Abstract
Complement factor H (CFH) is a negative regulator of the alternative pathway of complement, and properdin is the sole positive regulator. CFH-deficient mice (CFH−/−) develop uncontrolled C3 activation and spontaneous renal disease characterized by accumulation of C3 along the glomerular basement membrane, but the role of properdin in the pathophysiology is unknown. Here, we studied mice deficient in both CFH and properdin (CFH−/−.P−/−). Although CFH−/− mice had plasma depleted of both C3 and C5, CFH−/−.P−/− animals exhibited depletion of C3 predominantly, recapitulating the plasma complement profile observed in humans with properdin-independent C3 nephritic factors. Glomerular inflammation, thickening of the capillary wall, and glomerular C3 staining were significantly increased in CFH−/−.P−/− compared with CFH−/− mice. We previously reported that exogenous CFH ameliorates C3 staining of the glomerular basement membrane and triggers the appearance of mesangial C3 deposits in CFH−/− mice; here, we show that these effects require properdin. In summary, during uncontrolled activation of C3 driven by complete CFH deficiency, properdin influences the intraglomerular localization of C3, suggesting that therapeutic inhibition of properdin would be detrimental in this setting.
The complement system is an integral component of the host response to pathogens. Regulation of complement is achieved by a group of proteins located in plasma and on cell membranes that act collectively to prevent inappropriate complement activation on host tissues. Abnormalities in complement regulation are associated with renal disease.1,2 Specifically, there is a strong association between abnormal regulation of the complement alternative pathway (AP) and C3 glomerulopathy. C3 glomerulopathy refers to glomerular pathologies characterized by isolated glomerular accumulation of complement C3.1 Examples include dense deposit disease (DDD), C3 GN, and CFHR5 nephropathy. In these conditions, genetic and acquired factors that result in uncontrolled AP regulation may be seen.1,2 These factors include loss of function mutations in the AP regulatory protein complement factor H (CFH) and gain of function mutations in the AP activation protein, C3. Acquired factors include autoantibodies that stabilize the AP C3 convertase.
The mechanism through which uncontrolled C3 activation results in accumulation of C3 within the glomerulus has been partially elucidated through the study of murine models of AP dysregulation.3 Gene-targeted CFH-deficient mice (CFH−/−) develop uncontrolled AP activation, which results in secondary depletion of plasma C3.4 These animals develop marked accumulation of C3 along the glomerular basement membrane (GBM) and subsequently both morphologic changes to the GBM (subendothelial electron-dense C3-containing deposits) and glomerular inflammation (typically membranoproliferative GN [MPGN]). Similar pathology has also been described in a breed of pigs with spontaneous CFH deficiency.5 Importantly, renal C3 accumulation in the murine model was absolutely dependent on the ability to activate the AP: mice with deficiency of both CFH and the AP activation protein, factor B, did not develop renal injury or plasma C3 depletion. C3 accumulation along the GBM in the setting of CFH deficiency is also dependent on complement factor I (CFI). CFI is an enzyme that cleaves activated C3 (termed C3b) to iC3b. Experiments in mice with combined deficiency of CFH and CFI suggested that it is iC3b that targets the GBM during uncontrolled AP activation due to CFH deficiency.6
Properdin is the only positive regulator of complement activation.7 It stabilizes the AP C3 convertase, which in its absence rapidly and spontaneously decays. Properdin deficiency in humans is associated with increased susceptibility to Neisserial infections.8–10 Based on our current understanding of the pathogenesis of C3 glomerulopathy,1,2 enhancing properdin activity (e.g., through gain of function mutations) would, through increased AP activation, be predicted to exacerbate C3 glomerulopathy. Conversely, reducing properdin activity (e.g., through genetic or therapeutic manipulation) would, through reduced AP activation, be predicted to ameliorate C3 glomerulopathy. To date these scenarios have not been described in human pathology. Here, through the study of gene-targeted properdin-deficient mice, we investigate the contribution of properdin to both spontaneous and experimentally triggered renal disease in CFH−/− mice. Unexpectedly, our data demonstrated that coexisting properdin deficiency did not confer a beneficial effect on renal disease in CFH−/− animals. In fact, glomerular inflammation and accumulation of glomerular C3 were enhanced in mice with combined deficiency of CFH and properdin (CFH−/−.P−/−).
Results
Properdin Deficiency Exacerbated Spontaneous but not Antibody-Mediated Nephritis in CFH-Deficient Mice
Unrestricted AP convertase formation and C3 activation are critical for the development of renal disease in CFH−/− mice.4 To study the role of properdin in this disease, P−/− mice11 were intercrossed with CFH−/− mice to generate animals with combined deficiency of CFH and properdin (CFH−/−.P−/−). We then monitored cohorts of P−/− (n=11), CFH−/− (n=8), and CFH−/−.P−/− (n=15) animals over a 38-week period, at the end of which all animals were sacrificed and renal function and histology assessed. None of the P−/− animals had significant glomerular inflammation, albuminuria, hematuria, or elevated urea levels (Table 1). As expected, there was evidence of glomerular inflammation and capillary wall thickening in CFH−/− mice (Table 1 and Supplemental Figure 1A). Notably, both of these changes were significantly increased in the CFH−/−.P−/− animals and in many CFH−/−.P−/− mice a double contour appearance to the GBM was present (Table 1 and Supplemental Figure 1A). We examined the ultrastructural appearances of the GBM by electron microscopy in four CFH−/− and seven CFH−/−.P−/− animals from the cohort. Subendothelial electron-dense GBM deposits were present in all mice examined (Supplemental Figure 1B). Subepithelial electron-dense GBM deposits were also present in three of the CFH−/−.P−/− animals. Despite these glomerular changes, renal function remained intact in both CFH−/− and CFH−/−.P−/− mice at the time of sacrifice, although there was a trend to greater albuminuria in the CFH−/−.P−/− mice (Table 1). These data demonstrated that the absence of properdin exacerbated the spontaneous renal disease associated with CFH deficiency.
Table 1.
