Abstract
The structures of the medial temporal lobe (MTL) have been shown to be causally involved in episodic and recognition memory. However, recent work in a number of species has demonstrated that impairments in recognition memory seen following lesions of the perirhinal cortex can be accounted for by deficits in perceptual discrimination. These findings suggest that object representation, rather than explicit recognition memory signals, may be crucial to the mnemonic process. Given the large amount of visual information encountered by primates, there must be a reconsideration of the mechanisms by which the brain efficiently stores visually presented information. Previous neurophysiological recordings from MTL structures in primates have largely focused on tasks that implicitly define object familiarity (i.e., novel vs. familiar) or contain significant mnemonic demands (e.g., conditional associations between two stimuli), limiting their utility in understanding the mechanisms underlying visual object recognition and information storage. To clarify how different regions in the MTL may contribute to visual recognition we recorded from three rhesus macaques performing a passive viewing task. The task design systematically varies the relative familiarity of different stimuli enabling an examination of how neural activity changes as a function of experience. The data collected during this passive viewing task revealed that neurons in the MTL are generally not sensitive to the relative familiarity of a stimulus. In addition, when the specificity (i.e., which images a neuron was selective for) of individual neurons was analyzed, there was a significant dissociation between different medial temporal regions, with only neurons in TF, but not CA3 or the perirhinal cortex, altering their activity as stimuli became familiar. The implications of these findings are discussed in the context of how MTL structures process information during a passive viewing paradigm.
Keywords: macaque, sparse, medial temporal lobe, passive viewing, tuning
Introduction
The ability of an organism to classify a stimulus as familiar has been proposed to rely on parallel processes of accurate object identification and detection that an item was previously encountered (e.g., Mandler, 1980; Yonelinas, 2002). The behavioral signature of these processes can often be measured as a preferential viewing of novel over familiar images (Gunderson and Swartz, 1985; Fagan, 1972; Bachevalier et al., 1993; Buffalo et al., 1999; Pascalis and Bachevalier, 1999; Gothard et al., 2004; Richmond et al., 2007). Deficits in visual recognition memory are closely linked to the integrity of structures in the medial temporal lobe (Mahut et al., 1982; Zola-Morgan and Squire, 1985; Zola-Morgan et al., 1994). For example, lesions of the perirhinal cortex in humans (Buffalo et al., 1998; Yonelinas et al., 2002) and monkeys (Meunier et al., 1993; Tang et al., 1997; Buffalo et al., 2000) produce a significant deficit across different recognition memory tasks. Impairments in familiarity discrimination due to hippocampal (HC) lesions however are more ambiguous; with evidence both for (Beason-Held et al., 1999; Zola et al., 2000) and against (Murray and Mishkin, 1998a; Nemanic et al., 2004). Consistent with these observations, neurophysiological studies in primates have identified a putative mechanism by which the brain represents, and presumably tracks, stimulus familiarity. Subpopulations of neurons throughout the inferior temporal cortex (IT) and non-hippocampal structures of the medial temporal lobe (MTL; i.e., perirhinal cortex, PRh; parahippocal cortex, TF/TH; and the entorhinal cortex, EC) respond to the second presentation of a stimulus by reductions in their firing rate. This response suppression is seen across non-hippocampal regions of the MTL and ITC in a variety of mnemonic tasks (e.g., Miller et al., 1991; Riches et al., 1991; Fahy et al., 1993; Xiang and Brown, 1998). Single unit data from hippocampal recordings are equivocal in their support for a similar recognition memory signal (e.g., Brown et al., 1987; Rolls et al., 1993; Xiang and Brown, 1998; Jutras and Buffalo, 2010).
Critically however, the observed correlations between firing rate and recognition memory tasks may be confounded due to the statistical properties of task design. For example, during an active mnemonic task (e.g., delayed non-match to sample - DNMS) subjects are shown a stimulus and after a delay, are presented with a repeat of the original sample stimulus in addition to a non-matching stimulus. While these tasks undoubtedly contain a mnemonic component (i.e., working memory), it also contains a major statistical regularity involving the sample and sample-repeat nature of the task. It has been demonstrated that animals can use the simple strategy of detecting stimulus repetition rather than comparing stimulus content (Miller and Desimone, 1994). Importantly, the activity of IT neurons recorded during this task was suppressed by any stimulus repetition, even when the initial stimulus was already familiar (Miller and Desimone, 1994). Therefore, decrements in firing rate by PRh neurons are not necessarily evidence for an explicit recognition memory signal. Rather, it is possible that the activity of PRh neurons either reflects the statistical features of a task or an animal’s specific behavioral strategy. Thus it is possible that the response decrement signals observed in serial presentation or preferential looking tasks emerge because of the inherent sample/sample-repeat structure of those tasks and do not necessarily reflect the computational properties of the circuit.
In addition to possible confounds due to task design, a number experiments now suggest that deficits in recognition memory may be more specifically related to perceptual deficits. For example, rhesus monkeys with PRh lesions are impaired at identifying rotated views of familiar objects as well as objects in scenes (Buckley and Gaffan, 1998; Buckley et al., 2001). Monkeys and rodents with perirhinal lesions are similarly impaired in tests that systematically vary the amount of overlapping features between two simultaneously presented objects or image pairs (Bussey et al., 2002a, 2003; Norman and Eacott, 2004; Bartko et al., 2007). Difficulties on perceptual discrimination tasks, however, do not persist when animals are tested stimuli that are perceptually distinct (Eacott et al., 1994; Bussey et al., 2003). The observed patterns of deficits, as well as the anatomical connectivity with upstream cortical regions (Suzuki and Amaral, 1994; Lavenex et al., 2004), suggest that the PRh sits at the top of the ventral visual stream/object-analyzer pathway and acts to unify features of complex stimuli. Computationally, this process is thought to be supported by PRh neurons whose activity is modulated during the learning of conditional associations between two stimuli (Miyashita, 1988; Sakai and Miyashita, 1991; Erickson and Desimone, 1999; Fujimichi et al., 2010). This refined view of the PRh has been unified under the perceptual-mnemonic/feature-conjunctive model and raises a number of questions regarding the general encoding properties of PRh neurons (Miyashita, 1993; Sakai et al., 1994; Murray and Bussey, 2001; Murray and Richmond, 2001; Bussey et al., 2002b, Murray and Wise, this issue; Cowell, this issue)
Neurons in the ITC and MTL are selective to a wide range of stimuli, including naturalistic as well as geometrically complex (i.e., fractals) images (e.g., Desimone et al., 1984; Baylis et al., 1985; Gross, 1992; Miyashita, 1993; Ishai et al., 1999; Mormann et al., 2008). Single neurons are responsive to images from multiple different categories, with the final representation of a stimulus being distributed across a group of neurons (Gochin et al., 1994; Logothetis et al., 1995). The distributed activity pattern of these neurons suggests that these brain regions use a population coding scheme to store information (Hinton et al., 1986; Meunier et al., 1991). Population codes offer significant advantages for the storage of information because they are robust against interference and increase the number of patterns that can be stored in a network (Marr, 1971; Hinton et al., 1986; McNaughton and Morris, 1987; Amari, 1989; Rolls and Treves, 1990; Meunier et al., 1991). This encoding scheme however may become unstable due to network saturation or when multiple stimulus dimensions must be encoded simultaneously (Zhang and Sejnowski, 1999). From a theoretical perspective, interference between representations (i.e., overlapping groups of neurons) could be minimized by enhancing the sparseness of the population code (i.e., fewer neurons responding to a given stimulus; McNaughton and Morris, 1987; Meunier et al., 1991; Pouget et al., 1999).
At the level of a single neuron, changes in the signal-to-noise ratio of a population code can occur either via a decrease in the firing rate to stimuli which elicit sub-optimal responses (Baker et al., 2002; Freedman et al., 2006) or by increasing the firing rate to preferred images (Sakai et al., 1994; Kobatake et al., 1998). The direction of the firing rate change has been suggested to depend on whether a neuron is inhibitory or excitatory (Woloszyn and Sheinberg, 2012). Ultimately, either type of adaptation would increase the specificity of a neuron’s response to visual images. This enhanced specificity can be quantified by establishing a tuning profile that reflects a neuron’s sensitivity to different images or stimuli. For example, during learning of a perceptual discrimination task, neurons in the ITC are initially responsive to a large proportion of visually presented images. However, following learning, ITC neurons respond more selectively, resulting in a narrower tuning profile (Logothetis et al., 1995; Sigala and Logothetis, 2002; Freedman et al., 2006), although see Kobatake et al., (1998). Similarly, training on a variety of perceptual discrimination tasks also results in narrower tuning profiles in both lower level visual (Schoups et al., 2001; Vinje and Gallant, 2002; Yang and Maunsell, 2004) and somatosensory areas (Recanzone et al., 1992). Importantly, response accuracy closely matches changes in the tuning profiles of single neurons.
