mammalian Neks have divergent C termini, and the NEK8 C terminus contains multiple repeats that are homologous to RCC1, a guanine nucleotide exchange factor for Ran that is required for chromosome condensation,13–15 although NEK8 guanine nucleotide exchange factor activity has not been demonstrated. The jck mutation in Nek8 results in a glycine to valine change in the second RCC repeat of the protein. Subsequent studies have reported missense mutations in the RCC domain-encoding regions of both rat Nek8 in the Lewis PKD model16 and human NEK8, in which three independent mutations were identified from 700 NPHP patients, making it a candidate for NPHP type 9.11,17 Together, these data demonstrate that the NEK8 C terminus is critical for its function and the protein plays an important role in the maintenance of renal tubule integrity in the postnatal kidney. Of note, all of the reported mutations of Nek8 are missense mutations. We describe here the generation of a mouse carrying a null mutation of Nek8; in this line, mutant pups die shortly after birth and exhibit a variety of laterality defects and associated cardiac anomalies.
Mutations in several PKD-associated genes cause laterality defects in the developing mouse, and dysfunction of primary cilia is the critical link between the phenotypes. Kidney tubular epithelial cells each express a primary cilium that collectively act as mechanosensors of fluid flow through the tubules, transducing calcium-dependent signals hypothesized to keep the cells in a differentiated state.7 Early in development, the establishment of appropriate asymmetry in mammals requires the functions of two populations of cilia within the node, a pit in the ventral surface of the embryo. Motile cilia rotate and generate a leftward fluid flow, and primary cilia subsequently produce a left-sided calcium gradient.18,19 Mutations in Pkd2, Kif3A, and Tg737(Ift88) illustrate how cilia are critical signaling centers in the kidney and the node. Loss of Pkd2 from the adult kidney results in PKD, whereas Pkd2-null embryos exhibit left-right defects.20,21 Functionally, the PC1/PC2 complex mediates calcium influx in response to flow-induced sheer stress in cultured renal cells, and loss of either protein abolishes the response22; similarly, Pkd2-null embryos do not exhibit a left-sided calcium signal.19 Heterotaxy also occurs with loss of the Pkd1-related gene, Pkd1l1; the gene product localizes to cilia and interacts with PC2, forming a node-specific complex required for proper asymmetry.23,24
Alternatively, mutations that disrupt cilia assembly and/or structure cause both phenomena. PKD arises in the kidney-specific deletion of Kif3A, which encodes a kinesin subunit required for ciliogenesis, and in Tg737 (Ift88/polaris) hypomorphs that exhibit shorter cilia with bulbed tips.25,26 Laterality defects occur upon targeted disruption of Kif3A, Kif3B, or Tg737 (Ift88); each of these knockouts lack nodal cilia and die mid-gestation with randomized heart looping.18,27,28 Downstream of flow-induced calcium signals, left-sided determinants are expressed in the early embryo. The Nodal gene encodes a ligand of the TGF-β family and is expressed first in the node and subsequently in the left lateral plate mesoderm (LPM), and the transcription factor Pitx2 is later expressed in the left LPM; mutant alleles of each of these genes are embryonic lethal with laterality defects.29–32
A majority of proteins associated with human cystic diseases, including the polycystins and nephrocystins, localize to either the axoneme and/or the basal body of primary cilia.8,33 Similarly, NEK8 localizes to the base of renal cilia and is thus far the only kinase that has been localized to the ciliary axoneme.34,35 Cilia defects are indeed present in jck cystic renal epithelia and cells cultured from affected kidneys; mutant cilia are elongated and exhibit enhanced ciliary localization of PC1 and PC2 compared with wild-type cilia.36,37 In addition, PC2 is hyperphosphorylated in jck kidneys and both wild-type and mutant NEK8 coimmunoprecipitate with PC2 from kidney lysates, suggesting that these proteins perform critical cooperative functions.37
We originally hypothesized that the jck allele is hypomorphic because the only overt phenotype in homozygotes is PKD, mutant protein is expressed in the kidneys, and morpholino-mediated reduction of Nek8 in zebrafish causes pronephric cysts.11 Here, we characterize the Nek8-null mutant and determine that ciliogenesis is intact, but left-right patterning is perturbed. We revisit morpholino knockdown in zebrafish and demonstrate that Nek8 depletion causes laterality defects, confirming a conserved role for Nek8 in left-right patterning. Furthermore, we generated jck/Nek8− compound heterozygotes; surprisingly, renal cystic disease in these mutants is much less severe than in jck homozygotes, suggesting the jck allele of Nek8 encodes a gain-of-function protein. Finally, there is marked similarity of Nek8−/− and Pkd2−/− embryonic phenotypes and we determine that PC2-associated activity is abrogated in cells lacking NEK8.
Results
Generation of Nek8-Null Mouse Model
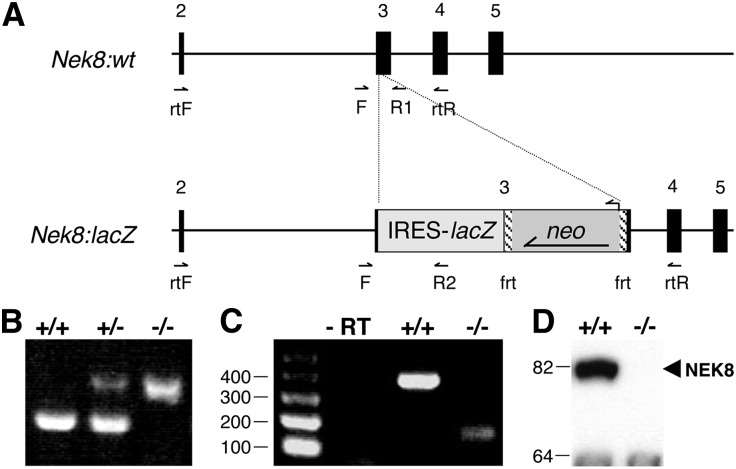
To generate a Nek8-null allele, we obtained a C57BL/6 (B6) bacterial artificial chromosome containing the Nek8 genomic locus. Nek8 exon 3 was targeted by recombineering using a cassette composed of an internal ribosomal entry sequence (IRES), the lacZ gene, and the neomycin resistance gene flanked by FLP recombinase recognition sites (Figure 1A). Nek8:lacZ was designed to encode a truncated protein lacking the kinase domain and a β-galactosidase reporter to recapitulate endogenous NEK8 expression. Nek8:lacZ-positive B6/129 hybrid embryonic stem cell38 clones were injected into B6 blastocysts, and the resulting chimeras were bred to obtain germline transmission of the Nek8:lacZ allele. Heterozygous Nek8:lacZ carriers are viable and fertile with no obvious phenotypes, whereas Nek8:lacZ homozygous pups die shortly after birth; carriers and homozygotes are obtained at expected 50% and 25% frequencies, respectively.
Figure 1.
Targeted disruption of Nek8 results in a true null allele. (A) Nek8 exon 3 is disrupted with an IRES-lacZ:neomycin cassette. FLP recombinase recognition sequences (frt) flank neomycin. (B) PCR genotyping distinguishes wild-type (+/+) embryos from heterozygous (+/−) and homozygous (−/−) lacZ-positive embryos. The wild-type band is amplified with F and R1 primers flanking exon 3. The mutant product is amplified with the F primer and R2 primer from the lacZ sequence. (C) RT-PCR analysis confirms that the lacZ reporter is spliced out of the mutant allele. rtF in exon 2 and rtR in exon 4 amplify a 400 bp product and a 150 bp product from wild-type and Nek8 mutant MEF cDNA, respectively. (D) No NEK8 is detected via Western blot analysis of MEF lysates.
Heterozygous- and homozygous-Nek8:lacZ embryos were analyzed for β-galactosidase activity, but enzyme activity was not detected in PCR-positive samples (Figure 1B and data not shown). We derived mouse embryo fibroblasts (MEFs) from E14.5 wild-type and homozygous embryos, analyzed the cDNA from the cells, and discovered the targeted exon 3 is spliced out of the mutant transcript, rendering the reporter ineffective (Figure 1C and data not shown). However, the aberrant splicing of exons 2–4 causes a frameshift and a creates premature stop codon resulting in a true null allele of Nek8, which we herein refer to as Nek8−, because no protein is detected in homozygotes by Western blot analysis (Figure 1D).