Renal function and glomerular histology at 38 weeks
| Genotype | Mice Assessed (n) | Renal Function | Glomerular Histology | |||
|---|---|---|---|---|---|---|
| Albuminuria (µg/24 h) | Plasma Urea (mmol/L) | Cellularity (0–3) | Capillary Wall Thickening (0–2) | Capillary Wall Double Contours (0–2) | ||
| P−/− | 11 | 0 (0–122) | 12.3 (9.9–16.3) | 1 (0–1) | 0 (0–1) | 0 (0–1) |
| CFH−/−.P−/− | 15 | 125 (29–10,601) | 12.6 (9.9–21.4) | 2 (2–3)a,b | 2 (1–2)a,b | 1 (0–2)a,b |
| CFH−/− | 8 | 34 (0–69) | 10.9 (9.5–16) | 2 (1–2)a | 1 (0–2)a | 0 (0–1) |
Values are presented as median (range).
P<0.001 versus P−/− mice, Bonferroni multiple comparison test.
P<0.05 versus CFH−/− mice, Bonferroni multiple comparison test.
We previously reported that CFH−/− mice are hypersensitive to experimentally triggered renal disease using heterologous nephrotoxic nephritis (NTN).12 To investigate whether properdin contributes to this injurious response we performed heterologous NTN in CFH−/− and CFH−/−.P−/− mice (Supplemental Figure 2). As we previously reported, increased glomerular neutrophil numbers developed in CFH−/− mice compared with wild-type animals after injection of the nephrotoxic serum.12 Similarly, glomerular neutrophil numbers were also significantly greater in the CFH−/−.P−/− mice compared with wild-type animals 24 hours after injection. However, there was no difference in glomerular neutrophil influx between CFH−/− and CFH−/−.P−/− mice. In both wild-type and P−/− mice, glomerular neutrophils did not increase at either time point after injection.
Properdin Deficiency Differentially Affected Plasma C3 and C5 Levels in CFH-Deficient Mice
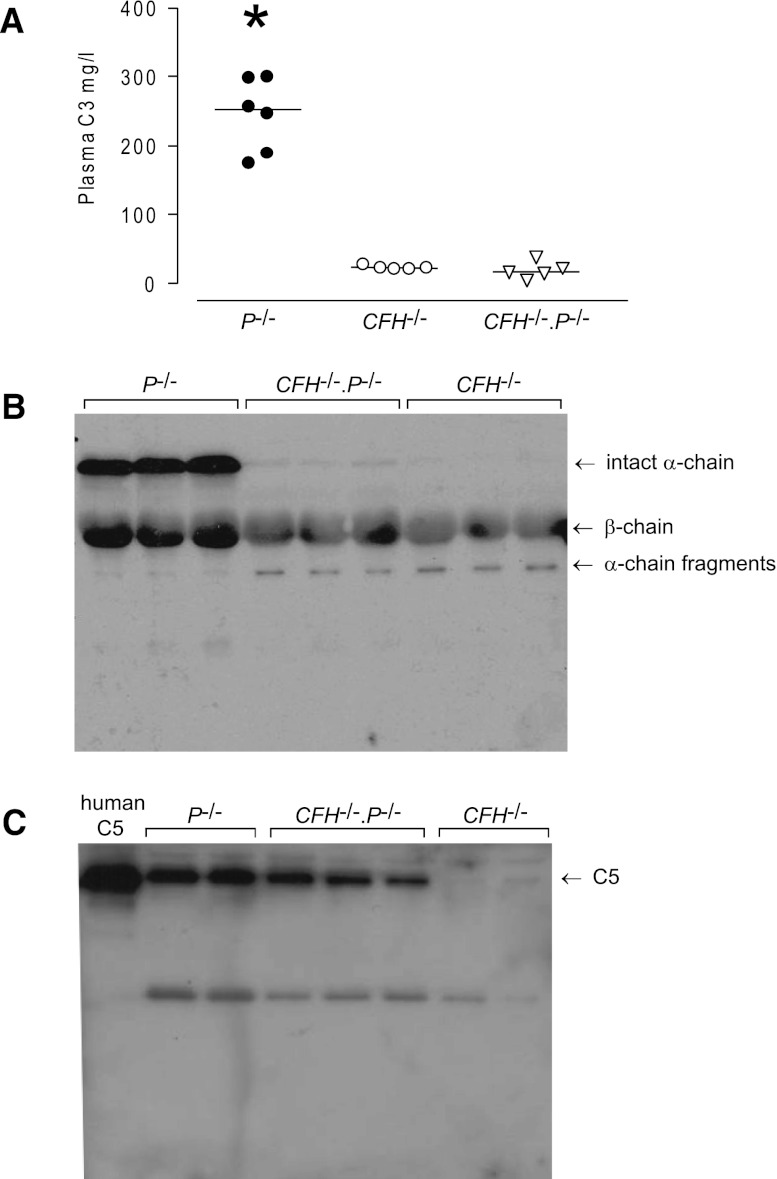
Both circulating C34 and C513 are severely reduced in CFH−/− mice. In contrast, plasma C3 levels in P−/− mice have previously been shown to be normal.11 To determine the contribution of properdin to these observations, we next examined C3 and C5 levels in CFH−/−.P−/− mice. Plasma C3 levels did not differ between CFH−/− (median 23 g/L; range, 21–28; n=5) and CFH−/−.P−/− mice (median 17 g/L; range, 5–38.3; n=5). In both groups, plasma C3 levels were severely reduced compared with that seen in the P−/− mice (median 252 g/L; range, 175.2–300.7; n=6; P<0.001 versus either CFH−/− or CFH−/−.P−/− mice, Bonferroni multiple comparison test) (Figure 1A). To determine the activation status of C3, we performed Western blot analysis of plasma samples under reducing conditions, enabling the visualization of α-chain, β-chain, and α-chain fragments (Figure 1B). In P−/− mice, both intact α-chain and β-chain were detectable. In contrast, α-chain fragments, but no intact α-chain, were seen in the plasma of CFH−/− animals. In CFH−/−.P−/− mice, intact α-chain was barely visible, whereas α-chain fragments were comparable with that seen in CFH−/− mice. Using densitometry, the intact α-chain intensity in CFH−/−.P−/− mice was 0.9% of the mean intensity of the α-chain in P−/− mice.
