Abstract
Prolonged prednisolone treatment for the initial episode of childhood nephrotic syndrome may reduce relapse rate, but whether this results from the increased duration of treatment or a higher cumulative dose remains unclear. We conducted a randomized, double-blind, placebo-controlled trial in 69 hospitals in The Netherlands. We randomly assigned 150 children (9 months to 17 years) presenting with nephrotic syndrome to either 3 months of prednisolone followed by 3 months of placebo (n=74) or 6 months of prednisolone (n=76), and median follow-up was 47 months. Both groups received equal cumulative doses of prednisolone (approximately 3360 mg/m2). Among the 126 children who started trial medication, relapses occurred in 48 (77%) of 62 patients who received 3 months of prednisolone and 51 (80%) of 64 patients who received 6 months of prednisolone. Frequent relapses, according to international criteria, occurred with similar frequency between groups as well (45% versus 50%). In addition, there were no statistically significant differences between groups with respect to the eventual initiation of prednisolone maintenance and/or other immunosuppressive therapy (50% versus 59%), steroid dependence, or adverse effects. In conclusion, in this trial, extending initial prednisolone treatment from 3 to 6 months without increasing cumulative dose did not benefit clinical outcome in children with nephrotic syndrome. Previous findings indicating that prolonged treatment regimens reduce relapses most likely resulted from increased cumulative dose rather than the treatment duration.
Nephrotic syndrome (NS) is the most common manifestation of glomerular disease in childhood. Despite its relatively low incidence of 1–7 in 100,000 children,1,2 NS poses recurring challenges to many clinicians.
Corticosteroids induce remission of proteinuria in 90%–95% of patients.3–6 Despite this high initial response rate, relapses occur in 60%–90% of the initial responders.6,7 The disease progresses to frequent relapses, often accompanied by steroid dependence, in around 20%–60% of patients. Recurrent or continuous corticosteroid therapy in these patients frequently results in corticosteroid toxicity.1 This finding calls for the improvement of existing treatment regimens, for which no international consensus currently exists.8
The present treatment modalities for initial childhood NS are mostly based on reports by the International Study of Kidney Disease in Children and the Arbeitsgemeinschaft für Pädiatrische Nephrologie. Currenty used regimens vary in dose and duration (Supplemental Table 1).6,9,10 The regimen prescribed in The Netherlands is made up of 60 mg/m2 prednisolone daily for 6 weeks followed by 40 mg/m2 prednisolone on alternate days for 6 weeks.10 The cumulative dose of this regimen is 3360 mg/m2.
In 2000, Hodson et al.11 performed a meta-analysis of corticosteroid therapy in childhood NS to evaluate the potential benefits of different corticosteroid regimens.11 Based on the analysis of seven clinical trials in patients with an initial episode of NS, it was concluded that the risk of relapse was significantly reduced by prednisolone regimens that were both longer and more intensive. Additional analysis suggested that the benefits were more likely to be related to the increased duration of the treatment than the higher cumulative dose. However, collinearity between treatment duration and dose prevented the work by Hodson et al.11 from drawing definite conclusions.11 A subsequent study by Hiraoka et al.12 comparing 3 months of prednisolone treatment to 6 month of treatment was also inconclusive. In this study, prolonged treatment reduced the relapse rate in children ages under 4 years; however, this intervention also consisted of a higher cumulative dose.12 The independent effects of treatment duration and cumulative dose, thus, remained undetermined.
Based on these data, we designed a study protocol to explore the independent effect of treatment duration. In the present study, we hypothesized that prolongation of a 3-month initial prednisolone treatment to 6 months using equal cumulative doses would reduce the occurrence of frequently relapsing NS (FRNS) without increasing adverse effects.
Results
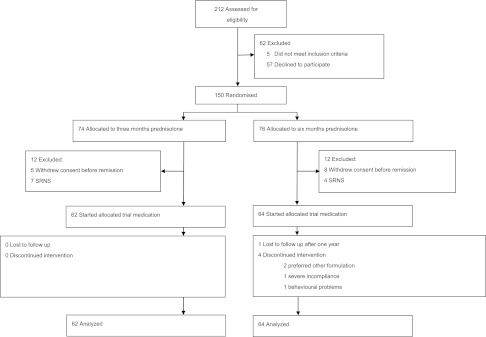
From February of 2005 to December of 2009, 212 patients were evaluated for eligibility. Participants and nonparticipants were similar in terms of sex and age at onset (Supplemental Table 2); 150 patients from 69 hospitals (60 general and 9 university hospitals) were randomized to either 3 months prednisolone followed by 3 months placebo or 6 months prednisolone (Figure 1). In both groups, 12 patients could not start trial medication because of either steroid resistance or withdrawn consent. These patients were excluded from the analysis. Median follow-up was 47 months in the 3-month group (interquartile range [IQR]=32–60) and 47 months in the 6-month group (IQR=37–60).
Figure 1.
Patients were analysed in a modified intention to treat analysis. All patients that started trial medication were analysed according to their allocated treatment. SRNS, steroid-resistant NS.
Induction therapy and trial medication were administered within a total of 24 weeks in both groups. The prescribed cumulative dose of prednisolone in the 6-month group depended on the number of days to remission, which is shown in Figure 2. Because the median number of days to remission was 10 days in both groups (IQR=8–14 and 7–14 days, respectively), the median prescribed cumulative prednisolone dose was 3360 mg/m2 in the 3-month group and 3390 mg/m2 in the 6-month group. Baseline characteristics revealed no relevant differences between the two groups (Table 1); 65% of the study population was of Western European descent.
Figure 2.
Treatment regimens were built up of comparable cumulative doses of prednisolone. The dotted line represents the median number of days to remission (10 days in both groups), the gray area represents the IQR. Doses are in mg/m2. AD, alternate days; D, daily.
Table 1.
