Abstract
CKD progresses more rapidly to ESRD among African Americans compared with Caucasians. Disordered mineral metabolism is more severe among African Americans with CKD, which might partially explain the accelerated progression of their kidney disease. Here, using data from the African American Study of Kidney Disease and Hypertension, we evaluated longitudinal changes in serum levels of fibroblast growth factor-23 (FGF23), parathyroid hormone (PTH), phosphate, and 25-hydroxyvitamin D in a subset of 420 participants followed for a median of 4 years. We also examined the association of baseline levels of mineral metabolites with risk for ESRD or death in 809 participants. FGF23, PTH, and phosphate levels rose over time; participants with faster rates of decline in measured GFR had the greatest increases in these parameters (P<0.01 for each). Higher baseline levels of FGF23, PTH, and phosphate each associated with increased risk for ESRD or death independent of GFR. FGF23 exhibited a dose–response relationship with outcomes (HR=1.30 per doubling, 95% CI=1.15–1.47; HR=2.24 for highest compared with lowest quartile, 95% CI=1.39–3.60), whereas PTH and phosphate showed nonlinear relationships. Vitamin D insufficiency (<30 ng/ml) was present in 95% of participants, but lower levels did not independently associate with outcomes. Using death-censored ESRD as the outcome produced qualitatively similar results. In conclusion, abnormalities of mineral metabolism worsen with progressive CKD and associate with higher risk for ESRD among African Americans with hypertensive nephrosclerosis.
African Americans represent approximately 14% of the US population, but they constitute 31% of patients undergoing treatment for ESRD.1,2 Genetic susceptibility to certain forms of CKD and more rapid rates of progression of established CKD contribute to the disproportionate burden of ESRD among African Americans compared with Caucasians.3,4 Even in the setting of optimal BP control using renin-angiotensin system antagonists that slow progression of CKD, African Americans experience unacceptably high rates of renal failure.5 To improve renal outcomes in this high-risk population, additional modifiable mechanisms of CKD progression must be identified.
Disordered mineral metabolism is associated with adverse outcomes in CKD, and cross-sectional studies suggest earlier onset and greater severity among African Americans compared with Caucasians. For example, at comparable levels of estimated GFR, African Americans manifest lower levels of 25-hydroxyvitamin D and higher levels of parathyroid hormone (PTH), serum phosphate, and fibroblast growth factor-23 (FGF23).6–8 Abnormal levels of these individual mineral metabolites have been associated with greater risks of mortality and cardiovascular disease,9–16 but analyses of risk of progression to ESRD yielded less consistent results, perhaps because of methodological limitations.13–20 Foremost among these limitations is difficulty discerning whether abnormal levels of mineral metabolites are true risk factors for CKD progression, or instead, if they simply identify individuals with more advanced and rapidly progressive CKD. Addressing this limitation requires the ability to control for precise assessments of baseline GFR and the baseline rate of GFR decline when mineral metabolism exposures are ascertained. Few, if any, previous studies of mineral metabolism and risk of ESRD incorporated longitudinal assessments of directly measured GFR, and few studies specifically evaluated a panel of mineral metabolites in high-risk African-American populations.
The African American Study of Kidney Disease and Hypertension (AASK) was a randomized clinical trial that examined the effect of BP management strategies on the risk of CKD progression in African Americans with hypertensive nephrosclerosis.21 After the trial, additional follow-up was collected in an observational cohort phase.22,23 By design, AASK maintained tight protocolized BP control and performed repeated gold-standard measurements of 125I-iothalamate GFR with long-term follow-up for clinical events, making it an ideal cohort to study risk factors for CKD progression. In this study, we measured serum levels of FGF23, PTH, phosphate, and 25-hydroxyvitamin D at baseline and longitudinally in AASK participants, and we evaluated the associations of longitudinal change in each mineral metabolite with changes in 125I-iothalamate GFR. Next, we tested the hypothesis that abnormal levels of mineral metabolites are associated with greater risk of progression to ESRD independent of direct measures of GFR.
Results
Study Population
The study population included 809 participants from AASK who had serum samples available for measurement of mineral metabolites at the 12-month follow-up visit of the trial phase. Characteristics of the study population and measures of mineral metabolites at the 12-month follow-up visit are described in Table 1. The mean 125I-iothalamate GFR at the 12-month visit was 45.2±17.9 ml/min per 1.73 m2. The mean annualized slope of decline in 125I-iothalamate GFR, beginning at month 3 postrandomization (as defined by the AASK study investigators21,24) and ending at month 12 (when mineral metabolites were first measured), was −1.55±2.20 ml/min per 1.73 m2 per year (mean=2.3 GFR measurements per participant).
Table 1.
Baseline characteristics of the study population (n=809)
| Characteristic | Mean ± SD or n |
|---|---|
| General | |
| Age (yr) | 55±10 |
| Female sex | 320 (39.6%) |
| Incomea | |
| <$15,000/yr | 392 (48.5%) |
| ≥$15,000/yr | 264 (32.6%) |
| Prior cardiovascular disease | 428 (52.9%) |
| Smoking | |
| Current | 240 (29.7%) |
| Past | 234 (28.9%) |
| Never | 335 (41.4%) |
| Body mass index (kg/m2) | 30.4±6.5 |
| Categories of body mass index (kg/m2) | |
| <25.0 | 169 (20.9%) |
| 25.0–29.9 | 264 (32.6%) |
| 30.0–34.9 | 198 (24.5%) |
| ≥35.0 | 178 (22.0%) |
| Serum albumin (mg/dl) | 4.1±0.3 |
| Kidney function | |
| 125I-iothalamate GFR (ml/min per 1.73 m2) | 45.2±17.9 |
| Stage of CKDa | |
| 2 (GFR≥60 ml/min per 1.73 m2) | 172 (21.3%) |
| 3a (GFR=45–59 ml/min per 1.73 m2) | 239 (29.5%) |
| 3b (GFR=30–44 ml/min per 1.73 m2) | 216 (26.7%) |
| 4–5 (GFR<30 ml/min per 1.73 m2) | 179 (22.1%) |
| 125I-iothalamate GFR slope (ml/min per 1.73 m2 per yr)b | −1.55±2.20 |
| UPCRc | 0.07 (0.03, 0.32) |
| Categories of UPCRa | |
| <0.22 | 553 (68.4%) |
| 0.22–0.99 | 146 (18.1%) |
| ≥1.00 | 84 (10.4%) |
| Mineral metabolites | |
| Serum FGF23 (pg/ml)c | 44.2 (30.7, 64.3) |
| Elevated (>50 pg/ml) | 332 (41.0%) |
| Intact PTH (pg/ml)c | 37 (24, 61) |
| Elevated (>65 pg/ml) | 177 (21.9%) |
| Serum phosphate (mg/dl) | 3.5±0.7 |
| Elevated (>4.6 mg/dl) | 38 (4.7%) |
| 25-hydroxyvitamin D (ng/ml)c | 13.4 (9.0, 19.2) |
| Insufficient (<30 ng/ml) | 766 (94.7%) |
| Deficient (<10 ng/ml) | 266 (32.9%) |
| Serum calcium (mg/dl) | 8.9±0.5 |
| Medication use | |
| 25-hydroxyvitamin D | 4 (0.5%) |
| Active vitamin D sterols | 6 (0.7%) |
| Phosphate binding medications | 25 (3.1%) |
Total does not sum to 100% because of missing data.