Nek8−/− Embryos Exhibit Randomization of Left-Right Asymmetry and Cardiac Defects
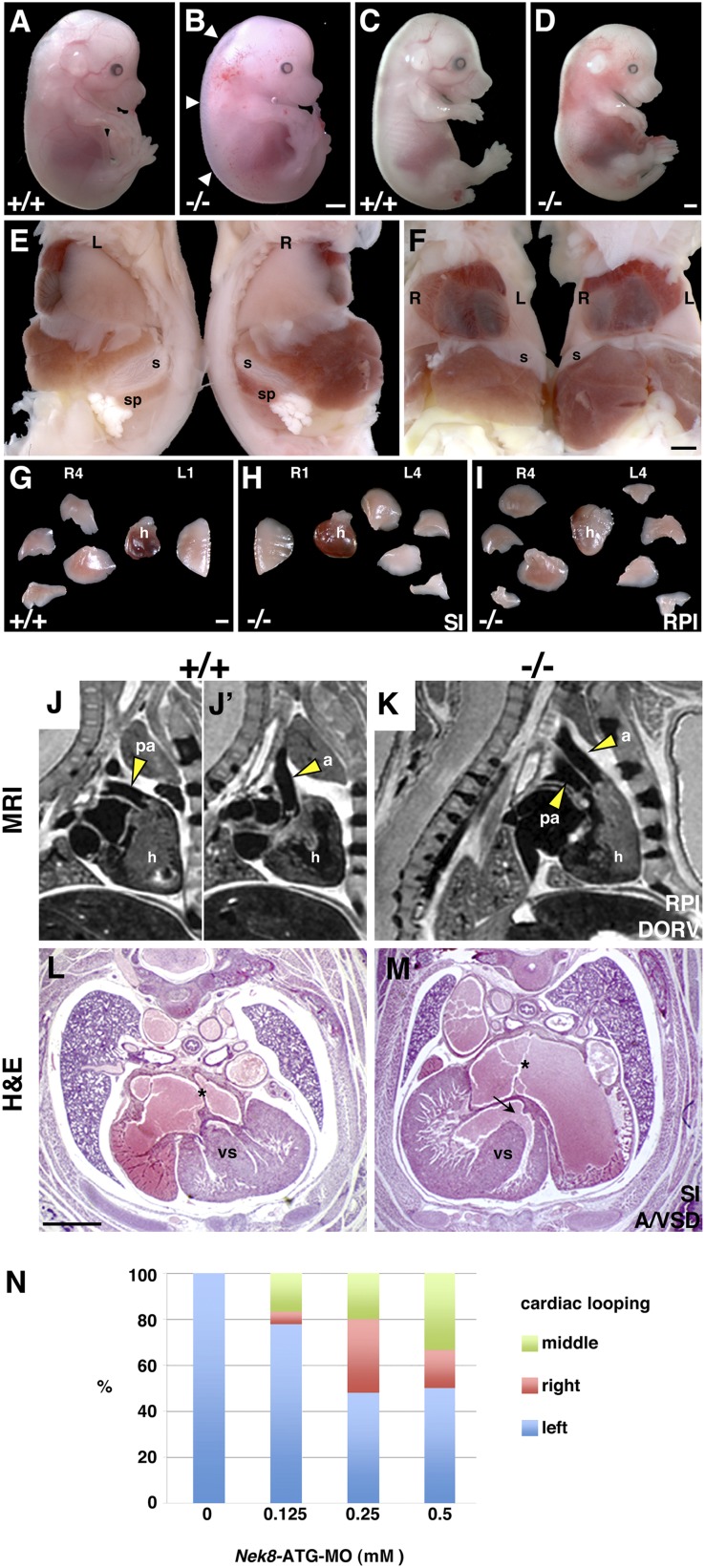
Nek8−/− mice die shortly after birth and mid-gestation mutants frequently exhibit edema and focal hemorrhages that are suggestive of cardiac defects (Figure 2, A–D). Upon autopsy, we observe randomization of left-right asymmetry in the mutants. The characteristic asymmetry of mouse body plan is defined in the thorax by a four-lobed right lung and single left lung lobe with a left-oriented heart apex; in the abdomen, the stomach and adjacent spleen reside on the left side (Figure 2, E–G). Approximately 15% of Nek8−/− mutants have normal body asymmetry, whereas 24% display situs inversus totalis, in which the body plan is a mirror image to that of a normal fetus (Figure 2, E, F, and H). However, a majority of Nek8−/− mutants (61%) exhibit right pulmonary isomerism (RPI), a “duplication” of the four-lobed right lung on the left side (Figure 2I); one-third of these mutants have normal abdominal situs, whereas two-thirds have inverted abdominal situs (Table 1).
Figure 2.
Nek8−/− mice exhibit laterality and cardiac defects. (A–D) Lateral views of littermates. E14.5 wild-type embryo (A) compared with Nek8−/− embryo (B) with edema (arrows) and focal hemorrhages. E16.5 wild-type embryo (C) is larger than the mutant (D). (E–H) E18.5 wild-type compared with a mutant with SI. (E) Lateral views of a wild-type embryo (left) and a null embryos (right) illustrate abdominal situs defect in the mutant. (F) Frontal views of the embryos in E, in which internal organs mirror each other. (G and H) Hearts and lung lobes dissected out of the thoracic cavities of embryos in E and F. (G) Wild-type lungs contain four right lobes and one left lobe (R4, L1). (H) The mutant with SI has one right lobe and four left lobes (R1, L4). (I) Heart and lungs dissected out of a mutant with RPI; right and left lungs are four-lobed (R4, L4). (J–M) Nek8−/− embryos exhibit cardiac defects. (J and K) MRI images through thoraces of a wild-type and a null littermate with RPI. Sagittal views show that wild-type pulmonary artery (J) and aorta (J’) are present in distinct images. (K) Mutant pulmonary artery and aorta run parallel in the same image. (L and M) H&E-stained transverse cardiac sections. (L) The wild-type heart has intact atrial (*) and ventricular septa. (M) The SI mutant heart exhibits atrial (*) and ventricular (arrow) septal defects. (N) Cardiac looping occurs in Nek8-ATG MO zebrafish embryos in a dose-dependent manner. Scale bars, 1.0 mm in A–I; 100 μm in J–M. SI, situs inversus; s, stomach; sp, spleen; R, right; L, left; h, heart; pa, pulmonary artery; a, aorta; vs, ventricular septa.
Table 1.
Summary of laterality defects in Nek8−/− embryos
| Abdominal Situs | Thoracic Situs | ||||
|---|---|---|---|---|---|
| SS | RPI | SI | |||
| SS | 20 (39) | 8 (15) | 12 (24) | 0 | |
| SI | 31 (61) | 0 | 19 (37) | 12 (24) | |
| Total | 51 (100) | 8 (15) | 31 (61) | 12 (24) | |
Data are shown as the number of embryos (% of total). SS, situs solitus; SI, situs inversus.
Mutants with laterality defects often have major structural cardiac anomalies.29,39–41 Therefore, magnetic resonance imaging (MRI) and histologic analyses were performed to assess the hearts of Nek8−/− embryos. In three mutants with RPI we identified double outlet right ventricle (DORV), a defect in which both the pulmonary artery and aorta exit the right ventricle of the heart, accompanied by atrial and ventricular septal defects (A/VSD) (Figure 2, J and K and data not shown). DORV is best exemplified in lateral MRI images: the pulmonary artery and aorta are captured in separate images in a wild-type embryo, but the structures run parallel to each other in the same image in the Nek8−/− mice. Although mice with situs inversus totalis can be viable, as in inversin mutant mice,42 no Nek8−/− mice survive the perinatal period and we found A/VSD in mutants with complete situs inversus (Figure 2, L and M).
To further examine the requirement for Nek8 in establishing normal patterning in a vertebrate system, we utilized morpholino knockdown of Nek8 in zebrafish. We previously used this approach to evaluate cystogenesis in adult fish,11 but laterality was not assessed. In this analysis, randomization of heart looping occurs in a dose-dependent manner in zebrafish embryos injected with a morpholino targeted to the initiator methionine codon of Nek8 (P<0.001; Figure 2N).
Early Laterality Markers Are Misexpressed in Nek8−/− Embryos but Nodal Cilia Are Intact
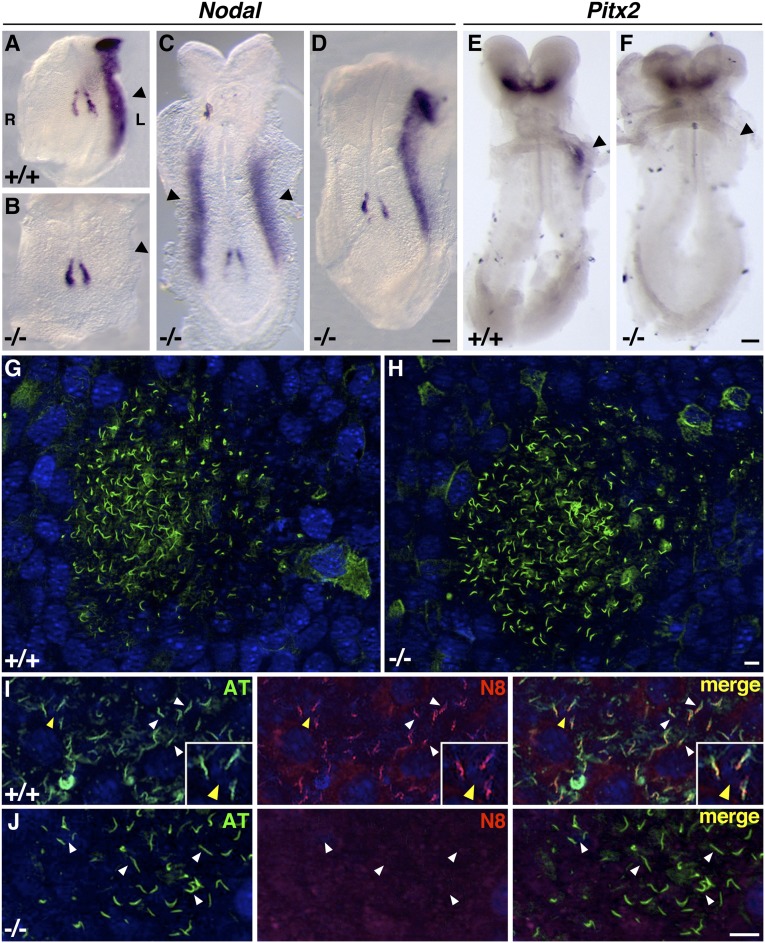
Left-right asymmetry mutants have defects in nodal signaling that are assessed via in situ hybridization of Nodal and Pitx2 between E8.0 and E8.5.43–45 In wild-type presomite embryos, Nodal expression is symmetrical in the node. By the 3–4 somite stage, it becomes asymmetrically expressed in the node, staining stronger on the left side than the right, and expression is restricted to the left LPM (Figure 3A). In contrast, in Nek8−/− embryos at the 3–4 somite stage, Nodal expression within the nodes was always symmetrical (n=9) and was absent from the LPM in seven of nine embryos, bilateral in one embryo, and expressed in the left LPM in one embryo (Figure 3, B–D and Supplemental Figure 1). We examined embryos at the 6–8 somite stage for Pitx2 expression, which is normally apparent in the headfolds and a discrete region of the left LPM of wild-type embryos (Figure 3E). Pitx2 was appropriately expressed in the headfolds of stage-matched Nek8−/− embryos (n=6), but was absent from the LPM in four of six mutants (Figure 3F), right-sided in one mutant, and correctly expressed in one mutant.