Baseline characteristics
| Overall (n=126) | 3 Months Prednisolone (n=62) | 6 Months Prednisolone (n=64) | |
|---|---|---|---|
| Male, n (%) | 86 (68) | 39 (63) | 47 (73) |
| Age (yr) median (IQR) | 4.2 (3.2–6.2) | 4.7 (3.2–5.8) | 3.8 (3.2–6.4) |
| BPa (mean ± SD) | |||
| Systolic, Z-value | 1.7±1.3b | 1.7±1.3c | 1.6±1.3d |
| Diastolic, Z-value | 1.6±1.1b | 1.7±1.3c | 1.6±1.0d |
| Serum albumin (g/L) median (IQR) | 14.0 (10.0–16.2) | 14.0 (10.0–17.0) | 13.4 (10.0–16.0) |
| Microscopic hematuriae, n (%) | 40 (33)f | 19 (32)g | 21 (34)h |
| Hospital, n (%) | |||
| University | 14 (11.1) | 5 (8.0) | 9 (14.1) |
| General | 112 (88.9) | 57 (92.0) | 55 (85.9) |
| Descent, n (%) | |||
| Western European | 83 (65.9) | 46 (74.2) | 37 (57.8) |
| Non-Western European | 16 (12.7) | 6 (9.7) | 10 (15.6) |
| Mixed | 13 (10.3) | 3 (4.8) | 10 (15.6) |
| Not reported | 14 (11.1) | 7 (11.3) | 7 (10.9) |
| Quarterly distribution of disease onset, n (%) | |||
| January to March | 25 (19.8) | 14 (22.6) | 11 (17.2) |
| April to June | 24 (19.0) | 11 (17.7) | 13 (20.3) |
| July to September | 40 (31.7) | 19 (30.6) | 21 (32.8) |
| October to December | 37 (29.4) | 18 (29.0) | 19 (29.7) |
Lowest BP reported in patient’s chart at diagnosis. Z-values are adjusted for sex, age, and height.33
Data available for 123 of 126 patients.
Data available for 61 of 62 patients.
Data available for 62 of 64 patients.
Defined as >5 erythrocytes/field; if cell count not available, ≥+ on dipstick analysis.
Data available for 121 of 126 patients.
Data available for 59 of 62 patients.
Data available for 62 of 64 patients.
FRNS was scored and analyzed according to strict definitions (strict FRNS) as well as a broader, clinically relevant definition (clinical FRNS) as explained below.
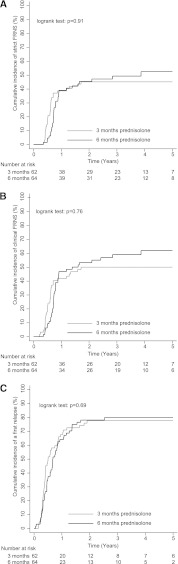
The cumulative incidences of FRNS did not reveal a benefit of the 6-month regimen, regardless of the definition used (Table 2). Strict FRNS was found in 28 of 62 children (45%) in the 3-month group and 32 of 64 children (50%) in the 6-month group (log rank test: P=0.91) (Figure 3A and Table 3). Three patients in the 3-month group and six patients in the 6-month group did not meet the strict criteria for FRNS, but they were characterized as having clinical FRNS (Supplemental Table 3B). Accordingly, clinical FRNS occurred in 31 of 62 children (50%) in the 3-month group versus 38 of 64 children (59%) in the 6-month group (log rank test: P=0.76) (Figure 3B).
Table 2.
Kaplan–Meier estimates of the cumulative incidences of strict and clinical FRNS
| 3-Month Group (n=62) | 6-Month Group (n=64) | Difference (%; 95% CI) | Log Rank Test | |
|---|---|---|---|---|
| Strict FRNS (%) | ||||
| 6 months | 14.5±4.5 | 3.1±2.2 | −11.40 (−21.20, −1.60) | |
| 1 year | 38.7±6.2 | 39.1±6.1 | 0.40 (−16.60, 17.40) | |
| 2 years | 45.2±6.3 | 45.6±6.2 | 0.40 (−16.90, 17.70) | |
| 3 years | 45.2±6.3 | 49.3±6.4 | 4.10 (−13.50, 21.70) | |
| 4 years | 45.2±6.3 | 52.5±6.7 | 7.30 (−10.70, 25.30) | |
| 5 years | 45.2±6.3 | 52.5±6.7 | 7.30 (−10.70, 25.30) | P=0.91 |
| Clinical FRNS (%) | ||||
| 6 months | 17.7±4.9 | 10.9±3.9 | −6.80 (−19.10, 5.50) | |
| 1 year | 41.9±6.3 | 46.9±6.2 | 5.00 (−9.10, 19.10) | |
| 2 years | 50.1±6.4 | 53.3±6.3 | 3.20 (−14.40, 20.80) | |
| 3 years | 50.1±6.4 | 59.4±6.4 | 9.30 (−8.40, 27.00) | |
| 4 years | 50.1±6.4 | 59.4±6.4 | 12.20 (−5.70, 30.10) | |
| 5 years | 50.1±6.4 | 62.3±6.5 | 12.20 (−5.70, 30.10) | P=0.76 |
Data are expressed as percentages ± SEMs at 6 months and yearly afterward. Between-group differences are expressed as percentages with 95% CIs. Log rank tests were performed on all available data at the end of follow-up. Clinical FRNS, FRNS according to the definition of strict FRNS or other indications for additional treatment measures (e.g., prednisolone maintenance therapy, ciclosporin, etc.); strict FRNS, FRNS based on more than or equal to two relapses within 6 months after initial treatment or four relapses within any 12 months.
Figure 3.
Initial prednisolone treatments of 3 and 6 months resulted in similar therapeutic outcome. Kaplan–Meier curves represent cumulative incidences of (A) strict FNRS based on more than or equal to two relapses within 6 months after initial treatment or four relapses within any 12 months, (B) clinical FRNS according to either the definition of strict FRNS or a clinical indication for additional treatment (e.g., prednisolone maintenance therapy, cyclophosphamide, etc.), and (C) cumulative incidence of a first relapse.
Table 3.
Distribution of patients according to three criteria for FRNS
| 3-Month Group (n=62) | 6-Month Group (n=64) | |
|---|---|---|
| A (two relapses within 6 months after ending first treatment), n | 23 (11 SD) | 18 (11 SD) |
| B (four relapses within any period of 12 months), n | 5 (3 SD) | 14 (10 SD) |
| C (need for additional treatment for other reasons than A or B), n | 3 (1 SD) | 6 (3 SD) |
| Strict FRNS (A or B) | 28 (45%) | 32 (50%) |
| Clinical FRNS (A, B, or C) | 31 (50%) | 38 (59%) |
Patients fulfilling criterion A or B were characterized as strict FRNS. Patients fulfilling criterion A, B, or C were characterized as clinical FRNS. Numbers of patients that also fulfilled criteria for steroid dependence are shown in parentheses. Detailed information on patients fulfilling only criterion C is presented in Supplemental Table 3. SD, steroid dependence.
