Abstract
The mechanisms underlying the insulin resistance that frequently accompanies CKD are poorly understood, but the retention of renally excreted compounds may play a role. One such compound is p-cresyl sulfate (PCS), a protein-bound uremic toxin that originates from tyrosine metabolism by intestinal microbes. Here, we sought to determine whether PCS contributes to CKD-associated insulin resistance. Administering PCS to mice with normal kidney function for 4 weeks triggered insulin resistance, loss of fat mass, and ectopic redistribution of lipid in muscle and liver, mimicking features associated with CKD. Mice treated with PCS exhibited altered insulin signaling in skeletal muscle through ERK1/2 activation. In addition, exposing C2C12 myotubes to concentrations of PCS observed in CKD caused insulin resistance through direct activation of ERK1/2. Subtotal nephrectomy led to insulin resistance and dyslipidemia in mice, and treatment with the prebiotic arabino-xylo-oligosaccharide, which reduced serum PCS by decreasing intestinal production of p-cresol, prevented these metabolic derangements. Taken together, these data suggest that PCS contributes to insulin resistance and that targeting PCS may be a therapeutic strategy in CKD.
The uremic syndrome is attributed to the progressive retention of numerous compounds, which in healthy individuals are normally excreted by the kidneys. At least 90 compounds, often referred to as uremic toxins, were described to accumulate in ESRD1 and to be harmful for biologic systems. In recent years, the dialysis community has paid great attention to improve clearance of water-soluble molecules, such as urea. Unfortunately, several studies, including the HEMO study in hemodialysis,2 and the ADEMEX study (Adequacy of Peritoneal Dialysis in Mexico),3 failed to significantly improve patient outcome. Protein-bound uremic toxins especially exert major toxic effects because of poor removal by the common dialysis techniques.4,5 p-Cresol is the mother compound of an important group of protein-bound retention solutes.6 It is produced in the gut from the metabolism of aromatic amino acids by putrefactive bacteria of the gut microbiota.7,8 As it crosses through the intestinal mucosa, p-cresol is then metabolized by a cytoplasmic sulfotransferase and therefore mainly circulates in blood as its sulfate conjugate, p-cresyl sulfate (PCS) (Supplemental Figure 1). PCS is excreted by the kidney mainly through proximal tubular secretion and therefore accumulates in serum of patients with renal dysfunction. Of note, p-cresol/PCS has been shown to be independently associated with mortality and cardiovascular disease in patients with CKD.9–11 There are now accumulating in vitro data on the harmful effects of p-cresol/PCS and related protein-bound retention solutes.7,9,12–15
CKD is associated with a large range of metabolic alterations.16 As established by the pioneering work of DeFronzo et al., insulin resistance is a well documented feature of CKD.17,18 Among patients with ESRD, insulin resistance is associated with higher prevalence of vascular diseases.19 Although the causes of CKD-associated insulin resistance remain poorly identified,20 several abnormalities associated with renal dysfunction were reported to interfere with insulin signaling.21,22 The exact mechanisms underlying insulin resistance in patients with CKD have not been clearly elucidated, but growing evidence suggests that the progressive retention of a large number of compounds, which under normal conditions are excreted by the healthy kidneys, could play a key role. In a recent study, D’Apolito et al.23 demonstrated that increased urea levels in CKD could induce insulin resistance. However, to date no study has ruled out the role of protein-bound uremic toxins in the development of insulin resistance and metabolic disturbances in CKD. We hypothesized that increased concentrations of PCS associated with CKD might drive insulin resistance and metabolic disturbances associated with CKD. In this study, we demonstrate that PCS administrated to mice with normal renal function induces insulin resistance and metabolic disturbances mimicking those reported in CKD. We further show that the reduction of p-cresol intestinal production (and thus plasma PCS) by the use of a prebiotic (arabino-xylo-oligosaccharide [AXOS]) prevented insulin resistance and dyslipidemia in a mouse model of CKD.
Results
Serum Concentration of PCS in PCS-Treated Mice
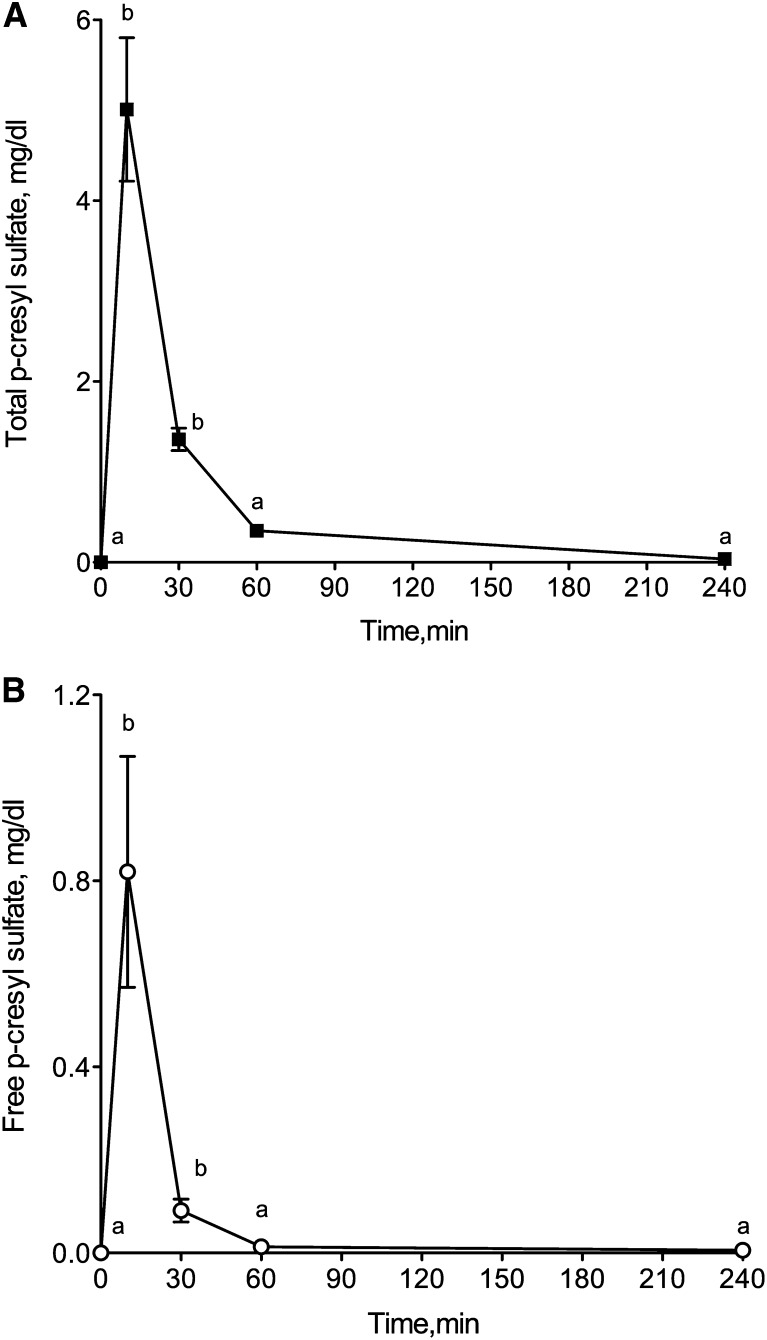
Intraperitoneal injection of PCS (10 mg/kg) in mice with normal renal function induced a transient increase in serum PCS concentration. Serum total concentration of PCS reached 5.01±1.59 mg/dl 10 minutes after injection and decreased to 0.35±0.04 mg/dl 1 hour after injection (Figure 1A). The mice exhibited free PCS serum levels of 0.82±0.24 mg/dl at 10 minutes and 0.01±0.03 mg/dl at 1 hour (Figure 1B). Four hours after injection, serum PCS concentration returned to baseline level. The peak concentrations were in the range of concentrations found in sera from patients with ESRD (2–4 mg/dl).24 In vehicle-treated mice, total PCS level was 0.03±0.01 mg/dl and free PCS remained undetectable. After 4 weeks of chronic administration of PCS, no accumulation was noticed (Supplemental Table 1). In mice, the in vivo plasma protein binding of PCS was 90.5%±2.0% (Supplemental Table 2).
Figure 1.
Intraperitoneal injection of PCS (10 mg/kg) transiently increases plasma PCS concentration in mice with normal renal function. Serum concentrations of (A) total and (B) free PCS. Results are expressed as mean ± SEM for n=4 mice. Different letters between time points indicate a significant difference at the P<0.05 level.
PCS Mice Exhibit Hyperglycemia and Hypercholesterolemia
To evaluate the in vivo activity of PCS, mice were given daily injections of PCS (10 mg/kg, twice a day) or vehicle for 4 weeks. Renal function was extensively evaluated in mice treated for 4 weeks with PCS (Table 1 and Supplemental Figure 2). It is worth noting that no difference in renal function or structure was observed between saline- and PCS-treated mice. The fasting plasma glucose concentration in PCS mice was 1.5-fold higher than in vehicle mice (P<0.001) (Table 1), suggesting the presence of insulin resistance. Remarkably, in PCS mice, plasma cholesterol levels were increased by >50% compared with vehicle mice, whereas a nonsignificant trend toward increase was observed for plasma triacylglycerols (Table 1). As shown in Table 1, these metabolic disturbances closely mimic the profile of metabolic changes observed in CKD mice (i.e., hyperglycemia and hypercholesterolemia).