Figure 3.
Laterality marker genes are misexpressed in Nek8−/− embryos but nodal cilia are intact. (A–F) In situ hybridization of laterality markers confirms signaling defects in Nek8−/− embryos (arrows indicate LPM). (A–D) Nodal is expressed in the node proper in 4–5 somite wild-type and null embryos, as well as in the wild-type left LPM shown in A. Mutant embryos lack LPM staining (B) or have bilateral (C) or left LPM expression (D). Pitx2 is expressed in 6 somite wild-type and null headfolds and in the wild-type left LPM (E), whereas mutant embryos usually lack LPM expression (F). Anti-acetylated tubulin immunofluorescence of E8.0 embryos shows that wild-type nodal cilia (G) (n=10) and null nodal cilia (n=5) (H) appear similar. High-magnification view of wild-type nodal cilia (I) (arrows) stained with anti-acetylated tubulin (green) and anti-NEK8 (red); NEK8 localizes to all node monocilia (merge; inset, NEK8 at base of cilia) and is not detected in Nek8−/− nodal cilia (J). Scale bars, 200 μm in A–I. R, right; L, left.
Several laterality mutants have short nodal cilia or lack them altogether, which disrupts asymmetric signaling downstream of nodal flow.18,27,28 Because NEK8 localizes to renal epithelial cilia, we examined the nodal cilia of E8.0 embryos using immunofluorescent detection of the ciliary marker acetylated α-tubulin and the anti-NEK8 antibody. Wild-type and Nek8−/− nodes are indistinguishable (Figure 3, G and H) and NEK8 is expressed in the nodal cilia of wild-type E8.0 embryos (Figure 3I). Taken together, the in situ hybridization and immunofluorescence data suggest that Nek8 performs a critical function in laterality determination, but is not directly required for ciliogenesis.
Nek8−/− Kidneys Do Not Develop PKD
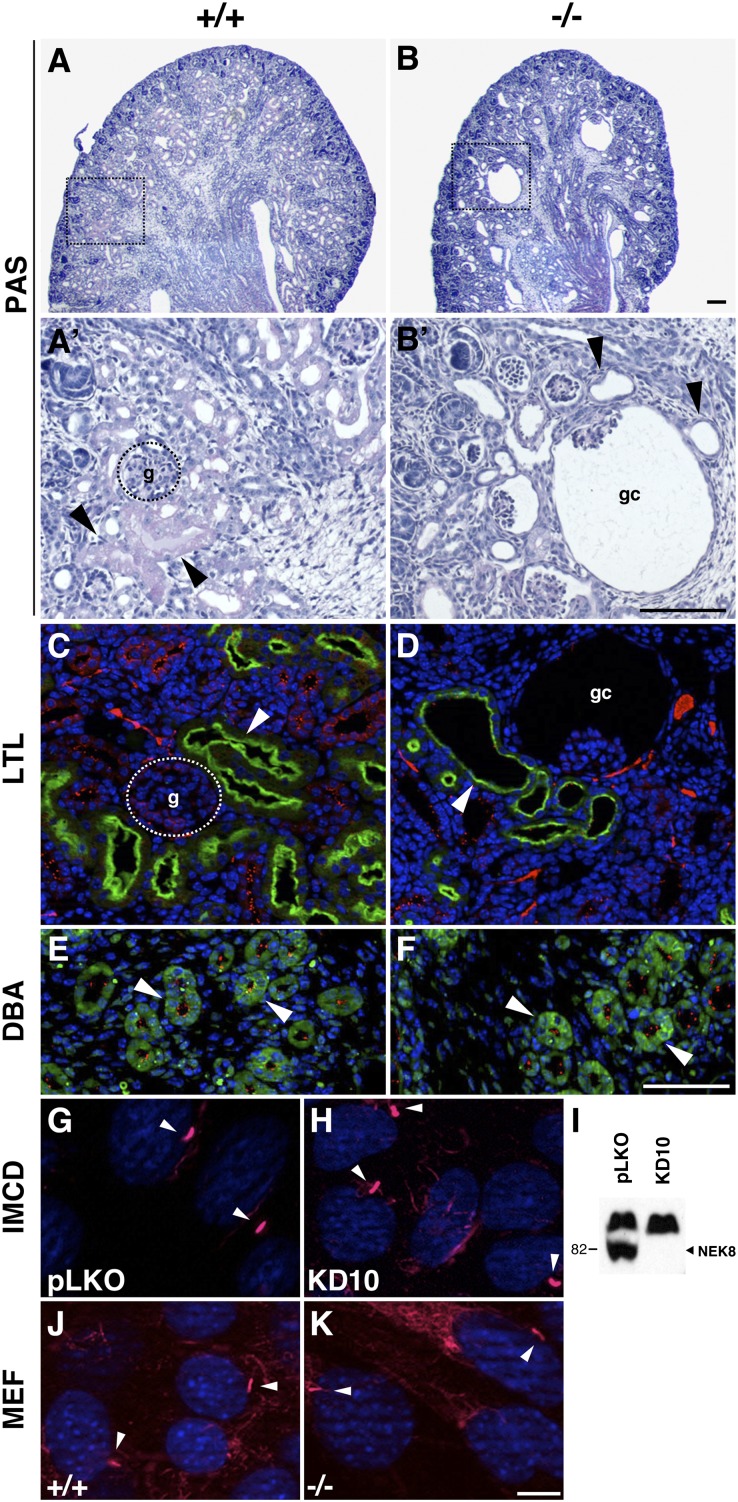
Nek8−/− pups die within hours of birth but they can produce urine, indicating that the kidney is functional. We examined renal development in Nek8−/− embryos and the mutant kidneys develop similarly to those of wild-type through E15.5; however, by E16.5 and through P0, glomerular cysts are apparent in periodic acid–Schiff (PAS)-stained samples (data not shown and Figure 4, A and B). In addition, P0 Nek8−/− proximal tubules appear thin-walled and slightly dilated compared with the wild-type proximal tubules, which are thicker due to intact brush borders on the apical surfaces. To further assess the tubular phenotype, we stained kidneys with the fluorescein-conjugated Lotus tetragonolobus lectin (LTL) and Dolichos biflorus agglutinin (DBA) to identify the proximal tubules and collecting ducts, respectively. LTL staining confirmed that the Nek8−/− proximal tubules are indeed dilated at P0 but not at E18.5, whereas the DBA staining showed that P0 mutant collecting ducts are largely unaffected (Figure 4, C–F and data not shown). Importantly, the abundance and appearance of cilia in wild-type and Nek8−/− tubules is similar (Figure 4, C–F, acetylated α-tubulin in red).
Figure 4.
Nek8−/− kidneys exhibit glomerular cysts and proximal tubule dilation. (A and B) PAS-stained kidneys from P0 pups. (A and A’) The wild-type kidney has normal glomeruli and proximal tubules stain pale purple (arrows). (B and B’) The mutant kidney develops glomerular cysts and proximal tubules are distended. FITC-conjugated LTL lectin marks proximal tubules (arrows) and confirms that the wild-type tubules are intact (C), whereas the mutant tubules adjacent to glomerular cysts are distended (D). (E and F) FITC-conjugated DBA lectin marks the collecting ducts (arrows). Wild-type tubules (E) and mutant tubules (F) are similar in size and shape and cilia are present in both (red puncta, anti-acetylated tubulin immunofluorescence). Cilia (arrows; red, anti-acetylated tubulin) of control (pLKO) (G) and Nek8-knockdown (N8KD10) (H) IMCD cells are of similar morphology and length. (I) Western analysis of IMCD lysates illustrates N8KD10 cells lack NEK8 (arrow) compared with the larger nonspecific band recognized by the antibody. Cilia of wild-type (J) and Nek8−/− MEFs (K) are of similar morphology and length. Scale bars, 100 μm in A–F; 10 μm in G, H, J, and K. Original magnification, ×10 in A and B; ×40 images of boxed region in A’ and B’. g, glomeruli; gc, glomerular cysts.