Figure 1.
Properdin differentially influences plasma C3 and C5 levels in CFH−/−.P−/− mice. (A) Plasma C3 levels in P−/−, CFH−/−, and CFH−/−.P−/− mice. Plasma C3 are measured by ELISA. Levels are normal in P−/− mice, whereas levels are dramatically reduced to a comparable extent in both CFH−/− and CFH−/−.P−/− animals. Horizontal bars denote median values. *P<0.001 versus either CFH−/− or CFH−/−.P−/− groups, Bonferroni multiple comparison test. (B) Western blot for C3 under reducing conditions using sera from P−/−, CFH−/−, and CFH−/−.P−/− mice. Intact C3 α-chain and β-chain are seen in P−/− mice. In contrast, absent and barely visible intact α-chain is seen in CFH−/− and CFH−/−.P−/− mice, respectively. In both strains, α-chain fragments are demonstrable. (C) Western blot for C5 under nonreducing conditions using sera from P−/−, CFH−/−, and CFH−/−.P−/− mice. Under these conditions, whereas plasma C5 is readily detectable in P−/− mice, it is absent in CFH−/− animals. C5 is readily detectable in CFH−/−.P−/− mice, although the intensity is less than that seen in P−/− animals. Lane one contains purified human C5. In both B and C, 1 μl of EDTA plasma from the examined strains is loaded on the gels.
We next assessed plasma C5 levels in CFH−/−.P−/− mice using Western blotting under nonreducing conditions (Figure 1C) because we do not have an ELISA to directly quantify mouse C5. Plasma C5 was readily detectable in P−/− mice but absent in CFH−/− animals. The latter is consistent with the absence of C5 hemolytic activity in CFH−/− mice.13 Strikingly, plasma C5 was readily detectable in the CFH−/−.P−/− animals. Using densitometry, compared with the mean intensity of the α-chain in P−/− mice, the mean C5 intensity was 18% in CFH−/−.P−/− mice (n=3; Figure 1C) and 0.55% in CFH−/− mice (n=2; Figure 1C). These results indicated that plasma C5 depletion in CFH deficiency is, at least in part, properdin dependent. In contrast, plasma C3 depletion was almost totally properdin independent. We next examined glomerular C3 in CFH−/−.P−/− mice.
Properdin Deficiency Increased Glomerular C3 but not C9 Accumulation in CFH-Deficient Mice
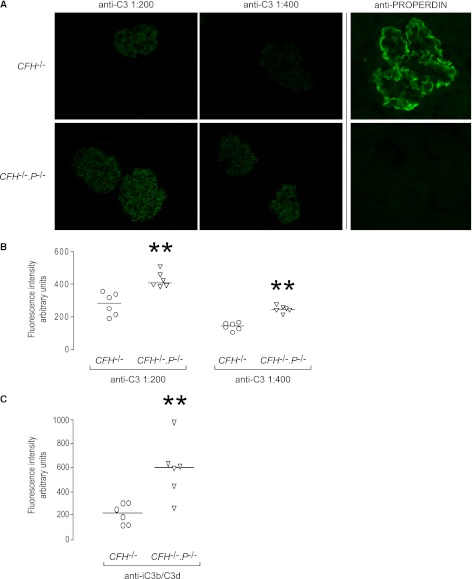
CFH−/− mice have large amounts of C3 along the GBM, which is readily visualized using immunofluorescence microscopy with FITC-conjugated polyclonal anti-mouse C3 antibodies.4,14 Using a FITC-conjugated goat anti-mouse C3 antibody, linear glomerular staining was evident in both CFH−/− and CFH−/−.P−/− animals (Figure 2A). Using a goat anti-human properdin antibody, properdin was readily detectable within glomeruli from untreated CFH−/− in a linear distribution similar to that of glomerular C3 (Figure 2A). As expected, no reactivity with this antibody was demonstrable in renal sections from CFH−/−.P−/− mice (Figure 2A). Unexpectedly, the intensity of glomerular C3 staining was significantly greater in CFH−/−.P−/− animals (Figure 2B). To determine if there was greater deposition of terminal complement components, we compared glomerular C9 staining between the two groups using a cross-reactive anti-rat C9 polyclonal antibody. Linear glomerular C9 staining was detected in both CFH−/− and CFH−/−.P−/− mice (Supplemental Figure 3A) but there was no difference in the median intensity between the two groups (Supplemental Figure 3B).
Figure 2.
Glomerular C3 reactivity is increased in CFH−/−.P−/− mice. (A) Representative images of glomerular C3 and properdin staining in CFH−/− and CFH−/−.P−/− mice. Two dilutions of anti-C3 antibody are used to determine if there is a difference in glomerular C3 staining. Glomerular properdin is present in CFH−/− mice in a pattern comparable with that seen for C3. As expected, no staining for properdin is seen in CFH−/−.P−/− mice. (B) Quantitative immunofluorescence demonstrates significantly greater glomerular C3 in CFH−/−.P−/− mice at both dilutions of anti-C3 antibody. (C) Quantitative immunofluorescence demonstrates significantly greater glomerular iC3b/C3d in CFH−/−.P−/− mice. Horizontal bars denote median values. **P<0.01 versus CFH−/− group, Mann–Whitney test. Original magnification, ×40.
To characterize the nature of the C3 fragments (C3b, iC3b, and C3dg) present along the GBM in CFH−/− and CFH−/−.P−/− animals, we analyzed glomerular C3 staining using two monoclonal anti-C3 antibodies that have differential reactivity to C3 fragments (i.e., reacting with either iC3b/C3c/C3b or iC3b/C3d) (Supplemental Figure 4). Using both monoclonal antibodies, linear glomerular C3 staining was demonstrable in CFH−/− and CFH−/−.P−/− animals. When we stained renal sections from CFI−/− mice that contain mesangial deposits of C3b,6 mesangial C3 was demonstrable using the polyclonal anti-C3 antibody and the anti-C3b/C3c/iC3b mAb but not the anti-iC3b/C3d mAb. This demonstrated that the anti-iC3b/C3d antibody did not recognize tissue-associated mouse C3b ex vivo. Using quantitative immunofluorescence, the glomerular staining intensity for iC3b/C3d was significantly greater in CFH−/−.P−/− versus CFH−/− animals (Figure 2C), similar to our findings with the polyclonal anti-C3 antibody (Figure 2B). Taken together, these findings demonstrated that the absence of properdin exacerbated glomerular iC3b/C3d accumulation in CFH deficiency.