Estimated from month 3 to 12 postrandomization using linear mixed models.
Presented as median (interquartile range).
Laboratory characteristics according to ascending quartiles of FGF23, PTH, serum phosphate, and 25-hydroxyvitamin D are presented in Supplemental Table 1. Unlike previous studies,13 PTH was more strongly correlated with 125I-iothalamate GFR (r = −0.55; P<0.001) than FGF23 (r = −0.39; P<0.001) or serum phosphate (r = −0.36; P<0.001); 25-hydroxyvitamin D was not correlated with GFR (P=0.90). In the AASK trial, participants were randomized in a 2×3 factorial design to a standard versus low BP goal and one of three primary antihypertensives (amlodipine, metoprolol, or ramipril).5 Levels of mineral metabolites were not associated with any of these randomized interventions (data not shown).
Longitudinal Change in Mineral Metabolites
We performed repeated measurements of mineral metabolites in a randomly selected subcohort of participants at the 24- (n=114), 36- (n=104), and 48-month follow-up visits (n=52). We also repeated the mineral metabolite assessment in all participants at entry into the AASK observational cohort phase, which followed the conclusion of the trial phase.22 In total, 420 of 809 participants had more than one panel of mineral metabolites performed, 311 participants had two measures, 37 participants had three measures, and 72 participants had four or five measures.
Overall, mean levels of FGF23, PTH, and serum phosphate rose over a median of 4 years of follow-up (P<0.001 each), and levels of 25-hydroxyvitamin D declined (P=0.01). The rate of rise in FGF23, PTH, and phosphate was greatest among individuals with more rapid decline in 125I-iothalamate GFR measured from 12 months postrandomization to the end of the trial phase (mean=7.7 GFR measures per participant), and it was particularly pronounced among participants with baseline GFR<45 versus ≥45 ml/min per 1.73 m2 (P for interactions<0.05) (Table 2). Longitudinal change in 25-hydroxyvitamin D was not associated with change in 125I-iothalamate GFR (P=0.90).
Table 2.
Overall and GFR-stratified annualized change in mineral metabolites by rate of GFR decline in the AASK trial
| Rate of 125I-iothalamate GFR Declinea | P Trend | P Interaction | |||
|---|---|---|---|---|---|
| Slow (<1 ml/min per 1.73 m2 per yr; n=127) | Moderate (1–3 ml/min per 1.73 m2 per yr; n=176) | Rapid (>3 ml/min per 1.73 m2 per yr; n=116) | |||
| Percent change in FGF23 (%) | |||||
| Overall (n=419b) | −1.1 (−4.4, 2.3) | 3.9 (−0.1, 8.1) | 14.9 (10.4, 19.7) | <0.01 | |
| Stratified by baseline GFR (ml/min per 1.73 m2) | |||||
| ≥45 (n=247) | −1.5 (−5.4, 2.5) | 1.3 (−4.2, 7.2) | 9.1 (4.0, 14.3) | 0.01 | 0.02 |
| <45 (n=172) | 0.3 (−5.8, 6.7) | 8.7 (3.2, 14.6) | 27.2 (17.9, 37.3) | <0.01 | |
| Percent change in PTH (%) | |||||
| Overall (n=419b) | 2.0 (0.0, 4.0) | 6.1 (4.1, 8.2) | 12.6 (9.2, 16.1) | <0.01 | |
| Stratified by baseline GFR (ml/min per 1.73 m2) | |||||
| ≥45 (n=247) | 3.0 (0.7, 5.4) | 3.6 (1.3, 5.9) | 9.1 (5.6, 12.8) | 0.01 | <0.01 |
| <45 (n=172) | −0.9 (−4.5, 2.8) | 9.2 (6.0, 12.5) | 19.1 (12.8, 25.8) | <0.01 | |
| Change in serum phosphate (mg/dl) | |||||
| Overall (n=419b) | −0.01 (−0.03, 0.01) | 0.04 (0.01, 0.06) | 0.12 (0.08, 0.17) | <0.01 | |
| Stratified by baseline GFR (ml/min per 1.73 m2) | |||||
| ≥45 (n=247) | −0.01 (−0.03, 0.02) | 0.01 (−0.02, 0.04) | 0.06 (0.03, 0.10) | <0.01 | <0.01 |
| <45 (n=172) | −0.01 (−0.06, 0.04) | 0.07 (0.02, 0.12) | 0.22 (0.13, 0.31) | <0.01 | |
| Change in 25-hydroxyvitamin D (ng/dl) | |||||
| Overall (n=419b) | −0.11 (−0.34, 0.12) | −0.28 (−0.49, −0.07) | −0.11 (−0.38, 0.15) | 0.93 | |
| Stratified by baseline GFR (ml/min per 1.73 m2) | |||||
| ≥45 (n=247) | −0.21 (−0.47, 0.06) | −0.12 (−0.44, 0.20) | −0.11 (−0.45, 0.22) | 0.55 | 0.39 |
| <45 (n=172) | 0.15 (−0.33, 0.63) | −0.44 (−0.75, −0.14) | −0.13 (−0.55, 0.29) | 0.48 | |
Measured from 12 months postrandomization to the completion of the trial.
One participant with repeated measures was missing information on GFR decline.
Association between Quartiles of Mineral Metabolites and Risk of Outcomes
During a median follow-up of 7.9 years (interquartile range=4.1–9.6 years) beginning from the 12-month follow-up visit and spanning the trial and observational phases of AASK, 234 participants developed ESRD (43/1000 patient-years), and 117 patients died before ESRD (21/1000 patient-years). Higher levels of FGF23, PTH, and serum phosphate were associated with a greater cumulative incidence of ESRD or death (P<0.001 for each) (Supplemental Figure 1, A–C). Adjustment for 125I-iothalamate GFR partially attenuated the association of FGF23, PTH, and phosphate with outcomes, but after full multivariable adjustment, higher quartiles of FGF23 (hazard ratio [HR]=2.24 for quartile 4 versus 1; 95% confidence interval [CI]=1.39–3.60), PTH (HR=1.65 for quartile 4 versus 2; 95% CI=1.17–2.32), and phosphate (HR=1.46 for quartile 4 versus 2; 95% CI=1.19–1.80) remained independently associated with greater risk of ESRD or death (Table 3). Lower quartiles of 25-hydroxyvitamin D were marginally associated with greater risk of ESRD or death in unadjusted (Supplemental Figure 1D) and GFR-adjusted analyses but not after adjustment for the urine protein to creatinine ratio (UPCR) (Table 3).
Table 3.