Table 1.
Renal function and metabolic profile in PCS-treated mice
| Variable | Control Mice | PCS Mice | CKD Mice |
|---|---|---|---|
| Mice (n) | 8 | 8 | 5 |
| Diuresis (ml/24 hr) | 0.89±0.14a | 0.95±0.2a | 2.4±0.4b |
| Water intake (ml/24 hr) | 3.2±0.7a | 2.6±0.5a | 8.4±0.6b |
| Proteinuria (mg/24 hr) | 1.07±0.21a | 1.12±0.25a | 7.1±2.8b |
| Urea (mM) | 6.1±0.2a | 5.9±0.3a | 34.1±3.2b |
| Creatinine (µM) | 9.6±1.1a | 10.3±1.2a | ND |
| Triacylglycerols (mg/dl) | 51±5a | 65±7a | 67±3b |
| Total cholesterol (mg/dl) | 98±14a | 152±17b | 170±9c |
| Fasting glucose (mg/dl) | 73±4a | 110±4b | 98±5b |
| MDA (µM) | 8.19±1.01a | 7.76±0.67a | ND |
| MCP-1 (pg/ml) | 21.6±3.1a | 19.2±2.5a | ND |
| TNF-α (pg/ml) | 18.0±2.4a | 16.7±2.9a | ND |
Data are expressed as mean ± SEM. ND, not determined; MDA, malondialdehyde; MCP-1, monocyte chemotactic protein-1. Different letters indicate a significant difference at the P<0.05 level.
Chronic Administration of PCS Induces Insulin Resistance in Mice and Interferes with Insulin Signaling Pathways in Skeletal Muscle
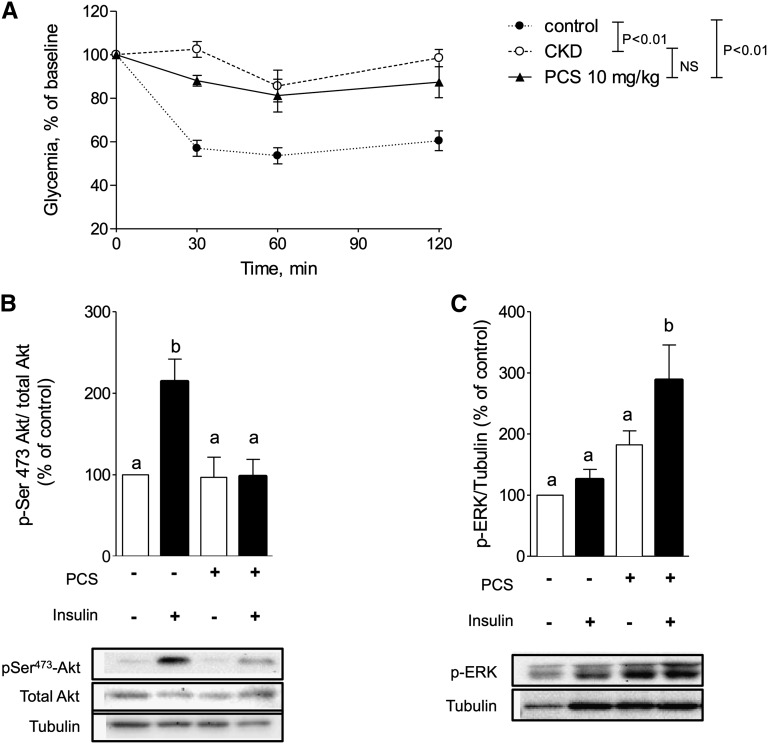
The results of the insulin tolerance test after 4 weeks of PCS administration are shown in Figure 2A. Sixty minutes after injection of PCS, insulin administration (0.5 IU/kg) triggered a significantly (P<0.01) larger hypoglycemic response in vehicle-treated mice (change, −47%) than in PCS-treated mice (change, −19%) or CKD mice (change, −14%). In vehicle-treated mice, insulin stimulation induced a 3.2-fold increase in PKB/Akt phosphorylation on Ser 473 (P<0.01). In contrast, insulin-induced PKB/Akt phosphorylation was impaired in PCS mice (P<0.01), indicating a disruption of the intracellular insulin signaling pathways (Figure 2B). Activation of stress protein kinases, such as c-Jun N-terminal kinase (JNK),25 and extracellular signal-regulated kinase (ERK1/2)26 are described to interfere with insulin signaling pathways; we therefore studied ERK and JNK activation in muscle of PCS-treated mice. PCS-treated mice exhibited a significant activation of ERK1/2 compared with vehicle-treated mice. Indeed, phosphorylation level of ERK1/2 was increased by 2.9-fold (P<0.05) compared with control in the insulin-stimulated condition (Figure 2C). In contrast, we failed to detect any phosphorylation of JNK in skeletal muscle of PCS-treated mice (data not shown). The effects of a single injection of PCS (10 mg/kg) on insulin sensitivity are shown in Supplemental Figure 3.
Figure 2.
PCS induces insulin resistance in mice by interfering with insulin signaling pathways. (A) For the insulin tolerance test, mice were injected intraperitoneally with insulin (0.5 IU/kg) and blood glucose concentration was measured. Data are mean ± SEM for n=5–8 mice. NS, not significant. Baseline glucose concentrations were 76±3, 103±5, and 94±2 mg/dl for saline, PCS, and CKD mice, respectively. (B) Inhibition of insulin-induced phosphorylation of PKB/Akt and (C) activation of phosphorylation of ERK1/2 in gastrocnemius muscle. Mice were stimulated with insulin (0.75 IU/kg intraperitoneally) before euthanasia. Data are mean ± SEM for n=4–6 mice. Different characters indicate a significant difference at P<0.05.
Chronic Treatment with PCS Decreases White Adipose Tissue Accretion and Induces Ectopic Lipid Redistribution
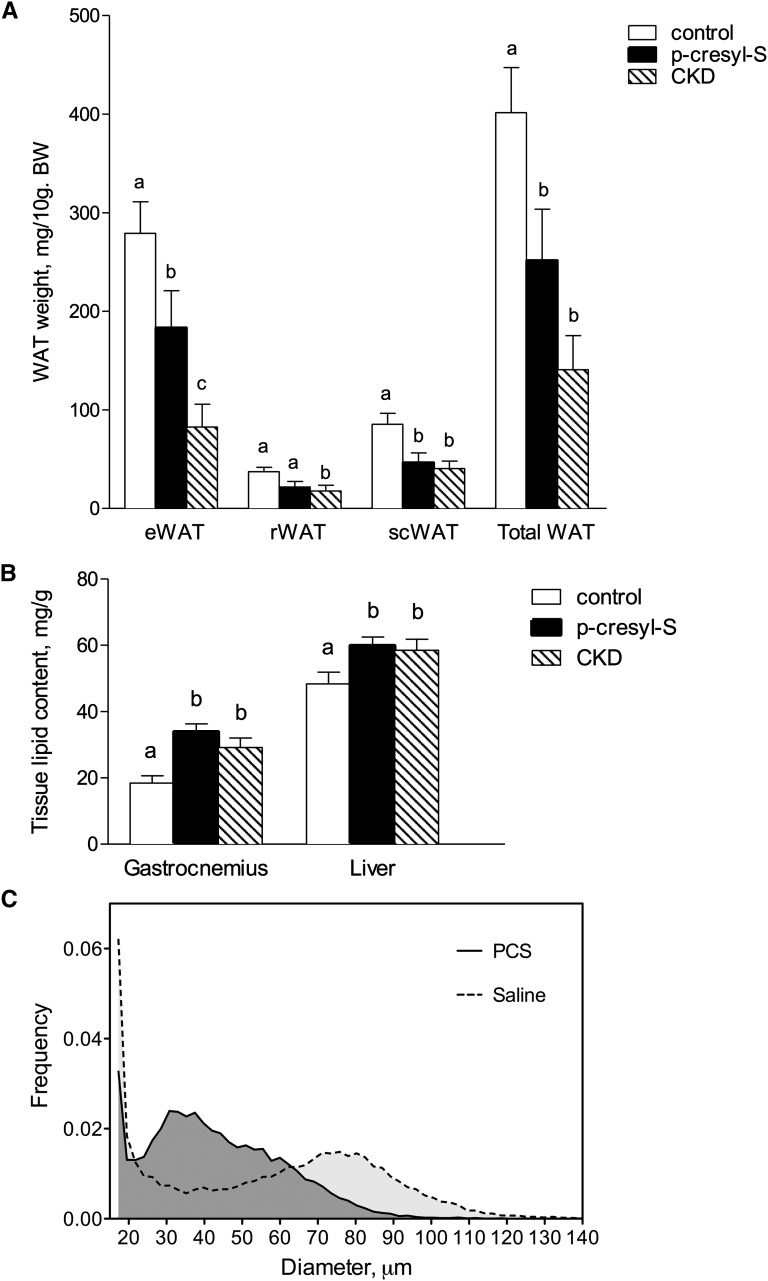
The mean daily food intake was 68.1±1.3 kJ/24 hours for mice treated with PCS and 69.1±2.1 kJ/24 hours in control animals (n=10, nonsignificant). Biometric data for each group are shown in Table 2. Total weight and lean body mass did not significantly differ between PCS mice and control mice after 4 weeks of treatment. PCS mice, however, exhibited a significant decrease in white adipose tissue accretion (change, −51%; P<0.05) (Figure 3A). PCS treatment significantly decreased white adipose tissue mass in the intraabdominal fat pads (e.g., retroperitoneal fat pads) (change, −42%; P<0.05) and the epididymal fat pads (change, −46%; P<0.05), as well as in the subcutaneous inguinal fat pad (change, −46%; P<0.05) (Figure 3A). The effect of PCS was restricted to white adipose tissue because the mass of other organs (liver, heart, and kidney) remained unaltered. On the other hand, ectopic lipid contents were increased in muscle (83% increase; P<0.001) and liver (23% increase; P<0.05) compared with vehicle (Figure 3B), suggesting lipotoxicity as a putative cause for insulin resistance. Of note, a similar loss of fat mass (change, −83%; P<0.05) and ectopic lipid redistribution were found in CKD mice. The distribution frequencies of adipocyte volume for saline- or PCS-treated mice are shown in Figure 3C. A significant shift leftward was observed in frequency distribution of adipocyte volumes of mice treated with PCS compared with control animals. The cellular characteristics of epididymal white adipose tissue in control and PCS-treated mice are shown in Table 3. Mean adipocyte diameter was reduced by 21% in PCS-treated mice (n=8; P<0.005) resulting in a 46% decrease in adipose cell weight (n=8; P<0.01). The total number of adipocytes per parametrial fat pad, calculated from cell weight, failed to show any difference between PCS and control mice. Thus, the reduction of white adipose tissue accretion resulted from a reduction in the volume of adipocytes (i.e., hypotrophia) rather than from a decrease in the total number of adipocytes per fat pad (i.e., hypoplasia).