Experience-dependent modifications of PRh neurons, however, have primarily been demonstrated during the learning of stimulus associations (e.g., during paired association tasks). One prediction arising from the perceptual-mnemonic/feature-conjunctive model is that PRh neurons should show task-related changes either when there is significant ambiguity between a set of visually presented items or when temporal associations must be encoded. The aim of the current experiment was to investigate whether passive viewing, in the absence of associative learning demands or stimulus ambiguity, modulates the activity of PRh neurons either as a function of stimulus novelty/familiarity or with respect to stimulus selectivity. Towards this end, a passive viewing task was developed that systematically varies stimulus novelty, establishing a gradient of familiarity. The design of this task incorporated sufficient repetition of individual stimuli so that experience-dependent changes in stimulus selectivity could be determined. Image presentation was randomized so that there was no association between stimulus type (i.e., novel or familiar) and either a motoric demand or reward delivery. By controlling presentation order, stimulus repetition, motoric components or reward associations, the present task eliminates these potential confounds.
Materials and Methods
Subjects
Three adult rhesus macaques (Macaca mulatta) were used in the present study. Monkey BZ (14.0 kg) was a male, Monkey NS (12.0 kg) and Monkey JN (7.5 kg) female. All subjects were part of a series of long-term behavioral and neurophysiological testing. All subjects were continuously housed at the California Regional Primate Center (CRPC) in Davis, CA. Animals were pair housed and kept on a 12-12 h light-dark cycle. Subjects were fed ad libitum chow and water. No water manipulation was used during experimental testing. All surgical and experimental procedures were approved by the IACUC at the University of California, Davis, CA.
Surgeries and Electrode implantation
The procedures for implanting the headpost and chronic microdrives are as described in detail by Skaggs et al. (2007). Prior to any surgical manipulations each animal received an MRI scan used for surgical planning, followed by a two-step surgical process. During the first procedure subjects were fitted with headposts for head restraint to enable eye tracking during the experiment. Following a recovery period, during which animals undergo behavioral training, animals were implanted with a chronic microdrive recording device (“hyperdrive”, Skaggs et al., (2007)). The position for the hyperdrive implant was calculated for each animal from presurgical MRI scans to target the middle hippocampus from plate 68 in Paxinos et al. (1999).
Each hyperdrive consists of 12 independently movable tetrodes, as well as one reference electrode and one ground electrode (Wilson and McNaughton, 1993). Tetrodes were custom manufactured from 30 μm polyimide-coated nichrome wire (Ro-800, Kanthal Precision Technology, Palm Coast, FL). Electrical impedances were adjusted to 100–200 KOhm by gold plating. Individual tetrodes were encased in 160 μm diameter silica tubing, which are supported by 28-gauge stainless steel guide cannulae. During the first surgery a recording well (Crist Instruments, Hagerstown, MD) and a headpost was attached to the skull with screws and dental cement. Before implantation of the hyperdrive, a craniotomy was drilled and the drive that holds the tetrode recording probes was lowered and secured in to this recording chamber with 3 set screws. During placement of the hyperdrive into the chamber, the tetrode/silica/cannulae assembly was lowered approximately 26 mm below the dorsal cortical surface, to just above medial temporal lobe targets. Individual tetrodes were attached to threaded micro-manipulator legs housed in the hyperdrive. A full turn of the shuttle nut moves the attached tetrode approximately 320 microns. The maximum travel distance for each tetrode varied by animal and structure targeted, but generally was between 11 – 13 mm.
Data Acquisition
Local field potential and multiple single units were recorded using the Neuralynx Cheetah data acquisition system (Neuralynx, Bozeman, MO). Eye movement data were collected using an infrared eye-tracking system – ISCAN (Boston, MA). Analog eye position data was also sent to the Cheetah data acquisition system. The behavioral task was controlled via a Cortex system (NIMH/SALK, dally.nimh.nih.gov) CORTEX software emits time locked codes corresponding to different image presentations or fixation events. These events are recorded by Cheetah via a custom serial interface board.
Single-unit data were recorded from each tetrode channel, amplified 2000 times and band-pass filtered between 600–6000 Hz and recorded at 32 KHz. Spike data were recorded from all four tetrode channels in 1 ms windows when the voltage on any given channel crossed a user specified threshold. Thresholds were optimized daily to improve single unit isolation. Single unit isolation was done offline using either Xclust (M.A. Wilson) or MClust (A.D. Redish). Tetrodes were generally moved between recording sessions, either to optimize the recordings or to collect data from a new set of cells. While it is possible that the same neuron was recorded on multiple days, we have found no reliable means to determine conclusively whether a neuron recorded on one day is exactly the same neuron as recorded on a following day. Presumably if the electrode is moved, different neurons will be recorded, and thus we have treated all recorded neurons as individual samples. Local Field Potential data were collected from 1 user defined tetrode channel, amplified 1000 times and band pass filtered between 1–475 Hz and recorded continuously at 1000 Hz. All data analysis was performed using custom software written in MATLAB (The Mathworks, Inc.). For all analyses described below, data from each animal was initially considered separately. However, as the pattern of results was similar across monkeys, neurons from all animals were pooled.
For each recording session animals were seated in a primate restraint chair (SAS Precision, Mason City, NE) and placed in a dimly lit sound attenuating chamber. Once in the chamber, animals were head fixed and a headstage with its associated cables was attached to the hyperdrive. Once the system was connected, data acquisition began. Each day began and ended with a 15–30 minute period of inactivity, the data for which were reported previously (Skaggs et al., 2007).
Histology and Reconstruction
Following the conclusion of an animal’s neurophysiological testing and a brief post experimental behavioral battery, all subjects were necropsied. All necropsy and histological procedures have been previously described in detail in (Skaggs et al., 2007). Briefly, following transcardial perfusions with paraformaldehyde, brains were removed and sectioned into three coronal blocks and cryoprotected. Once cryoprotected, the tissue of interest was sectioned at 30 μm and divided into four series for processing. One series from each subject was Nissl stained to visualize electrode tracks. Nissl-stained sections were examined under a light microscope and the portions of the sections containing visible electrode tracks were photographed at 100x magnification. Post-surgical CT scans are used to visualize individual cannula from the hyperdrive. The cannula/tetrode trajectory from the CT scan, is used to identify different electrode tracks seen in the histological material. When combined with the daily logs indicating the distance each electrode was moved, we derive highly accurate electrode positions. In addition to the histological reconstruction, physiological parameters such as baseline firing rates and signature events in the local field potential (e.g., hippocampal sharpwaves) are used to further verify our locations (Bartho et al., 2004; Skaggs et al., 2007).
Behavioral task and training
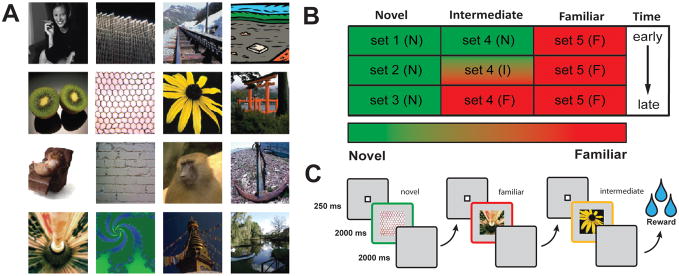
The current experiment utilized a passive viewing task called VARNOV (VARiable NOVelty). Subjects were presented with a sequence of images which varied with respect to how familiar they were. Each VARNOV session consisted of 50 images assigned to one of three categories (Figure 1B). A total of 30, previously unseen, images were assigned to the novel group. Images in the novel group were divided into three sets (Sets 1, 2, and 3, Figure 1B), with each of the 10 images in a set being presented a total of 5 times. The familiar category (Set 5, Figure 1B) consisted of 10 images that were highly familiar to the animal and had been seen hundreds of times previously. The third, or intermediate, set of images (Set 4, Figure 1B) contained 10 images that were completely novel at the start of the day, but were repeated 30 times during the course of a recording session. This experimental design allows neuronal responses to completely novel stimuli (Set 1, 2, 3) to be obtained and compared to those from completely familiar stimuli (Set 5). Additionally it allows activity of neurons to be monitored during the evolution of a given stimulus as it becomes familiar within a given day (intermediate category, Set 4).
Figure 1. Visual Stimuli and VARNOV task structure.