Although ciliogenesis appears normal in Nek8 mutant nodes and kidneys, it is difficult to identify overt length or structural defects via immunofluorescence in these tissues. Therefore, we analyzed cilia in control and Nek8-deficient mouse inner medullary collecting duct (IMCD) cells (Figure 4, G and H) as well as in wild-type and null MEFs (Figure 4, J and K). To generate Nek8-depleted IMCD cells, small hairpin RNA (shRNA) against Nek8 was introduced and individual clones were isolated and expanded; NEK8 is not detected via Western blot in line N8KD10 compared with the vector control clonal (pLKO) line (Figure 4I). There are no morphologic or length differences between the cilia of wild-type and Nek8-deficient cells in culture: pLKO and N8KD10 cilia lengths are 3.3±1 μm and 3.0±0.6 μm, respectively; wild-type and Nek8−/− MEF cilia lengths are 2.9±0.7 and 2.8±0.9 μm, respectively. Statistical analysis using a nonparametric ANOVA test reveals that these differences are not significant, suggesting that ciliogenesis can occur normally in the absence of NEK8.
jck/Nek8− Compound Heterozygotes Have Less Severe Cystic Disease Than jck Homozygotes
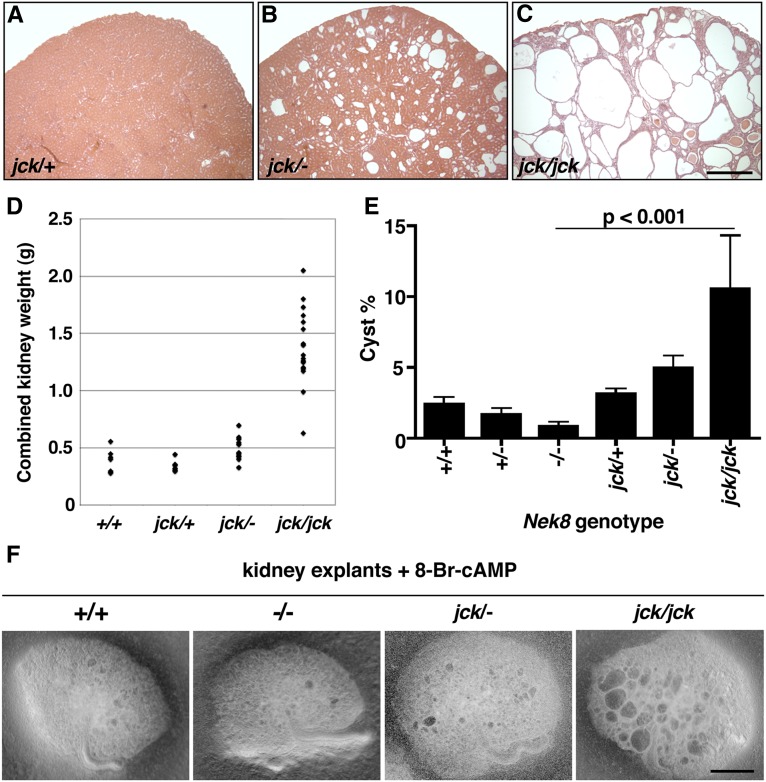
We hypothesized that the jck allele of Nek8 is hypomorphic, and to test this we intercrossed jck/+ and Nek8+/− mice to generate compound heterozygous jck/Nek8− animals. Wild-type, jck/+, jck/Nek8−, and jck/jck mice were sacrificed at 7 weeks of age, the kidneys were weighed and histology was performed to assess disease severity. Surprisingly, jck/Nek8− animals develop very mild renal cystic disease and the combined kidney weights are not much greater than those of wild-type mice (Figure 5, A–D).
Figure 5.
jck/Nek8-compound heterozygotes exhibit moderate PKD and Nek8−/− kidneys do not develop cysts. (A–C) H&E stained kidneys at 7 weeks. (A) jck/+ kidneys do not develop PKD. jck/Nek8− kidneys (B) develop moderate PKD compared with the severe PKD of jck/jck mutants (C). (D) Comparison of combined kidney weights between wild-type (n=7), jck/+(n=8), jck/Nek8− (n=8), and jck/jck (n=15) animals. (E and F) Embryonic kidney explant culture cyst formation assay results. Nek8−/− kidneys do not develop many cysts, jck/Nek8− kidneys develop moderate cysts, and jck/jck cystogenic potential is the greatest when cultured with 8-Br-cAMP. Error bars in E represent SEM. Scale bars, 0.5 mm in A–C and F.
Because Nek8−/− pups die at birth, we analyzed the cystogenic potential of null, jck, and compound mutant embryonic kidneys in an explant culture assay.46,47 Kidneys were removed from E14.5 embryos and cultured for 5 days with or without a cyst-inducing cAMP analog. In the presence of 8-Bromo-cAMP, jck/jck kidneys develop the greatest numbers of cysts, whereas Nek8−/− kidneys have the least cystogenic potential of all the genotypes (Figure 5, E and F). Taken together, the findings that jck/Nek8− adult and cultured embryonic kidneys develop less severe cystic disease than homozygous jck kidneys, and that the Nek8-null kidney explants do not develop tubular cysts, suggest the jck mutation results in a gain-of-function protein.
Nek8−/− Phenotypes Resemble Those Caused by Loss of PC2 Function
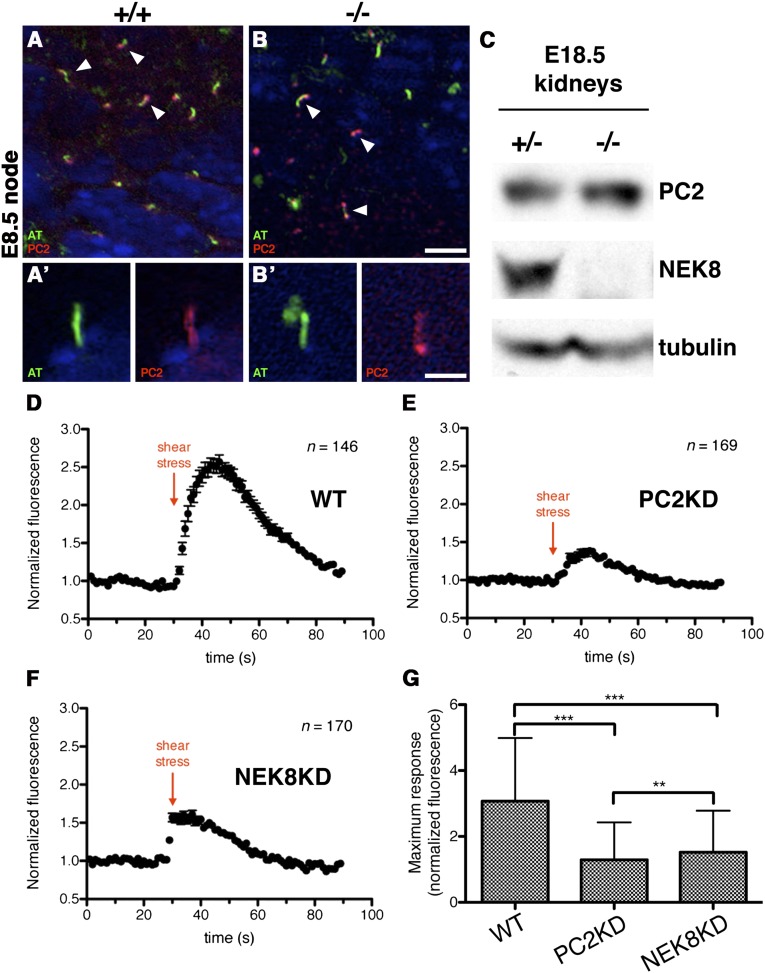
The Nek8−/− phenotypes are remarkably similar to those of Pkd2−/− embryos, including randomization of left-right asymmetry, RPI, cardiac malformations, and glomerular cysts in late-gestation kidneys,21,39 and NEK8 and PC2 have been shown to interact.37 Therefore, we sought to determine whether the Nek8−/− defects are due to loss of PC2 expression or a change in localization. PC2 localizes to all node monocilia19 and we observe its localization is identical in E7.75 wild-type and Nek8−/− embryo nodes (Figure 6, A and B). In addition, we do not detect gross differences in PC2 levels via Western blot between littermatched E18.5 wild-type and Nek8−/− kidney lysates (Figure 6C).
Figure 6.