Table 2.
Biometric data
| Variable | Control Mice | PCS Mice | CKD Mice |
|---|---|---|---|
| Mice (n) | 8 | 8 | 8 |
| Initial body weight (g) | 36±2 | 37±1 | 32±3 |
| Final body weight (g) | 32±1 | 30±1 | 28±1 |
| Body length (cm) | 10.2±0.2 | 10.1±0.1 | 9.7±0.2 |
| Lee index (×103) | 313±5.0 | 309±5.0 | 313±5.5 |
| Liver weight (mg) | 1255±49 | 1047±60 | 1226±92 |
| Heart weight (mg) | 141±7 | 151±5 | 160±12 |
| Kidney weight (mg) | 338±99a | 338±14a | 205±145b |
| Gastrocnemius weight (mg) | 161±5 | 153±8 | 144±5 |
| Total WAT weight (mg) | 1711±135a | 871±202b | 402±98c |
| Total WAT (mg/10 g body weight) | 534±33a | 277±58b | 141±35c |
Data are expressed as mean ± SEM. Lee index was calculated as the cubic square of body weight divided by naso-anal length. WAT, white adipose tissue. Different letters indicate a significant difference at the P<0.05 level.
Figure 3.
Chronic treatment with PCS decreases white adipose tissue accretion in mice and induces ectopic lipid redistribution. (A) Weights of different pads of white adipose tissue (WAT). e, epididymal; r, retroperitoneal; sc, subcutaneous. (B) Tissue lipid content in gastrocnemius and liver. Note ectopic lipid redistribution in liver and gastrocnemius muscle of PCS and CKD mice. (C) Typical frequency distribution of adipocyte diameters in PCS and vehicle-treated mice. Data are mean ± SEM for n=8 mice. Different characters indicate a significant difference at P<0.05.
Table 3.
Cellular characteristics of epididymal white adipose tissue in vehicle- and PCS-treated mice
| Variable | Control Mice | PCS Mice | Change (%) | P Value |
|---|---|---|---|---|
| Mice (n) | 8 | 8 | ||
| Epididymal WAT (mg) | 985±85 | 534±133 | −46 | 0.03 |
| Cell diameter (µm) | 60.7±2,6 | 48.1±2.2 | −21 | 0.003 |
| Cell weight (ng) | 154.1±21.4 | 81.9±11.7 | −47 | 0.01 |
| No. of cells (×106) | 6.85±0.69 | 7.45±1.71 | 9 | 0.76 |
| DNA content (µg/pad) | 67.9±9.5 | 60.1±8.3 | −12 | 0.60 |
Data are mean ± SEM. Data were compared using t test and, when appropriate, Welch correction for variance inhomogeneity. Differences were considered significant at the P<0.05 level. WAT, white adipose tissue.
PCS Inhibits Lipogenesis and Increases Lipolysis in Adipocytes
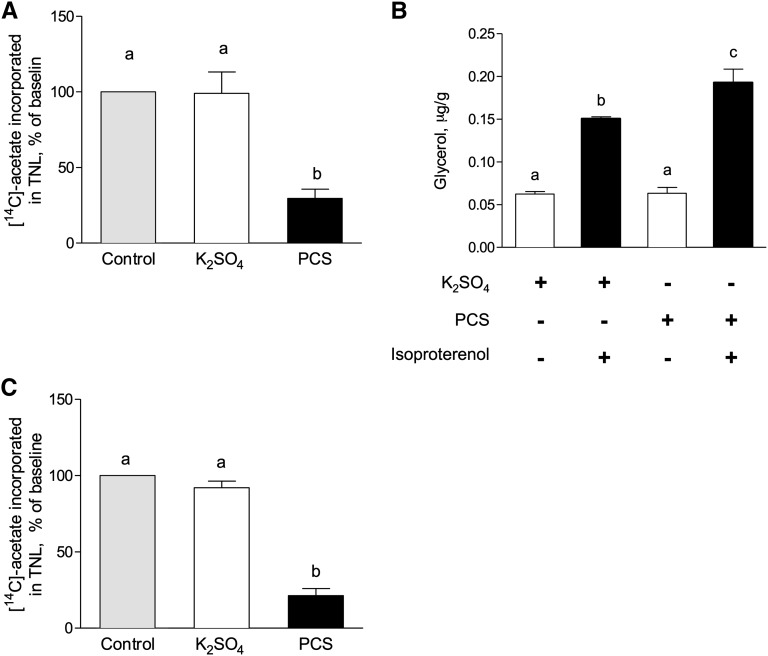
To obtain an insight into the mechanisms of PCS action on adipose cells, we measured the lipolytic response to the β-adrenergic agonist isoproterenol and de novo lipogenesis. To this end, human isolated adipocytes and 3T3-L1 preadipocytes were incubated in the presence of PCS (40 µg/ml). In 3T3-L1 adipose cells, PCS decreased lipogenesis by 78% compared with control (Figure 4A). In the same model, incubation with PCS did not change basal lipolysis (Figure 4B) but increased isoproterenol-stimulated lipolysis (26%; P<0.05). In human isolated adipose cells, PCS inhibited lipogenesis by roughly 80% compared with vehicle-treated cells, thus validating our results in 3T3-L1 cells (Figure 4C).
Figure 4.
PCS decreases lipogenesis and stimulates lipolysis in 3T3-L1 adipocytes and isolated human adipose cells. (A) Lipogenesis measured on 3T3-L1 adipocytes as the incorporation of [14C]-acetate into total lipids. (B) Lipolysis measured in 3T3-L1 adipocytes as the glycerol released in presence or absence of isoproterenol. (C) PCS inhibited lipogenesis on human isolated adipocytes. Data are mean ± SEM for n=4 mice. Different characters indicate a significant difference at P<0.05. TNL, total neutral lipids.