No abnormal glomerular C3 staining was seen in P−/− mice (Supplemental Figure 4). Tubulointerstitial C3 staining is a normal finding in wild-type mice. C3 activation along proximal tubular epithelium has been considered to be properdin dependent.15 However, we could not detect any difference in the degree of tubulointerstitial staining between wild-type and P−/− mice (Supplemental Figure 5). We also noted that both CFH−/− and CFH−/−.P−/− mice had abnormal, but comparable, C3 staining within hepatic sinusoids and central veins (Supplemental Figure 5). The significance of the hepatic C3 staining in CFH−/− and CFH−/−.P−/− mice is unclear but is clearly properdin independent.
Properdin Deficiency Enhanced the Ability of a Single Injection of Human CFH to Increase Plasma C3 Levels in CFH-Deficient Mice
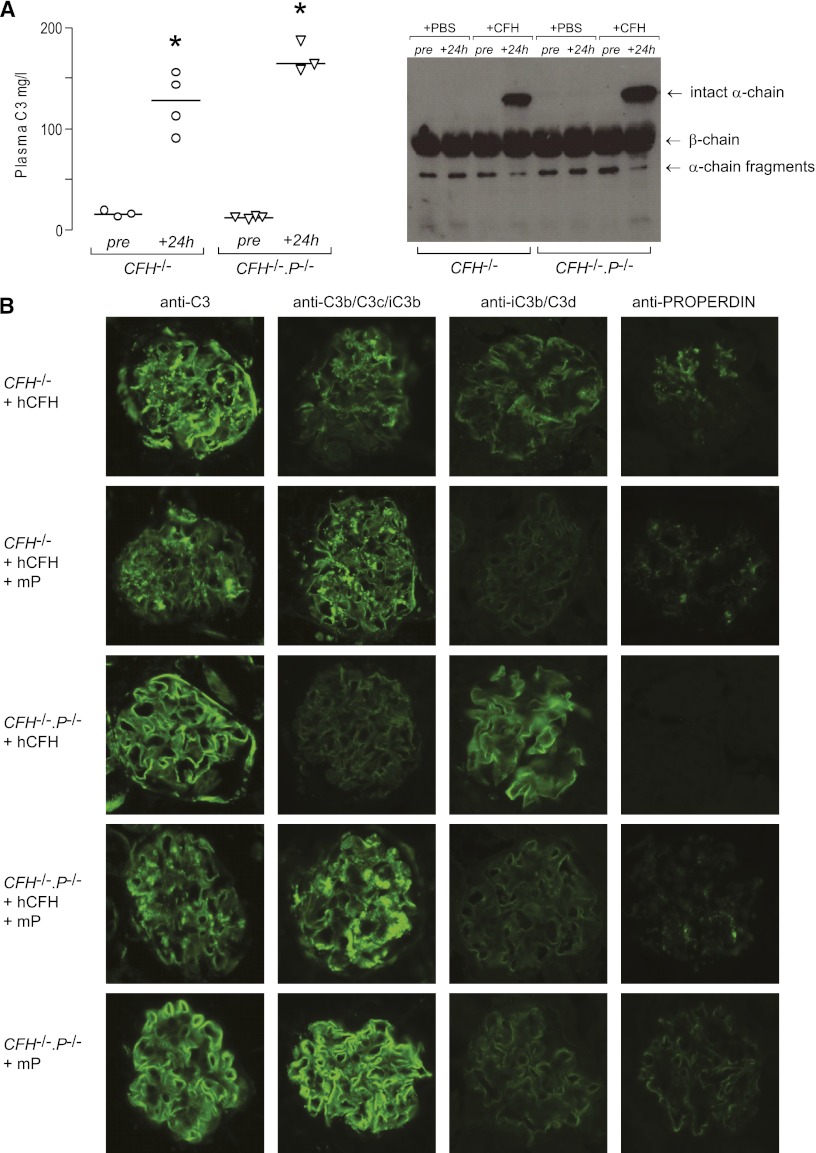
We previously reported that administration of either mouse14 or human16 CFH to CFH−/− mice results in a rapid rise in plasma C3 levels. We next examined the contribution of properdin to this response by measuring plasma C3 levels in CFH−/−.P−/− animals 24 hours after a single 0.5 mg intraperitoneal injection of human CFH. Plasma C3 levels increased in both CFH−/− and CFH−/−.P−/− mice treated with human CFH (Figure 3A), with the appearance of intact C3 α-chain in both groups (Figure 3A). However, the increase in plasma C3 was significantly greater in the CFH−/−.P−/− animals. This indicated that in the presence of endogenous properdin, the exogenous CFH was less potent at downregulating C3 convertase activity in CFH deficiency.
Figure 3.
Properdin influences the response of CFH−/− mice to human CFH. (A) Plasma C3 levels in CFH−/− and CFH−/−.P−/− mice measured by ELISA. Median C3 levels significantly rise in both CFH−/− and CFH−/−.P−/− mice 24 hours after administration of human CFH but the magnitude of this rise is significantly greater for CFH−/−.P−/− animals. Horizontal bars denote median values. *P<0.001 for both CFH−/− and CFH−/−.P−/− mice 24-hour values versus their respective pretreatment values, P<0.05 for CFH−/− versus CFH−/−.P−/− at 24 hours. P values derived from Bonferroni multiple comparison testing. Western blotting for C3 under reducing conditions using sera from CFH−/− and CFH−/−.P−/− mice. Intact C3 α-chain is seen in both groups 24 hours after administration of human CFH. (B) Representative images of glomerular C3 and properdin immunostaining 24 hours after administration of human CFH (hCFH) with and without mouse properdin (mP) to CFH−/− and CFH−/−.P−/− mice. Reduction in linear C3 staining together with the appearance of mesangial C3 staining is seen in CFH−/− but not CFH−/−.P−/− mice after hCFH. The pattern of glomerular properdin staining in the CFH−/− mice after human CFH is mesangial distinct from the linear properdin staining seen in unmanipulated CFH−/− mice (Figure 2A). Importantly, administration of both mouse properdin and human CFH to CFH−/−.P−/− mice results in both mesangial C3 reactivity and reduction in GBM C3 staining. Original magnification, ×40.
