Abstract
Recently there has been a dramatic rise in the abuse of so-called “bath salts” products that are purchased as legal alternatives to illicit drugs like cocaine and 3,4-methylenedioxymethamphetamine (MDMA). Baths salts contain one or more synthetic derivatives of the naturally-occurring stimulant cathinone. Low doses of bath salts produce euphoria and increase alertness, but high doses or chronic use can cause serious adverse effects such as hallucinations, delirium, hyperthermia and tachycardia. Owing to the risks posed by bath salts, the governments of many countries have made certain cathinones illegal, namely: 4-methylmethcathinone (mephedrone), 3,4-methylenedioxymethcathinone (methylone) and 3,4-methylenedioxypyrovalerone (MDPV). Similar to other psychomotor stimulants, synthetic cathinones target plasma membrane transporters for dopamine (i.e., DAT), norepinephrine (i.e., NET) and serotonin (i.e, SERT). Mephedrone and methylone act as non-selective transporter substrates, thereby stimulating non-exocytotic release of dopamine, norepinephrine and serotonin. By contrast, MDPV acts as a potent blocker at DAT and NET, with little effect at SERT. Administration of mephedrone or methylone to rats increases extracellular concentrations of dopamine and serotonin in the brain, analogous to the effects of MDMA. Not surprisingly, synthetic cathinones elicit locomotor activation in rodents. Stimulation of dopamine transmission by synthetic cathinones predicts a high potential for addiction and may underlie clinical adverse effects. As popular synthetic cathinones are rendered illegal, new replacement cathinones are appearing in the marketplace. More research on the pharmacology and toxicology of abused cathinones is needed to inform public health policy and develop strategies for treating medical consequence of bath salts abuse.
Index words: cathinone, designer drug, dopamine, serotonin, monoamine transporter
1. “Bath salts” products contain synthetic cathinones
In the past few years, there has been an alarming increase in the abuse of so-called “bath salts” products sold on the internet and in retail shops. These products have no legitimate use as bath additives. Instead, they are purchased as “legal highs” that mimic the effects of illicit drugs like cocaine, methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA) (Coppola and Mondola, 2012; Prosser and Nelson, 2012). Bath salts are given evocative names - “Ivory Wave”, “Bliss”, “White Lightning” - to entice consumers, and they are labeled “not for human consumption” as a ploy to circumvent laws governing the sale of psychoactive substances (Shanks et al., 2012; Spiller et al., 2011). Baths salts powders are usually self-administered by insufflation, but oral and intravenous (i.v.) routes are also used. Clinical reports indicate that recreational doses of bath salts enhance mood and increase alertness, whereas higher doses or repeated use can lead to dangerous neurological and cardiovascular complications requiring emergency medical care (Borek and Holstege, 2012; Kyle et al., 2011; Ross et al., 2011; Spiller et al., 2011). Data from poison control centers in the US reveal a dramatic spike in the reports of bath salts overdose since 2010 (Centers for Disease and Prevention, 2011; Spiller et al., 2011).