The cumulative incidences of first relapses were similar in the two treatment groups. At least one relapse occurred in 48 of 62 children (77%) in the 3-month group and 51 of 64 children (80%) in the 6-month group. Median survival time from randomization to the first relapse was 6 months (95% confidence interval [CI]=4.00–8.00) in the 3-month group and 8 months (95% CI=6.00–10.00) in the 6-month group (log rank test: P=0.69) (Figure 3C).
Children allocated to the 6-month group experienced more relapses during follow-up compared with the 3-month group, although differences were not statistically significant. The median total number of relapses during follow-up was 2.5 (IQR=1.0–5.0) in the 3-month group and 4.0 (IQR=1.0–6.0) in the 6-month group (P=0.13). The median number of relapses per year of follow-up was 0.6 (IQR=0.2–1.4) and 1.0 (IQR=0.3–1.6), respectively (P=0.16). Simultaneous evaluation (performed with Poisson regression) of relapse rates in relation to treatment, sex, age category, and follow-up period (I, II, and III) showed no significant difference between treatments. The adjusted overall relative relapse rate (RRR) for the 3- compared with 6-month group was 0.81 (95% CI=0.60–1.09; P=0.16). The RRR was highest in the period between 6 and 12 months after diagnosis (1.5; P=0.008). The effect of treatment did not differ between the three follow-up periods (P=0.46).
Steroid dependence was noted less often in the 3-month group: 15 of 62 children (24%) versus 24 of 64 children (38%) in the 6-month group (Table 3). The difference did not reach statistical significance (log rank test, P=0.10).
Cox regression analysis revealed that boys tended to develop FRNS more often than girls, although differences were not statistically significant. For strict FRNS, the male versus female hazard ratio (HR) was 1.68 (95% CI=0.92–3.01; P=0.09); a similar HR was found for clinical FRNS: HR=1.72 (95% CI=0.98–3.03; P=0.06) (Table 4). Interaction between sex and treatment group was not significant, indicating that neither boys nor girls benefitted more from one treatment over the other. During follow-up, boys tended to have higher relapse rates than girls (RRR=1.4, P=0.05). Sex was not associated with the incidences of a first relapse or steroid dependence (Table 4). Age at onset (<4 or ≥4 years) had no effect on any of the therapeutic outcome events; the same was true for the number of days to remission (Table 4). Hematuria and BP at presentation were not related to development of any of the therapeutic outcome events (data not shown). Interestingly, five patients achieved remission after more than 4 weeks of daily prednisolone treatment. Of these patients, four patients had only one relapse, and one patient had no relapses at all during follow-up.
Table 4.
Adjusted multivariate analysis of treatment group, sex, age, and time to remission
| HR (95% CI) | ||||
|---|---|---|---|---|
| First Relapse | Strict FRNS | Clinical FRNS | SDNS | |
| Treatment: 3 versus 6 months | 1.11 (0.74–1.64) | 1.08 (0.65–1.80) | 0.97 (0.60–1.56) | 0.62 (0.32–1.18) |
| Sex: male versus female | 1.19 (0.77–1.84) | 1.68 (0.92–3.06) | 1.77 (0.98–3.03) | 1.96 (0.90–4.28) |
| Age: <4 versus ≥4 yr | 1.22 (0.82–1.82) | 0.97 (0.59–1.62) | 0.97 (0.60–1.56) | 1.30 (0.69–2.44) |
| Time to remission (per day) | 1.01 (0.99–1.04) | 0.96 (0.92–1.01) | 0.98 (0.95–1.02) | 0.98 (0.93–1.03) |
SDNS, steroid-dependent NS.
Secondary steroid resistance was noted in two patients allocated to the 3-month regimen and one patient allocated to the 6-month regimen.
Adverse effects were mostly transient and similar between the two groups (Table 5). Evaluation of height SD scores showed a significant decrease of growth at 3-months follow-up compared with baseline (P<0.01), which was restored within 1 year after the start of initial treatment. Growth did not differ between treatment groups (P=0.58) (Supplemental Figure 1). Overall height SD scores at baseline were lower than anticipated (−0.35±0.90). This observation was irrespective of descent (P=0.83).
Table 5.
Adverse effects
| 3 Months Prednisolone | 6 Months Prednisolone | P Value | |
|---|---|---|---|
| BP≥P95 | |||
| At diagnosis | 36/61 (59%) | 28/62 (45%) | 0.15 |
| At 3 months FU | 12/57 (21%) | 7/60 (12%) | 0.21 |
| At 6 months FU | 8/55 (14%) | 10/52 (19%) | 0.61 |
| Cushingoid appearance at 6 months FU | |||
| Cushing (moon face) | 14/59 (23.7%) | 21/58 (36.2%) | 0.14 |
| Striae | 3/58 (5.2%) | 4/60 (6.7%) | 1.00 |
| Ophtalmological abnormalities at 6 months FU | |||
| Glaucoma | 0/51 (0.0%) | 0/45 (0.0%) | — |
| Cataract | 1/53(1.9%)a | 0/46 (0.0%) | 1.00 |
| Severe infections | |||
| Pneumonia | 1/62 (1.6%) | 6/64 (9.4%) | 0.16 |
| Meningitis | 0/62 (0.0%) | 0/64 (0.0%) | — |
| Osteomyelitis | 0/62 (0.0%) | 0/64 (0.0%) | — |
| VZV reactivation | 2/62 (3.2%) | 1/64 (1.6%) | 0.62 |
| Whooping cough | 0/62 (0.0%) | 2/64 (3.1%) | 0.50 |
| Miscellaneousb | 3/62 (4.8%) | 1/64 (1.6%) | 0.36 |
| Overall | 6/62 (9.7%) | 10/64 (15.6%) | 0.42 |
| Dyspepsia | 1/62 (1.6%) | 2/64 (3.1%) | 1.00 |
| Thrombosis | 0/62 (0.0%) | 0/64 (0.0%) | — |
Data are expressed as number of events/number analyzed (percentages). FU, follow-up; VZV, Varicella Zoster virus.