PC2 is expressed in Nek8-deficient embryos and cells but flow sensing is perturbed. Whole-mount immunofluorescence of E7.75 embryos shows PC2 localization in both wild-type (A) and Nek8−/− nodal cilia (B). (C) Western blot analysis shows that PC2 is expressed at similar levels in E18.5 wild-type and Nek8−/− kidney lysates. (D–G) Effects of fluid shear stress exposed to cultured IMCD cells on PC2-dependant intracellular Ca2+ influx. The representative time traces of GCaMP3 fluorescence intensity (normalized by the initial fluorescence measured before introducing the sheer stress to the cells) are shown for Ca2+-depleted wild-type PC2-knockdown (E) and NEK8-knockdown (N8KD10) IMCD cells (F). The live cell media contains 1.25 mM CaCl2 at the time of the flow stimulation. Error bars represent SEM. (G) The maximum (peak) values of normalized GCaMP3 fluorescence intensity were obtained from multiple single time traces and averaged over the cell population. Error bars represent the SD of multiple single cell measurements. Nonparametric t tests were performed on the selected data sets (***P<0.001, **P<0.01). All of the measurements were carried out under identical conditions. Scale bars, 10 μm in A and B; 5 μm in A’ and B’.
Because PC2 expression/localization is intact in Nek8-null tissues, we hypothesized that PC2 activity may be perturbed in NEK8-deficient cells. To investigate PC2 function, we subjected cultured wild-type and N8KD10 (knockdown) IMCD cells to fluid shear stress and measured changes in PC2-dependent intracellular Ca2+ influx across the membrane using live cell microscopy; PC2 is properly localized in wild-type and N8KD10 cilia under the conditions used in the assay (Supplemental Figure 2). The cells stably express a genetically encoded calcium ion indicator, GCaMP3, used to monitor changes in intracellular Ca2+ levels.48,49 Live fluorescence image time series were acquired while Ca2+ depleted cells were exposed to fluid shear stress. As expected, wild-type IMCD cells exhibit a robust response to fluid shear stress (Figure 6, D and G). PC2-depleted IMCD cells50 were used as a negative control and show significantly reduced fluid flow response (Figure 6, E and G). The N8KD10 cells also have a reduced response to flow, suggesting that PC2 function may be perturbed in the absence of Nek8 (Figure 6, F and G).
Discussion
The Nek8 gene, which is mutated in the jck mouse model of PKD, encodes the only kinase that to date has been localized to the ciliary axoneme. In this study we report that its targeted disruption in the mouse is lethal and causes laterality defects, further demonstrating a link between cystogenesis and left-right asymmetry. Importantly, there is a conserved requirement for the gene in the specification of vertebrate left-right asymmetry as knockdown of Nek8 expression in zebrafish causes randomization of heart looping, a result described both here and by an independent group.51
A small number of genes implicated in human renal cystic diseases cause both PKD and laterality defects when disrupted in the mouse. Three such examples include Pkd2, inversin, and Nphp3; PKD2 is mutated is 15% of human ADPKD cases and mutations in INVS (inversin) and NPHP3 cause NPHP types 2 and 3, respectively.1,52,53 NEK8 has been identified as an interacting partner of each of the mouse protein counterparts: NEK8 and PC2 reciprocally coimmunoprecipitate from kidney and cell lysates,37 and NEK8 has been shown to colocalize with inversin and NHPH3 in the base of the cilium in an inversin-dependent manner.35 The association of NEK8 with each of these proteins is intriguing due to the similarities and some of the differences we identified in the jck and Nek8-null mouse models compared with Pkd2, inversin, and Nphp3 mutants.
With regard to left-right asymmetry, Pkd2−/− embryos die in utero and 90% of the mutants exhibit RPI,39,21,54 whereas a majority of inversin mutants exhibit situs inversus.55–57 The observation that Pkd2−/− early embryo nodes fail to elicit an asymmetric calcium signal in response to flow19 likely accounts for the absence of Nodal and Pitx2 expression in the LPM, causing the high percentage of RPI in mutant embryos. Interestingly, inversin nodal cilia produce a weak net leftward flow much slower than wild type,58 which may cause right-sided Nodal expression,44 more frequently lead to inversion of the body plan, and preclude the development of RPI in the thorax. A majority of Nek8-null embryos exhibit RPI whereas only 24% have situs inversus, and both Nodal and Pitx2 are frequently absent from the left LPM of mutants; thus, Nek8−/− mutants are more similar to Pkd2−/− than to the inversin mutant mice. We determined that Nek8-null phenotypes are not due to loss of PC2, because the protein is expressed and properly localized in mutant embryos, tissues, and cells. Therefore, we utilized a fluid flow assay to measure PC2-dependent mechanosensitive activity in cells lacking NEK8. IMCD cells stably knocked down for Pkd2 or Nek8 exhibit decreased calcium influx in response to shear stress compared with wild-type cells, suggesting PC2 is not fully functional in the absence of NEK8. Given that PC2 is required for both the IMCD cell responses to flow and the calcium gradients detected in the mouse embryo node, we hypothesize that the ability of PC2 to “sense” nodal flow and elicit a left-sided signal is diminished in nodes lacking NEK8. Perhaps NEK8 is a critical component of the PC2 channel complex that is required to mediate the signaling processes downstream of nodal calcium flux that are indispensable for the establishment of appropriate left-right asymmetry.
Similarly to other heterotaxy mutants, Nek8−/− embryos exhibit severe cardiac malformations that include DORV coupled with A/VSD.59 It is not surprising to find such abnormalities in animals with RPI, because aberrant heart development accompanies the inappropriate symmetry in the thoracic cavity. However, we identified cardiac septal defects in mutants with complete situs inversus, a condition that is not lethal if the inverted body plan is appropriately established, suggesting that Nek8 may play a role in cardiac morphogenesis independent of the initial left-right asymmetry defect, as is the case for inversin mutants. It was originally reported that nearly 100% of inversin mutants display situs inversus totalis; therefore, the thoracic situs was “normal reversed” compared with wild-type littermates. However, a later study of inversin homozygotes found that 90% of the pups did have situs inversus, but 10% were identified with situs solitus; furthermore, cardiac malformations including outflow tract and septal defects were found in 37% of the pups, including those few with normal thoracic situs.60 Perhaps Nek8 and inversin are required in the cardiac cilia present in mid-gestation mouse embryos,61 a biologic question best addressed by conditional deletion of the gene.
Our original observations that PKD develops in jck homozygotes, affected animals express the mutant protein, and morpholino knockdown of zebrafish Nek8 causes pronepephric cysts all suggested the jck allele represented a partial loss of function. This hypothesis was consistent with analysis of the Ift88 gene, which initially established the critical link between renal cysts and left-right asymmetry, because the hypomorphic Tg737 allele causes PKD, whereas the null mutation causes laterality defects.26,28 Similarly, the pcy mouse carries a missense mutation in the Nphp3 gene and develops slowly progressive PKD, whereas null mutants die mid-gestation with laterality and severe, complex cardiac defects.52,62
Of note, pcy/Nphp3− compound heterozygous mice are viable and develop severely cystic kidneys by week 12. This intermediate phenotype indicates that the pcy allele of the gene is hypomorphic compared with the null allele.62 We crossed the Nek8-null allele with jck carriers anticipating a similar result; that is, compound heterozygous mice would have a PKD phenotype more severe than that of jck homozygous mice. On the contrary, jck/Nek8− compound heterozygotes develop less severe PKD than the homozygous jck mutants. The null pups die shortly after birth and the kidneys do have glomerular cysts and dilated proximal tubules, but the defect is likely due to impaired glomerular function rather than early signs of NEK8-dependent PKD. Supporting this notion is that jck mutant glomeruli and proximal tubules do not develop cysts; it is the distal DBA-positive collecting ducts that are affected.36,37,63 Therefore, we utilized embryonic kidney explant culture to further explore the cystic potential of the mutant kidneys.46 This in vitro system recapitulates what we observe in the mice: Nek8−/− kidneys do not develop tubular cysts, whereas jck/jck homozygous kidneys are dramatically affected and jck/− kidneys have an intermediate phenotype.
The data therefore suggest that the jck allele encodes a gain-of-function protein, a hypothesis not initially considered because jck/+ adults do not exhibit PKD. However, Natoli et al. performed explant cultures with kidneys harvested a day earlier than in our study, and observed a significant difference between wild-type and jck/+ kidneys, reporting cyst percentages of 2.5% for wild-type and 7.5% for jck/+ compared with 15% for jck/jck kidneys.47 In the adult jck/+ kidney, perhaps the presence of wild-type NEK8 is protective against the deleterious effect of the mutant protein. It is currently unclear how the jck allele may result in a gain-of-function protein. A recent study demonstrated that wild-type NEK8 and the jck-equivalent NEK8G442V variant were similarly active in in vitro kinase assays toward β-casein and histone, but it remains unknown whether NEK8G442V exhibits altered protein interactions and/or substrate specificity in vivo that would affect its function.64 Another potential consequence of the mutation is the mislocalization of jck-specific NEK8 proteins; both Smith et al. and Zalli et al. suggest the mutant NEK8 fails to localize to cilia and centrioles in cells cultured from jck/jck kidneys and RPE cells, respectively.36,64 However, Sohara et al. demonstrate that in sections of intact jck homozygous kidneys, ciliary expression of the mutant protein is detected along the entire axoneme, and is not confined to the proximal region in which it is normally localized.37 Further studies are therefore required to understand the consequences of the jck missense mutation.