A. Example of visual stimuli used in the Variable Novelty (VARNOV) task. Images were collected and modified from a wide range of sources and adjusted to be 300×300 pixels (or 10 DVA) in size. Stimuli were presented in the center of the display and monkeys were free to explore the images after stimulus onset. B. Diagram of the familiarity gradients inherent in the VARNOV task. Note that the “Novel” category consists of 3 sets of unique images. Once images in this category are no longer novel (i.e., after 5 presentations), images are refreshed with stimuli from another set until all novel images are exhausted. Novel and familiar contrasts are measured between images in columns 1 and 3 as well as between early and late trials in column 2. C. Example of one VARNOV trial, one image from each category is presented. After three image presentations subjects receive a small juice reward. Image order is randomized so no specific association can be made with respect to reward delivery.
The basic structure of a trial in the VARNOV task involves the sequential presentation of one image randomly selected from each category (Figure 1C; i.e., novel/familiar/intermediate). Importantly, the category order of the presented images is randomized within each trial. Image presentation is preceded by the appearance of a fixation square (white square - Figure 1C). When an animal has fixated for 500 ms, an image is presented for two seconds after which the screen is reset. Animals are free to explore the image within a given boundary during the presentation period; however if the animal moves its eyes outside of this boundary, the trial is aborted. Following a 2 sec delay the fixation/presentation cycle starts over with an image from a different category (Figure 1C). Once one trial (3 fixation/presentation cycles) is complete, the screen resets and subjects receive a small juice reward (Crist Instruments, Hagerstown, MD). When images in a novel set have been exhausted a new set is introduced, thereby maintaining stimulus novelty.
All experiments were conducted while subjects were seated in a primate restraint chair in a dimly illuminated sound proof recording chamber. Images were presented on a CRT monitor placed approximately 50 cm from the subjects’ eyes. As indicated above no behavioral response, other than a brief fixation, was required from our subjects. The stimulus dataset used in these experiments consisted of 1000 complex visual images from various sources. Individual images were often cropped from larger images, and included complex geometric patterns, scenes, objects and animals. Examples of image types can be seen in Figure 1A.
Results
A total of 428 stable and well isolated neurons were recorded from 3 animals performing the VARNOV task (CA3 n = 252, PRh n=101, TF = 75). To determine whether neurons were visually responsive, the average firing rate during the pre-stimulus fixation period was compared to the average firing rate during the period of 75 ms – 450 ms post stimulus onset. Neurons were categorized as visually responsive if the difference in pre-fixation firing rate vs. the presentation firing rate from all trials was less than p <.05 (student’s t-test).
Lack of response suppression
A number of studies have reported that changes in the firing rate of individual neurons across the MTL are associated with the relative novelty or familiarity of a stimulus (Brown et al., 1987; Miller et al., 1991; Fahy et al., 1993; Li et al., 1993; Xiang and Brown, 1998; Jutras and Buffalo, 2010). These changes in firing rates have been suggested to serve as a recognition memory signal. Notably, the majority of these tasks were active mnemonic tasks in which the animal was trained to make a response to a given stimulus. Relatively few studies have examined the responses of neurons in the absence of explicit mnemonic demands. To determine whether neurons in different MTL regions signaled stimulus novelty, the activity of all visually responsive neurons to the first and second presentation of all images in sets 1–4 was analyzed – yielding a total of 80 responses per neuron (40 responses to first presentations and 40 to second presentations). A two-tailed paired t-test was used to compare the firing rates between the first and the second condition. Individual neurons were classified as sensitive to stimulus novelty if this difference was significant at p < .05. The sign of the difference between the mean firing rates between novel and familiar images was used to indicate whether a neuron incremented or decremented it’s firing rate. The percentages of neurons which by the above measure are sensitive to familiarity are summarized in Table 1.
Table 1. Percentage of visually responsive neurons whose activity is significantly modulated by stimulus novelty.
Summary table of the percentages of neurons in CA3, perirhinal (PRh) and area TF that change their activity in response to stimulus novelty. Firing rate modulations were deemed significant if p < .05 in a two-tailed paired t-test. Neurons that, on average, showed a decrease in firing rate to the second presentation of a stimulus were classified as decrementing, while neurons that increased their firing rate were classified as incrementing.
| Decrementing | Incrementing | |
|---|---|---|
| CA3 | 5% (4/79) | 8% (6/79) |
| PRh | 3% (2/67) | 2% (1/67) |
| TF | 5% (3/51) | 2% (1/51) |
A critical component for stable representations in the nervous system is the reliability with which a neuron transmits a given message. From the perspective of a downstream decoder neuron, response reliability translates into signal fidelity. Consequently, it may be hypothesized that if a neuron is signaling the familiarity or novelty of a stimulus, then that signal should be highly reliable. The reliability of neurons sensitive to stimulus familiarity was estimated by determining the proportion of repeated stimuli for which a neuron showed increased or decreased firing rates. It was reasoned that if a neuron reliably detected novelty then it would do so for more than 75% of stimuli. The criterion value of 75% was used to allow for small fluctuations of selectivity to novelty or familiarity. Across all neurons that passed the statistical cutoff of p < .05, none met the 75% criteria. Regional averages of response reliability for incrementing/decrementing responses are as follows: CA3 (45%/44%), PRh (61%/45%), TF (47%,60%). These data, showing a lack of sensitivity to the stimulus familiarity (or novelty), are consistent with the responses of PRh neurons recorded from rodents passively encountering 3D objects while running on a track (Burke et al., this issue).
Differential effects of experience on the tuning profiles of medial temporal lobe neurons
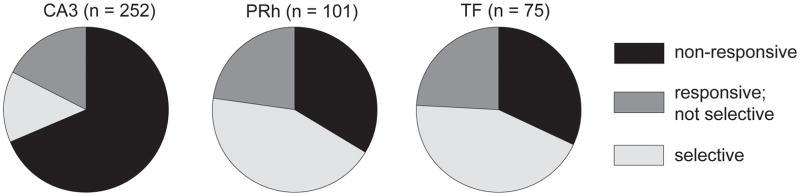
Of the 428 recorded neurons 193 (45%) showed differential activity (i.e., were generically responsive) to visual stimuli in the VARNOV task (CA3 n = 79, PRh n=67, TF = 51). For each neuron the average firing rate response between 75 – 450ms after initiation of stimulus presentation was computed. These values were used in a one way ANOVA, evaluated at p < .05, to determine whether neurons that were visually responsive were specifically active to a subset of the presented images. The proportion of neurons that showed response specificity was: CA3 (14%; 35/252), PRh (44%; 44/101), TH (44%; 33/75); Figure 2.
Figure 2. Proportion of Responsive Neurons.
The relative proportions of the different kinds of neuronal response types across the three brain regions targeted by our recordings. Neurons in the CA3 region of the hippocampus were significantly less visually responsive than were neurons in either PRh or area TF.
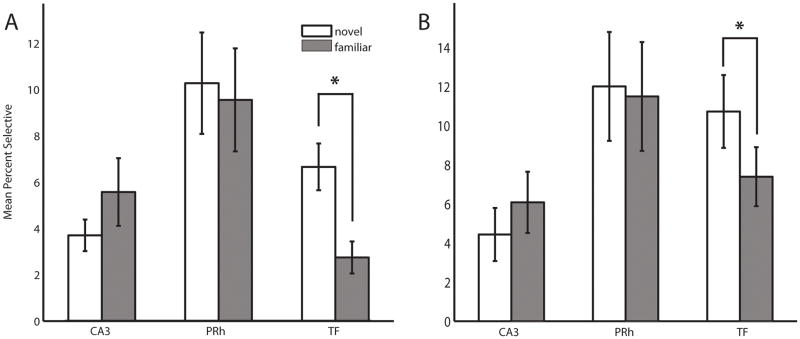
The specific subset of images that a given neuron is selective for were identified using a posthoc Tukey HSD test—establishing a neuron’s tuning profile. Using this tuning profile, the percentage of novel and familiar stimuli that a neuron was specifically selective for was estimated. The percent-selective measure reveals how narrowly or broadly tuned a neuron is (e.g., a widely tuned neuron would respond to many images, while a narrowly tuned neuron would respond to only a small set of images). Neurons that were responsive, but not selective, were not included in the analysis; though the results below are robust even when non-selective neurons are included. There was no difference in the tuning breadth of CA3 or PRh neurons between the novel and familiar condition (Wilcoxon two-sample sign rank test, p=.22 n = 35 and p = .699, n = 44 respectively). However, neurons in area TF were significantly more specific in their selectivity to familiar stimuli, compared to novel stimuli (Wilcoxon two-sample sign rank test, significant P < 0.005, n= 33) (Figure 3A).
Figure 3. Effects of experience on stimulus selectivity across MTL regions.
(A) Average selectivity for all neurons within a region in the pure familiar and novel conditions (columns 1 and 3, Figure 1 B). The responses of neurons in area TF are significantly more specific in the familiar condition than in the novel condition (Wilcoxon signrank test p < .05). (B) As in A, estimates of stimulus familiarity are based on the first 4 and last 4 presentations of a stimulus in the intermediate category. TF continues to show a significant decrease in response specificity (i.e. selective to fewer images; Wilcoxon signrank test p < .05). Error bars – standard error of the mean.