Mild cataract, which was absent at diagnosis.
Three-month group: n=1 cellulitis, n=1 muscle abscess, n=1 intracranial abscess. Six-month group: n=1 appendicitis.
No effect of treatment was observed in the behavioral visual analog scales at any time. Compared with baseline, children scored significantly higher on eating, overactive behavior, and aggressive behavior at 3-months follow-up (all P value<0.01). These scores returned to baseline within 1 year in both groups. Scores for happiness temporarily dropped in the first 6 months, while scores for sleeping remained relatively stable over the whole observation period.
Bone mineral density (BMD) at 6 months was not different from baseline in both groups. Mean change in Z-scores of lumbar spine BMD was +0.09 (−0.17 to 0.36) and +0.33 (−0.06 to 0.71) in the 3- (n=17) and 6-month group (n=19), respectively (P=0.35). Mean change in Bone Health Index SD scores was −0.10 (−0.35 to 0.14) in the 3-month group (n=33) and −0.03 (−0.16 to 0.11) in the 6-month group (n=30; P=0.56).
Discussion
Our study shows that prolongation of initial prednisolone treatment from 3 to 6 months, while maintaining an equal cumulative dose, does not reduce the risk of frequent relapses in childhood NS. This finding challenges the previous assumption that prolonged treatment duration improves clinical outcome.
The high relapse rate in childhood NS initiated research aimed at improving prednisolone treatment regimens. A Cochrane meta-analysis of seven clinical trials by Hodson et al.7,11 last updated in 2007 showed that prednisolone regimens with both higher cumulative doses and longer treatment durations (up to 7 months and 5235 mg/m2) resulted in a reduction of relapses compared with a standard 2-month regimen (2240 mg/m2). The works by Hodson et al.7,11 assumed that longer duration of treatment was of greater importance than increased dose and suggested at least 3 months prednisolone should be given for the first episode of NS.7,11 Unfortunately, the existing studies have not led to international consensus. Two matters still deserved attention. First, the independent effects of treatment duration and dose remained unproven. Second, studies comparing 3-month regimens with longer regimens were of limited methodological quality. The present study addresses both issues for the first time.
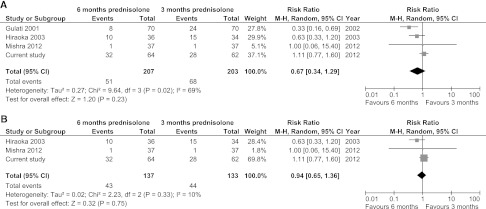
The main strength of our study is its design. To review our results in the context of other reports, we searched for studies comparing 3- with (approximately) 6-months prednisolone use for the initial episode of NS. Four studies had been reported in the work by Hodson et al.7 We found one additional study by Mishra et al.13 Characteristics of the five previous studies revealed several limitations (Supplemental Table 4). None of the studies included a placebo or blinding in their design12–16; allocation concealment was inadequate or not reported in three studies.13,14,16 In at least one study, patients who did not complete study medication were excluded from the analysis after randomization.13 Interestingly, two studies were never fully published. Before our study, the Japanese trial by Hiraoka et al.12 was the only published study reporting adequate concealment of allocation. This work found a therapeutic benefit of the 6-month regimen only in a small subgroup of children aged less than 4 years; overall relapse rate and FRNS did not differ significantly between the two groups.12 We evaluated the occurrence of FRNS in a meta-analysis, of which the results are shown in Figure 4. Four studies, including our study, reported FRNS. Overall analysis revealed no significant benefit of long versus short regimens; however, significant heterogeneity was present (Figure 4A). Heterogeneity was no longer significant when only fully published studies and our study were included (Figure 4B). Nonetheless, these studies are still quite different from each other with respect to administered dose, design, definitions, and observation time; therefore, overall results of this meta-analysis should be interpreted with caution.
Figure 4.
Meta-analyses of studies comparing 3 months of prednisolone to 6 months of prednisolone do not reveal a benefit of prolonged treatment duration. (A) All four available studies (B) Two fully published studies. In both analyses, numbers of FRNS of the current study correspond with numbers of strict FRNS. Analyses were performed with Review Manager (RevMan) version 5.1 for Windows (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark, 2011).
The incidences of both strict and clinical FRNS in our study population were higher than anticipated: 60/126 (48%) and 69/126 (55%), respectively. In previous studies, FRNS was reported in 32%–78% of patients who received 2-month prednisolone treatment (2240 mg/m2)10,17–21 and 18%–44% of patients who received prednisolone for 3 months (3360 mg/m2).10,12,20 This variation may, in part, be explained by regional differences or variations in definitions of FRNS, length of observation, and relapse treatments.
Based on our data, a benefit of the 6-month regimen cannot be excluded if the study had been performed with larger sample sizes. However, the CIs that we found for the difference in FRNS between the two groups exclude a clinically relevant difference in favor of the experimental regimen (Table 2). At 5 years, the difference between the two groups for strict FRNS was 7.30%, with a 95% CI ranging from −10.70% to 25.30%. At best, the experimental 6-month treatment was 10.7 percent points better than the standard 3-month treatment. For clinical FRNS, which in our opinion, represents an even more relevant group for clinicians, this difference was 12.20%, with a 95% CI ranging from −5.70% to 30.10%. Accordingly, applying the 6-month regimen would gain 5.7 percent points at most. The cumulative incidences of steroid dependence at 5 years further illustrate these statements, because they were 24.90%±5.6 and 40.10%±6.80 respectively, corresponding with a between-group difference of 15.20% (95% CI=−2.10% to 32.50%). Based on these results, we are confident that a clinically relevant difference in favor of the 6-month regimen is unlikely.
Previous studies have differed in observing and reporting (frequent) relapses from either the start or end of initial therapy. We chose a transitional type of observation to make a fair comparison but still include early relapses during treatment. We did verify that observing strictly from the end of initial treatment did not lead to differences between the two treatments (data not shown).