The fact that jck homozygotes do not exhibit laterality defects suggests that the missense mutation does not abrogate PC2 activity. However, the PKD phenotype of jck mice may still be a consequence of PC2 dysregulation, because PC2 is upregulated and mislocalized in jck cystic tissues.37 Similar to loss of Pkd2, its transgenic overexpression in the kidney causes cysts65; perhaps the excess PC2 in jck kidneys underlies the progressive cystogenesis we observe in these mice. Overall, our data provide in vivo support for the importance of the biochemical interaction identified between NEK8 and PC2 and compel additional studies to determine the nature of the relationship between these two proteins, both of which are critical for the establishment of left-right asymmetry and are required later in life for the maintenance of renal epithelial integrity.
Concise Methods
Generation of the Nek8:lacZ Allele, Mouse Husbandry, and Genotyping
EL350 E. coli were transformed with a C57BL/6 (B6) BAC (RP23–163A8) containing the Nek8 genomic locus and exon 3 was targeted with an IRES-lacZ cassette via gap repair.66 Nek8:lacZ was electroporated into B6/129 hybrid embryonic stem cells38,67 and a positive clone was injected at the Brigham and Women’s Hospital Transgenic Core. Chimeric males were crossed to B6 females and germline transmission of the Nek8:lacZ allele was confirmed by PCR. Male and female Nek8:lacZ carriers were intercrossed and homozygotes were not present at weaning. Matings were set, embryos were harvested at various gestational time points, and tails were used for genotyping to confirm homozygous offspring. A three-primer genotyping strategy was utilized as follows: a forward primer that anneals to both wild-type and mutant loci 5′-GACACCATTAGGCGCCTTCC-3′ was combined with a wild-type–specific reverse primer 5′-CTTCTCAAAGCAGGCCTTGG-3′ and a mutant-specific reverse primer 5′-GGGGATCCATATTATCATCG-3′. Maintenance and genotyping for the jck mouse line has been described previously,11 and intercrosses were performed between jck/+ females and Nek8+/− males to obtain compound heterozygotes. All animals were housed in accordance with Harvard Medical School Animal Resources and Comparative Medicine regulations.
Zebrafish Morpholino Injection and Phenotype Analyses
For Nek8 morpholino knockdowns, wild-type strain TU-AB zebrafish embryos were injected at the one- to four-cell stage with 4.6 nl of a 0.125 mM, 0.25 mM, or 0.5mM solution of a Nek8 ATG translation blocking oligo: 5′-CTTCTCATACTTCTCCATGTTTTCG-3′. Heart looping was determined at 29 hours postfertilization. Uninjected embryos or embryos injected with a scrambled control morpholino 5′-CCTCTTACCTCAGTTACAATTTATA-3′ exhibited normal development.
Cell Lines, RT-PCR, and Western Blot Analyses
MEFs were derived from E14.5 embryos using standard procedures67 and subsequently immortalized via retroviral infection of the large T antigen.68 Transcript analysis was performed on RNA isolated with Trizol (Invitrogen) from wild-type and mutant MEF lines; Superscript One-step RT-PCR (Invitrogen) was used to amplify transcripts with primers specific to Nek8 exon 2 (5′-CAACCACCCCAACGTCATCG-3′) and exon 4 (5′-GCTACTCTTCTGGTTGTAGGG-3′).
Nek8 knockdown was performed in IMCD cells50 by lentiviral infection of shRNA-expressing constructs that were generated by annealing complementary primers and cloning into the pLKO-puromycin vector (shRNA target sequences were obtained from the Broad Institute shRNA Consortium). A hairpin targeting nucleotide 1570 (sh1570) conferred the most robust knockdown when analyzed in a single infection; we subsequently isolated clonal lines and screened for the loss of NEK8 expression by Western blot and identified line N8KD10.
For fluid shear stress experiments, stable wild-type, N8KD10, and PC2-deficient IMCD cell lines expressing GCaMP3 were generated using a blasticidin-resistant retroviral vector as previously described.69 GCaMP3-positive cells were grown in MatTek chambers (35-mm petri dishes with a bottom coverslip insert) to 40%–50% confluency before serum starvation (0.5% FBS) (MatTek, No. 1.5). Cells were serum starved for 96 hours before fluid shear stress experiments in order to reach their highest frequency of ciliation.
For Western blot analyses, cell and tissue lysates were subjected to SDS-PAGE. Blots were incubated with primary antibodies overnight at 4°C at the following concentrations: anti-NEK8 (1:500011), anti-PC2 (1:100070), and anti-tubulin (1:500; Sigma).
MRI
A multi-channel 7.0-T MRI scanner (Varian Inc.) with a 6-cm inner bore diameter insert gradient set was used to acquire anatomic images of E18.5 mouse embryos. Before imaging, samples were immersed in 2 mM ProHance in PBS (gadoteridol; Bracco Diagnostics Inc.) for a week, and were then placed into 2 mM ProHance in low-melting-point agarose (Fisher). Parameters used in the scans were optimized for contrast within the mature mouse embryo as follows: a T2-weighted, three-dimensional fast spin-echo sequence, with repetition time/echo time (TR/TE) of 325/30 milliseconds, eight averages, field of view of 14×14×25 mm, and matrix size of 348×348×624, giving an image with 40 μm isotropic voxels.
Histology and Immunofluorescence
Embryos and kidneys were fixed in Bouin’s solution and were embedded in paraffin, and 6-μm sections were cut for hematoxylin and eosin (H&E) staining. Kidneys were fixed in 4% paraformaldehyde and were embedded in paraffin, and 8-μm sections were cut for PAS staining and immunofluorescence/lectin procedures. PAS staining (Sigma) was performed according to the manufacturer’s instructions. For immunofluorescence and lectin staining, slides were subjected to citrate buffer antigen retrieval and incubated with antiacetylated tubulin antibody (Sigma) at a 1:10,000 dilution combined with either FITC-conjugated LTL or DBA (Vector Labs) at 4°C overnight. Goat anti-mouse Alexa Fluor 594 (Invitrogen) was used to visualize the tubulin and slides were mounted with Vectashield (Vector Labs).
Whole-mount immunofluorescence was performed on E7.75–E8.0 embryos with anti-NEK8 (1:500) or anti-PC2 (1:100071) antibodies overnight at 4°C. Embryos were then incubated with antiacetylated tubulin for 1 hour at room temperature followed by goat anti-rabbit Alexa Fluor 488 and anti-mouse Alexa Fluor 647 secondary antibodies (Invitrogen). Embryos were mounted under individual coverslips with Vectashield (Vector Labs) and processed for genotyping after imaging.
For cilia length analysis, IMCD cells and MEFs were plated on glass coverslips (VWR Scientific); at approximately 30% confluence, the cells were serum-deprived for 48 hours before incubation with antiacetylated tubulin and Alexa Fluor 647. A nonparametric ANOVA test was carried out to compare the cilium length distributions obtained.
Whole-Mount In Situ Hybridization and Fixed Tissue Genotyping
E8.0–E8.5 embryos were harvested and fixed overnight in 4% paraformaldehyde at 4°C, and were then dehydrated through a graded methanol series. For hybridization, embryos were rehydrated and taken through the whole-mount in situ procedure described by Wilkinson.72 Embryos were hybridized with digoxigenin-labeled mPitx2c73 and nodal44 probes overnight at 65°C, incubated with antidigoxigenin antibody (Roche) conjugated with alkaline phosphatase and probes were visualized with BM purple substrate (Roche). Whole litters were processed simultaneously; after images were obtained, DNA was prepared from the embryos for genotyping. The forward primer is the same as described above, whereas the reverse primers wild-type 5′-CAGAGCCAGCAGGATCTGCAC-3′ and mutant 5′-CGGCTTCGGCCAGTAACGTTAG-3′ efficiently amplify DNA target sequences from fixed tissue.
Renal Explant Culture
Renal explant culture was performed as described by Natoli et al.47 Kidneys were harvested from E14.5 embryos and transferred to Corning transwell filters suspended in DMEM/F12/10% FBS. At 24 hours, media were supplemented with either DMSO or 100 μM 8-bromo-cAMP (Sigma) and changed daily for 4 days. Cyst percentage was calculated from images by obtaining the total area of the cysts divided by the area of the kidneys, as determined using ImageJ software and data were analyzed with Prism software. The SEM was calculated from the cyst percentages of pooled data from at least four independent experiments (n=10 kidneys per genotype), and the P values were determined using the Bonferroni multiple comparison test.
Microscopy and Imaging
Whole-mount embryos and renal explants were analyzed on a Leica DM125 dissecting microscope and images were captured with Leica FireCam software. Histologic and immunofluorescent experiments were analyzed on a Zeiss Imager.Z1 microscope and images were captured with AxioVision software; immunofluorescence slides were imaged with the ApoTome engaged for better resolution of cilia and nuclei.
Fluid Shear Stress Experiments and Statistical Analyses
GCaMP3-positive IMCD cells were introduced to CO2 independent Hanks balanced salt solution media supplemented with HEPES (25 mM final concentration), nonessential amino acids, sodium pyruvate, glucose and GlutaMAX. Intracellular calcium stores were purged by challenging cells with 2 mM EGTA and 100 µM ATP before Ca2+ influx measurements. Cells were introduced back to EGTA-free Hanks balanced salt solution–based media and the cell chambers were mounted on the stage of a Nikon Eclipse Ti inverted microscope equipped with a Nikon Plan Apo 20×A 0.75 NA objective lens and a CoolSnap-HQ (Photometrics). The Nikon FITC cube was used to efficiently reflect 488 nm wavelength and pass the emission wavelengths into the charge-coupled device camera detection channel. While fluorescence image time series were acquired, cells were challenged with fluid shear stress via the controlled addition of Ca2+ media. The acquisition settings were kept constant for all samples so that valid comparisons could be made between measurements from different data sets. Acquisition parameters were set within the linear range of the charge-coupled device camera detection.