The Ability of a Single Injection of Human CFH to Ameliorate Glomerular C3 Deposition and Trigger Mesangial C3 Deposits in CFH-Deficient Mice is Properdin Dependent
We previously reported that, 24 hours after a single intraperitoneal injection of either human or mouse CFH to CFH−/− mice, there was a reduction in the preexisting linear glomerular C3 staining together with the concomitant appearance of mesangial C3 staining.14,16 We next examined the contribution of properdin to this response by assessing glomerular C3 staining in CFH−/−.P−/− animals 24 hours after a single 0.5 mg intraperitoneal injection of human CFH (Figure 3B). Consistent with our previous report,16 glomerular C3 staining in the injected CFH−/− mice showed a reduction in linear staining together with the appearance of mesangial C3 reactivity (Figure 3B). Twenty-four hours after human CFH administration, glomerular properdin remained detectable in CFH−/− animals but the staining pattern was now mesangial with little reactivity along capillary walls (Figure 3B) in contrast to the linear pattern seen in the unmanipulated CFH−/− animals (Figure 2A). Unexpectedly, injected CFH−/−.P−/− mice did not show either reduction in the preexisting linear C3 staining (Figure 3B) or appearance of mesangial C3 reactivity. Strikingly, when we administered exogenous mouse properdin (mP) in combination with human CFH to CFH−/−.P−/− mice, both mesangial C3 reactivity and reduction in GBM C3 staining were demonstrable (Figure 3B). Administration of properdin alone to CFH−/−.P−/− mice did not alter glomerular C3 distribution at 24 hours but we were able to detect properdin staining in an identical distribution to that of C3 (Figure 3B). Taken together, these data indicated that both the reduction in glomerular iC3b/C3d and appearance of mesangial C3 after administration of CFH to CFH−/− animals are properdin-dependent phenomena.
Discussion
Our data demonstrate that properdin deficiency exacerbated renal injury in mice lacking CFH. This is an intriguing finding because previous reports have demonstrated that properdin deficiency protects mice against complement-mediated tissue injury.17 Properdin deficiency has been shown to prevent complement-mediated embryonic lethality in Crry-deficient embryos,17 ameliorate joint injury in an antibody-mediated, AP-dependent arthritis model (K/BxN),17 and prevent the complement-mediated extravascular lysis of Crry-deficient erythrocytes.18 In our study, properdin deficiency did not influence the response of wild-type or CFH−/− mice to experimentally triggered renal disease using the heterologous NTN model.
Although properdin has long been recognized to function as a stabilizer for the AP C3 convertase, it has also been suggested that properdin may initiate AP activation on surfaces (reviewed in Kemper et al.7) by interacting with surface AP C3 convertase19 or microbial antigens.20 In support of the latter observation, murine studies have shown that properdin is essential for LPS-induced AP activation18 and influences susceptibility to polymicrobial sepsis.11 However, for some pathogens (e.g., Neisseria gonorrhoeae and Neisseria meningitidis), it is clear that properdin does not interact directly with the pathogen but is required for stabilization of the AP C3 convertase and hence optimal pathogen opsonisation.21
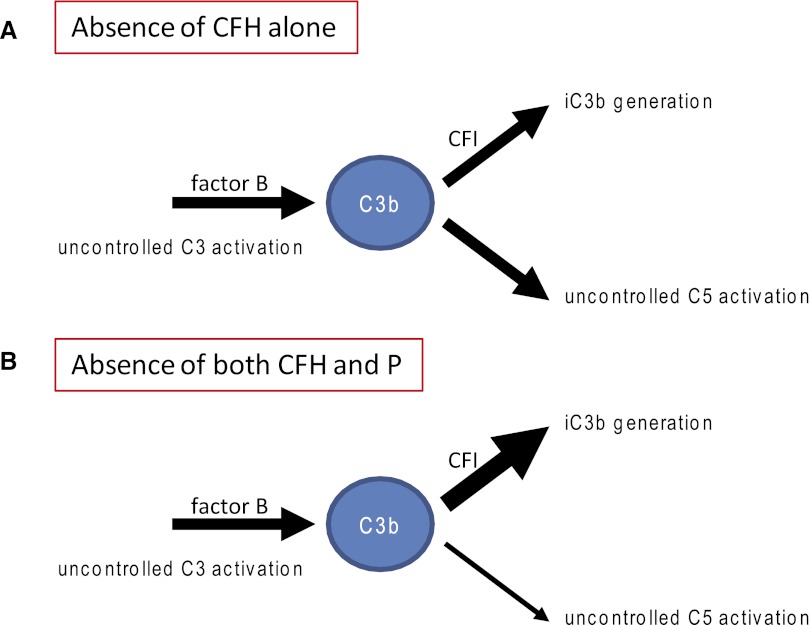
Our previous studies in CFH−/− mice have shown that activation of C3 is critical for the development of renal disease.4,12 The prevention of conversion of C3b to iC3b, achieved through genetic deficiency of CFI, prevented C3 accumulation along the GBM in CFH−/− mice.6 When mice with combined deficiency of CFH and CFI were given a source of CFI, the preexisting circulating C3b in these animals was rapidly converted to iC3b with the concomitant appearance of GBM C3 accumulation. GBM-associated C3 in CFH−/− mice contains iC3b.14 Our present data show that properdin deficiency exacerbated glomerular C3 accumulation and renal injury in CFH−/− mice. We expected that the AP C3 convertase would also be less efficient in the absence of properdin. Consistent with this, although there was no significant difference in the plasma C3 levels between the CFH−/− and CFH−/−.P−/− animals, intact C3-α chain was only detectable in plasma from CFH−/−.P−/− animals. Plasma C5 levels were significantly increased in CFH−/−.P−/− mice, suggesting that the absence of properdin reduced C5 convertase efficacy. We speculate that the increased availability of intact C3 and reduced utilization of C3b by C5 convertase may have increased the availability of plasma iC3b fragments to accumulate along the GBM in the CFH−/−.P−/− mice (Figure 4). In this respect, it is relevant that the production of iC3b in the fluid-phase by CFI with cofactor was properdin independent in vitro.22 The increased availability of C5 in CFH−/−.P−/− animals, through enhanced terminal pathway activation, may have significantly contributed to the increased glomerular inflammation seen in this strain.