Analysis of covariates in our study revealed findings of clinical interest, although not supported by statistical significance. Boys tended to have worse outcomes than girls in terms of frequent relapses and RRR. In the few studies that observed an effect of sex on the clinical course of NS, males were at a disadvantage.20,22 It would be interesting to further explore whether boys and girls benefit from different treatment regimens in studies with larger sample sizes. We found no effect of age at onset. The influence of age at onset is still debated, because several studies have reported young age to be associated with FRNS and/or steroid dependence.3,20,22,23 However, others did not find an effect of age on the clinical course of NS.21,24,25
Side effects were equally distributed over the two treatment groups. Cushingoid side effects, high BP, and behavioral changes were clearly present but transient in the vast majority of patients. Ophtalmological complications were rare in our study. Cataract and glaucoma have previously most often been reported in Japanese patients12,26; in general, these complications are rare.7 Our findings indicate that there is no need for standard ophtalmological screening in children with NS at an early stage. The same applies to measurements of BMD, which remained stable over the first 6 months. We found severe infections in a clinically relevant proportion of both treatment groups. This observation is consistent with previous reports7 and justifies awareness of and early therapeutic intervention in children with NS facing infectious diseases.
Our prospective growth data noticeably illustrated how growth velocity significantly dropped in the first months during highly dosed prednisolone treatment and subsequently returned to its baseline within 1 year. Although this study was not designed to assess a causal relationship, this temporary effect corresponds with previous retrospective studies that describe a dose-dependent effect of corticosteroids on growth in children with NS.27–29 It is unclear why baseline height SD scores were relatively low in our study population. A similar observation was reported in the work by Schärer et al.,30 whereas others described normal height SD scores at diagnosis of NS.27
In countries where a 2-month prednisolone regimen is applied for the first episode of NS, children who do not achieve remission within 4 weeks of daily prednisolone are generally characterized as steroid-resistant. Steroid resistance is associated with increased risk of renal failure and entails more aggressive immunosuppressive therapy.1 Intriguingly, all five patients in our study who achieved remission after 4–6 weeks of prednisolone treatment subsequently experienced a mild clinical course. As argued in the work by Ehrich et al.,31 this finding suggests that patients who do not respond within 4 weeks of daily prednisolone should be offered at least another 2 weeks of daily prednisolone to prevent late responders from undergoing unnecessary and potentially harmful interventions.
A limitation of our study is the fact that participants were observed and treated at their local hospital. Adverse effects were scored by multiple observers, and ophtalmological and radiologic assessments were not available for all patients. A more centralized approach could have prevented these issues to some extent; however, the setting that we chose made participation feasible throughout the country. We were able to include at least one half of all newly diagnosed patients with NS in The Netherlands.2 By including patients in a nationwide setting, we believe that we have sufficiently avoided selection bias.7
Frequent relapses remain a major challenge in the treatment of childhood NS. In our opinion, FRNS, rather than the occurrence of relapses in general, should be the focus of ongoing research. Broader uniform definitions for FRNS that take into account other clinically relevant aspects besides relapse frequency per se should be considered to facilitate a more evidence-based approach to both treatment and research. A possible effect of higher cumulative prednisolone dose during initial treatment needs additional exploration, because it may explain better outcomes in some of the reported prolonged treatment regimens.7
In contrast to what was previously assumed but unproven, the present study shows that extending initial prednisolone treatment from 3 to 6 months, while maintaining an equal cumulative dose, does not improve clinical outcomes in children with NS. We believe that our results offer an important contribution to more evidence-based treatment of this disease.
Concise Methods
Trial Design
A double-blind, randomized, placebo-controlled, parallel-group trial was carried out in 84 of 87 (97%) general hospitals in The Netherlands along with 1 Belgian and all 8 Dutch university hospitals. The trial was approved by the medical ethics committee of Erasmus University Medical Centre in Rotterdam and registered at The Netherlands Trial Register (www.trialregister.nl; registration number NTR255). Detailed information regarding median inclusion rates per hospital and reasons for not participating can be found in Supplemental Table 5, A and B, respectively.
Participants
Children with a first episode of NS ages 9 months to 17 years were assessed for eligibility. NS was defined as >200 mg protein/mmol creatinine in urine and albumin<25 g/L in serum. Renal biopsy was not required to establish the diagnosis, because it is generally not indicated at this stage of childhood NS.1 Patients with underlying disease, such as Henoch–Schönlein purpura or postinfectious GN, were excluded. Remission was defined as urinary protein excretion<20 mg/L or negative trace on dipstick analysis on 3 consecutive days. Patients who did not achieve remission within 6 weeks of 60 mg/m2 daily prednisolone were characterized as steroid-resistant. Relapse was defined as proteinuria≥++ on dipstick analysis or >200 mg protein/mmol creatinine for 3 consecutive days after previously achieved remission. When milder proteinuria was present, pediatricians were instructed to hold off corticosteroid treatment, particularly when signs of mild infection were present. In these patients, relapse treatment was indicated when spontaneous remission became unlikely: continued proteinuria for more than 10 days, marked edema, or decrease of serum albumin to less than 30 g/L. Relapses were treated with prednisolone (60 mg/m2 per day) until remission followed by prednisolone (40 mg/m2) on alternate days for 4 weeks.
For our study, the definition of FRNS was originally restricted to commonly used criteria: (A) Two or more relapses within 6 months after completing initial treatment, or (B) Four relapses within any period of 12 months, including relapses during initial treatment. However, during the blinded data collection phase, it became clear that the use of this definition posed difficulties in some cases. Five patients displayed secondary steroid resistance and/or steroid dependency within 3–6 months after diagnosis. Consequently, they experienced their first relapses before the end of trial therapy; additional treatment measures were taken before these patients could even meet criterion A or B. Four additional patients experienced several relapses within short periods of time but did not fulfill criterion A or B. The high burden of multiple relapses within a relatively short period of time, the prospect of experiencing another relapse in the near future, and several signs of steroid toxicity resulted in a clinical indication for additional measures in these patients. Because we found all of these patients to be clinically relevant, we decided to add a third criterion: (C) FRNS based on a clinical decision that included additional treatment of prednisolone maintenance therapy (>3 months) or other immunosuppressive agents. Detailed information on patients characterized as FRNS based on criterion C can be found in Supplemental Table 3A. We analyzed both modalities of FRNS: strict FRNS (criterion A or B) to facilitate comparison with other studies and clinical FRNS (criterion A, B, or C) to report all clinically relevant outcomes.