Risk of ESRD or death during the AASK trial and cohort by quartiles of mineral metabolites
| Incidence per 1000 person-yr | HR | ||||||
|---|---|---|---|---|---|---|---|
| Age, Sex, and Randomizationa (n=809) | +GFR (n=806) | +UPCR (n=782) | Multivariableb (n=780) | +Preceding GFR Slope (n=773) | +Mineral Metabolites (n=772) | ||
| FGF23 (pg/ml) | |||||||
| ≤30.7 | 26.2 | Ref | Ref | Ref | Ref | Ref | Ref |
| 30.8–44.2 | 47.4 | 1.80 (1.34, 2.43) | 1.40 (0.98, 1.99) | 1.57 (1.07, 2.29) | 1.47 (0.98, 2.20) | 1.44 (0.96, 2.15) | 1.49 (0.94, 2.36) |
| 44.3–64.3 | 70.0 | 2.71 (1.79, 4.09) | 1.58 (0.99, 2.52) | 1.64 (1.05, 2.56) | 1.67 (1.04, 2.69) | 1.65 (1.02, 2.68) | 1.78 (1.04, 3.06) |
| ≥64.4 | 141.3 | 5.57 (3.94, 7.89) | 2.17 (1.39, 3.39) | 2.24 (1.43, 3.51) | 2.24 (1.39, 3.60) | 2.22 (1.37, 3.58) | 2.26 (1.34, 3.83) |
| P trend | — | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| PTH (pg/ml) | |||||||
| ≤24.0 | 35.6 | 0.93 (0.68, 1.27) | 1.22 (0.92, 1.63) | 1.25 (0.88, 1.77) | 1.24 (0.88, 1.76) | 1.21 (0.86, 1.72) | 1.17 (0.80, 1.72) |
| 24.1–37.0 | 38.6 | Ref | Ref | Ref | Ref | Ref | Ref |
| 37.1–60.7 | 71.0 | 1.84 (1.33, 2.54) | 1.25 (0.91, 1.72) | 1.22 (0.87, 1.70) | 1.25 (0.90, 1.75) | 1.24 (0.89, 1.74) | 1.05 (0.72, 1.52) |
| ≥60.8 | 138.2 | 3.72 (2.71, 5.10) | 1.67 (1.25, 2.24) | 1.66 (1.17, 2.37) | 1.65 (1.17, 2.32) | 1.64 (1.16, 2.32) | 1.35 (0.91, 2.01) |
| P trend | — | <0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.26 |
| Serum phosphate (mg/dl) | |||||||
| ≤3.1 | 50.7 | 1.03 (0.74, 1.42) | 1.17 (0.86, 1.61) | 1.20 (0.86, 1.68) | 1.13 (0.79, 1.62) | 1.12 (0.78, 1.60) | 1.14 (0.77, 1.68) |
| 3.2–3.5 | 49.2 | Ref | Ref | Ref | Ref | Ref | Ref |
| 3.6–3.9 | 63.4 | 1.31 (0.99, 1.75) | 0.95 (0.63, 1.42) | 0.90 (0.57, 1.41) | 0.95 (0.61, 1.46) | 0.93 (0.60, 1.43) | 0.94 (0.63, 1.41) |
| ≥4.0 | 112.7 | 2.37 (1.86, 3.02) | 1.51 (1.12, 2.04) | 1.43 (1.15, 1.78) | 1.46 (1.19, 1.80) | 1.44 (1.18, 1.76) | 1.39 (1.15, 1.67) |
| P trend | — | <0.01 | 0.09 | 0.21 | 0.08 | 0.09 | 0.19 |
| 25-hydroxyvitamin D (ng/dl)c | |||||||
| Quartile 1 | 69.8 | 1.41 (1.03, 1.92) | 1.61 (1.16, 2.21) | 1.36 (0.97, 1.90) | 1.25 (0.88, 1.78) | 1.23 (0.87, 1.75) | 1.39 (0.89, 2.18) |
| Quartile 2 | 71.8 | 1.42 (1.15, 1.75) | 1.70 (1.31, 2.21) | 1.49 (0.97, 2.29) | 1.50 (0.98, 2.27) | 1.46 (0.96, 2.23) | 1.55 (0.97, 2.46) |
| Quartile 3 | 66.1 | 1.33 (1.02, 1.73) | 1.46 (1.07, 1.98) | 1.28 (0.93, 1.78) | 1.27 (0.96, 1.68) | 1.26 (0.95, 1.67) | 1.35 (1.01, 1.81) |
| Quartile 4 | 49.8 | Ref | Ref | Ref | Ref | Ref | Ref |
| P trend | — | 0.02 | <0.01 | 0.08 | 0.20 | 0.23 | 0.16 |
Adjusted for age, sex, and randomized treatment assignment and clustered by clinical center. +GFR, additionally adjusted for 125I-iothalamate GFR; +UPCR, additionally adjusted for UPCR.
Multivariable model adjusted for age, sex, randomized treatment assignment, 125I-iothalamate GFR, UPCR, income, prior heart disease, smoking, serum albumin, and categories of body mass index. This model is the primary model of interest. + preceding GFR slope, additionally adjusted for the slope of 125I-iothalamate GFR in the 9 months preceding measurement of mineral metabolites; + mineral metabolites, additionally adjusted for serum calcium, phosphate, PTH, FGF23 and 25-hydroxyvitamin D.
Ranges for 25-hydroxyvitamin D quartiles (Q) are season-specific as follows: winter (Q1, ≤8.4; Q2, 8.5–10.9; Q3, 11.0–16.2; Q4, ≥16.3 ng/ml); spring (Q1, ≤7.9; Q2, 8.0–10.9; Q3, 11.0–16.5; Q4, ≥16.6 ng/ml); summer (Q1, ≤10.0; Q2, 10.1–15.2; Q3, 15.3–20.6; Q4, ≥20.7 ng/ml); and fall (Q1, ≤11.0; Q2, 11.1–14.7; Q3, 14.8–22.8; Q4, ≥22.9 ng/ml).
To evaluate for residual confounding by the rate of CKD progression before measurement of mineral metabolites, we performed secondary analyses adjusted for the slope of GFR decline from month 3 to 12 postrandomization (mean=2.3 GFR measurements per participant). Associations between each mineral metabolite and outcomes were unchanged after this adjustment (Table 3). The highest quartiles of FGF23 and serum phosphate remained independently associated with outcomes after additional adjustment for all of the other mineral metabolites, but the association between PTH and outcomes attenuated (Table 3).
To confirm that the findings using our composite end point were because of associations with CKD progression and not primarily driven by death, we analyzed risk of death-censored ESRD and death-censored ESRD or doubling of serum creatinine from trial baseline. The point estimates for FGF23, PTH, and phosphate were similar; however, 25-hydroxyvitamin D levels were not associated with the renal end points in unadjusted or adjusted models (Table 4). All results were qualitatively similar in sensitivity analyses restricted to the trial phase only and when we analyzed time-varying exposures updated at baseline of the observational cohort phase (data not shown).
Table 4.