While compelling, it must be considered whether the observed experience-dependent changes arise simply by chance due to the stimuli used. One advantage of the VARNOV task is that neurons in the intermediate category are repeatedly presented throughout the session–establishing a gradient of familiarity. Tuning breadth was estimated as described above, however, novel and familiar response data were drawn from the first four vs. last four presentations to a given stimulus set. As above, neurons in TF were significantly more narrowly tuned in the familiar than the novel condition (Wilcoxon two-sample sign rank test, significant P < 0.05, N= 33) (Figure 3B). While the purely novel and familiar conditions are presented in an interleaved manner throughout the experiment, the familiarity gradient in the intermediate condition has a necessary temporal gradient. Consequently, it is possible that there occurred a change in the baseline firing rate over the course of the experiment, which could influence estimates of stimulus selectivity. Baseline firing rates were estimated from the start of the experiment until the last presentation of a stimulus associated with the novel-intermediate group. Similarly, baseline firing rates were computed from the first presentation of a stimulus associated with the familiar-intermediate group to the end of the recording session. Difference scores for tuning breadth and baseline firing rates were correlated in a linear regression. The results of the regression suggest that only ~2% of the variance in tuning can be accounted for by changes in baseline firing rate, effectively ruling out contamination of our results due to differences in rate (r2=0.0269, df =228; p = .0128).
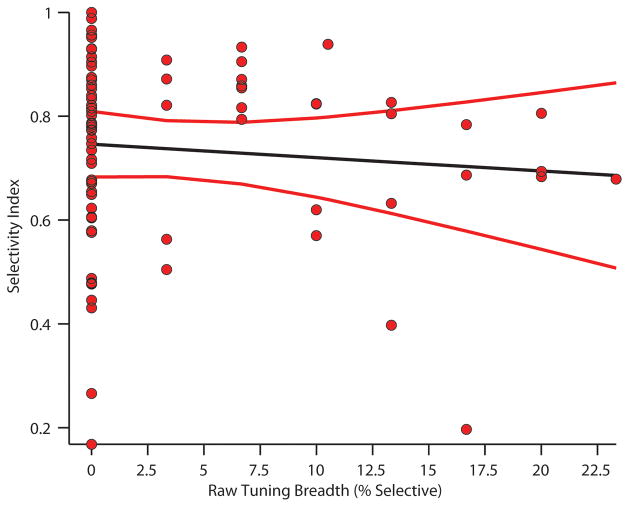
The lack of enhanced selectivity (i.e., narrower tuning) of hippocampal neurons contrasts with a previous study by Yanike et al. (2004). The study by Yanike et al. (2004) used a different measure of tuning known as the selectivity index (SI). The selectivity index quantifies the proportion of image-related responses that approach the maximum firing rate of a given neuron to any image, producing values between 0 and 1. The index will be 1 if only a single image is equal to the maximum rate. Alternatively if, for example, 9 out of 10 images are close to the maximum firing rate the index will tend towards 0. The selectivity index however can be difficult to interpret for two reasons. First, the method weighs extremely low firing neurons exactly as much as neurons with very high rates. This weighting confound introduces the possibility that noise fluctuations in low firing rate cells are treated are overemphasized. This is particularly problematic for principal cells in the hippocampus that have inherently low firing rates. In addition, the selectivity index does not take in to account the statistical variability of neuronal responses, introducing the possibility of intrinsic fluctuations producing an effect. In contrast, our approach of using raw data in a Tukey based approximation (discussed above), fully takes in to account the firing rate statistics of a neuron to individual images. This latter approach is much more indicative of whether the responses of a neuron effectively discriminate different images. When the data from the CA3 region collected in the present experiment is analyzed using the SI measure there is a highly significant difference in tuning between novel and familiar images (t(156) = 6.4, p < 10−8). However, a linear regression between the SI and our percent-selective measure showed no significant relationship between the two measures (r2=−0.0026, t(77) = −0.78, p = .4367, Figure 4). The lack of a correlation suggests that these measures quantify different aspects of stimulus selectivity, although the possibility exists that issues not related to the analytic approach could account for the discrepancy (see discussion).
Figure 4. Comparison of Tuning measures.
Results of a linear regression comparing the results from different tuning breadth (or selectivity) measures. Raw tuning breadth (used in the current study) was estimated via Tukey post-hoc test, a measure effectively based on firing rate statistics across multiple image presentations, vs. the selectivity index, which is based on dispersion measures. The results of the regression indicate that there is no statistical relationship between the two measures, suggesting that the selectivity index does not adequately capture tuning properties of individual neurons. Red lines - standard errors of the regression.
Discussion
The main novel finding in this experiment is that, during passive viewing, neurons across the medial temporal lobe are not sensitive to stimulus novelty. When the activity of single neurons was examined more closely however, our results revealed a significant dissociation of stimulus selectivity between subregions of the medial temporal lobe (MTL). Of the three MTL regions examined, only neurons in temporal area F (TF) exhibit significant experience-dependent adaptations in their selectivity to visual stimuli. These results suggest that familiarity responses and changes in visual selectivity are dissociable processes. The current experiment utilized chronically implanted microdrives that allowed us to fully reconstruct the path of individual electrodes, establishing with a high degree of certainty the location of all recorded neurons. Similar reconstructions are more difficult in acute recording preparations, often resulting in pooling of neural responses across multiple regions. The approach used in this study yielded 428 well isolated neurons recorded from three monkeys across regions CA3, PRh (perirhinal cortex), and TF. As discussed more completely below, these findings have implications for understanding the mechanisms underlying novelty detection and stimulus recognition across the MTL.
Lack of Novelty/Familiarity Response
A number of neurophysiological studies in humans and non-human primates have demonstrated that a subpopulation of neurons in both the inferior temporal cortex and medial temporal lobe can show decrements (or increments) in their firing rate upon the second presentation of a stimulus. With respect to the CA3 data, our findings are in agreement with previous studies in primates showing that neurons in this region are not reliably sensitive to stimulus novelty (Xiang and Brown, 1998; Yanike et al., 2004; cf. Viskontas et al., 2006 and Jutras and Buffalo, 2010). Neurons in the PRh and TF recorded in the current experiment also failed to demonstrate a consistent modulation by stimulus novelty. Previous neurophysiological studies have reported that a significant proportion of neurons in the MTL and ITC show decreases in their firing rates when a novel image is presented a second time (e.g., Brown et al., 1987; Riches et al., 1991; Miller et al., 1991; Xiang and Brown, 1998; Hölscher et al., 2003). While our data do contain a small proportion (~5%) of neurons that are classified as sensitive to stimulus novelty based on a students t-tests, none of the neurons examined in the present study reliably responded to stimulus novelty more than ~75% of the time.
The absence of novelty-sensitive neurons in hippocampal recordings is largely in agreement with the previous literature. However, it has been suggested (Jutras and Buffalo 2010), that previous studies failed to find response modulation in hippocampal neurons because those studies did not to use complex naturalistic stimuli and did not properly control for levels of novelty (i.e., stimuli only had to be novel on a given day, but may have been seen during previous days). The present results, however, cannot be explained by either of these suggestions, as the stimuli used in the VARNOV task can be broadly classified as complex naturalistic stimuli and truly novel images were introduced during every recording session.
The current data are consistent with previous studies that indicate that the hippocampus may not be necessary for simple recognition memory tasks (Murray and Mishkin, 1998b; Nemanic et al., 2004), although see (Beason-Held et al., 1999; Zola et al., 2000; Manns et al., 2003). One crucial element that has received relatively little attention in the human and primate literature is that the activity of neurons across multiple subregions of the hippocampus is often pooled for analysis. However, it is known from a variety of species that neurons in different subregions of the hippocampus may perform distinct computational functions (e.g., Sybirska et al., 2000; Lee et al., 2004; Vazdarjanova and Guzowski, 2004). Thus, it might be suggested that inconsistencies in the literature may emerge due to mixing of neurons from different regions. The results from the present experiment indicate that during passive viewing, neurons in the CA3 region of the primate hippocampus are not differentially active during any phase of the recognition memory process. While studies across species have implicated the hippocampus in spatial and episodic processing, the computational demands required to successfully perform these tasks differ significantly from the simple viewing task used here. The current results suggest that while CA3 circuitry of the hippocampal network is clearly not engaged by simple recognition memory tasks it may well be required when mnemonic demands increase or when task demands are spatial and episodic in nature.