Steroid dependence was defined as two or more consecutive relapses either during or within 2 weeks after cessation of prednisolone. All patients were diagnosed and treated according to the study protocol at their local hospital by their own pediatrician. Participants’ descent was obtained from self-reported countries of birth of parents and grandparents.
Procedures
A statistician provided the central trial pharmacy with a computer-generated random number table. Allocation to 3 months of prednisolone plus 3 months of placebo (referred to as the 3-month group) or 6 months of prednisolone was stratified for type of hospital (general or university) and balanced with a ratio of 1:1 in fixed blocks of four patients. The central trial pharmacy fabricated trial medication, controlled allocation concealment, allocated patients, and distributed trial medication after informed consent was obtained. Participants, health care providers, data collectors, and researchers were blinded to group allocation. Trial medication was sent prepackaged to local pharmacies and consisted of identical tasteless capsules containing either prednisolone or placebo. Trial medication was dispensed in five containers, each with a fixed blinded dose and a preset time frame. Although doses of the containers differed between treatment groups, container time frames were exactly the same. Container 1 was used from remission to week 6, 2 was used from weeks 7 to 10, 3 was used from weeks 11 to 12, 4 was used from weeks 13 to 14, and 5 was used from weeks 15 to 24. The first patient was randomized in February of 2005, and the last patient was randomized in December of 2009. Follow-up started at diagnosis and was truncated at either 5 years after diagnosis or July of 2011, at which time the last enrolled patients had a minimum follow-up of 18 months. The randomization code was subsequently broken in September of 2011.
All children diagnosed with NS started induction therapy of 60 mg/m2 oral prednisolone one time daily. Participants switched to trial medication only after remission was achieved. If remission was not achieved within 6 weeks of 60 mg/m2 daily prednisolone, patients were characterized as steroid-resistant, and trial medication was not started. Both treatment regimens are shown in detail in Figure 2. In both groups, induction therapy and trial medication were administered within a total of 24 weeks. The prescribed cumulative dose of prednisolone in the 3-month group was 3360 mg/m2. Depending on the number of days to remission, the prescribed cumulative dose of prednisolone in the 6-month group was 3320–3710 mg/m2, corresponding with 99%–110% of the cumulative dose in the 3-month group. Prescribed cumulative doses did not include potential relapse treatments during trial medication, because the occurrence of a relapse and the total dose administered for that particular relapse could not be anticipated. In the event of a relapse occurring during the period of trial medication, relapse treatment temporarily replaced trial medication to maintain a 24-week schedule duration.
Outcomes
The primary outcome event was FRNS. Secondary outcome parameters were cumulative incidences of a first relapse, steroid dependence, number of relapses per patient per year, and adverse effects. Height SD scores, BP, Cushingoid appearance (moon face or striae), dyspepsia, thrombosis, severe infections, and behavior were noted at diagnosis and after 3 and 6 months and 1 and 2 years. Height SD scores were calculated with Dutch pediatric reference data.32 High BP was defined as systolic and/or diastolic BP more than or equal to the 95th percentile for sex, age, and height.33 Severe infections were defined as nonself-limiting infections requiring hospital admission. Behavior was scored by parents on visual analog scales for overactive and aggressive behavior, happiness, eating, and sleeping. At diagnosis and after 6 months, participants were screened for cataract and glaucoma by an ophthalmologist; at the same time points, BMD was assessed. Using dual energy x-ray absorptiometry, Z-scores of lumbar spine BMD were calculated according to local reference data. Changes in individual Z-scores over time were calculated from paired measurements. As an additional indicator of BMD, Bone Health Index SD scores from hand x-rays was calculated with BoneXpert.34
Statistical Analyses
Primary outcome events were originally defined as the cumulative incidences of first relapses and FRNS. Subsequently, at the time the study was still blinded, FRNS was chosen as the sole primary outcome, because we considered FRNS to be the most relevant parameter. Incidence of a first relapse became the secondary outcome. For the cumulative incidence of FRNS to decrease by 20% points, 72 patients per treatment arm were sufficient (80% power, α=0.05).
A modified intention-to-treat principle was applied in such a way that all patients who started trial medication were included in the analysis. Participants who were subsequently lost to follow-up or in whom trial medication was stopped prematurely were analyzed according to their allocated groups.
Cumulative event rates are expressed as Kaplan–Meier estimates with SEMs. Treatment group, sex, age at onset, and number of days to remission were included as covariates in the Cox regression analysis. Age at onset was stratified as <4 and ≥4 years.23
For comparison of relapses within time intervals between treatments, follow-up was categorized into three periods (period I, 0–6 months; period II, 6–12 months; period III, >12 months after randomization), and within each period, the number of relapses was counted. Poisson regression was used to evaluate relapse rates in relation to treatment, sex, age category, and period. Calculations were done using Generalized Estimation Equations with a log link. Longitudinal data concerning height SD scores and behavior were analyzed with linear mixed models that included treatment, age strata, sex, time, baseline values, and interaction between time and treatment as fixed effects. For the remaining variables, continuous outcome was analyzed with either the t or Mann–Witney test, and categorical outcome was analyzed with either the Pearson chi-squared or Fisher exact test. P values<0.05 were considered statistically significant. All analyses were performed with SPSS (version 17.0).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the children and their parents participating in this study, the central trial pharmacy, and the Hans Mak Institute for meticulous work during the recruitment and data monitoring phases. We acknowledge the Working Group Nephrotic Syndrome (WINS), which provided advice on the study design.
This study was funded by Dutch Kidney Foundation Grant C03.2072 and the Vrienden van het Sophia Foundation.
An abstract containing data from this study was submitted and accepted for presentation at the European Society of Pediatric Nephrology 2012 Meeting, September 6–8 2012, Cracow, Poland.