Risk of the secondary renal end points, death-censored ESRD, and death-censored ESRD or doubling of serum creatinine by quartiles of mineral metabolites
| Secondary End Points | ||||||
|---|---|---|---|---|---|---|
| ESRD | ESRD or Doubling of Serum Creatinine | |||||
| Incidence per 1000 person-yr | Age, Sex, and Randomization Adjusted HR | Multivariable Adjusteda HR | Incidence per 1000 person-yr | Age, Sex, and Randomization Adjusted HR | Multivariable Adjusteda HR | |
| FGF23 (pg/ml) | ||||||
| ≤30.7 | 11.2 | Ref | Ref | 26.4 | Ref | Ref |
| 30.8–44.2 | 26.1 | 2.29 (1.45, 3.62) | 1.43 (0.77, 2.65) | 42.3 | 1.60 (1.21, 2.10) | 1.28 (0.90, 1.81) |
| 44.3–64.3 | 44.9 | 3.95 (2.38, 6.57) | 1.77 (1.00, 3.12) | 62.8 | 2.33 (1.53, 3.53) | 1.28 (0.77, 2.14) |
| ≥64.4 | 114.6 | 9.86 (6.68, 14.53) | 2.46 (1.35, 4.49) | 136.9 | 5.05 (3.47, 7.35) | 2.12 (1.26, 3.57) |
| P trend | — | <0.01 | <0.01 | — | <0.01 | 0.01 |
| PTH (pg/ml) | ||||||
| ≤24.0 | 14.6 | 0.72 (0.45, 1.13) | 1.16 (0.69, 1.94) | 28.8 | 0.81 (0.58, 1.12) | 1.13 (0.78, 1.64) |
| 24.1–37.0 | 21.3 | Ref | Ref | 37.4 | Ref | Ref |
| 37.1–60.7 | 48.6 | 2.25 (1.57, 3.22) | 1.48 (0.90, 2.42) | 69.5 | 1.85 (1.39, 2.46) | 1.35 (0.96, 1.89) |
| ≥60.8 | 111.4 | 5.28 (3.70, 7.52) | 1.80 (1.15, 2.82) | 130.2 | 3.52 (2.56, 4.83) | 1.60 (1.09, 2.33) |
| P trend | — | <0.01 | <0.01 | — | <0.01 | 0.04 |
| Serum phosphate (mg/dl) | ||||||
| ≤3.1 | 24.2 | 0.73 (0.52, 1.05) | 0.88 (0.55, 1.40) | 46.4 | 1.01 (0.69, 1.48) | 1.12 (0.71, 1.76) |
| 3.2–3.5 | 33.9 | Ref | Ref | 47.5 | Ref | Ref |
| 3.6–3.9 | 46.0 | 1.28 (0.90, 1.83) | 0.82 (0.45, 1.48) | 64.7 | 1.30 (0.90, 1.88) | 0.97 (0.64, 1.46) |
| ≥4.0 | 85.3 | 2.48 (1.88, 3.28) | 1.19 (0.93, 1.53) | 93.1 | 1.96 (1.43, 2.67) | 1.35 (0.98, 1.85) |
| P trend | — | <0.01 | 0.09 | — | <0.01 | 0.35 |
| 25-hydroxyvitamin D (ng/dl)b | ||||||
| Quartile 1 | 48.3 | 1.20 (0.77, 1.89) | 0.95 (0.60, 1.51) | 69.8 | 1.25 (0.90, 1.74) | 1.11 (0.78, 1.57) |
| Quartile 2 | 49.4 | 1.23 (0.99, 1.54) | 1.20 (0.78, 1.84) | 61.9 | 1.11 (0.93, 1.32) | 1.11 (0.74, 1.66) |
| Quartile 3 | 39.7 | 1.06 (0.72, 1.56) | 0.89 (0.61, 1.30) | 56.5 | 1.08 (0.79, 1.48) | 1.04 (0.73, 1.47) |
| Quartile 4 | 34.4 | Ref | Ref | 49.7 | Ref | Ref |
| P trend | — | 0.32 | 0.86 | — | 0.16 | 0.51 |
Adjusted for age, sex, income, prior heart disease, smoking, categories of body mass index, 125I-iothalamate GFR, UPCR, and randomized treatment assignment and clustered by clinical center
Ranges for 25-hydroxyvitamin D quartiles (Q) are season-specific as follows: winter (Q1, ≤8.4; Q2, 8.5–10.9; Q3, 11.0–16.2; Q4, >16.2 ng/ml); spring (Q1, ≤7.9; Q2, 8.0–10.9; Q3, 11.0–16.5; Q4, ≥16.6 ng/ml); summer (Q1, ≤10.0; Q2, 10.1–15.2; Q3, 15.3–20.6; Q4, ≥20.7 ng/ml); and fall (Q1, ≤11.0; Q2, 11.1–14.7; Q3, 14.8–22.8; Q4, ≥22.9 ng/ml).
Association between Continuous Levels of Mineral Metabolites and Outcomes
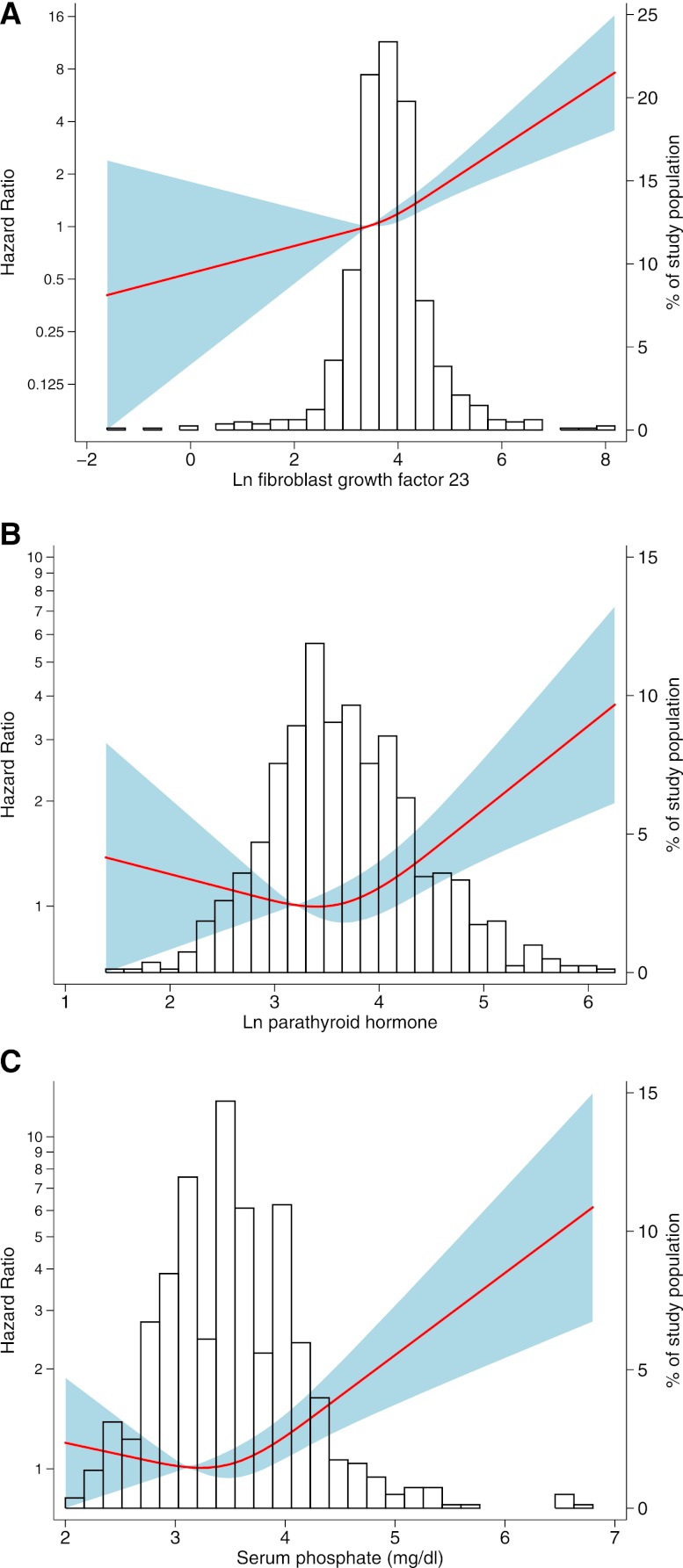
To more completely characterize the nature of the relationships between FGF23, PTH, phosphate, and outcomes, we modeled each as a continuous variable using restricted cubic splines (Figure 1). Based on these graphs, we modeled log-transformed FGF23 as a continuous linear predictor and log-transformed PTH and phosphate with piecewise linear splines, including knots at their means (PTH=39.3 pg/ml, phosphate=3.5 mg/dl; both knots significant at P<0.05). FGF23 was associated with a 30% increased risk of ESRD or death per doubling after multivariable adjustment (HR=1.30, 95% CI=1.15–1.47). Neither PTH nor phosphate was associated with outcomes in the lower range, but within their higher ranges, both PTH (HR=1.29 per doubling of PTH>39.3 pg/ml, 95% CI=1.14–1.45) and phosphate (HR=1.31 per 0.5 mg/dl higher serum phosphate>3.5 mg/d, 95% CI=1.19–1.45) were independently associated with greater risk in the primary multivariable-adjusted model.