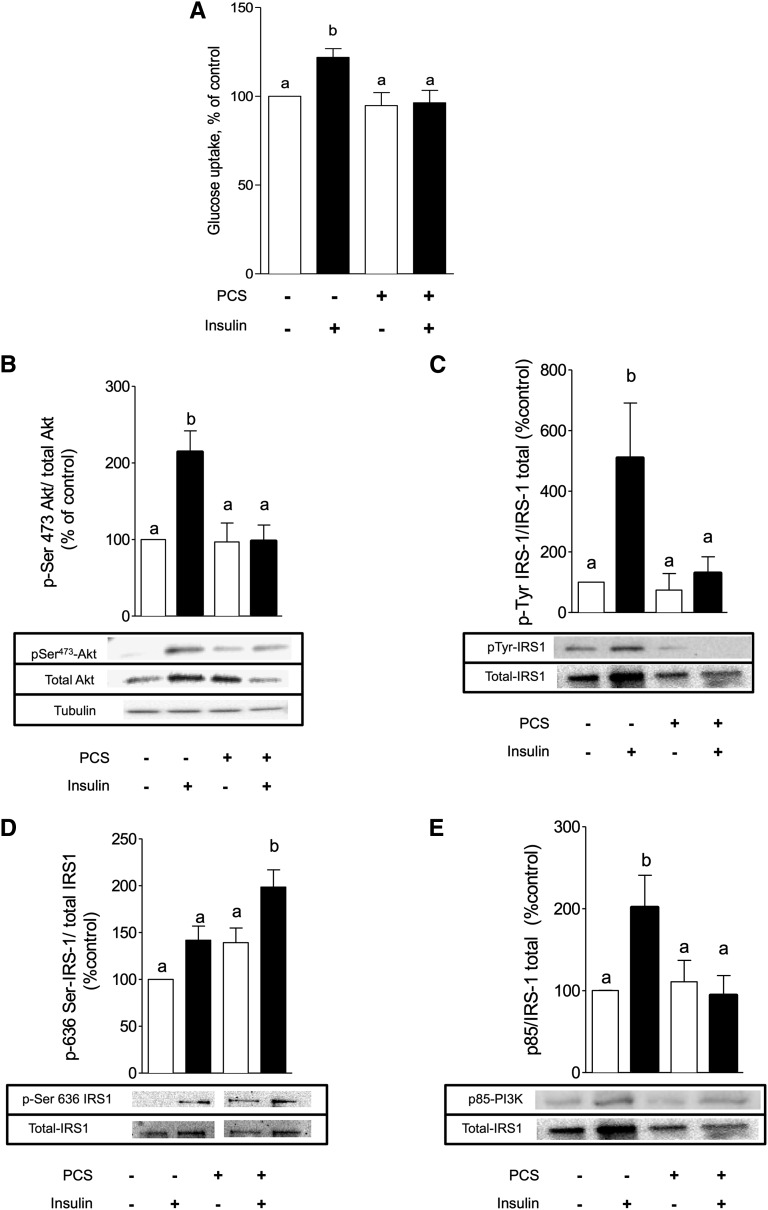
PCS Impairs Insulin-Induced Glucose Uptake and Signaling in C2C12 Myotubes
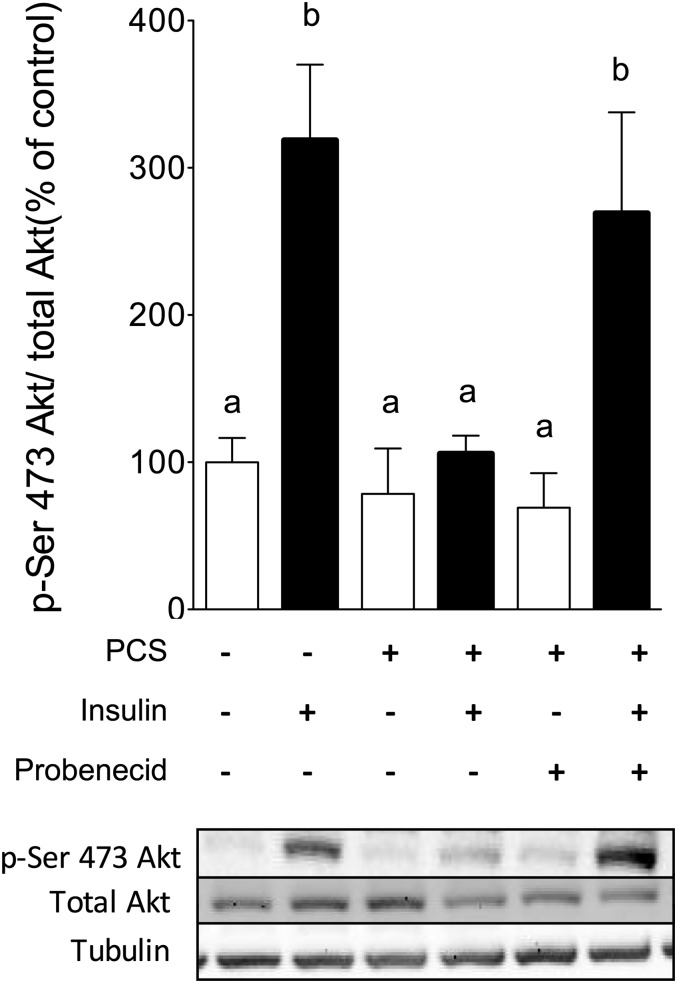
Skeletal muscle accounts for a large percentage of the whole-body glucose uptake and is a major site for insulin resistance in type 2 diabetes.27,28 To get insight into the molecular mechanisms of PCS-induced insulin resistance in skeletal muscle, C2C12 myotubes were treated with PCS. No significant difference of cell viability was observed between control and PCS treated cells for both tests (Supplemental Table 3). To determine whether PCS could impair glucose uptake, we studied the [3H]-2-deoxy-d-glucose transport in C2C12 myotubes. In control cells, stimulation with 100 nM insulin for 20 minutes induced a 25% increase in [3H]-2-deoxy-d-glucose uptake (P<0.05). A 30-minute pretreatment with PCS abolished the insulin-stimulated glucose uptake without affecting the basal glucose uptake (Figure 5A). To evaluate the mechanisms responsible for the inhibition of glucose uptake, we analyzed the insulin signaling pathways in the absence or presence of PCS. Activation of the downstream kinase PKB/Akt was estimated through the insulin-induced phosphorylation of serine 473, which was increased 2.1-fold after a treatment by 100 nM insulin for 20 minutes (Figure 5B). This effect was abolished after pretreatment with PCS from a concentration of 10 to 80 µg/ml (Supplemental Figure 4). The early molecular event IRS-1 phosphorylation was compared in the absence or presence of PCS. In control cells, insulin triggered a 5.6-fold increase in tyrosine phosphorylation of IRS-1 (Figure 5C). Pretreatment with PCS did not affect the basal tyrosine phosphorylation of IRS-1 but significantly impaired its insulin-stimulated phosphorylation. In parallel, PCS treatment was associated with increased inhibitory phosphorylation on IRS-1 on serine 636 residues (Figure 5D). In good agreement, insulin stimulation induces a marked increase in the level of p85 protein co-immunoprecipitated with IRS-1 (1.7-fold compared with unstimulated cells), whereas PCS significantly decreased the level of p85 protein co-immunoprecipitated with IRS-1 (Figure 5E). Miyamoto et al.29 recently proposed that in renal cells, PCS enters the cell via organic anion transporters (OAT), especially OAT3. We pretreated C2C12 muscle cells with probenecid (1 mM, 1 hour), a potent inhibitor of OAT family, before incubation with PCS. Pretreatment with probenecid abrogated PCS-induced disruption of insulin signaling pathways, as evidenced by the measurement of insulin-induced PKB/Akt phosphorylation (Figure 6).
Figure 5.
PCS inhibits insulin-stimulated glucose uptake and disrupts insulin signaling pathways in C2C12 myotubes. C2C12 myotubes were incubated with PCS (40 µg/ml) for 30 minutes and stimulated by 100 nM insulin for 20 minutes. (A) Glucose uptake was measured as the incorporation of tritiated 2-deoxy-glucose uptake into cells. The insulin pathway was explored by Western blotting. Also shown are the effects of PCS on (B) serine 473 phosphorylation of PKB/Akt, (C) tyrosine-phosphorylation of IRS-1, and (D) serine phosphorylation (Ser 636) of IRS-1, as well as (E) p85 subunit of PI3K co-immunoprecipitation. Data are mean ± SEM for n=4–5 experiments. Different letters indicate a significant difference at P<0.05.
Figure 6.
Probenecid prevents PCS-induced disruption of insulin signaling pathways in C2C12 myotubes. C2C12 myotubes were incubated with probenecid (1 mM, 1 hour), a potent inhibitor of organic anion transporters, before incubation with PCS (40 µg/ml, 30 minutes) and stimulation by insulin (100 nM, 20 minutes). Effect of PCS on serine 473 phosphorylation of PKB/Akt. Data are mean ± SEM for n=6 mice. Different letters indicate a significant difference at P<0.05.