Pediatricians from participating centers: Albert Schweitzer Hospital E de Klein, Amphia Hospital S de Pont, Atrium Medical Centre Parkstad P Theunissen and H Sijstermans, Beatrix Hospital M Visser, Canisius-Wilhelmina Hospital BA Semmekrot, Catharina Hospital JE Bunt, Diaconessenhuis Utrecht AJ Kok, Diakonessenhuis Leiden DAJP Haring, Diakonessenhuis Meppel FJ Kloosterman-Eygenraam, Diakonessenhuis Zeist AJ Kok, Dr. JH Jansen Hospital WP Vogt, Elkerliek Hospital MA Breukels, Academic Medical Centre- Emma Children’s Hospital JC Davin, Flevo Hospital MAJM Trijbels-Smeulders, Franciscus Hospital ND van Voorst Vader-Boon, Gelre Hospital HFH Thijs and DJ Pot, Gemini Hospital MC Wallis-Spit, BovenIJ Hospital N Menelik, Groene Hart Hospital EHG van Leer, Hofpoort Hospital C Dorrepaal, IJsselland Hospital AAM Leebeek, Ikazia Hospital C Aleman, Isala Klinieken JME Quak, Jeroen Bosch Hospital, AHPM Essink and PE Jira, Haga Hospital-Juliana Children’s hospital P Vos, Kennemergasthuis A Adeel, VU University Medical Centre JAE van Wijk and A Bökenkamp, Maastricht University Medical Centre FAPT Horuz-Engels, Radboud University Medical Centre L Koster-Kamphuis, Lange Land Hospital ED Stam, Leiden University Medical Centre Sukhai, Maas Hospital A Verhoeven-van Lieburg, Maasland Hospital JWCM Heynens, Martini Hospital HJ Waalkens, Maxima Medical Centre SHJ Zegers, Maasstad Hospital JG Brinkman, Meander Medical Centre MR Ernst-Kruis, Alkmaar Medical Centre WWM Hack, Leeuwarden Medical Centre TE Faber, Medisch Spectrum Twente EWD ten Kate-Westerhof, St. Antonius Hospital HE Blokland-Loggers and MMJ van der Vorst, Onze Lieve Vrouwe Gasthuis J van Andel, Oosterschelde Hospital AGH Poot, Refaja Hospital SS Nowak, Reinier de Graaf Gasthuis LC ten Have, Rijnland Hospital EA Schell-Feith, Rode Kruis Hospital K Olie, Röpcke-Zweers Hospital IFM Fagel, Ruwaard van Putten Hospital D Birnie, Scheper Hospital A Colijn, Vlietland Hospital NJ Langendoen, Slingeland Hospital MAM Jacobs, Slotervaart Hospital JHM Budde, Erasmus Medical Centre-Sophia Children’s Hospital EM Dorresteijn, Spaarne Hospital JP de Winter, St. Anna Hospital MCG Beeren, Antonius Hospital AH van der Vlugt, St. Elisabeth Hospital RA de Moor, St. Franciscus Gasthuis HTM Jongejan, St. Jans Gasthuis M van Helvoirt-Jansen, St. Jansdal Hospital W Peelen, St. Laurentius Hospital ST Potgieter, St. Lucas Andreas Hospital MK Sanders, St. Lucas Hospital GHC van Weert, Deventer Hospital J van der Deure, Streekziekenhuis Koningin Beatrix AJM van Kuppevelt, Streekziekenhuis Midden-Twente A van der Wagen, Ter Gooi Hospital AJ van der Kaaden and CA Lasham, TweeSteden Hospital JAC van Lier, Twenteborg Hospital IT Merth, Groningen University Medical Centre M Kömhoff, Utrecht University Medical Centre-Wilhelmina Children’s Hospital MR Lilien, Vie Curi Medical Centre AAM Haagen and CML van Dael, Waterland Hospital F Veenstra, West-Fries Gasthuis BJ Tuitert, Wilhelmina Hospital R Meekma, Zaans Medical Centre JM Karperien, Amstelland Hospital L Spanjerberg-Rademaker, Bernhoven Hospital JP Leusink and EML Rammeloo, Bethesda Hospital Chr van Ingen, De Sionsberg Hospital J Karsten, De Tjongerschans Hospital AI Kistemaker, Dirksland Hospital IN Snoeck, De Gelderse Vallei Hospital M Koppejan-Stapel, Lievensberg Hospital AJJ van der Linden, Nij Smellinghe Hospital WA van Asselt, Rijnstate Hospital DGJW Creemers, Rivierenland Hospital CS Barbian, Admiraal de Ruyter Hospital JG van Keulen, ZorgSaam Hospital WS Corijn, IJsselmeer Hospital WP Vogt, University Medical Centre Leuven EN Levtchenko.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Corticosteroid Therapy for Steroid-Sensitive Nephrotic Syndrome in Children: Dose or Duration?,” on pages 7–9.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012070646/-/DCSupplemental.