Figure 4.
Comparison of uncontrolled C3 activation in CFH and combined CFH and properdin deficiency states. (A) In CFH-deficient mice, there is uncontrolled activation of both C3 and C5. As a consequence, plasma levels of both C3 and C5 are severely reduced. Uncontrolled activation of C3 results in the generation of C3b fragments such as iC3b, a process that is dependent on the enzyme factor I. We have previously shown that the absence of either factor B or factor I but not C5 prevented the accumulation of C3 along the GBM. (B) In CFH-deficient mice lacking properdin uncontrolled C3 activation persists, whereas plasma C5 depletion, although present, is less severe (thinner arrow). Glomerular C3 accumulation is increased in these animals. One explanation could be that reduced C3b utilization by the C5 convertase increases the amount of C3b that could be cleaved to iC3b and deposit along the GBM.
Important related insights are derived from characterization of mice (CFHM/M) expressing low levels of a mutant CFH protein lacking SCR domains 19 and 20.23 Like CFH−/− animals, CFHM/M mice have severe and comparable reduction in circulating C3 levels due to the fact that only very low amounts of this protein are expressed (Supplemental Figure 6).23 Properdin deficiency exacerbated renal injury in both CFH−/− and CFHM/M animals, although this was most marked for the CFHM/M.P−/− strain, which all died prematurely from lethal C3 GN resembling human DDD.23 Interestingly, compared with CFH−/− and CFH−/−.P−/− mice, CFHM/M and CFHM/M.P−/− mice have higher plasma C3 levels (Supplemental Figure 6). Furthermore, CFHM/M but not CFH−/− mice have abnormal mesangial C3 staining that is properdin dependent (Supplemental Figure 6). Here we show that mesangial C3 staining triggered by the administration of human CFH to CFH−/− mice was also properdin dependent. Properdin therefore critically influences the intraglomerular distribution of C3 during C3 dysregulation secondary to CFH dysfunction. We speculate that mesangial staining may reflect surface activation that requires properdin and/or properdin may interact with C3 fragments directly and influence whether C3 deposits within the mesangium or along the GBM.
How do these data contribute to our understanding of human C3 glomerulopathy? DDD is strongly associated with uncontrolled plasma C3 activation, which is most commonly due to C3 nephritic factor (C3NeF), an IgG autoantibody that stabilizes the AP C3 convertase (reviewed in Smith et al.2). It has long been recognized that C3NeF are heterogeneous with respect to their ability to activate complement.24 Some were able to activate C3 potently without any effect on terminal pathway activation, whereas others potentiated both C3 and terminal pathway activation. The former were properdin independent (P-indepC3NeF), whereas the latter were properdin dependent (P-depC3NeF).24–26 P-depC3NeF were predominantly detected in patients with MPGN types 1 and 3, whereas P-indepC3NeF was typically detected in patients with MPGN type 2 (now termed DDD).25,27 Predominant C3 depletion was seen in the CFH−/−.P−/− animals and is analogous to the complement profile seen in humans with P-indepC3NeF. Depletion of both C3 and C5 was seen in CFH−/− animals and is analogous to the complement profile seen in humans with P-depC3NeF. These data would suggest that CFH−/−.P−/− mice, in which there is predominant depletion of C3, would better model the complement dysregulation and renal pathology seen in human DDD than CFH−/− animals. Our data demonstrated predominant C3 depletion in CFH−/−.P−/− mice (Figure 1) and, compared with CFH−/− mice, significantly greater capillary wall thickening and GBM double contours (Table 1).
It is also intriguing to speculate that factors that alter properdin levels among individuals with CFH mutations may contribute to renal injury by changing the intraglomerular C3 distribution. Exacerbation of renal injury is common in individuals with CFH and or CFH-related protein mutations during infection, sometimes leading to the phenomenon of synpharyngitic macroscopic hematuria.28 Perhaps pathogens that activate complement may, through sequestration, temporally alter properdin levels and precipitate or enhance GBM complement deposition.
In conclusion, we have shown that properdin deficiency enhanced glomerular C3 accumulation and renal injury in CFH-deficient mice and recapitulated the complement profile reported in humans with P-indepC3NeF. Our data together with those of Lesher et al.23 also show that properdin influenced the intraglomerular fate of C3 during C3 dysregulation. Most importantly, these data indicated that therapeutic inhibition of properdin in situations of uncontrolled fluid-phase complement activation could be deleterious.
Concise Methods
Mice
CFH−/− 4 and properdin-deficient (Cfptm1cmst, herein denoted by P−/−)11 mice were generated as previously described and were back-crossed onto the C57BL/6 genetic background. C57BL/6 CFH−/−.P−/− mice were developed by intercrossing the CFH−/− and P−/− strains. All studies and protocols were performed in accordance with institutional guidelines and were approved by the United Kingdom Home Office.
C3 Quantification by ELISA
Coating antibody was a goat anti-mouse C3 polyclonal antibody (MP Biomedical LLC, France) used at a dilution of 1:8000 in 0.1 M carbonate buffer, pH 9.6. Captured mouse C3 was detected using a horseradish peroxidase-conjugated goat anti-mouse C3 polyclonal antibody (MP Biomedicals, France) used at a dilution of 1:25000 PBS/Tween. Plates were developed using TMB substrate (Sigma-Aldrich, UK). Concentration of plasma C3 was estimated by reference to a calibration curve constructed using reference sera containing a known amount of mouse C3 (Serum Amyloid P mouse standard; Calbiochem, Germany).