In contrast to the largely equivocal data implicating the hippocampal formation in recognition memory tasks, lesion studies consistently implicate upstream regions in the recognition memory processes (e.g., Mishkin, 1982; Horel et al., 1987; Zola-Morgan et al., 1989; Gaffan and Murray, 1992; Meunier et al., 1993). A principle candidate mechanism supporting recognition memory and familiarity judgements has been the firing rate response decrements to stimulus repetition in those regions. Consequently, the observed lack of response decrements in the current study warrants consideration. To begin it should be noted that, while compelling, the evidence for a causal role of the response decrement in the recognition memory process has not been fully established. For example, local infusion of scopolamine into the ITC has been shown to significantly disrupt performance on recognition memory tasks in both rodents and primates (Miller and Desimone, 1993; Tang et al., 1997; Warburton et al., 2003). However, when single unit responses are recorded from the injection site, response decrements still occur and do not mirror the behavioral impairment of the subject (Miller and Desimone, 1993). Moreover, response decrements can be seen while animals are under general anesthesia (Miller et al., 1991; Vogels et al., 1995; Zhu and Brown, 1995; Zhu et al., 1997). Finally, a recent study in rodents using naturalistic objects in a navigation task (effectively a passive encounter paradigm) found results comparable to those presented in the current study for monkeys (Burke et. al this issue). These, and other findings, argue that decremental firing of neurons in the PRh is not the sole mechanism used by animals to represent the familiarity of a given stimulus. However, there are at least two alternative explanations for the discrepancy between ours and previous studies.
First, it is possible that due to the highly discriminable and unique nature of the stimuli used, subjects in our study were able to easily recognize each stimulus, without the use of familiarity detection mechanisms. This interpretation would suggest that recognition memory processes may rely more on object recognition than on explicit novelty detection. Secondly, it is possible that neurons recorded in previous studies that responded selectively to stimulus novelty are simply showing training-related adaptations and that these adaptations do not appear when animals are performing a passive viewing task. As detailed in the introduction, tasks used to study recognition memory often rely on a sample/sample-repeat design, which introduces stimulus repetition as a major statistical regularity in the animal’s environment. Moreover, studies in which subjects must actively indicate whether a stimulus has been previously seen (often 100’s of times within a session), likely do not reflect the ethological and cognitive demands encountered in the natural world. Future studies, using refined versions of standard recognition memory tasks, will be required to determine the extent to which training influences these responses and whether the sensitivity of MTL neurons is causally related to the recognition memory process.
Information processing and storage in the medial temporal lobe
The ventral visual system is comprised of a hierarchical processing arrangement in which successive stages of processing are tasked with extracting and combining different features of visually presented information (Hubel and Wiesel, 1959; Barlow, 1961; Wurtz, 1969; Mishkin et al., 1983; Tanaka et al., 1991; Gallant et al., 1993; Miyashita, 1993; Brincat and Connor, 2006). This process of feature extraction and unification culminates with the generation of view invariant responses to faces and objects. Recent data from rodents and primates have suggested that the final anatomical endpoint of this hierarchy is the PRh (see Murray and Wise this issue for review).
The current study found a significant dissociation between the numbers of visually responsive neurons in three medial temporal lobe regions. A large proportion (~44%) of neurons in both PRh and TF were visually responsive, in contrast only 14% of cells that were visually responsive in the CA3 region of the hippocampus (Figure 2). The large proportion of active neurons in PRh and TF is unsurprising, given their known involvement in recognition memory and visual object processing. However, as hippocampal involvement in recognition memory remains under debate, two possible explanations for the observed level of hippocampal activity must be considered. First, theoretical and empirical studies have posited that hippocampal processing produces a sparse code that may ultimately drive more efficient storage in cortical regions targeted by its back-projections. Alternatively, and as will be discussed in detail below, the CA3 network may not be engaged by simple visual recognition memory tasks. Finally, it should be noted that reports of the proportions of cells firing during active behavior in the primate hippocampus varies significantly across the literature (mean: 47%, min: 2% max: 88%), making it unclear what activity level may be expected from this region in primates. Importantly, as noted previously, studies often pool neurons across hippocampal regions, even though imaging studies in rodents have demonstrated significantly different levels of activity in the different regions (e.g., Guzowski et al., 1999; Vazdarjanova and Guzowski, 2004). This further underscores the need for accurate histological reconstructions of electrode position.
From the perspective of object representation, it has been proposed that accuracy on recognition memory tasks may rely on the relative strength or weakness of a memory (Squire et al., 2007). Numerous neurophysiological studies now support earlier theoretical work suggesting that at the neural level, stimulus representation is distributed across multiple simultaneously active neurons (i.e., a population code). One interpretation of such a system of encoding would be that the precision (i.e., memory strength) with which a stimulus can be recalled depends on the specificity of the representation within a given population of neurons.
Theoretically, the specificity with which a stimulus is represented at the neuronal level can be enhanced by narrowing the tuning profile of individual neurons; a view supported by multiple neurophysiological studies (e.g., Logothetis et al., 1995; Sigala and Logothetis, 2002; Freedman et al., 2006). The observed enhancement in the specificity of the neuronal population has been suggested to facilitate perceptual decision making by reducing the time required for the population to settle on the state associated with a given stimulus (Desimone, 1996). Our results extend these findings by demonstrating that the population of TF neurons becomes significantly more specific as a function of experience. It is interesting to note that experience-dependent increases in selectivity occur largely in regions that are primarily unimodal in origin (although TF does receive some inputs from cingulate, parietal lobe, and retrosplenial cortex), while PRh and the hippocampus are decidedly polymodal (Suzuki and Amaral, 1994, 2004). This anatomical dissociation suggests that stimulus specific tuning enhancements may be critical for exact object representation but that similar mechanisms are not as critical when multiple features must be associated in higher order association areas (e.g., PRh and Hippocampus).
The observation that, as a population, PRh neurons do not become more selective as a function of visual experience may be interpreted as follows. Animals with PRh lesions are significantly impaired at learning conditional association tasks across sensory modalities (Zola-Morgan et al., 1989; Meunier et al., 1993; Suzuki et al., 1993; Murray and Bussey, 1999; Kholodar-Smith et al., 2008). In passive paired associate tasks where animals must learn a behavioral response dependent on the relationship of two sequentially presented images, the activity of neurons in PRh closely matches the performance of animals on this task. Specifically, during learning, neurons gradually become selective to both the paired item and its conditioned associate (Miyashita, 1988; Sakai and Miyashita, 1991; Erickson and Desimone, 1999). Consistent with theoretical models (Murray and Bussey, 1999; Bussey and Saksida, 2002), a recent physiological study demonstrated that representations of stimuli in a conditional association task become increasingly unified, at the neuronal level within the PRh (Fujimichi et al., 2010). It may be speculated that If, as the data in the literature suggest, neural activity in PRh represents unified complex associations between lower level representations, then in fact, reducing the overall selectivity in the population could result in the destruction of previously established associations. Importantly, from the perspective of the feature-conjunctive model of PRh function, the removal of units from a representation may lead to a disassociation of previously associated features, resulting in stimulus misclassification. While the current data cannot directly speak to this possibility, the data are at least consistent with this conjecture. An alternative possibility is that the stimuli used in the current study never engage plasticity mechanisms in PRh, because virtually no effort needs to be expended to make the perceptual discriminations required under these circumstances.
As noted previously, in contrast to the PRh and area TF, only 14% of hippocampal neurons were visually responsive. Within this small population of selective cells, there was no effect of experience on the population selectivity. However, spatial responses of hippocampal pyramidal cells in rodents have been shown to undergo an experience-dependent expansion in their response profile, thought to instantiate a mechanism that could facilitate the sequential binding of information (Mehta et al., 1997; Ekstrom et al., 2001; Burke et al., 2008). To date, the only evidence for an experience-dependent change in the selectivity of primate hippocampal neurons for visually presented information comes from a location-scene association task (Yanike et al., 2004).The measure used by Yanike et al. (2004) is a ratio measure, that only partially characterizes the stimulus selective properties of individual neurons. When the current data were analyzed using this measure, there exists a significant effect of experience on the “selectivity” of individual neurons (Figure 4). Critically however, it is demonstrated that there exists no relationship between the measure used by Yanike et al. (2004) and our measure, which identifies the proportion of images that a given neuron is selective to. This suggests that under passive viewing conditions representations in the hippocampus do not undergo experience-dependent changes. However, there are two alternative explanations that could account for these differences. First, we used a significantly different approach to recording neural activity in our subjects. The use of chronic recording electrodes may have captured the activity of neurons that are not routinely recorded in acute preparations due to their low firing rates. As normalized rates and no waveform data are available from the Yannike study, this possibility cannot be determined. Second, it may be that since the task used in Yanike et.al. (2004) had an active spatial association component, mnemonic demands under these conditions were sufficient to engage hippocampal plasticity mechanisms. The data from the current experiment are consistent with the idea that passive viewing behaviors may not drive the CA3 region of the primate hippocampus. However, it is possible that under different passive viewing conditions, such as visual paired comparison tasks, CA3 may be engaged. Although the possibility remains that the CA3 region in the primate may only be involved when there are significant spatial, mnemonic, or episodic (conjunctions of items in space) demands (Lavenex and Amaral, 2000; Nadel et al., 2003; Leutgeb et al., 2005; Quiroga et al., 2005, 2008; Rolls et al., 2005; Bachevalier and Nemanic, 2007; Chadwick et al., 2010; Naya and Suzuki, 2011).