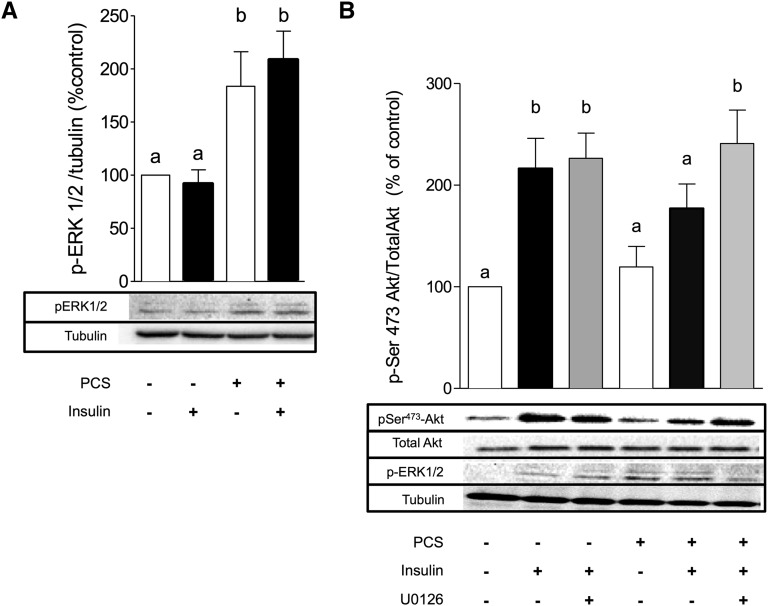
PCS Impairs Insulin Signaling in C2C12 Myotubes through Activation of the ERK Kinases
ERK can induce inhibitory phosphorylation of IRS-1 on serine residue. To further explore the role of ERK1/2 in PCS-induced muscle insulin resistance, C2C12 cells were incubated with PCS and phosphorylation status of ERK1/2 was studied. C2C12 myotubes preincubated with PCS without or with insulin exhibited a twofold increase in ERK1/2 phosphorylation (P<0.05) (Figure 7A). Pretreatment of the cells with the ERK1/2 inhibitor U0126 (10 µM, 1 hour) before PCS totally inhibited PCS-induced ERK1/2 activation (data not shown) and restored insulin-induced PKB/Akt phosphorylation (Figure 7B), pointing out the specific role of ERK1/2 in mediating the effects of PCS in C2C12 myotubes. In contrast, PCS triggered only modest phosphorylation of JNK (data not shown), but pretreatment of C2C12 with a potent JNK inhibitor (SP600125, 10 µM, 1 hour) failed to reverse the inhibitory effect of PCS on insulin-induced phosphorylation of PKB/Akt (data not shown), thereby excluding a role of JNK in PCS-induced insulin resistance.
Figure 7.
Role of ERK1/2 in PCS-induced insulin resistance in C2C12 muscle cells. C2C12 myotubes were incubated with PCS (40 µg/ml) for 30 minutes and stimulated by 100 nM insulin for 20 minutes. (A) PCS induces phosphorylation of ERK1/2. (B) ERK1/2 inhibitor U0126 (10 µM, 1 hour) reverses the effect of PCS on insulin-stimulated PKB/Akt phosphorylation. Data are mean ± SEM for n=6 mice. Different letters indicate a significant difference at P<0.05.
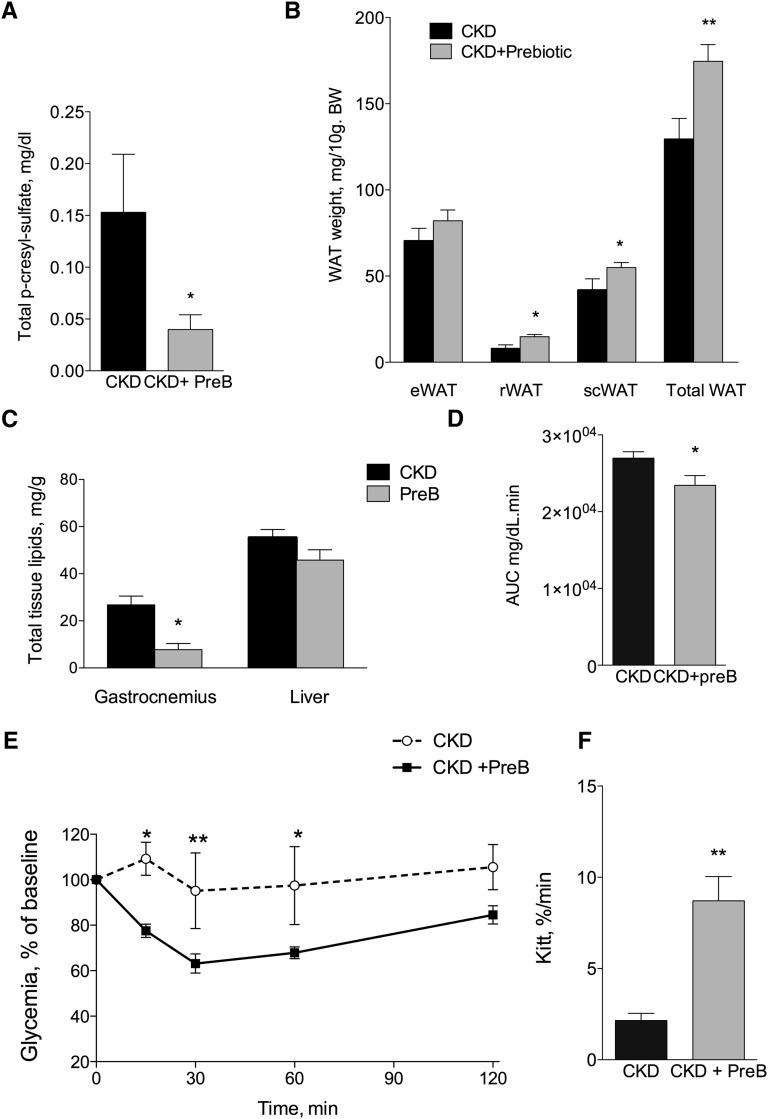
Treatment of Uremic Mice with a Prebiotic AXOS Reduces Fat Mass Loss and Ectopic Lipids Associated with CKD
Because of its tight protein binding, PCS is poorly removed by most dialysis techniques. We therefore tested a prebiotic strategy aimed at reducing intestinal production of p-cresol in an attempt to prevent metabolic disturbances associated with serum PCS accumulation in CKD. AXOS are prebiotics that promote intestinal growth of bifidobacteria (instead of fermentative bacteria), which were shown to significantly reduce the intestinal production of p-cresol.30,31 To determine in vivo the effect of the PCS decrease on insulin resistance and glucose tolerance, CKD mice were treated for 4 weeks with the prebiotic AXOS (5% wt/wt in diet; see Supplemental Table 4 for diet composition). CKD mice treated with AXOS exhibited a significant decrease in serum total PCS (change, −74%; P=0.03) compared with CKD mice fed the control diet (Figure 8A). After subtotal nephrectomy, mice receiving prebiotics exhibited a steady but nonsignificant improvement in weight compared with control CKD mice (P=0.09) (Supplemental Table 5). The energy intake was not significantly different between the two groups. Prebiotic treatment reduced the loss of fat mass observed in CKD, as shown by the weight of epididymal (16%; P=0.24), subcutaneous (31%; P=0.06), retroperitoneal (84%; P<0.05), and total white adipose tissue (33%; P<0.05) (Figure 8B). The prebiotic treatment prevented the ectopic lipid redistribution associated with CKD (Figure 8C).
Figure 8.
The prebiotic AXOS reduces plasma PCS and improves metabolic measures in CKD mice. Nephrectomized mice were fed for 4 weeks with a diet containing 5% (wt/wt) of AXOS prebiotic as described in the Concise Methods section. (A) Effect of AXOS on plasma PCS concentration (n=4–5 mice). (B) Weight of different pads of white adipose tissue (WAT). e, epididymal; r, retroperitoneal; sc, subcutaneous. (C) Tissue lipid content in gastrocnemius and liver (n=8). (D) Glucose tolerance test (intraperitoneal); n=6–8 mice. Baseline glucose concentrations were 87±2 and 75±2 mg/dl for control and prebiotic-fed CKD mice, respectively (P<0.001). AUC, area under the curve. (E and F) Insulin tolerance test (n=5 mice). Baseline glucose concentrations were 88±3 and 74±2 mg/dl for control and prebiotic-fed CKD mice, respectively (P<0.001). Data are mean ± SEM. *P<0.05; **P<0.01; ***P<0.001.
Treatment of Uremic Mice with a Prebiotic AXOS Improves Dyslipidemia, Glucose Tolerance, and Insulin Resistance in CKD Mice
Prebiotic administration had no effect on plasma urea, adiponectin, or insulin levels in CKD mice (Table 4). In contrast, prebiotic treatment completely prevented the expected increase in glycemia, total cholesterol, and triglycerides associated with CKD (Table 4). The intraperitoneal glucose tolerance test showed significantly improved glucose control in the prebiotic group compared with the CKD group (Figure 8D). Finally, an insulin tolerance test was performed. After 3 weeks, insulin sensitivity was significantly improved in the prebiotic group compared with the CKD group receiving standard diet (Figure 8, E and F).
Table 4.
Metabolic profile in CKD mice fed or not fed with an AXOS prebiotic–enriched diet
| Diet | Standard Mice | AXOS Mice | Change (%) | P Value |
|---|---|---|---|---|
| Fasting insulin (pg/ml) | 60.5±11 | 38±17 | −37 | 0.12 |
| Fasting glucose (mg/dl) | 97±5 | 81±2 | −16 | 0.002 |
| HOMA-IR | 1.8±0.4 | 1.0±0.2 | −44 | 0.09 |
| Triacylglycerols (mg/dl) | 118±12 | 76±10 | −36 | 0.02 |
| Total cholesterol (mg/dl) | 123±11 | 80±10 | −35 | 0.01 |
| Adiponectin (μg/ml) | 25±3 | 26±1 | 4 | 0.47 |
| Urea (mmol/L) | 34±3 | 30±3 | −13 | 0.29 |
| MDA (μM) | 10.3±3.9 | 11.1±2.7 | 8 | 0.73 |
Data are mean ± SEM and variation compared with control group. Differences were considered significant at the P<0.05 level using ANOVA test. n=5–6 mice for each group. HOMA-IR, homeostasis model assessment insulin resistance; MDA, malondialdehyde.