Figure 1.
Association between mineral metabolites and adjusted hazard of ESRD or death. Models were performed using restricted cubic splines with knots at the 10th, 50th, and 90th percentiles. (A) Natural log-transformed (ln) FGF23, (B) ln PTH, and (C) serum phosphate. Solid line represents estimated HR, shaded area represents the 95% CI, and histogram represents the distribution of the mineral metabolite in the study population. Models are adjusted for age, sex, income, prior heart disease, smoking, UPCR, GFR, randomized treatment groups, categories of body mass index, and serum albumin, and they are clustered by clinical center.
To evaluate for effect modification by CKD severity that has been previously reported,13 we analyzed continuous models stratified by GFR (GFR<45 versus GFR≥45 ml/min per 1.73 m2) and proteinuria (UPCR<0.22 versus ≥0.22). The association between FGF23 and outcomes was not modified by GFR or proteinuria (P for interactions=0.25 and 0.11). In contrast, the association between PTH in the upper range and outcomes was present only among those patients with higher proteinuria (UPCR≥0.22; P for interaction<0.01), and the association between high levels of serum phosphate and outcomes was present only in those patients with lower GFR (P for interaction=0.01).
Risk of Outcomes According to the Number of Mineral Metabolism Abnormalities
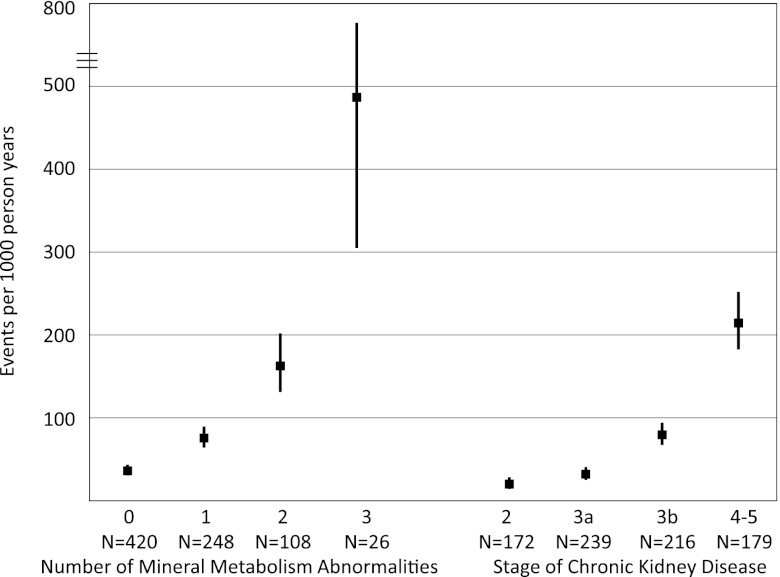
Given the independent relationships between FGF23, PTH, serum phosphate, and outcomes, we assessed the association between the total number of these abnormalities at baseline and risk of ESRD or death during follow-up. Clinical characteristics according to the number of mineral metabolite abnormalities are presented in Supplemental Table 2. The hazard of ESRD or death progressively increased with a greater number of mineral metabolism abnormalities. Compared with participants with no abnormalities, risk was 2.15-fold higher among participants with one abnormality (95% CI=1.61–2.88), 4.79-fold higher among participants with two abnormalities (95% CI=3.58–6.42), and 16.42-fold higher among participants with all three abnormalities (95% CI=8.92–30.22). The increased risk persisted after adjustment for 125I-iothalamate GFR (HR=3.85 for those patients with three compared with zero abnormalities, 95% CI=1.95–7.58). The overall incidence of ESRD or death was 487/1000 person-years for participants with abnormal levels of all three mineral metabolites, which was qualitatively higher than the incidence among participants with stages 4–5 CKD (Figure 2).
Figure 2.
Incidence of ESRD or death by the number of mineral metabolism abnormalities versus stage of CKD. Black squares represent incidence rate estimates, and vertical lines represent the 95% CIs calculated using a Poisson variance distribution.
Discussion
In this large cohort of African Americans with hypertensive nephrosclerosis, tight BP control, and long-term follow-up, we observed that higher levels of FGF23, PTH, and serum phosphate were associated with greater risk of progression to ESRD independent of directly measured 125I-iothalamate GFR. In contrast, low levels of 25-hydroxyvitamin D were highly prevalent but not associated with progression of CKD. A novel aspect of this study is the longitudinal assessment of mineral metabolites within individual CKD patients, which showed that the rates of increase in serum levels of FGF23, PTH, and phosphate are tightly linked to the concomitant change in GFR. To our knowledge, this study is the first to document longitudinal changes in multiple mineral metabolites within a large cohort of CKD patients. Another novel finding was that adjusting for the slope of directly measured 125I-iothalamate GFR during the 9 months before ascertainment of mineral metabolites did not attenuate the results of the outcome analyses. Although we acknowledge that these slope estimates were based on a limited time period and did not account for possible nonlinear trajectories of GFR decline,25 our findings suggest that abnormal levels of mineral metabolites convey additional clinically relevant information for assessing severity of CKD beyond static and longitudinal trends in GFR that clinicians already monitor routinely. Mechanistically, our data suggest that disordered mineral metabolism is not only a consequence of worsening kidney function, but perhaps contributes to progressive renal injury. Interventional studies are needed to determine whether therapeutic strategies that delay or attenuate the severity of disordered mineral metabolism can slow progression of CKD.