Acknowledgments
Grant Sponsor/Grant number: AG003376, McKnight Brain Research Foundation
We thank W.E. Skaggs, M. Permenter, J. Vogt, M. Archibeque for their assistance with recordings and behavior and Bruce McNaughton for assistance with implanting the hyperdrives.
References
- Amari S. Characteristics of sparsely encoded associative memory. Neural Networks. 1989;2:451–457. [Google Scholar]
- Bachevalier J, Brickson M, Hagger C. Limbic-dependent recognition memory in monkeys develops early in infancy. Neuroreport. 1993;4:77–80. doi: 10.1097/00001756-199301000-00020. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Nemanic S. Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus. 2007;18:64–80. doi: 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- Baker CI, Behrmann M, Olson CR. Impact of learning on representation of parts and wholes in monkey inferotemporal cortex. Nat Neurosci. 2002;5:1210–1216. doi: 10.1038/nn960. [DOI] [PubMed] [Google Scholar]
- Barlow H. Sensory Communication. MIT Press; 1961. Possible principles underlying the transformation of sensory messages; pp. 217–234. [Google Scholar]
- Bartho P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsaki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. Journal of neurophysiology. 2004;92:600. doi: 10.1152/jn.01170.2003. [DOI] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ. Perirhinal cortex resolves feature ambiguity in configural object recognition and perceptual oddity tasks. Learn Mem. 2007;14:821–832. doi: 10.1101/lm.749207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis GC, Rolls ET, Leonard CM. Selectivity between faces in the responses of a population of neurons in the cortex in the superior temporal sulcus of the monkey. Brain Res. 1985;342:91–102. doi: 10.1016/0006-8993(85)91356-3. [DOI] [PubMed] [Google Scholar]
- Beason-Held LL, Rosene DL, Killiany RJ, Moss MB. Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus. 1999;9:562–574. doi: 10.1002/(SICI)1098-1063(1999)9:5<562::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Brincat SL, Connor CE. Dynamic Shape Synthesis in Posterior Inferotemporal Cortex. Neuron. 2006;49:17–24. doi: 10.1016/j.neuron.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Brown MW, Wilson FAW, Riches IP. Neuronal evidence that inferomedial temporal cortex is more important than hippocampus in certain processes underlying recognition memory. Brain Research. 1987;409:158–162. doi: 10.1016/0006-8993(87)90753-0. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Booth MC, Rolls ET, Gaffan D. Selective perceptual impairments after perirhinal cortex ablation. J Neurosci. 2001;21:9824–9836. doi: 10.1523/JNEUROSCI.21-24-09824.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley MJ, Gaffan D. Perirhinal Cortex Ablation Impairs Visual Object Identification. The Journal of Neuroscience. 1998;18:2268–2275. doi: 10.1523/JNEUROSCI.18-06-02268.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalo EA, Ramus SJ, Clark RE, Teng E, Squire LR, Zola SM. Dissociation between the effects of damage to perirhinal cortex and area TE. Learn Mem. 1999;6:572–599. doi: 10.1101/lm.6.6.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalo EA, Ramus SJ, Squire LR, Zola SM. Perception and recognition memory in monkeys following lesions of area TE and perirhinal cortex. Learn Mem. 2000;7:375–382. doi: 10.1101/lm.32100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalo EA, Reber PJ, Squire LR. The human perirhinal cortex and recognition memory. Hippocampus. 1998;8:330–339. doi: 10.1002/(SICI)1098-1063(1998)8:4<330::AID-HIPO3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Burke SN, Maurer AP, Yang Z, Navratilova Z, Barnes CA. Glutamate receptor-mediated restoration of experience-dependent place field expansion plasticity in aged rats. Behav Neurosci. 2008;122:535– 548. doi: 10.1037/0735-7044.122.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. The organization of visual object representations: a connectionist model of effects of lesions in perirhinal cortex. Eur J Neurosci. 2002;15:355–364. doi: 10.1046/j.0953-816x.2001.01850.x. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. Perirhinal cortex resolves feature ambiguity in complex visual discriminations. Eur J Neurosci. 2002a;15:365–374. doi: 10.1046/j.0953-816x.2001.01851.x. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. The role of perirhinal cortex in memory and perception: conjunctive representations for object identification. In: Witter M, Wouterlood F, editors. The Parahippocampal Region: Organization and Role in Cognitive Functions. 1. Oxford University Press; USA: 2002b. [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. Impairments in visual discrimination after perirhinal cortex lesions: testing “declarative” vs. “perceptual-mnemonic” views of perirhinal cortex function. Eur J Neurosci. 2003;17:649–660. doi: 10.1046/j.1460-9568.2003.02475.x. [DOI] [PubMed] [Google Scholar]
- Chadwick MJ, Hassabis D, Weiskopf N, Maguire EA. Decoding Individual Episodic Memory Traces in the Human Hippocampus. Current Biology. 2010;20:544–547. doi: 10.1016/j.cub.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R. Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci USA. 1996;93:13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Albright TD, Gross CG, Bruce C. Stimulus-selective properties of inferior temporal neurons in the macaque. J Neurosci. 1984;4:2051–2062. doi: 10.1523/JNEUROSCI.04-08-02051.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacott MJ, Gaffan D, Murray EA. Preserved recognition memory for small sets, and impaired stimulus identification for large sets, following rhinal cortex ablations in monkeys. Eur J Neurosci. 1994;6:1466–1478. doi: 10.1111/j.1460-9568.1994.tb01008.x. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Meltzer J, McNaughton BL, Barnes CA. NMDA receptor antagonism blocks experience-dependent expansion of hippocampal “place fields. Neuron. 2001;31:631–638. doi: 10.1016/s0896-6273(01)00401-9. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Desimone R. Responses of macaque perirhinal neurons during and after visual stimulus association learning. J Neurosci. 1999;19:10404–10416. doi: 10.1523/JNEUROSCI.19-23-10404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan JF., 3rd Infants’ recognition memory for faces. J Exp Child Psychol. 1972;14:453–476. doi: 10.1016/0022-0965(72)90065-3. [DOI] [PubMed] [Google Scholar]
- Fahy FL, Riches IP, Brown MW. Neuronal activity related to visual recognition memory: long-term memory and the encoding of recency and familiarity information in the primate anterior and medial inferior temporal and rhinal cortex. Exp Brain Res. 1993;96:457–472. doi: 10.1007/BF00234113. [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Experience-dependent sharpening of visual shape selectivity in inferior temporal cortex. Cereb Cortex. 2006;16:1631–1644. doi: 10.1093/cercor/bhj100. [DOI] [PubMed] [Google Scholar]
- Fujimichi R, Naya Y, Koyano KW, Takeda M, Takeuchi D, Miyashita Y. Unitized representation of paired objects in area 35 of the macaque perirhinal cortex. Eur J Neurosci. 2010;32:659–667. doi: 10.1111/j.1460-9568.2010.07320.x. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Murray EA. Monkeys (Macaca fascicularis) with rhinal cortex ablations succeed in object discrimination learning despite 24-hr intertrial intervals and fail at matching to sample despite double sample presentations. Behav Neurosci. 1992;106:30–38. doi: 10.1037//0735-7044.106.1.30. [DOI] [PubMed] [Google Scholar]
- Gallant JL, Braun J, Van Essen DC. Selectivity for Polar, Hyperbolic, and Cartesian Gratings in Macaque Visual Cortex. Science. 1993;259:100–103. doi: 10.1126/science.8418487. [DOI] [PubMed] [Google Scholar]
- Gochin PM, Colombo M, Dorfman GA, Gerstein GL, Gross CG. Neural ensemble coding in inferior temporal cortex. J Neurophysiol. 1994;71:2325–2337. doi: 10.1152/jn.1994.71.6.2325. [DOI] [PubMed] [Google Scholar]
- Gothard KM, Erickson CA, Amaral DG. How do rhesus monkeys (Macaca mulatta) scan faces in a visual paired comparison task? Anim Cogn. 2004;7:25–36. doi: 10.1007/s10071-003-0179-6. [DOI] [PubMed] [Google Scholar]
- Gross CG. Representation of visual stimuli in inferior temporal cortex. Philos Trans R Soc Lond, B, Biol Sci. 1992;335:3–10. doi: 10.1098/rstb.1992.0001. [DOI] [PubMed] [Google Scholar]
- Gunderson VM, Swartz KB. Visual recognition in infant pigtailed macaques after a 24-hour delay. American journal of primatology. 1985;8:259–264. doi: 10.1002/ajp.1350080309. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Hinton GE, McClelland JL, Rumelhart DE. Distributed representations. Parallel distributed processing: Explorations in the microstructure of cognition. 1986;1:77–109. [Google Scholar]