Discussion
To our knowledge, this study is the first to show that a major protein-bound uremic toxin, PCS, induces insulin resistance in vivo in mice (Figure 2), as well as in vitro in C2C12 myotubes (Figure 6). The disruption of insulin signaling associated with CKD was described in 1955,32 and the effects of kidney disease on renal uptake and excretion of insulin were reported in 1970.33 Insulin resistance in CKD was evidenced by DeFronzo et al.17 using the “gold standard” euglycemic hyperinsulinemic clamp technique. In CKD, the decline of renal function is associated with the development of insulin resistance, and insulin resistance is recognized as an independent risk factor for cardiovascular morbidity and mortality in patients with CKD.19 However, the exact causes of insulin resistance in CKD are still poorly defined.20,22,23,34,35 McCaleb et al.36 demonstrated that isolated rat adipocytes had inhibition of insulin-stimulated glucose uptake after incubation with serum from patients with CKD, whereas serum from obese patients or those with type 2 diabetes with normal renal function showed no effect. This suggested that one or several unknown circulating molecules unique to uremia can induce insulin resistance and prompted numerous fractionation studies that attempted to decipher the precise molecular nature of this factor. A central role for uremic solutes generated from protein breakdown in the gastrointestinal tract was suggested when decreased plasma glucose concentrations and reduced insulin requirements in uremic rats were reported after installment of a low-protein diet37 and after ingestion of an oral sorbent of protein degradation products.38 PCS is one of these products, resulting from the transformation of tyrosine by gut bacterial fermentation. Free PCS9,10 and insulin resistance are two independent factors associated with cardiovascular mortality in CKD. In this study, we explored the causal relationship between insulin resistance and PCS accumulation in cellular and animal models. To our knowledge, this study is the first to show that PCS induces insulin resistance; as a result, this compound can be classified as one of the major uremic toxins.
PCS chronically administered to mice with normal renal function triggered a striking insulin resistance and fat mass loss. Of importance, the PCS concentration after administration was in the range of levels observed in patients with ESRD.10,39 The ectopic lipid redistribution and reduction of fat accretion in PCS-treated mice were the result of a direct effect of PCS treatment rather than malnutrition because food intake and body weight were not altered. Fat mass loss should be related to the fact that we observed a decrease in the volume of adipocytes, which may reflect adipocyte dysfunction. Axelsson et al. showed that an undefined circulating factor in patients with CKD increased basal lipolysis in human adipocytes in vitro,40 and we indeed demonstrated that PCS-treated adipocytes exhibit inhibited lipogenesis and stimulated lipolysis. In PCS-treated mice, circulating lipids that could no longer be stored into adipose tissue accumulate in the muscle and liver, leading to an ectopic lipid redistribution that matched the one observed in CKD mice. Because intramuscular lipids are correlated with insulin resistance,41–43 it is likely that lipotoxicity phenomena induced by PCS could contribute to insulin resistance, as described in CKD mice. Ectopic lipids disrupt the insulin signaling pathway, which induces a reduction in tyrosine phosphorylation of IRS1.44 We demonstrated that PCS in concentrations observed in ESRD (40 µg/ml)1,24,45,46 can impair glucose uptake as well as insulin pathway at the level of IRS-1 and p-PKB/Akt in C2C12 myotubes. In type 2 diabetes, insulin resistance is related to a defect in elements involved in insulin signal transduction, such as IRS-1, which are modulated by several kinases (serine and tyrosine kinases).47 IRS-1 phosphorylation on serine residues impedes activating phosphorylation on tyrosine residues and shuts down the insulin signal transduction. Serine phosphorylation is a mechanism used by many diabetogenic factors (such as fatty acids, TNF-α, hyperinsulinemia) to inhibit insulin signaling. The stress signaling pathways have been shown to contribute to the development of insulin resistance, and IRS-1 contains many potential sites of inhibitory phosphorylation by mitogen-activated protein kinase.47 We demonstrated that in both mice and C2C12, PCS is responsible for an increase in the mitogen-activated protein kinase–signaling ERK but not JNK. ERK1/2 involvement in the PCS noxious effects was evidenced by the use of an ERK inhibitor (U0126), which totally restored the impaired insulin-induced p-AKT/PKB phosphorylation. Thus, PCS can directly impair the insulin signaling pathway by activating ERK. PCS has repeatedly been demonstrated (in various cell types) to exert its toxic effects by triggering intracellular oxidative stress. However, we failed to observe any increase in intracellular reactive oxygen species production or lipid peroxidation in C2C12 muscle cells (Supplemental Figure 5) excluding that oxidative stress was responsible for the impaired response to insulin.
Recent studies demonstrated that the bacterial flora of the intestine may play a key role in glucose homeostasis and obesity.48 Prebiotics are oligofructose or inulin polymers that selectively stimulate growth and activity of a limited number of beneficial bacteria in the colon.49 The prebiotics demonstrated their effectiveness to improve satiety and glycemic control in patients with diabetes50 and plasma lipid profiles.51 Several studies reported a reduction in urinary p-cresol with the use of prebiotics, such as oligo-fructose, in healthy persons,30 as well as in hemodialysis patients.52 Treatment with the prebiotic AXOS decreased PCS, and prevented CKD-induced insulin resistance, the loss of adipose tissue and the accumulation of ectopic lipids in muscle and liver. Prebiotics probably exert pleiotropic effects that, besides suppressing protein fermentation and blunting p-cresol production, could also contribute to improve metabolic status of CKD mice.
Taken together, our data demonstrate that a possible cause of insulin resistance in the chronically PCS-treated animal is adipocyte dysfunction associated with accumulation of ectopic lipids and that under these conditions, PCS alone is sufficient to induce the same degree of insulin resistance as that reported in CKD mice. In summary, we have provided the first in vivo and in vitro evidence that PCS induces muscle insulin resistance accompanied by a defect in the IRS/PI3K/Akt pathway, suggesting a causative link between PCS and insulin resistance (Figure 8). Furthermore, we showed that PCS can directly activate ERK1/2 kinase in vivo and in vitro, leading to disruption of the insulin pathway. Finally, we identified a mechanism of lipotoxicity after long-term administration of PCS, which reproduces the metabolic abnormalities observed in CKD mice and could be reversed by prebiotic treatment, through decreased plasmatic levels of PCS. Because insulin resistance is an important cardiovascular risk factor, novel therapeutic approaches such as prebiotics, which could decrease PCS more substantially than with the currently available strategies, must be developed, especially because this toxin is not very efficiently removed by hemodialysis.53
Concise Methods
PCS
All in vitro experiments were performed according to the standard approach and the recommendations for handling uremic retention solutes published by the European Uremic Toxin Work Group (EUTox, http://www.uremic-toxins.org).1 The potassium salt of PCS was synthesized as described by Feigenbaum and Neuberg.54 In the in vitro study, concentration of PCS was 40 µg/ml (212 µM), which is the concentration found in humans with ESRD.45,46,55 Because PCS was synthesized as a potassium salt, a solution of 35 µg/ml (200 µM) K2SO4 in saline was chosen as control to equal the potassium concentration in the K-salt of PCS. Because PCS is mainly protein-bound in biologic systems, all in vitro experiments were performed in medium supplemented with 35 g/L BSA according to the recommendations of EUTox.1
Animal Experiments
Animal experiments were performed under authorization no. 69–266–0501 (CarMeN Laboratory, Direction Départementale des Services Vétérinaires du Rhône). All experiments were carried out according to the guidelines laid down by the French Ministère de l’Agriculture (no. 87–848) and the European Union Council Directive for the Care and Use of Laboratory Animals of November 24, 1986 (86/609/EEC). CD1 Swiss and C57BL/6J mice were purchased from Janvier SA (Le Genest-Saint-Isle, France) and housed in an air-conditioned room with a controlled environment of 21°C±0.5°C and 60%–70% humidity, under a 12-hour light/dark cycle (light on from 07:00 to 19:00) with free access to food and water. Moderate CKD was induced by 5/6 nephrectomy with a two-step surgical procedure. NaHCO3 (80 mM) was added to the drinking water of CKD mice to prevent metabolic acidosis.56 CD1 Swiss mice were randomly assigned to receive twice daily (8:00 a.m. and 6:00 p.m.) intraperitoneal injections of PCS (10 mg/kg) or vehicle for control mice, sham mice, and CKD mice, for 4 weeks.
Metabolic Challenges
After an overnight fast, an intraperitoneal glucose tolerance test (glucose, 1 g/kg body weight) and insulin tolerance test (insulin, 0.50 IU/kg body weight) were performed. Blood glucose values were determined from a drop of blood sampled from the terminal portion of the tail, using an automatic glucose monitor (Accu-Check Performa, Roche, Meylan, France).