A custom MATLAB (The MathWorks Inc) subroutine was written to analyze acquired image time series. The mean intensity of the background noise was calculated from empty dark regions in the images and each frame was individually corrected. Regions of interest (ROIs) from individual cells were chosen for the subsequent analysis. The mean intensity values measured from the first 30 frames (before exposing cells to fluid shear stress) of acquired image time series defined ROIs and used to normalize the values of fluorescence intensity to unity. The SDs of the recovered mean values were obtained from the analysis of multiple ROIs in multiple independent experiments.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank A. Kumar and M. Keuhn for the nodal in situ hybridization probe and N. Kurpios and C. Tabin for the mPitx2c in situ hybridization probe. PC2 antibodies were provided by G. Pazour and T. Benjamin. We thank R. Bronson at the Harvard Medical School Rodent Histopathology Core for analysis of cardiac serial sections and W. Pu for MRI assistance.
Support for this work was provided by grants from the National Institutes of Health to J.V.S. (NIH P50 DK074030 and Genzyme GRIP), I.D. (NIH R01 DK053093), and D.R.B. (NIH R01 DK66370), as well as grants from the PKD Foundation and the Harvard Center for PKD Research (NIH P50 DK074030).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012050490/-/DCSupplemental.
References
- 1.Wilson PD: Mouse models of polycystic kidney disease. Curr Top Dev Biol 84: 311–350, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Parnell SC, Magenheimer BS, Maser RL, Rankin CA, Smine A, Okamoto T, Calvet JP: The polycystic kidney disease-1 protein, polycystin-1, binds and activates heterotrimeric G-proteins in vitro. Biochem Biophys Res Commun 251: 625–631, 1998 [DOI] [PubMed] [Google Scholar]
- 3.González-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF: Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci U S A 98: 1182–1187, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vassilev PM, Guo L, Chen XZ, Segal Y, Peng JB, Basora N, Babakhanlou H, Cruger G, Kanazirska M, Ye Cp, Brown EM, Hediger MA, Zhou J: Polycystin-2 is a novel cation channel implicated in defective intracellular Ca(2+) homeostasis in polycystic kidney disease. Biochem Biophys Res Commun 282: 341–350, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Delmas P, Nomura H, Li X, Lakkis M, Luo Y, Segal Y, Fernández-Fernández JM, Harris P, Frischauf AM, Brown DA, Zhou J: Constitutive activation of G-proteins by polycystin-1 is antagonized by polycystin-2. J Biol Chem 277: 11276–11283, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S: Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol 4: 191–197, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Zhou J: Polycystins and primary cilia: Primers for cell cycle progression. Annu Rev Physiol 71: 83–113, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Hildebrandt F, Attanasio M, Otto E: Nephronophthisis: Disease mechanisms of a ciliopathy. J Am Soc Nephrol 20: 23–35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simms RJ, Hynes AM, Eley L, Sayer JA: Nephronophthisis: A genetically diverse ciliopathy. Int J Nephrol 2011: 527137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benzing T, Schermer B: Clinical spectrum and pathogenesis of nephronophthisis. Curr Opin Nephrol Hypertens 21: 272–278, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Lu W, Obara T, Kuida S, Lehoczky J, Dewar K, Drummond IA, Beier DR: A defect in a novel Nek-family kinase causes cystic kidney disease in the mouse and in zebrafish. Development 129: 5839–5846, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Osmani SA, Pu RT, Morris NR: Mitotic induction and maintenance by overexpression of a G2-specific gene that encodes a potential protein kinase. Cell 53: 237–244, 1988 [DOI] [PubMed] [Google Scholar]
- 13.Nishimoto T, Eilen E, Basilico C: Premature of chromosome condensation in a ts DNA- mutant of BHK cells. Cell 15: 475–483, 1978 [DOI] [PubMed] [Google Scholar]
- 14.Bischoff FR, Ponstingl H: Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature 354: 80–82, 1991 [DOI] [PubMed] [Google Scholar]
- 15.O’Connell MJ, Krien MJ, Hunter T: Never say never. The NIMA-related protein kinases in mitotic control. Trends Cell Biol 13: 221–228, 2003 [DOI] [PubMed] [Google Scholar]
- 16.McCooke JK, Appels R, Barrero RA, Ding A, Ozimek-Kulik JE, Bellgard MI, Morahan G, Phillips JK: A novel mutation causing nephronophthisis in the Lewis polycystic kidney rat localises to a conserved RCC1 domain in Nek8. BMC Genomics 13: 393, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otto EA, Trapp ML, Schultheiss UT, Helou J, Quarmby LM, Hildebrandt F: NEK8 mutations affect ciliary and centrosomal localization and may cause nephronophthisis. J Am Soc Nephrol 19: 587–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N: Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95: 829–837, 1998 [DOI] [PubMed] [Google Scholar]
- 19.McGrath J, Somlo S, Makova S, Tian X, Brueckner M: Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 114: 61–73, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Wu G, D’Agati V, Cai Y, Markowitz G, Park JH, Reynolds DM, Maeda Y, Le TC, Hou H, Jr, Kucherlapati R, Edelmann W, Somlo S: Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell 93: 177–188, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Pennekamp P, Karcher C, Fischer A, Schweickert A, Skryabin B, Horst J, Blum M, Dworniczak B: The ion channel polycystin-2 is required for left-right axis determination in mice. Curr Biol 12: 938–943, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J: Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Field S, Riley KL, Grimes DT, Hilton H, Simon M, Powles-Glover N, Siggers P, Bogani D, Greenfield A, Norris DP: Pkd1l1 establishes left-right asymmetry and physically interacts with Pkd2. Development 138: 1131–1142, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamura K, Kobayashi D, Uehara Y, Koshida S, Iijima N, Kudo A, Yokoyama T, Takeda H: Pkd1l1 complexes with Pkd2 on motile cilia and functions to establish the left-right axis. Development 138: 1121–1129, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P: Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci U S A 100: 5286–5291, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoder BK, Richards WG, Sweeney WE, Wilkinson JE, Avener ED, Woychik RP: Insertional mutagenesis and molecular analysis of a new gene associated with polycystic kidney disease. Proc Assoc Am Physicians 107: 314–323, 1995 [PubMed] [Google Scholar]
- 27.Takeda S, Yonekawa Y, Tanaka Y, Okada Y, Nonaka S, Hirokawa N: Left-right asymmetry and kinesin superfamily protein KIF3A: New insights in determination of laterality and mesoderm induction by kif3A-/- mice analysis. J Cell Biol 145: 825–836, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murcia NS, Richards WG, Yoder BK, Mucenski ML, Dunlap JR, Woychik RP: The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development 127: 2347–2355, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Kitamura K, Miura H, Miyagawa-Tomita S, Yanazawa M, Katoh-Fukui Y, Suzuki R, Ohuchi H, Suehiro A, Motegi Y, Nakahara Y, Kondo S, Yokoyama M: Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra- and periocular mesoderm and right pulmonary isomerism. Development 126: 5749–5758, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Gage PJ, Suh H, Camper SA: Dosage requirement of Pitx2 for development of multiple organs. Development 126: 4643–4651, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Lowe LA, Yamada S, Kuehn MR: Genetic dissection of nodal function in patterning the mouse embryo. Development 128: 1831–1843, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Kumar A, Lualdi M, Lewandoski M, Kuehn MR: Broad mesodermal and endodermal deletion of Nodal at postgastrulation stages results solely in left/right axial defects. Dev Dyn 237: 3591–3601, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoder BK, Hou X, Guay-Woodford LM: The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13: 2508–2516, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Mahjoub MR, Trapp ML, Quarmby LM: NIMA-related kinases defective in murine models of polycystic kidney diseases localize to primary cilia and centrosomes. J Am Soc Nephrol 16: 3485–3489, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Shiba D, Manning DK, Koga H, Beier DR, Yokoyama T: Inv acts as a molecular anchor for Nphp3 and Nek8 in the proximal segment of primary cilia. Cytoskeleton (Hoboken) 67: 112–119, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith LA, Bukanov NO, Husson H, Russo RJ, Barry TC, Taylor AL, Beier DR, Ibraghimov-Beskrovnaya O: Development of polycystic kidney disease in juvenile cystic kidney mice: Insights into pathogenesis, ciliary abnormalities, and common features with human disease. J Am Soc Nephrol 17: 2821–2831, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Sohara