Western Blot Analyses of Mouse C3 and C5
Blood was collected from CFH−/−, P−/−, and CFH−/−.P−/− mice by cardiac puncture in the presence of EDTA, promptly chilled on ice and the plasma separated. The samples were separated using SDS-PAGE: 7.5% gel under nonreducing conditions for C5 and 10% gel under reducing conditions for C3 analysis. Detection antibodies were as follows: goat anti-serum to mouse C3 (MP Biomedicals) and goat anti-serum to human C5 (Quidel, US). Secondary antibody was HRP-conjugated anti-goat Ig (Sigma-Aldrich, UK). Blots were visualized using Pierce ECL Western Blotting substrate (Thermo Scientific, Belgium). Band densitometry was assessed using scanned auto-radiographs and ImageJ free software version 1.45 for Windows (Image Processing and Analysis in Java; National Institutes of Health, US).
Renal Immunostaining
Cryosections (5 µm) from snap-frozen kidneys were fixed in acetone for 10 minutes. C3 was visualized using a FITC-conjugated goat anti-mouse C3 polyclonal antibody (MP Biomedicals), a biotinylated mouse anti-human iC3b/C3d mAb (gift from Professor Santiago Rodríguez de Cordoba, Centro de Investigaciones Biológicas, Madrid), and a rat anti-mouse C3b/C3c/iC3b mAb (Hycult, The Netherlands). The latter was visualized using streptavidin Alexa Fluor 488 (Life Technologies, UK). Mouse C9 was visualized using a rabbit anti-rat C9 polyclonal antibody that cross-reacts with mouse C9 (gift from Professor B. Paul Morgan, Cardiff University, UK) and a FITC-conjugated mouse anti-rabbit IgG mAb (Sigma-Aldrich,). Quantitative immunofluorescence staining was performed as previously described.29 Ten glomeruli were assessed per section and fluorescence intensity expressed in arbitrary units. Properdin was visualized using FITC-conjugated goat anti-human properdin polyclonal antibody (Nordic Immunologic Laboratories, The Netherlands).
Renal Histology
For light microscopy, kidneys were fixed in Bouin’s solution and embedded in paraffin. Sections were stained with periodic acid–Schiff reagent. Histologic analysis was performed in a blinded manner and 50 glomeruli per section were scored as previously described.4 For electron microscopy, 1-mm cubed renal cortical tissue fragments were fixed in 3% glutaraldehyde and incubated in cacodylate buffer. The samples were embedded in Spurr resin. Ultrathin sections were impregnated with heavy metals using 1% aqueous uranyl acetate and Reynold’s lead citrate.
Renal Function
Albuminuria was measured as described previously.12 Briefly, mice were placed in metabolic cages overnight to enable accurate collection and volume measurement. Urinary albumin was measured by radial immunodiffusion using a rabbit anti-mouse albumin antibody (AbD Serotec, UK). Purified mouse albumin (Sigma-Aldrich) was used to generate a reference curve. Plasma urea was measured using an enzymatic ultraviolet method (R-Biopharm Rhone Ltd, Germany) according to the manufacturer’s instructions.
Administration of Human CFH, Mouse Properdin, and Induction of Nephrotoxic Nephritis
Human CFH was prepared and administered intraperitoneally as previously described.16 Recombinant mouse properdin (160 μg) was administered intraperitoneally. The mice were sacrificed and tissue samples harvested 24 hours after injection. Induction of heterologous serum nephrotoxic nephritis was also performed as previously described.12
Recombinant Properdin
The coding sequence of murine properdin was amplified from mouse liver cDNA using the following primers: (1) 5′- AAG CTT ATG CCT GCT GAA ATG CAA GCC C-3′ and (2) 5′-CTC GAG AGT AGG GTT TCT TCT CTT CTG GGT CTT T-3′. These primers introduced restriction sites for HindIII and XhoI for subsequent cloning into pSecTag2/HygroB in a frame with and immediately upstream of nucleotides encoding 6-histidine tag and stop codon. Primer 2 replaced the stop codon (TAA) of the murine properdin cDNA. CHO-k1 cells were transfected using GeneJuice reagent (Novagen) according to the manufacturer’s protocol. Stable transfected cell line was generated by selection on Hygromycin B (300 μg/ml, Invitrogen).
Statistical Analyses
Data were analyzed using GraphPad Prism software (version 3.0; GraphPad, US). The Mann–Whitney test was used for comparison of two groups. The Bonferroni multiple comparison test was used for comparison of three or more groups.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Professor Santiago Rodríguez de Cordoba, Centro de Investigaciones Biológicas, Madrid, for the anti-human iC3b/C3dg antibody; Professor B. Paul Morgan, Cardiff University, United Kingdom, for the anti-rat C9 antibody; and Dr. Jill Moss for the electron microscopy expertise.
M.C.P. is a Wellcome Trust Senior Fellow in Clinical Science (WT098476MA) and M.M.R. is funded by this fellowship. K.A.V. is a Kidney Research UK Clinical Fellow (TF8/2009). A.M.L. is supported by a predoctoral fellowship from the American Heart Association. W.C.S. is supported by grants from the National Institutes of Health (AI085596, AI44970, AI49344) and a grant from the Kidneeds Foundation. C.M.S. was funded by a grant from the Medical Research Council (G0400300).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Unexpected Role for Properdin in Complement C3 Glomerulopathies,” on pages 5–7.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012060571/-/DCSupplemental.