Euthanasia and Tissue Dissection
To study insulin signaling in skeletal muscle, mice were intraperitoneally injected with insulin (Actrapid, 0.75 IU/kg) or saline solution 60 minutes after the last administration of PCS or vehicle. Thirty minutes after insulin injection mice were anesthetized with sodium pentobarbital (35 mg/kg intraperitoneally). Blood (750 µl) was collected through cardiac puncture in heparinized tubes, centrifuged for 2 minutes at 3500 g to separate plasma and stored at −80°C. Liver; heart; kidneys; gastrocnemius muscle; and epididymal, retroperitoneal, and subcutaneous inguinal white adipose tissue were dissected out, weighed, and snap-frozen in liquid nitrogen.
Biochemical Measurements
Free and total PCS were quantified in serum by using reverse-HPLC coupled to a fluorescence detector as previously described.15,45,57 The plasma concentration of cholesterol, triacylglycerols, adiponectin, and insulin were determined using commercial assays. Muscle (gastrocnemius) and liver lipids were extracted using chloroform-methanol (2:1, vol/vol),58 and total lipid content was estimated gravimetrically.
Cellularity Study: Measurement of Adipocyte Size and Number
Adipose tissue was fixed in osmium tetroxide, and cell size was determined essentially as described by Etherton et al.59 DNA content in epididymal white adipose tissue was measured, after delipidation of the samples, by a standard fluorometric method using bisbenzimide and calf thymus DNA as standard.
Differentiation of C2C12 and 3T3-L1 Cells
C2C12 myoblasts, from the American Type Culture Collection (LGC Standard, Molsheim, France), were grown and differentiated to myotubes as recommended by the provider. Mouse 3T3-L1 fibroblasts were obtained from the American Type Culture Collection (reference CL-173) and differentiated to adipocytes. PCS cytotoxicity was determined by measuring cell viability using a 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyltetrazolium (Cell Proliferation Kit I, Roche) assay and lactate dehydrogenase activity (In Vitro Toxicology Assay Kit, Lactic Dehydrogenase based, Sigma Aldrich).
Protein Isolation, Immunoprecipitation, and Immunoblotting
C2C12 myotubes were washed and solubilized as described previously.60 Lysates were kept on ice for 20 minutes, and insoluble material was removed by centrifugation at 14,000 g for 20 minutes. Protein concentration was determined by colorimetric assay (Bio-Rad). For immunoprecipitation and immunoblots, antibodies were used as previously described.60 Immunoreactive proteins were detected using horseradish peroxidase–linked secondary antibodies and enhanced chemiluminescence according to the manufacturer's instructions. Signal intensities were measured with Quantity One software (Bio-Rad).
2-Deoxyglucose Uptake and Lipogenesis
C2C12 myotubes were incubated in serum-free DMEM containing 0.2% (wt/vol) BSA for 12 hours and treated or not with PCS. For glucose uptake and lipogenesis, cells were incubated and treated as previously described.60,61 Radioactivity was counted, and samples were normalized to protein concentration.
Adipose Tissue Collection for Lipogenesis Assay
Human adipose tissue was obtained from an ongoing study approved by the Ethical Committee (CPP Lyon Sud-Est IV) of Lyon University Hospital (MODAIR study, ref D-09–17). Subcutaneous abdominal adipose tissue (1–2 g) was collected from four nonobese men undergoing elective urologic surgery (radical prostatectomy). All patients gave written informed consent for the study, and all procedures were in accordance with the principles of the Declaration of Helsinki. Adipocytes were isolated using collagenase and lipogenesis was measured as previously described.61
Statistical Analyses
Data are expressed as mean ± SEM. All data were analyzed using GraphPad Prism, version 5.0, software (GraphPad, La Jolla, CA). Differences were considered significant at the P<0.05 level.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Dr. E. Kalbacher for human adipose tissue collection and Dr. F. Guebre-Egziabher for fruitful discussions.
L.K. held a fellowship from Fondation pour la Recherche Médicale and. C.P. held a grant from Société Française de Néphrologie. N.J.P., M.L.C., and R.E.V. were supported by grants from the French Ministère de l’Education Nationale, de la Recherche et de la Technologie. This work was supported by Institut National de la Santé et de la Recherche Médicale (INSERM) and Institut National des Sciences Appliquées de Lyon (INSA-Lyon).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012050503/-/DCSupplemental.
References
- 1.Cohen G, Glorieux G, Thornalley P, Schepers E, Meert N, Jankowski J, Jankowski V, Argiles A, Anderstam B, Brunet P, Cerini C, Dou L, Deppisch R, Marescau B, Massy Z, Perna A, Raupachova J, Rodriguez M, Stegmayr B, Vanholder R, Hörl WH, European Uremic Toxin Work Group (EUTox) : Review on uraemic toxins III: recommendations for handling uraemic retention solutes in vitro—towards a standardized approach for research on uraemia. Nephrol Dial Transplant 22: 3381–3390, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R, Hemodialysis (HEMO) Study Group : Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 347: 2010–2019, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, Mujais S, Mexican Nephrology Collaborative Study Group : Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol 13: 1307–1320, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Bammens B, Evenepoel P, Verbeke K, Vanrenterghem Y: Removal of middle molecules and protein-bound solutes by peritoneal dialysis and relation with uremic symptoms. Kidney Int 64: 2238–2243, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Bammens B, Evenepoel P, Verbeke K, Vanrenterghem Y: Removal of the protein-bound solute p-cresol by convective transport: A randomized crossover study. Am J Kidney Dis 44: 278–285, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Liabeuf S, Drüeke TB, Massy ZA: Protein-bound uremic toxins: new insight from clinical studies. Toxins (Basel) 3: 911–919, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Smet R, Van Kaer J, Van Vlem B, De Cubber A, Brunet P, Lameire N, Vanholder R: Toxicity of free p-cresol: A prospective and cross-sectional analysis. Clin Chem 49: 470–478, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Curtius HC, Mettler M, Ettlinger L: Study of the intestinal tyrosine metabolism using stable isotopes and gas chromatography-mass spectrometry. J Chromatogr A 126: 569–580, 1976 [DOI] [PubMed] [Google Scholar]
- 9.Bammens B, Evenepoel P, Keuleers H, Verbeke K, Vanrenterghem Y: Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int 69: 1081–1087, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Liabeuf S, Barreto DV, Barreto FC, Meert N, Glorieux G, Schepers E, Temmar M, Choukroun G, Vanholder R, Massy ZA, European Uraemic Toxin Work Group (EUTox) : Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant 25: 1183–1191, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Wu I-W, Hsu K-H, Hsu H-J, Lee C-C, Sun C-Y, Tsai C-J, Wu MS: Serum free p-cresyl sulfate levels predict cardiovascular and all-cause mortality in elderly hemodialysis patients—a prospective cohort study. Nephrol Dial Transplant 27: 1169–1175, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Dou L, Bertrand E, Cerini C, Faure V, Sampol J, Vanholder R, Berland Y, Brunet P: The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int 65: 442–451, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Meijers BKI, Bammens B, De Moor B, Verbeke K, Vanrenterghem Y, Evenepoel P: Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int 73: 1174–1180, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Lin C-J, Wu C-J, Pan C-F, Chen Y-C, Sun F-J, Chen H-H: Serum protein-bound uraemic toxins and clinical outcomes in haemodialysis patients. Nephrol Dial Transplant 25: 3693–3700, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Meert N, Schepers E, Glorieux G, Van Landschoot M, Goeman JL, Waterloos M-A, Dhondt A, Van der Eycken J, Vanholder R: Novel method for simultaneous determination of p-cresylsulphate and p-cresylglucuronide: Clinical data and pathophysiological implications. Nephrol Dial Transplant 27: 2388–2396, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Kurella M, Lo JC, Chertow GM: Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol 16: 2134–2140, 2005 [DOI] [PubMed] [Google Scholar]
- 17.DeFronzo RA, Alvestrand A, Smith D, Hendler R, Hendler E, Wahren J: Insulin resistance in uremia. J Clin Invest 67: 563–568, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeFronzo RA, Tobin JD, Rowe JW, Andres R: Glucose intolerance in uremia. Quantification of pancreatic beta cell sensitivity to glucose and tissue sensitivity to insulin. J Clin Invest 62: 425–435, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bodlaj G, Berg J, Pichler R, Biesenbach G: Prevalence, severity and predictors of HOMA-estimated insulin resistance in diabetic and nondiabetic patients with end-stage renal disease. J Nephrol 19: 607–612, 2006 [PubMed] [Google Scholar]
- 20.Takenaka T, Kanno Y, Ohno Y, Suzuki H: Key role of insulin resistance in vascular injury among hemodialysis patients. Metabolism 56: 153–159, 2007 [DOI] [PubMed] [Google Scholar]