References
- 1.Eddy AA, Symons JM: Nephrotic syndrome in childhood. Lancet 362: 629–639, 2003 [DOI] [PubMed] [Google Scholar]
- 2.El Bakkali L, Rodrigues Pereira R, Kuik DJ, Ket JC, van Wijk JA: Nephrotic syndrome in The Netherlands: A population-based cohort study and a review of the literature. Pediatr Nephrol 26: 1241–1246, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trompeter RS, Lloyd BW, Hicks J, White RH, Cameron JS: Long-term outcome for children with minimal-change nephrotic syndrome. Lancet 1: 368–370, 1985 [DOI] [PubMed] [Google Scholar]
- 4.Bruneau S, Dantal J: New insights into the pathophysiology of idiopathic nephrotic syndrome. Clin Immunol 133: 13–21, 2009 [DOI] [PubMed] [Google Scholar]
- 5.International Study of Kidney Disease in Children : The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J Pediatr 98: 561–564, 1981 [DOI] [PubMed] [Google Scholar]
- 6.Tarshish P, Tobin JN, Bernstein J, Edelmann CM, Jr: Prognostic significance of the early course of minimal change nephrotic syndrome: Report of the International Study of Kidney Disease in Children. J Am Soc Nephrol 8: 769–776, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Hodson EM, Willis NS, Craig JC: Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev 4: CD001533, 2007 [DOI] [PubMed] [Google Scholar]
- 8.MacHardy N, Miles PV, Massengill SF, Smoyer WE, Mahan JD, Greenbaum L, Massie S, Yao L, Nagaraj S, Lin JJ, Wigfall D, Trachtman H, Hu Y, Gipson DS: Management patterns of childhood-onset nephrotic syndrome. Pediatr Nephrol 24: 2193–2201, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Frange P, Frey MA, Deschênes G: Immunity and immunosuppression in childhood idiopathic nephrotic syndrome. Arch Pediatr 12: 305–315, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Ehrich JH, Brodehl J: Long versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Arbeitsgemeinschaft für Pädiatrische Nephrologie. Eur J Pediatr 152: 357–361, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Hodson EM, Knight JF, Willis NS, Craig JC: Corticosteroid therapy in nephrotic syndrome: A meta-analysis of randomised controlled trials. Arch Dis Child 83: 45–51, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiraoka M, Tsukahara H, Matsubara K, Tsurusawa M, Takeda N, Haruki S, Hayashi S, Ohta K, Momoi T, Ohshima Y, Suganuma N, Mayumi M, West Japan Cooperative Study Group of Kidney Disease in Children : A randomized study of two long-course prednisolone regimens for nephrotic syndrome in children. Am J Kidney Dis 41: 1155–1162, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Mishra OP, Thakur N, Mishra RN, Prasad R: Prolonged versus standard prednisolone therapy for initial episode of idiopathic nephrotic syndrome. J Nephrol 25: 394–400, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Ksiazek J, Wyszyńska T: Short versus long initial prednisone treatment in steroid-sensitive nephrotic syndrome in children. Acta Paediatr 84: 889–893, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Gulati S, Ahmed M, Sharma R, Gupta A, Pokhariyal S: Comparison of abrupt withdrawal versus slow tapering regimen of prednisolone therapy in the management of first episode of steroid responsive childhood idiopathic nephrotic syndrome [Abstract]. Nephrol Dial Transplant 16: A87, 2001 [Google Scholar]
- 16.Pecoraro C, Caropreso M, Malgieri G, Ferretti A, Raddi G, Piscitelli A, Nuzzi F: Therapy of first episode of steroid responsive nephrotic syndrome: A randomised controlled trial [Abstract]. Pediatr Nephrol 19: C72, 2004 [Google Scholar]
- 17.Bagga A, Hari P, Srivastava RN: Prolonged versus standard prednisolone therapy for initial episode of nephrotic syndrome. Pediatr Nephrol 13: 824–827, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Arbeitsgemeinschaft für Pädiatrische Nephrologie : Short versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Arbeitsgemeinschaft für Pädiatrische Nephrologie. Lancet 1: 380–383, 1988 [PubMed] [Google Scholar]
- 19.Koskimies O, Vilska J, Rapola J, Hallman N: Long-term outcome of primary nephrotic syndrome. Arch Dis Child 57: 544–548, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen RF, Thrane N, Noergaard K, Rytter L, Jespersen B, Rittig S: Early age at debut is a predictor of steroid-dependent and frequent relapsing nephrotic syndrome. Pediatr Nephrol 25: 1299–1304, 2010 [DOI] [PubMed] [Google Scholar]
- 21.International Study of Kidney Disease in Children : Early identification of frequent relapsers among children with minimal change nephrotic syndrome. A report of the International Study of Kidney Disease in Children. J Pediatr 101: 514–518, 1982 [DOI] [PubMed] [Google Scholar]
- 22.Lewis MA, Baildom EM, Davis N, Houston IB, Postlethwaite RJ: Nephrotic syndrome: From toddlers to twenties. Lancet 1: 255–259, 1989 [DOI] [PubMed] [Google Scholar]
- 23.Kabuki N, Okugawa T, Hayakawa H, Tomizawa S, Kasahara T, Uchiyama M: Influence of age at onset on the outcome of steroid-sensitive nephrotic syndrome. Pediatr Nephrol 12: 467–470, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Yap HK, Han EJ, Heng CK, Gong WK: Risk factors for steroid dependency in children with idiopathic nephrotic syndrome. Pediatr Nephrol 16: 1049–1052, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Takeda A, Takimoto H, Mizusawa Y, Simoda M: Prediction of subsequent relapse in children with steroid-sensitive nephrotic syndrome. Pediatr Nephrol 16: 888–893, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Hiraoka M, Tsukahara H, Haruki S, Hayashi S, Takeda N, Miyagawa K, Okuhara K, Suehiro F, Ohshima Y, Mayumi M, The West Japan Cooperative Study of Kidney Disease in Children : Older boys benefit from higher initial prednisolone therapy for nephrotic syndrome. Kidney Int 58: 1247–1252, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Donatti TL, Koch VH: Final height of adults with childhood-onset steroid-responsive idiopathic nephrotic syndrome. Pediatr Nephrol 24: 2401–2408, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Simmonds J, Grundy N, Trompeter R, Tullus K: Long-term steroid treatment and growth: A study in steroid-dependent nephrotic syndrome. Arch Dis Child 95: 146–149, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Hung YT, Yang LY: Follow-up of linear growth of body height in children with nephrotic syndrome. J Microbiol Immunol Infect 39: 422–425, 2006 [PubMed] [Google Scholar]
- 30.Schärer K, Essigmann HC, Schaefer F: Body growth of children with steroid-resistant nephrotic syndrome. Pediatr Nephrol 13: 828–834, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Ehrich JH, Geerlings C, Zivicnjak M, Franke D, Geerlings H, Gellermann J: Steroid-resistant idiopathic childhood nephrosis: Overdiagnosed and undertreated. Nephrol Dial Transplant 22: 2183–2193, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Talma H, Schönbeck Y, Bakker B, Hirasing R, van Buuren S: Growth Diagrams 2010: Manual for Measuring and Weighing Children and the Use of Growth Diagrams, Leiden, The Netherlands, TNO kwaliteit van leven, 2010 [Google Scholar]
- 33.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents : The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114[Suppl 4th Report]: 555–576, 2004 [PubMed] [Google Scholar]
- 34.Thodberg HH, van Rijn RR, Tanaka T, Martin DD, Kreiborg S: A paediatric bone index derived by automated radiogrammetry. Osteoporos Int 21: 1391–1400, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.