References
- 1.Fakhouri F, Frémeaux-Bacchi V, Noël LH, Cook HT, Pickering MC: C3 glomerulopathy: A new classification. Nat Rev Nephrol 6: 494–499, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Smith RJ, Harris CL, Pickering MC: Dense deposit disease. Mol Immunol 48: 1604–1610, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pickering MC, Cook HT: Translational mini-review series on complement factor H: Renal diseases associated with complement factor H: Novel insights from humans and animals. Clin Exp Immunol 151: 210–230, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pickering MC, Cook HT, Warren J, Bygrave AE, Moss J, Walport MJ, Botto M: Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet 31: 424–428, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Høgåsen K, Jansen JH, Mollnes TE, Hovdenes J, Harboe M: Hereditary porcine membranoproliferative glomerulonephritis type II is caused by factor H deficiency. J Clin Invest 95: 1054–1061, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rose KL, Paixao-Cavalcante D, Fish J, Manderson AP, Malik TH, Bygrave AE, Lin T, Sacks SH, Walport MJ, Cook HT, Botto M, Pickering MC: Factor I is required for the development of membranoproliferative glomerulonephritis in factor H-deficient mice. J Clin Invest 118: 608–618, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kemper C, Atkinson JP, Hourcade DE: Properdin: Emerging roles of a pattern-recognition molecule. Annu Rev Immunol 28: 131–155, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Sjöholm AG, Kuijper EJ, Tijssen CC, Jansz A, Bol P, Spanjaard L, Zanen HC: Dysfunctional properdin in a Dutch family with meningococcal disease. N Engl J Med 319: 33–37, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Söderström C, Sjöholm AG, Svensson R, Ostenson S: Another Swedish family with complete properdin deficiency: Association with fulminant meningococcal disease in one male family member. Scand J Infect Dis 21: 259–265, 1989 [DOI] [PubMed] [Google Scholar]
- 10.Späth PJ, Sjöholm AG, Fredrikson GN, Misiano G, Scherz R, Schaad UB, Uhring-Lambert B, Hauptmann G, Westberg J, Uhlén M, Wadelius C, Truedsson L: Properdin deficiency in a large Swiss family: Identification of a stop codon in the properdin gene, and association of meningococcal disease with lack of the IgG2 allotype marker G2m(n). Clin Exp Immunol 118: 278–284, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stover CM, Luckett JC, Echtenacher B, Dupont A, Figgitt SE, Brown J, Männel DN, Schwaeble WJ: Properdin plays a protective role in polymicrobial septic peritonitis. J Immunol 180: 3313–3318, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Pickering MC, Warren J, Rose KL, Carlucci F, Wang Y, Walport MJ, Cook HT, Botto M: Prevention of C5 activation ameliorates spontaneous and experimental glomerulonephritis in factor H-deficient mice. Proc Natl Acad Sci U S A 103: 9649–9654, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Jorge EG, Macor P, Paixão-Cavalcante D, Rose KL, Tedesco F, Cook HT, Botto M, Pickering MC: The development of atypical hemolytic uremic syndrome depends on complement C5. J Am Soc Nephrol 22: 137–145, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paixão-Cavalcante D, Hanson S, Botto M, Cook HT, Pickering MC: Factor H facilitates the clearance of GBM bound iC3b by controlling C3 activation in fluid phase. Mol Immunol 46: 1942–1950, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaarkeuken H, Siezenga MA, Zuidwijk K, van Kooten C, Rabelink TJ, Daha MR, Berger SP: Complement activation by tubular cells is mediated by properdin binding. Am J Physiol Renal Physiol 295: F1397–F1403, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Fakhouri F, de Jorge EG, Brune F, Azam P, Cook HT, Pickering MC: Treatment with human complement factor H rapidly reverses renal complement deposition in factor H-deficient mice. Kidney Int 78: 279–286, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura Y, Zhou L, Miwa T, Song WC: Genetic and therapeutic targeting of properdin in mice prevents complement-mediated tissue injury. J Clin Invest 120: 3545–3554, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimura Y, Miwa T, Zhou L, Song WC: Activator-specific requirement of properdin in the initiation and amplification of the alternative pathway complement. Blood 111: 732–740, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hourcade DE: The role of properdin in the assembly of the alternative pathway C3 convertases of complement. J Biol Chem 281: 2128–2132, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Spitzer D, Mitchell LM, Atkinson JP, Hourcade DE: Properdin can initiate complement activation by binding specific target surfaces and providing a platform for de novo convertase assembly. J Immunol 179: 2600–2608, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Agarwal S, Ferreira VP, Cortes C, Pangburn MK, Rice PA, Ram S: An evaluation of the role of properdin in alternative pathway activation on Neisseria meningitidis and Neisseria gonorrhoeae. J Immunol 185: 507–516, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farries TC, Lachmann PJ, Harrison RA: Analysis of the interactions between properdin, the third component of complement (C3), and its physiological activation products. Biochem J 252: 47–54, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lesher AM, Zhou L, Kimura Y, Sato S, Gullipalli D, Herbert AP, Barlow PN, Eberhardt HU, Skerka C, Zipfel PF, Hamano T, Miwa T, Tung KS, Song WC: Lethal murine C3 glomerulonephritis resembling human dense deposit disease caused by combined fH C-terminal mutation and properdin deficiency. J Am Soc Nephrol 24: 53–65, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mollnes TE, Ng YC, Peters DK, Lea T, Tschopp J, Harboe M: Effect of nephritic factor on C3 and on the terminal pathway of complement in vivo and in vitro. Clin Exp Immunol 65: 73–79, 1986 [PMC free article] [PubMed] [Google Scholar]
- 25.Clardy CW, Forristal J, Strife CF, West CD: A properdin dependent nephritic factor slowly activating C3, C5, and C9 in membranoproliferative glomerulonephritis, types I and III. Clin Immunol Immunopathol 50: 333–347, 1989 [DOI] [PubMed] [Google Scholar]
- 26.Tanuma Y, Ohi H, Hatano M: Two types of C3 nephritic factor: Properdin-dependent C3NeF and properdin-independent C3NeF. Clin Immunol Immunopathol 56: 226–238, 1990 [DOI] [PubMed] [Google Scholar]
- 27.Varade WS, Forristal J, West CD: Patterns of complement activation in idiopathic membranoproliferative glomerulonephritis, types I, II, and III. Am J Kidney Dis 16: 196–206, 1990 [DOI] [PubMed] [Google Scholar]
- 28.Gale DP, Pickering MC: Regulating complement in the kidney: Insights from CFHR5 nephropathy. Dis Model Mech 4: 721–726, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robson MG, Cook HT, Botto M, Taylor PR, Busso N, Salvi R, Pusey CD, Walport MJ, Davies KA: Accelerated nephrotoxic nephritis is exacerbated in C1q-deficient mice. J Immunol 166: 6820–6828, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.