- 21.May RC, Kelly RA, Mitch WE: Mechanisms for defects in muscle protein metabolism in rats with chronic uremia. Influence of metabolic acidosis. J Clin Invest 79: 1099–1103, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey JL, Zheng B, Hu Z, Price SR, Mitch WE: Chronic kidney disease causes defects in signaling through the insulin receptor substrate/phosphatidylinositol 3-kinase/Akt pathway: Implications for muscle atrophy. J Am Soc Nephrol 17: 1388–1394, 2006 [DOI] [PubMed] [Google Scholar]
- 23.D’Apolito M, Du X, Zong H, Catucci A, Maiuri L, Trivisano T, Pettoello-Mantovani M, Campanozzi A, Raia V, Pessin JE, Brownlee M, Giardino I: Urea-induced ROS generation causes insulin resistance in mice with chronic renal failure. J Clin Invest 120: 203–213, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandenburg VM, Schlieper G, Heussen N, Holzmann S, Busch B, Evenepoel P, Vanholder R, Meijers B, Meert N, Fassbender WJ, Floege J, Jahnen-Dechent W, Ketteler M: Serological cardiovascular and mortality risk predictors in dialysis patients receiving sevelamer: a prospective study. Nephrol Dial Transplant 25: 2672–2679, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GS: Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc Natl Acad Sci U S A 103: 10741–10746, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bost F, Aouadi M, Caron L, Even P, Belmonte N, Prot M, Dani C, Hofman P, Pagès G, Pouysségur J, Le Marchand-Brustel Y, Binétruy B: The extracellular signal-regulated kinase isoform ERK1 is specifically required for in vitro and in vivo adipogenesis. Diabetes 54: 402–411, 2005 [DOI] [PubMed] [Google Scholar]
- 27.DeFronzo RA, Ferrannini E, Sato Y, Felig P, Wahren J: Synergistic interaction between exercise and insulin on peripheral glucose uptake. J Clin Invest 68: 1468–1474, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeFronzo RA, Gunnarsson R, Björkman O, Olsson M, Wahren J: Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest 76: 149–155, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyamoto Y, Watanabe H, Noguchi T, Kotani S, Nakajima M, Kadowaki D, Otagiri M, Maruyama T: Organic anion transporters play an important role in the uptake of p-cresyl sulfate, a uremic toxin, in the kidney. Nephrol Dial Transplant 26: 2498–2502, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Cloetens L, Broekaert WF, Delaedt Y, Ollevier F, Courtin CM, Delcour JA, Rutgeerts P, Verbeke K: Tolerance of arabinoxylan-oligosaccharides and their prebiotic activity in healthy subjects: A randomised, placebo-controlled cross-over study. Br J Nutr 103: 703–713, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Lecerf J-M, Dépeint F, Clerc E, Dugenet Y, Niamba CN, Rhazi L, Cayzeele A, Abdelnour G, Jaruga A, Younes H, Jacobs H, Lambrey G, Abdelnour AM, Pouillart PR: Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br J Nutr 23: 1–12, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Runyan JW, Jr, Hurwitz D, Robbins SL: Effect of Kimmelstiel-Wilson syndrome on insulin requirements in diabetes. N Engl J Med 252: 388–391, 1955 [DOI] [PubMed] [Google Scholar]
- 33.Rabkin R, Simon NM, Steiner S, Colwell JA: Effect of renal disease on renal uptake and excretion of insulin in man. N Engl J Med 282: 182–187, 1970 [DOI] [PubMed] [Google Scholar]
- 34.May RC, Clark AS, Goheer MA, Mitch WE: Specific defects in insulin-mediated muscle metabolism in acute uremia. Kidney Int 28: 490–497, 1985 [DOI] [PubMed] [Google Scholar]
- 35.Kraus LM, Traxinger R, Kraus AP: Uremia and insulin resistance: N-carbamoyl-asparagine decreases insulin-sensitive glucose uptake in rat adipocytes. Kidney Int 65: 881–887, 2004 [DOI] [PubMed] [Google Scholar]
- 36.McCaleb ML, Izzo MS, Lockwood DH: Characterization and partial purification of a factor from uremic human serum that induces insulin resistance. J Clin Invest 75: 391–396, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rigalleau V, Combe C, Blanchetier V, Aubertin J, Aparicio M, Gin H: Low protein diet in uremia: effects on glucose metabolism and energy production rate. Kidney Int 51: 1222–1227, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Okada K, Takahashi Y, Okawa E, Onishi Y, Hagi C, Aoki K, Shibahara H, Higuchi T, Nagura Y, Kanmatsuse K, Takahashi S: Relationship between insulin resistance and uremic toxins in the gastrointestinal tract. Nephron 88: 384–386, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Lin C-J, Chen H-H, Pan C-F, Chuang C-K, Wang T-J, Sun F-J, Wu CJ: p-Cresylsulfate and indoxyl sulfate level at different stages of chronic kidney disease. J Clin Lab Anal 25: 191–197, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Axelsson J, Aström G, Sjölin E, Qureshi AR, Lorente-Cebrián S, Stenvinkel P, Rydén M: Uraemic sera stimulate lipolysis in human adipocytes: role of perilipin. Nephrol Dial Transplant 26: 2485–2491, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Kelley DE, Goodpaster BH: Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care 24: 933–941, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI: Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350: 664–671, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Itani SI, Ruderman NB, Schmieder F, Boden G: Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-α. Diabetes 51: 2005–2011, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI: Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277: 50230–50236, 2002 [DOI] [PubMed] [Google Scholar]
- 45.de Loor H, Meijers BKI, Meyer TW, Bammens B, Verbeke K, Dehaen W, Evenepoel P: Sodium octanoate to reverse indoxyl sulfate and p-cresyl sulfate albumin binding in uremic and normal serum during sample preparation followed by fluorescence liquid chromatography. J Chromatogr A 1216: 4684–4688, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Meijers BKI, Van Kerckhoven S, Verbeke K, Dehaen W, Vanrenterghem Y, Hoylaerts MF, Evenepoel P: The uremic retention solute p-cresyl sulfate and markers of endothelial damage. Am J Kidney Dis 54: 891–901, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Tanti J-F, Jager J: Cellular mechanisms of insulin resistance: role of stress-regulated serine kinases and insulin receptor substrates (IRS) serine phosphorylation. Curr Opin Pharmacol 9: 753–762, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Ley RE: Obesity and the human microbiome. Curr Opin Gastroenterol 26: 5–11, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Broekaert WF, Courtin CM, Verbeke K, Van de Wiele T, Verstraete W, Delcour JA: Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylan-oligosaccharides, and xylooligosaccharides. Crit Rev Food Sci Nutr 51: 178–194, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Nelson RW: Dietary management of diabetes mellitus. J Small Anim Pract 33: 213–217, 1992 [Google Scholar]
- 51.Diez M, Hornick JL, Baldwin P, Istasse L: Influence of a blend of fructo-oligosaccharides and sugar beet fiber on nutrient digestibility and plasma metabolite concentrations in healthy beagles. Am J Vet Res 58: 1238–1242, 1997 [PubMed] [Google Scholar]
- 52.Meijers BKI, De Preter V, Verbeke K, Vanrenterghem Y, Evenepoel P: p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol Dial Transplant 25: 219–224, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Krieter DH, Hackl A, Rodriguez A, Chenine L, Moragues HL, Lemke H-D, Wanner C, Canaud B: Protein-bound uraemic toxin removal in haemodialysis and post-dilution haemodiafiltration. Nephrol Dial Transplant 25: 212–218, 2010 [DOI] [PubMed] [Google Scholar]
- 54.Feigenbaum J, Neuberg C: J Am Chem Soc 63: 3529–3530, 1941 [Google Scholar]
- 55.Martinez AW, Recht NS, Hostetter TH, Meyer TW: Removal of P-cresol sulfate by hemodialysis. J Am Soc Nephrol 16: 3430–3436, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Bailey JL, Wang X, England BK, Price SR, Ding X, Mitch WE: The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway. J Clin Invest 97: 1447–1453, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meert N, Eloot S, Schepers E, Lemke H-D, Dhondt A, Glorieux G, Van Landschoot M, Waterloos MA, Vanholder R: Comparison of removal capacity of two consecutive generations of high-flux dialysers during different treatment modalities. Nephrol Dial Transplant 26: 2624–2630, 2011 [DOI] [PubMed] [Google Scholar]
- 58.Folch J, Lees M, Sloane Stanley GH: A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957 [PubMed] [Google Scholar]
- 59.Etherton TD, Thompson EH, Allen CE: Improved techniques for studies of adipocyte cellularity and metabolism. J Lipid Res 18: 552–557, 1977 [PubMed] [Google Scholar]
- 60.Pillon NJ, Croze ML, Vella RE, Soulère L, Lagarde M, Soulage CO: The lipid peroxidation by-product 4-hydroxy-2-nonenal (4-HNE) induces insulin resistance in skeletal muscle through both carbonyl and oxidative stress. Endocrinology 153: 2099–2111, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Zarrouki B, Pillon NJ, Kalbacher E, Soula HA, Nia N’Jomen G, Grand L, Chambert S, Geloen A, Soulage CO: Cirsimarin, a potent antilipogenic flavonoid, decreases fat deposition in mice intra-abdominal adipose tissue. Int J Obes (Lond) 34: 1566–1575, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.