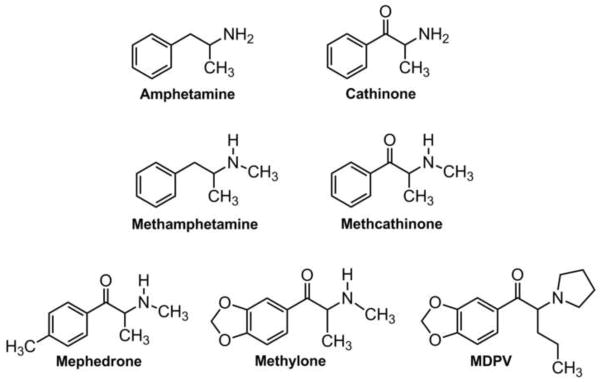
The psychoactive compounds in bath salts powders have been identified as synthetic derivatives of cathinone, a structural analog of amphetamine found in the khat plant (Shanks et al., 2012; Spiller et al., 2011). While the recent rise in synthetic cathinone use is unprecedented, the stimulant properties of khat have been known for centuries (Kalix, 1992), and non-medical use of the synthetic cathinone analog, methcathinone, was prevalent in Russia and Eastern Europe during the 1990s (Rosenbaum et al., 2012). Figure 1 illustrates the chemical structures of cathinone, methcathinone and 3 popular bath salts constituents: 4-methylmethcathinone (mephedrone), 3,4-methylenedioxymethcathinone (methylone), and 3,4-methylenedioxypyrovalerone (MDPV). Note that all bath salts compounds share a β-ketophenethylamine moiety as part of their chemical structure; MDPV is unique among the compounds due to the presence of a nitrogen-containing pyrrolidine ring. Some bath salts products consist of single cathinones while others contain a mixture of compounds. MDPV is the chief substance detected in blood and urine from patients hospitalized for bath salts overdose in the US (Spiller et al., 2011), while mephedrone is more commonly associated with adverse clinical outcomes in Europe (James et al., 2011). Owing to public health risk posed by bath salts, the governments of many countries have passed laws to render mephedrone, methylone and MDPV illegal (Drug Enforcement Administration, 2011; Schifano et al., 2011). Unfortunately, a new wave of cathinone derivatives has appeared in the marketplace to replace those drugs now subject to regulatory control (Brandt et al., 2010; Shanks et al., 2012), and the introduction of “replacement” cathinones is expected to continue.
Figure 1.
Chemical structures of cathinone, methcathinone and synthetic bath salts cathinones.
2. Bath salts cathinones target monoamine transporters
Despite the widespread use of bath salts, there is limited information about the mechanism of action underlying the physiological and behavioral effects produced by most synthetic cathinone derivatives. Emerging evidence indicates that bath salts cathinones interact with plasma membrane transporters for dopamine (i.e., DAT), norepinephrine (i.e., NET) and serotonin (i.e., SERT) (Baumann et al., 2012a; Cozzi et al., 1999; Hadlock et al., 2011; Lopez-Arnau et al., 2012; Martinez-Clemente et al., 2012; Nagai et al., 2007; Simmler et al., 2012; Sogawa et al., 2011). This is not surprising given that cathinone and methcathinone are known substrates (i.e., releasers) at monoamine transporters (Glennon et al., 1987; Kalix, 1992; Rothman and Baumann, 2003; Rothman et al., 2003). On the other hand, there is disagreement in the literature regarding the precise nature of drug-transporter interactions for specific cathinone compounds. Drugs that target monoamine transporters can be classified generally as either substrates (i.e., like amphetamine) or blockers (i.e., like cocaine), and this mechanistic distinction is important to consider for at least two reasons: 1) substrates, but not blockers, are translocated into cells where they disrupt vesicular storage and stimulate non-exocytotic release of neurotransmitters by reversing the normal direction of transporter flux (Rothman and Baumann, 2003; Sitte and Freissmuth, 2010), and 2) substrates can produce persistent deficits in monoamine neurons, including depletion of neurotransmitters and loss of functional transporters (Baumann et al., 2007; Fleckenstein et al., 2007). Thus, transporter substrates and blockers display critical differences in their acute and long-term effects.
Several studies have reported that mephedrone and methylone inhibit the uptake of monoamine neurotransmitters in brain tissue and in cells, suggesting these two cathinones function as transporter blockers (Cozzi et al., 1999; Hadlock et al., 2011; Lopez-Arnau et al., 2012; Martinez-Clemente et al., 2012; Simmler et al., 2012). Data from our laboratory, summarized in Table 1, confirm that mephedrone and methylone block the uptake of [3H]dopamine, [3H]norepinephrine and [3H]serotonin into rat brain synaptosomes (Baumann et al., 2012b). However, it must be clarified that traditional uptake-inhibition assays cannot discriminate between drugs acting as transporter substrates versus those acting as blockers, since both types of drugs prevent the accumulation of [3H]neurotransmitters into tissue. To address this problem, we and others have developed in vitro release assays in rat brain synaptosomes which can distinguish between these two types of drugs (Nagai et al., 2007; Rothman and Baumann, 2003; Rothman et al., 2001).
Table 1.