- Hölscher C, Rolls ET, Xiang J. Perirhinal cortex neuronal activity related to long-term familiarity memory in the macaque. Eur J Neurosci. 2003;18:2037–2046. doi: 10.1046/j.1460-9568.2003.02903.x. [DOI] [PubMed] [Google Scholar]
- Horel JA, Pytko-Joiner DE, Voytko ML, Salsbury K. The performance of visual tasks while segments of the inferotemporal cortex are suppressed by cold. Behavioural Brain Research. 1987;23:29–42. doi: 10.1016/0166-4328(87)90240-3. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of single neurones in the cat’s striate cortex. J Physiol. 1959;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV. Distributed Representation of Objects in the Human Ventral Visual Pathway. PNAS. 1999;96:9379–9384. doi: 10.1073/pnas.96.16.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutras MJ, Buffalo EA. Recognition memory signals in the macaque hippocampus. Proceedings of the National Academy of Sciences. 2010a;107:401. doi: 10.1073/pnas.0908378107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutras MJ, Buffalo EA. Recognition memory signals in the macaque hippocampus. Proc Natl Acad Sci USA. 2010b;107:401–406. doi: 10.1073/pnas.0908378107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholodar-Smith DB, Allen TA, Brown TH. Fear conditioning to discontinuous auditory cues requires perirhinal cortical function. Behav Neurosci. 2008;122:1178–1185. doi: 10.1037/a0012902. [DOI] [PubMed] [Google Scholar]
- Kobatake E, Wang G, Tanaka K. Effects of Shape-Discrimination Training on the Selectivity of Inferotemporal Cells in Adult Monkeys. Journal of Neurophysiology. 1998;80:324–330. doi: 10.1152/jn.1998.80.1.324. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Amaral DG. Hippocampal-neocortical interaction: a hierarchy of associativity. Hippocampus. 2000;10:420–430. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: Intrinsic projections and interconnections. The Journal of Comparative Neurology. 2004;472:371–394. doi: 10.1002/cne.20079. [DOI] [PubMed] [Google Scholar]
- Lee I, Rao G, Knierim JJ. A Double Dissociation between Hippocampal Subfields: Differential Time Course of CA3 and CA1 Place Cells for Processing Changed Environments. Neuron. 2004;42:803–815. doi: 10.1016/j.neuron.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser M-B. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Li L, Miller EK, Desimone R. The representation of stimulus familiarity in anterior inferior temporal cortex. Journal of neurophysiology. 1993;69:1918. doi: 10.1152/jn.1993.69.6.1918. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Poggio T. Shape representation in the inferior temporal cortex of monkeys. Curr Biol. 1995;5:552–563. doi: 10.1016/s0960-9822(95)00108-4. [DOI] [PubMed] [Google Scholar]
- Mahut H, Zola-Morgan S, Moss M. Hippocampal Resections Impair Associative Learning and Recognition Memory in the Monkey. J Neurosci. 1982;2:1214–1220. doi: 10.1523/JNEUROSCI.02-09-01214.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandler G. Recognizing: The judgment of previous occurrence. Psychological Review. 1980;87:252–271. [Google Scholar]
- Manns JR, Hopkins RO, Reed JM, Kitchener EG, Squire LR. Recognition memory and the human hippocampus. Neuron. 2003;37:171–180. doi: 10.1016/s0896-6273(02)01147-9. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond, B, Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Morris RGM. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends in Neurosciences. 1987a;10:408–415. [Google Scholar]
- McNaughton BL, Morris RGM. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends in Neurosciences. 1987b;10:408–415. [Google Scholar]
- Mehta MR, Barnes CA, McNaughton BL. Experience-dependent, asymmetric expansion of hippocampal place fields. Proc Natl Acad Sci USA. 1997;94:8918–8921. doi: 10.1073/pnas.94.16.8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier C, Yanai H-F, Amari S-I. Sparsely coded associative memories: capacity and dynamical properties. Network. 1991;2:469–487. [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M, Murray E. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. The Journal of Neuroscience. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Desimone R. Scopolamine affects short-term memory but not inferior temporal neurons. Neuroreport. 1993;4:81–84. doi: 10.1097/00001756-199301000-00021. [DOI] [PubMed] [Google Scholar]
- Miller EK, Desimone R. Parallel Neuronal Mechanisms for Short-Term Memory. Science. 1994;263:520– 522. doi: 10.1126/science.8290960. [DOI] [PubMed] [Google Scholar]
- Miller EK, Gochin PM, Gross CG. Habituation-like decrease in the responses of neurons in inferior temporal cortex of the macaque. Vis Neurosci. 1991a;7:357–362. doi: 10.1017/s0952523800004843. [DOI] [PubMed] [Google Scholar]
- Miller EK, Gochin PM, Gross CG. Habituation-Like Decrease in the Responses of Neurons in Inferior Temporal Cortex of the Macaque. Visual Neuroscience. 1991b;7:357–362. doi: 10.1017/s0952523800004843. [DOI] [PubMed] [Google Scholar]
- Miller EK, Gochin PM, Gross CG. Habituation-like decrease in the responses of neurons in inferior temporal cortex of the macaque. Vis Neurosci. 1991c;7:357–362. doi: 10.1017/s0952523800004843. [DOI] [PubMed] [Google Scholar]
- Mishkin M. A memory system in the monkey. Philos Trans R Soc Lond, B, Biol Sci. 1982;298:83–95. doi: 10.1098/rstb.1982.0074. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG, Macko KA. Object vision and spatial vision: two cortical pathways. Trends in Neurosciences. 1983;6:414–417. [Google Scholar]
- Miyashita Y. Neuronal correlate of visual associative long-term memory in the primate temporal cortex. Nature. 1988;335:817–820. doi: 10.1038/335817a0. [DOI] [PubMed] [Google Scholar]
- Miyashita Y. Inferior Temporal Cortex: Where Visual Perception Meets Memory. Annu Rev Neurosci. 1993;16:245–263. doi: 10.1146/annurev.ne.16.030193.001333. [DOI] [PubMed] [Google Scholar]
- Mormann F, Kornblith S, Quiroga RQ, Kraskov A, Cerf M, Fried I, Koch C. Latency and Selectivity of Single Neurons Indicate Hierarchical Processing in the Human Medial Temporal Lobe. The Journal of Neuroscience. 2008;28:8865–8872. doi: 10.1523/JNEUROSCI.1640-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, Bussey Perceptual-mnemonic functions of the perirhinal cortex. Trends Cogn Sci (Regul Ed) 1999;3:142–151. doi: 10.1016/s1364-6613(99)01303-0. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ. Consolidation and the medial temporal lobe revisited: methodological considerations. Hippocampus. 2001;11:1–7. doi: 10.1002/1098-1063(2001)11:1<1::AID-HIPO1014>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. J Neurosci. 1998;18:6568–6582. doi: 10.1523/JNEUROSCI.18-16-06568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Richmond BJ. Role of perirhinal cortex in object perception, memory, and associations. Curr Opin Neurobiol. 2001;11:188–193. doi: 10.1016/s0959-4388(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Nadel L, Ryan L, Hayes S, Gilboab A, Moscovitchb M. The role of the hippocampal complex in longterm episodic memory. Cognition and emotion in the brain: selected topics of the International Symposium on Limbic and Association Cortical Systems; Toyama, Japan. 7–12 October 2002; 2003. pp. 215–234. [Google Scholar]
- Naya Y, Suzuki WA. Integrating What and When Across the Primate Medial Temporal Lobe. Science. 2011;333:773–776. doi: 10.1126/science.1206773. [DOI] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Bachevalier J. The Hippocampal/Parahippocampal Regions and Recognition Memory: Insights from Visual Paired Comparison versus Object-Delayed Nonmatching in Monkeys. The Journal of Neuroscience. 2004;24:2013–2026. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman G, Eacott M. Impaired object recognition with increasing levels of feature ambiguity in rats with perirhinal cortex lesions. Behavioural Brain Research. 2004;148:79–91. doi: 10.1016/s0166-4328(03)00176-1. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Bachevalier J. Neonatal aspiration lesions of the hippocampal formation impair visual recognition memory when assessed by paired-comparison task but not by delayed nonmatching-to-sample task. Hippocampus. 1999;9:609–616. doi: 10.1002/(SICI)1098-1063(1999)9:6<609::AID-HIPO1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang X-F, Toga AW. The Rhesus Monkey Brain in Stereotaxic Coordinates. 1. Academic Press; 1999. [Google Scholar]