Among the mineral metabolites that we studied, elevated FGF23 was most strongly associated with outcomes before and after adjustment for confounders. FGF23 showed a biologic dose–response relationship with graded risk across the full range of values that was robust to all modeling strategies. One possibility is that the strong association that we observed between FGF23 and adverse clinical outcomes is caused by its potency as a marker of kidney function. However, in this study, PTH was more tightly correlated with GFR than FGF23, but FGF23 was most strongly associated with disease progression. A second possibility is that FGF23 promotes renal injury indirectly (for example, by reducing 1,25-dihydroxyvitamin D levels) or through other unknown mechanisms. We were not able to measure 1,25-dihydroxyvitamin D in this study to assess this hypothesis. A third possibility is that FGF23 may induce renal injury directly, analogous to its direct hypertrophic effects on cardiac myocytes.26 Experimental studies are needed to differentiate between these possibilities and evaluate potential mechanisms of injury.
In contrast to the current study, elevated FGF23 was associated with CKD progression only among participants with estimated GFR>30 ml/min per 1.73 m2 in the Chronic Renal Insufficiency Cohort (CRIC) Study.13 There are several potential reasons for this discrepancy. Whereas the CRIC study recruited a racially diverse study population with various etiologies of CKD and a range of proteinuria, AASK included a more homogenous population of African Americans with hypertensive nephrosclerosis and only modest proteinuria, and it specifically excluded participants with diabetes. Mineral metabolism abnormalities are more severe among CKD patients with high-grade proteinuria27 and diabetes,28 and therefore, the results of this study may be applicable only to the specific group studied. Alternatively, protocol-based optimization of BP using regimens that featured renin-angiotensin system antagonists may have mitigated the impact of powerful risk factors for CKD progression that were present in the less selected and noninterventional CRIC population. These design elements may have helped to expose effects of FGF23 in advanced CKD that remained hidden in the CRIC study. In support of this view, FGF23 was also associated with progression to ESRD in the randomized Homocysteinemia in Kidney and End Stage Renal Disease trial, which enrolled participants with late-stage CKD only.14
In the current study, PTH and phosphate were also independently associated with ESRD or death, but in contrast to FGF23, the associations showed threshold effects, with increased risks observed only within their upper ranges. It is possible that the nonlinear shape of these relationships highlights a distinct pathophysiology, in which overt increases are necessary to induce toxic effects. Alternatively, this finding may be because of differences in their corresponding relationships to kidney function. Serum phosphate was previously identified as one of a few baseline predictors of renal outcomes in the AASK study.20 The current analysis extends these findings by showing nonlinear effects and accounting for other mineral metabolites that are correlated with phosphate. Furthermore, high levels of all three mineral metabolites were associated with risk independent of each other, and therefore, the combination of all three abnormalities was a strong indicator of risk, even after adjustment for directly measured GFR. When interpreting this finding, it is important to recognize that the incidence rate of ESRD or death according to the number of mineral metabolism abnormalities depends on the cut points used to define abnormalities. Although these cut points were prespecified and informed by prior literature and clinical practice guidelines, strong evidence for optimal cut points for these measures is lacking.
Similarly, although the optimal vitamin D level has not been definitively established, the vast majority (95%) of AASK participants had 25-hydroxyvitamin D levels<30 ng/ml, which is the threshold promoted in CKD-specific guidelines.29 Coupled with limited use of vitamin D supplements, this cohort provided a unique opportunity to investigate vitamin D deficiency as a risk factor for CKD progression without interference by treatment. In the current study, low levels of 25-hydroxyvitamin D were associated with risk of ESRD or death in unadjusted analyses, but the effect was fully attenuated after adjustment for proteinuria. The latter may be an overadjustment; vitamin D deficiency may promote proteinuria, and higher-grade proteinuria can increase urinary loss of vitamin D bound to its binding protein.30,31 However, low 25-hydroxyvitamin D levels were only modestly associated with outcomes before adjustment for proteinuria. Furthermore, low 25-hydroxyvitamin D levels were not associated with death-censored renal end points in any models. This finding suggests that the unadjusted association between 25-hydroxyvitamin D and the composite primary end point was primarily driven by an association with death rather than ESRD. It is important to emphasize that we should interpret these predominantly negative results with caution given that the low number of individuals with normal vitamin D stores in the referent group may have limited our ability to define the true hazard associated with deficiency. Despite this limitation, the clinical implications of low 25-hydroxyvitamin D levels have been questioned in African Americans.7 The current results emphasize the need for additional outcomes studies specifically in African-American populations.
The CKD staging system was developed to standardize nomenclature as a means to enhance patient care and research.32 Although more advanced CKD stage is associated with greater risk of adverse outcomes,33 the prognostic use of the current GFR-based staging system has limitations. Age-related reductions in GFR can introduce false-positive diagnoses of CKD.34 Compensatory glomerular hyperfiltration that raises GFR can mislead clinicians to underestimate disease severity. Interpretation of GFR is clouded in patients treated with renin-angiotensin system antagonists, which reduce GFR in the short-term period, despite long-term renoprotective benefits.21 In our study, FGF23, PTH, and phosphate were associated with risk of ESRD independent of directly measured GFR. Although it is yet to be determined if interventions targeting mineral metabolism will improve outcomes in CKD, mineral metabolites may be useful markers of kidney disease severity above and beyond GFR. These findings are consistent with a recent study that documented improvement in ESRD risk prediction in advanced CKD by incorporating measures of CKD complications, including some mineral metabolites.35 Future studies should investigate whether incorporation of functional measurements of mineral metabolism into emerging diagnostic and therapeutic algorithms might enhance management and outcomes in CKD.
Concise Methods
Study Design and Population
AASK was a randomized trial of BP control strategies in 1094 African Americans with hypertensive kidney disease. Adult participants with 125I-iothalamate GFR between 20 and 65 ml/min per 1.73 m2, UPCR<2.5, and no apparent cause of CKD other than hypertension were enrolled from 1995 to 1998. Full inclusion and exclusion criteria have been described previously.21
Participants were randomized to intensive versus standard BP control (mean arterial pressure<92 versus 102–107 mmHg) and one of three primary antihypertensive agents (ramipril, metoprolol, or amlodipine) in a 2×3 factorial design. The primary end point of the trial was the slope of GFR decline beginning at 3 months postrandomization derived from repeated 125I-iothalamate GFR measurements. Secondary end points were incident ESRD or halving of 125I-iothalamate GFR from trial baseline. After the conclusion of the trial in September of 2001, 691 participants (89% of eligible participants) who had not developed ESRD continued into a prospective observational cohort phase, during which BP was managed to guideline-driven BP targets according to a common protocol based on the results of the trial.22 The AASK cohort study was specifically designed to provide additional follow-up of participants initially enrolled in the AASK trial and identify long-term risk factors for CKD progression.