E, Luo Y, Zhang J, Manning DK, Beier DR, Zhou J: Nek8 regulates the expression and localization of polycystin-1 and polycystin-2. J Am Soc Nephrol 19: 469–476, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.George SH, Gertsenstein M, Vintersten K, Korets-Smith E, Murphy J, Stevens ME, Haigh JJ, Nagy A: Developmental and adult phenotyping directly from mutant embryonic stem cells. Proc Natl Acad Sci U S A 104: 4455–4460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu G, Markowitz GS, Li L, D’Agati VD, Factor SM, Geng L, Tibara S, Tuchman J, Cai Y, Park JH, van Adelsberg J, Hou H, Jr, Kucherlapati R, Edelmann W, Somlo S: Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat Genet 24: 75–78, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Tan SY, Rosenthal J, Zhao XQ, Francis RJ, Chatterjee B, Sabol SL, Linask KL, Bracero L, Connelly PS, Daniels MP, Yu Q, Omran H, Leatherbury L, Lo CW: Heterotaxy and complex structural heart defects in a mutant mouse model of primary ciliary dyskinesia. J Clin Invest 117: 3742–3752, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aune CN, Chatterjee B, Zhao XQ, Francis R, Bracero L, Yu Q, Rosenthal J, Leatherbury L, Lo CW: Mouse model of heterotaxy with single ventricle spectrum of cardiac anomalies. Pediatr Res 63: 9–14, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Morgan D, Turnpenny L, Goodship J, Dai W, Majumder K, Matthews L, Gardner A, Schuster G, Vien L, Harrison W, Elder FF, Penman-Splitt M, Overbeek P, Strachan T: Inversin, a novel gene in the vertebrate left-right axis pathway, is partially deleted in the inv mouse. Nat Genet 20: 149–156, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Collignon J, Varlet I, Robertson EJ: Relationship between asymmetric nodal expression and the direction of embryonic turning. Nature 381: 155–158, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Lowe LA, Supp DM, Sampath K, Yokoyama T, Wright CV, Potter SS, Overbeek P, Kuehn MR: Conserved left-right asymmetry of nodal expression and alterations in murine situs inversus. Nature 381: 158–161, 1996 [DOI] [PubMed] [Google Scholar]
- 45.Yoshioka H, Meno C, Koshiba K, Sugihara M, Itoh H, Ishimaru Y, Inoue T, Ohuchi H, Semina EV, Murray JC, Hamada H, Noji S: Pitx2, a bicoid-type homeobox gene, is involved in a lefty-signaling pathway in determination of left-right asymmetry. Cell 94: 299–305, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Magenheimer BS, St John PL, Isom KS, Abrahamson DR, De Lisle RC, Wallace DP, Maser RL, Grantham JJ, Calvet JP: Early embryonic renal tubules of wild-type and polycystic kidney disease kidneys respond to cAMP stimulation with cystic fibrosis transmembrane conductance regulator/Na(+),K(+),2Cl(-) co-transporter-dependent cystic dilation. J Am Soc Nephrol 17: 3424–3437, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Natoli TA, Gareski TC, Dackowski WR, Smith L, Bukanov NO, Russo RJ, Husson H, Matthews D, Piepenhagen P, Ibraghimov-Beskrovnaya O: Pkd1 and Nek8 mutations affect cell-cell adhesion and cilia in cysts formed in kidney organ cultures. Am J Physiol Renal Physiol 294: F73–F83, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Pologruto TA, Yasuda R, Svoboda K: Monitoring neural activity and [Ca2+] with genetically encoded Ca2+ indicators. J Neurosci 24: 9572–9579, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, Bargmann CI, Jayaraman V, Svoboda K, Looger LL: Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods 6: 875–881, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Besschetnova TY, Kolpakova-Hart E, Guan Y, Zhou J, Olsen BR, Shah JV: Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation. Curr Biol 20: 182–187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fukui H, Shiba D, Asakawa K, Kawakami K, Yokoyama T: The ciliary protein Nek8/Nphp9 acts downstream of Inv/Nphp2 during pronephros morphogenesis and left-right establishment in zebrafish. FEBS Lett 586: 2273–2279, 2012 [DOI] [PubMed] [Google Scholar]
- 52.Olbrich H, Fliegauf M, Hoefele J, Kispert A, Otto E, Volz A, Wolf MT, Sasmaz G, Trauer U, Reinhardt R, Sudbrak R, Antignac C, Gretz N, Walz G, Schermer B, Benzing T, Hildebrandt F, Omran H: Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis. Nat Genet 34: 455–459, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Otto EA, Schermer B, Obara T, O’Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, Foreman JW, Goodship JA, Strachan T, Kispert A, Wolf MT, Gagnadoux MF, Nivet H, Antignac C, Walz G, Drummond IA, Benzing T, Hildebrandt F: Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet 34: 413–420, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim I, Ding T, Fu Y, Li C, Cui L, Li A, Lian P, Liang D, Wang DW, Guo C, Ma J, Zhao P, Coffey RJ, Zhan Q, Wu G: Conditional mutation of Pkd2 causes cystogenesis and upregulates beta-catenin. J Am Soc Nephrol 20: 2556–2569, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yokoyama T, Copeland NG, Jenkins NA, Montgomery CA, Elder FF, Overbeek PA: Reversal of left-right asymmetry: A situs inversus mutation. Science 260: 679–682, 1993 [DOI] [PubMed] [Google Scholar]
- 56.Morishima M, Yasui H, Nakazawa M, Ando M, Ishibashi M, Takao A: Situs variation and cardiovascular anomalies in the transgenic mouse insertional mutation, inv. Teratology 57: 302–309, 1998 [DOI] [PubMed] [Google Scholar]
- 57.Mochizuki T, Saijoh Y, Tsuchiya K, Shirayoshi Y, Takai S, Taya C, Yonekawa H, Yamada K, Nihei H, Nakatsuji N, Overbeek PA, Hamada H, Yokoyama T: Cloning of inv, a gene that controls left/right asymmetry and kidney development. Nature 395: 177–181, 1998 [DOI] [PubMed] [Google Scholar]
- 58.Okada Y, Nonaka S, Tanaka Y, Saijoh Y, Hamada H, Hirokawa N: Abnormal nodal flow precedes situs inversus in iv and inv mice. Mol Cell 4: 459–468, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Basu B, Brueckner M: Cilia multifunctional organelles at the center of vertebrate left-right asymmetry. Curr Top Dev Biol 85: 151–174, 2008 [DOI] [PubMed] [Google Scholar]
- 60.McQuinn TC, Miga DE, Mjaatvedt CH, Phelps AL, Wessels A: Cardiopulmonary malformations in the inv/inv mouse. Anat Rec 263: 62–71, 2001 [DOI] [PubMed] [Google Scholar]
- 61.Slough J, Cooney L, Brueckner M: Monocilia in the embryonic mouse heart suggest a direct role for cilia in cardiac morphogenesis. Dev Dyn 237: 2304–2314, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bergmann C, Fliegauf M, Brüchle NO, Frank V, Olbrich H, Kirschner J, Schermer B, Schmedding I, Kispert A, Kränzlin B, Nürnberg G, Becker C, Grimm T, Girschick G, Lynch SA, Kelehan P, Senderek J, Neuhaus TJ, Stallmach T, Zentgraf H, Nürnberg P, Gretz N, Lo C, Lienkamp S, Schäfer T, Walz G, Benzing T, Zerres K, Omran H: Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet 82: 959–970, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trapp ML, Galtseva A, Manning DK, Beier DR, Rosenblum ND, Quarmby LM: Defects in ciliary localization of Nek8 is associated with cystogenesis. Pediatr Nephrol 23: 377–387, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zalli D, Bayliss R, Fry AM: The Nek8 protein kinase, mutated in the human cystic kidney disease nephronophthisis, is both activated and degraded during ciliogenesis. Hum Mol Genet 21: 1155–1171, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park EY, Sung YH, Yang MH, Noh JY, Park SY, Lee TY, Yook YJ, Yoo KH, Roh KJ, Kim I, Hwang YH, Oh GT, Seong JK, Ahn C, Lee HW, Park JH: Cyst formation in kidney via B-Raf signaling in the PKD2 transgenic mice. J Biol Chem 284: 7214–7222, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu P, Jenkins NA, Copeland NG: A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res 13: 476–484, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joyner AL: Gene Targeting: A Practical Approach Oxford, UK, Oxford University Press, 2000
- 68.Hahn WC, Dessain SK, Brooks MW, King JE, Elenbaas B, Sabatini DM, DeCaprio JA, Weinberg RA: Enumeration of the simian virus 40 early region elements necessary for human cell transformation. Mol Cell Biol 22: 2111–2123, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Besschetnova TY, Roy B, Shah JV: Imaging intraflagellar transport in mammalian primary cilia. Methods Cell Biol 93: 331–346, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB: Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol 12: R378–R380, 2002 [DOI] [PubMed] [Google Scholar]
- 71.Tian Y, Kolb R, Hong JH, Carroll J, Li D, You J, Bronson R, Yaffe MB, Zhou J, Benjamin T: TAZ promotes PC2 degradation through a SCFbeta-Trcp E3 ligase complex. Mol Cell Biol 27: 6383–6395, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilkinson DG: In Situ Hybridization: A Practical Approach, Oxford, UK, IRL Press, 1992 [Google Scholar]
- 73.Yu X, St Amand TR, Wang S, Li G, Zhang Y, Hu YP, Nguyen L, Qiu MS, Chen YP: Differential expression and functional analysis of Pitx2 isoforms in regulation of heart looping in the chick. Development 128: 1005–1013, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.