- Pouget A, Deneve S, Ducom JC, Latham PE. Narrow versus wide tuning curves: What’s best for a population code? Neural Comput. 1999;11:85–90. doi: 10.1162/089976699300016818. [DOI] [PubMed] [Google Scholar]
- Quiroga RQ, Kreiman G, Koch C, Fried I. Sparse but not Grandmother-cell coding in the medial temporal lobe. Trends in Cognitive Sciences. 2008;12:87–91. doi: 10.1016/j.tics.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Quiroga RQ, Reddy L, Kreiman G, Koch C, Fried I. Invariant visual representation by single neurons in the human brain. Nature. 2005;435:1102–1107. doi: 10.1038/nature03687. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Schreiner CE. Changes in the distributed temporal response properties of SI cortical neurons reflect improvements in performance on a temporally based tactile discrimination task. J Neurophysiol. 1992;67:1071–1091. doi: 10.1152/jn.1992.67.5.1071. [DOI] [PubMed] [Google Scholar]
- Riches I, Wilson F, Brown M. The effects of visual stimulation and memory on neurons of the hippocampal formation and the neighboring parahippocampal gyrus and inferior temporal cortex of the primate. The Journal of Neuroscience. 1991;11:1763–1779. doi: 10.1523/JNEUROSCI.11-06-01763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond J, Colombo M, Hayne H. Interpreting visual preferences in the visual paired-comparison task. J Exp Psychol Learn Mem Cogn. 2007;33:823–831. doi: 10.1037/0278-7393.33.5.823. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Cahusac PM, Feigenbaum JD, Miyashita Y. Responses of single neurons in the hippocampus of the macaque related to recognition memory. Exp Brain Res. 1993;93:299–306. doi: 10.1007/BF00228398. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Treves A. The relative advantages of sparse versus distributed encoding for associative neuronal networks in the brain. Network: Computation in Neural Systems. 1990;1:407–421. [Google Scholar]
- Rolls ET, Xiang J, Franco L. Object, Space, and Object-Space Representations in the Primate Hippocampus. Journal of Neurophysiology. 2005;94:833–844. doi: 10.1152/jn.01063.2004. [DOI] [PubMed] [Google Scholar]
- Sakai K, Miyashita Y. Neural organization for the long-term memory of paired associates. Nature. 1991;354:152–155. doi: 10.1038/354152a0. [DOI] [PubMed] [Google Scholar]
- Sakai K, Naya Y, Miyashita Y. Neuronal tuning and associative mechanisms in form representation. Learn Mem. 1994;1:83–105. [PubMed] [Google Scholar]
- Schoups A, Vogels R, Qian N, Orban G. Practising orientation identification improves orientation coding in V1 neurons. Nature. 2001;412:549–553. doi: 10.1038/35087601. [DOI] [PubMed] [Google Scholar]
- Sigala N, Logothetis NK. Visual categorization shapes feature selectivity in the primate temporal cortex. Nature. 2002;415:318–320. doi: 10.1038/415318a. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Permenter M, Archibeque M, Vogt J, Amaral DG, Barnes CA. EEG sharp waves and sparse ensemble unit activity in the macaque hippocampus. Journal of neurophysiology. 2007;98:898. doi: 10.1152/jn.00401.2007. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Functional neuroanatomy of the medial temporal lobe memory system. Cortex. 2004;40:220–222. doi: 10.1016/s0010-9452(08)70958-4. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Zola-Morgan S, Squire LR, Amaral DG. Lesions of the perirhinal and parahippocampal cortices in the monkey produce long-lasting memory impairment in the visual and tactual modalities. J Neurosci. 1993;13:2430–2451. doi: 10.1523/JNEUROSCI.13-06-02430.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sybirska E, Davachi L, Goldman-Rakic PS. Prominence of direct entorhinal-CA1 pathway activation in sensorimotor and cognitive tasks revealed by 2-DG functional mapping in nonhuman primate. J Neurosci. 2000;20:5827–5834. doi: 10.1523/JNEUROSCI.20-15-05827.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Saito H, Fukada Y, Moriya M. Coding Visual Images of Objects in the Inferotemporal Cortex of the Macaque Monkey. J Neurophysiol. 1991;66:170–189. doi: 10.1152/jn.1991.66.1.170. [DOI] [PubMed] [Google Scholar]
- Tang Y, Mishkin M, Aigner TG. Effects of muscarinic blockade in perirhinal cortex during visual recognition. Proc Natl Acad Sci USA. 1997;94:12667–12669. doi: 10.1073/pnas.94.23.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, Guzowski JF. Differences in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. J Neurosci. 2004;24:6489–6496. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinje WE, Gallant JL. Natural Stimulation of the Nonclassical Receptive Field Increases Information Transmission Efficiency in V1. The Journal of Neuroscience. 2002;22:2904–2915. doi: 10.1523/JNEUROSCI.22-07-02904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viskontas IV, Knowlton BJ, Steinmetz PN, Fried I. Differences in mnemonic processing by neurons in the human hippocampus and parahippocampal regions. Journal of cognitive neuroscience. 2006;18:1654–1662. doi: 10.1162/jocn.2006.18.10.1654. [DOI] [PubMed] [Google Scholar]
- Vogels R, Sáry G, Orban GA. How task-related are the responses of inferior temporal neurons? Vis Neurosci. 1995;12:207–214. doi: 10.1017/s0952523800007884. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Koder T, Cho K, Massey PV, Duguid G, Barker GR, Aggleton JP, Bashir ZI, Brown MW. Cholinergic Neurotransmission Is Essential for Perirhinal Cortical Plasticity and Recognition Memory. Neuron. 2003;38:987–996. doi: 10.1016/s0896-6273(03)00358-1. [DOI] [PubMed] [Google Scholar]
- Wilson M, McNaughton B. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Woloszyn L, Sheinberg DL. Effects of Long-Term Visual Experience on Responses of Distinct Classes of Single Units in Inferior Temporal Cortex. Neuron. 2012;74:193–205. doi: 10.1016/j.neuron.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz RH. Visual receptive fields of striate cortex neurons in awake monkeys. J Neurophysiol. 1969;32:727–742. doi: 10.1152/jn.1969.32.5.727. [DOI] [PubMed] [Google Scholar]
- Xiang J-Z, Brown MW. Differential neuronal encoding of novelty, familiarity and recency in regions of the anterior temporal lobe. Neuropharmacology. 1998;37:657–676. doi: 10.1016/s0028-3908(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Yang T, Maunsell JHR. The effect of perceptual learning on neuronal responses in monkey visual area V4. J Neurosci. 2004;24:1617–1626. doi: 10.1523/JNEUROSCI.4442-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanike M, Wirth S, Suzuki WA. Representation of well-learned information in the monkey hippocampus. Neuron. 2004;42:477–487. doi: 10.1016/s0896-6273(04)00193-x. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The Nature of Recollection and Familiarity: A Review of 30 Years of Research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Kroll NEA, Quamme JR, Lazzara MM, Sauvé M-J, Widaman KF, Knight RT. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nature Neuroscience. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- Zhang K, Sejnowski TJ. Neuronal Tuning: To Sharpen or Broaden? Neural Computation. 1999;11:75–84. doi: 10.1162/089976699300016809. [DOI] [PubMed] [Google Scholar]
- Zhu X, McCabe B, Aggleton J, Brown M. Differential activation of the rat hippocampus and perirhinal cortex by novel visual stimuli and a novel environment. Neuroscience Letters. 1997;229:141–143. doi: 10.1016/s0304-3940(97)00437-0. [DOI] [PubMed] [Google Scholar]
- Zhu XO, Brown MW. Changes in neuronal activity related to the repetition and relative familiarity of visual stimuli in rhinal and adjacent cortex of the anaesthetised rat. Brain Res. 1995;689:101–110. doi: 10.1016/0006-8993(95)00550-a. [DOI] [PubMed] [Google Scholar]
- Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE. Impaired recognition memory in monkeys after damage limited to the hippocampal region. Journal of Neuroscience. 2000;20:451. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire L, Amaral D, Suzuki W. Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. The Journal of Neuroscience. 1989;9:4355–4370. doi: 10.1523/JNEUROSCI.09-12-04355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR. Medial temporal lesions in monkeys impair memory on a variety of tasks sensitive to human amnesia. Behav Neurosci. 1985;99:22–34. doi: 10.1037//0735-7044.99.1.22. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Ramus SJ. Severity of memory impairment in monkeys as a function of locus and extent of damage within the medial temporal lobe memory system. Hippocampus. 1994;4:483–495. doi: 10.1002/hipo.450040410. [DOI] [PubMed] [Google Scholar]