Because of limited sample availability at trial baseline, this analysis included 809 participants who had serum available at the NIDDK Repository for measurement of mineral metabolites at the 12-month follow-up visit of the trial phase. AASK was approved by institutional review boards at each participating institution, and all participants provided written informed consent. This ancillary study was approved by the institutional review board at the University of Miami Miller School of Medicine.
Data Collection
Demographics, medical history, and clinical measurements were collected at study entry. GFR was measured directly as clearance of 125I-iothalamate at baseline, 3 months, and 6 months and then, every 6 months thereafter throughout the trial phase. Proteinuria was quantified as UPCR. We analyzed laboratory covariates from the 12-month follow-up visit to coincide with the measurement of the mineral metabolism exposures. In 22 participants with missing GFR and 84 participants with missing UPCR measurements at the 12-month visit, we used the closest result within 6 months. To quantify the rate of disease progression before and after the time when mineral metabolites were measured, we used linear mixed models to estimate each participant's slope of GFR from month 3 to 12 postrandomization and from month 12 to the end of the trial phase. The AASK study investigators previously defined the chronic slope of GFR decline beginning at month 3 postrandomization.21,24 The first 3 months after randomization were excluded from the slope calculations because of acute changes in GFR after initiation of therapy. We used the 125I-iothalamate GFR slope from month 12 to the end of the trial phase to categorize rates of CKD progression subsequent to measurement of mineral metabolites as slow, moderate, or rapid (<1, 1–3, or >3 ml/min per 1.73 m2 per year) according to thresholds used in the literature.36
We measured serum intact FGF23 (Kyowa Medex, Japan; coefficient of variation [CV]<10%), intact total PTH (DiaSorin, Stillwater, MN; CV<5%), and 25-hydroxyvitamin D (DiaSorin, Stillwater, MN; CV<5%) in samples from the 12-month follow-up visit in all participants, a randomly selected subcohort annually, and all study participants with stored samples available at entry into the observational cohort phase. Annual serum phosphate, calcium, and albumin levels were previously measured in all participants.
Exposures and Outcomes
The primary exposures were FGF23, PTH, phosphate, and 25-hydroxyvitamin D. After correction for serum albumin, serum calcium was not associated with outcomes in univariate analyses and therefore, not evaluated further. The primary outcome for this analysis was incident ESRD or death spanning the trial and cohort phases from 12 months postrandomization to June 30, 2007. ESRD was defined as initiation of dialysis or kidney transplantation. Secondary outcomes included death-censored ESRD and death-censored ESRD or doubling of serum creatinine from trial baseline.
Statistical Analyses
We compared clinical characteristics across quartiles of each mineral metabolite using standard descriptive statistics. Longitudinal changes in mineral metabolites were assessed in those patients with at least two measurements and compared across strata of initial 125I-iothalamate GFR and rate of 125I-iothalamate GFR decline after the 12-month visit using linear mixed models.
We categorized baseline FGF23, PTH, and phosphate in quartiles; 25-hydroxyvitamin D was classified in quartiles using season-specific cut points. We classified abnormal levels of mineral metabolites as FGF23>50 pg/ml, PTH>65 pg/ml, phosphate>4.6 mg/dl, and 25-hydroxyvitamin D<30 ng/ml.29,37,38
We compared the cumulative incidence of primary and secondary outcomes across quartiles of mineral metabolites using log rank tests, and we performed multivariable Cox proportional hazards analyses to adjust for confounding. We hierarchically adjusted for age, sex, and randomized group; GFR; UPCR; and income, prior heart disease, smoking status, serum albumin, and categories of body mass index in the full multivariable model. All models were clustered by clinical center. In additional analyses, we also adjusted for the slope of GFR before measurement of mineral metabolites to assess the potential confounding effects of the preceding rate of CKD progression followed by adjustment for all other mineral metabolites (serum FGF23, PTH, phosphate, 25-hydroxyvitamin D, and calcium).
In sensitivity analyses, we repeated the main analysis restricting follow-up to the trial phase only (median follow-up=3.5 years) and using time-varying measures of mineral metabolites updated at baseline in the observational phase. We explored the association between continuous levels of mineral metabolites and events using Cox regression with restricted cubic splines after multivariable adjustment and fit-adjusted continuous models including linear spline terms as necessary. We stratified models by GFR (<45 versus ≥45 ml/min per 1.73 m2) and presence of proteinuria (UPCR≥0.22 versus <0.22) using cut points previously reported in AASK studies.23,39
We calculated incidence rates of ESRD or death according to the number of abnormalities in these mineral metabolites that each participant manifested (zero, one, two, or three). We compared these rates with the corresponding incidence rates according to CKD stage (CKD stage 2, GFR≥60; stage 3a, GFR=45–59; stage 3b, GF=30–44; and stages 4–5, GFR<30 ml/min per 1.73 m2), assuming a Poisson distribution for variance estimates. We used Cox proportional hazards models to adjust for randomized treatment assignment and GFR.
We used Stata Special Edition 11.0 (College Station, TX) for all analyses and considered two-sided α<0.05 significant. PTH and 25-hydroxyvitamin D assays were donated and performed by DiaSorin. FGF23 measurements were performed by Kyowa Medex.
Disclosures
T.I. has served as a consultant and received honoraria from Shire and Genzyme. M.W. has served as a consultant or received honoraria from Abbott, Amgen, Diasorin, Genzyme, Kai, Luitpold, Mitsubishi, and Shire.
Supplementary Material
Acknowledgments
J.J.S. was supported in part by National Institutes of Health Grants T32DK00732, KL2RR025006, and K23DK095949. B.C.A. was supported in part by National Institutes of Health Grant R21DK078218. M.W. was supported by National Institutes of Health Grants R01DK076116, R01DK081374, and K24DK093723. African American Study of Kidney Disease and Hypertension (AASK) received additional support from the Office of Research in Minority Health (now the National Center on Minority Health and Health Disparities) and National Institutes of Health Institutional Grants M01 RR-00080, M01 RR-00071, M0100032, P20-RR11145, M01 RR00827, M01 RR00052, 2P20 RR11104, RR029887, and DK 2818-02.
This work was previously reported in abstract form at the American Society of Nephrology’s Kidney Week on November 1, 2012, in San Diego, California.
AASK was conducted by the AASK Investigators and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The data and samples from the AASK study reported here were supplied by the NIDDK Central Repositories. This manuscript was not prepared in collaboration with Investigators of the AASK study and does not necessarily reflect the opinions or views of the AASK study, the NIDDK Central Repositories, or the NIDDK.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012070713/-/DCSupplemental.
References
- 1.United States Census Bureau: 2010 Census Briefs, The Black Population: 2010, Table 1, 2010. Available at: http://2010.census.gov/2010census/data/ Accessed April 26, 2012
- 2.United States Renal Data System : USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2011 [Google Scholar]
- 3.Hsu C-Y, Lin F, Vittinghoff E, Shlipak MG: Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol 14: 2902–2907, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appel LJ, Wright JT, Jr, Greene T, Kusek JW, Lewis JB, Wang X, Lipkowitz MS, Norris KC, Bakris GL, Rahman M, Contreras G, Rostand SG, Kopple JD, Gabbai FB, Schulman GI, Gassman JJ, Charleston J, Agodoa LY, African American Study of Kidney Disease and Hypertension Collaborative Research Group : Long-term effects of renin-angiotensin system-blocking therapy and a low blood pressure goal on progression of hypertensive chronic kidney disease in African Americans. Arch Intern Med 168: 832–839, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CAM, Lash JP, Hsu CY, Leonard MB, Wolf M: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutiérrez OM, Isakova T, Andress DL, Levin A, Wolf M: Prevalence and severity of disordered mineral metabolism in Blacks with chronic kidney disease. Kidney Int 73: 956–962, 2008 [DOI] [PubMed] [Google Scholar]
- 8.De Boer IH, Gorodetskaya I, Young B, Hsu C-Y, Chertow GM: The severity of secondary hyperparathyroidism in chronic renal insufficiency is GFR-dependent, race-dependent, and associated with cardiovascular disease. J Am Soc Nephrol 13: 2762–2769, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K: Secondary hyperparathyroidism is associated with higher mortality in men with moderate to severe chronic kidney disease. Kidney Int 73: 1296–1302, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, Shlipak MG, Whooley MA, Ix JH: The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: The Heart and Soul Study. Ann Intern Med 152: 640–648, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, Rifkin D, Siscovick DS, Sarnak MJ, Shlipak MG: Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J Am Coll Cardiol 60: 200–207, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, Chonchol M, HOST Investigators : FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 22: 1913–1922, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin A, Djurdjev O, Beaulieu M, Er L: Variability and risk factors for kidney disease progression and death following attainment of stage 4 CKD in a referred cohort. Am J Kidney Dis 52: 661–671, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Voormolen N, Noordzij M, Grootendorst DC, Beetz I, Sijpkens YW, van Manen JG, Boeschoten EW, Huisman RM, Krediet RT, Dekker FW, PREPARE study group : High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant 22: 2909–2916, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Zoccali C, Ruggenenti P, Perna A, Leonardis D, Tripepi R, Tripepi G, Mallamaci F, Remuzzi G, REIN Study Group : Phosphate may promote CKD progression and attenuate renoprotective effect of ACE inhibition. J Am Soc Nephrol 22: 1923–1930, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellasi A, Mandreoli M, Baldrati L, Corradini M, Di Nicolò P, Malmusi G, Santoro A: Chronic kidney disease progression and outcome according to serum phosphorus in mild-to-moderate kidney dysfunction. Clin J Am Soc Nephrol 6: 883–891, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, König P, Kraatz G, Mann JF, Müller GA, Köhler H, Riegler P, MMKD Study Group : Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: The Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol 18: 2600–2608, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Norris KC, Greene T, Kopple J, Lea J, Lewis J, Lipkowitz M, Miller P, Richardson A, Rostand S, Wang X, Appel LJ: Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol 17: 2928–2936, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agodoa LY, Appel L, Bakris GL, Beck G, Bourgoignie J, Briggs JP, Charleston J, Cheek D, Cleveland W, Douglas JG, Douglas M, Dowie D, Faulkner M, Gabriel A, Gassman J, Greene T, Hall Y, Hebert L, Hiremath L, Jamerson K, Johnson CJ, Kopple J, Kusek J, Lash J, Lea J, Lewis JB, Lipkowitz M, Massry S, Middleton J, Miller ER, 3rd, Norris K, O’Connor D, Ojo A, Phillips RA, Pogue V, Rahman M, Randall OS, Rostand S, Schulman G, Smith W, Thornley-Brown D, Tisher CC, Toto RD, Wright JT, Jr, Xu S, African American Study of Kidney Disease and Hypertension (AASK) Study Group : Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: A randomized controlled trial. JAMA 285: 2719–2728, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Appel LJ, Middleton J, Miller ER, 3rd, Lipkowitz M, Norris K, Agodoa LY, Bakris G, Douglas JG, Charleston J, Gassman J, Greene T, Jamerson K, Kusek JW, Lewis JA, Phillips RA, Rostand SG, Wright JT: The rationale and design of the AASK cohort study. J Am Soc Nephrol 14[Suppl 2]: S166–S172, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Appel LJ, Wright JT, Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML, Gabbai FB, Gassman JJ, Hebert LA, Jamerson KA, Kopple JD, Kusek JW, Lash JP, Lea JP, Lewis JB, Lipkowitz MS, Massry SG, Miller ER, Norris K, Phillips RA, Pogue VA, Randall OS, Rostand SG, Smogorzewski MJ, Toto RD, Wang X, AASK Collaborative Research Group : Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 363: 918–929, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG, African American Study of Kidney Disease and Hypertension Study Group : Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK trial. JAMA 288: 2421–2431, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Li L, Astor BC, Lewis J, Hu B, Appel LJ, Lipkowitz MS, Toto RD, Wang X, Wright JT, Jr, Greene TH: Longitudinal progression trajectory of GFR among patients with CKD. Am J Kidney Dis 59: 504–512, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St. John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malluche HH, Goldstein DA, Massry SG: Osteomalacia and hyperparathyroid bone disease in patients with nephrotic syndrome. J Clin Invest 63: 494–500, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wahl P, Xie H, Scialla J, Anderson CA, Bellovich K, Brecklin C, Chen J, Feldman H, Gutierrez OM, Lash J, Leonard MB, Negrea L, Rosas SE, Anderson AH, Townsend RR, Wolf M, Isakova T, Chronic Renal Insufficiency Cohort Study Group : Earlier onset and greater severity of disordered mineral metabolism in diabetic patients with chronic kidney disease. Diabetes Care 35: 994–1001, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Kidney Foundation: KDOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–S202, 2003 [PubMed] [Google Scholar]
- 30.de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving H-H, Pritchett Y, Remuzzi G, Ritz E, Andress D: Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): A randomised controlled trial. Lancet 376: 1543–1551, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Agarwal R: Vitamin D, proteinuria, diabetic nephropathy, and progression of CKD. Clin J Am Soc Nephrol 4: 1523–1528, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G, National Kidney Foundation : National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med 139: 137–147, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT, Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindeman RD, Tobin J, Shock NW: Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 33: 278–285, 1985 [DOI] [PubMed] [Google Scholar]
- 35.Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, Levin A, Levey AS: A predictive model for progression of chronic kidney disease to kidney failure. JAMA 305: 1553–1559, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, Newman AB, Sarnak MJ: Rapid kidney function decline and mortality risk in older adults. Arch Intern Med 168: 2212–2218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S: Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab 87: 4957–4960, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 113: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Scialla JJ, Appel LJ, Astor BC, Miller ER, 3rd, Beddhu S, Woodward M, Parekh RS, Anderson CAM, African American Study of Kidney Disease and Hypertension Study Group : Net endogenous acid production is associated with a faster decline in GFR in African Americans. Kidney Int 82: 106–112, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.