Effects of synthetic cathinones and comparison test drugs on transporter-mediated inhibition of uptake and stimulation of release in rat brain synaptosomes
| Mephedrone | Methylone | MDPV | MDMA | Amphetamine | Cocaine | |
|---|---|---|---|---|---|---|
| DAT uptake IC50 (nM ± S.E.M.) |
762 ± 79 | 1232 ± 133 | 4.1 ± 0.5 | 1009 ± 39 | 93 ± 17 | 211 ± 19 |
| NET uptake IC50 (nM ± S.E.M.) |
487 ± 66 | 1031 ± 162 | 26 ± 8 | 450 ± 30 | 67 ± 16 | 292 ± 34 |
| SERT uptake IC50 (nM ± S.E.M.) |
422 ± 26 | 1017 ± 59 | 3349 ± 305 | 125 ± 11 | 3418 ± 314 | 313 ± 17 |
| DAT release EC50 (nM ± S.E.M.) [Emax %] |
51 ± 5 [102 ± 2] | 117 ± 12 [96 ± 1] | Inactive | 42 ± 2 [100 ± 4] | 5.8 ± 0.4 [102 ± 1] | Inactive |
| NET release EC50 (nM ± S.E.M.) [Emax %] |
58 ± 11 [99 ± 4] | 140 ± 17 [94 ± 2] | Inactive | 53 ± 7 [95 ± 2] | 6.6 ± 0.7 [92 ± 1] | Inactive |
| SERT release EC50 (nM ± S.E.M.) [Emax %] |
122 ± 10 [101 ± 1] | 234 ± 35[98 ± 2] | Inactive | 39 ± 5 [103 ± 4] | 698 ± 71 [97 ± 2] | Inactive |
Uptake and release data are modified from Baumann et al. (2012b), with the exception of MDMA data, which are unpublished. Values are given as nM ± S.E.M. for N=3–4 experiments per drug. Emax % refers to percentage of maximal release response. Compounds are considered inactive in the release assay if they fail to elicit >30% of the maximal response.
Results from release assays reveal that mephedrone and methylone function as substrates at monoamine transporters, thereby stimulating the release of [3H]1-methyl-4-phenylpyridinium ([3H]MPP+) via DAT and NET, and release of [3H]serotonin via SERT (Baumann et al., 2012a; Nagai et al., 2007). The data in Table 1 show that mephedrone, methylone, and MDMA are non-selective transporter substrates (i.e., non-selective releasers), while amphetamine is a selective substrate at DAT and NET. Mephedrone displays similar releasing potency at all three transporters and is about twice as potent as methylone. Mephedrone, methylone, MDMA, and amphetamine are fully efficacious in the release assays (i.e., Emax close to 100%), while MDPV and cocaine are inactive as releasers. The findings from assays using synaptosomes are consistent with the evidence demonstrating mephedrone and methylone function as transportable substrates in assays utilizing transfected cells expressing human DAT, NET and SERT (Eshleman et al., unpublished; Simmler et al., 2012).
Recent data from our laboratory and others reveal that MDPV displays a novel pharmacological profile when compared to other bath salts cathinones (Baumann et al., 2012b; Simmler et al., 2012). Specifically, MDPV is a potent uptake blocker at DAT and NET with no measurable substrate activity (see Table 1). The transporter blocking properties of MDPV are analogous to those of the structurally-related compound pyrovalerone (Heron et al., 1994; Meltzer et al., 2006). When compared to the prototypical transporter blocker cocaine: MDPV is 50-fold more potent at DAT, 10-fold more potent at NET, and 10-fold less potent at SERT. Taken together, the in vitro results indicate that mephedrone and methylone are non-selective transporter substrates, whereas MDPV is a pure catecholamine-selective transporter blocker.
3. Synthetic cathinones produce stimulant effects in animals
A number of studies have examined the in vivo pharmacology of baths salts compounds in rodent models, though the majority of available data pertains to the effects of mephedrone (Angoa- Perez et al., 2012; Baumann et al., 2012a; Hadlock et al., 2011; Huang et al., 2012; Kehr et al., 2011; Lisek et al., 2012; Lopez-Arnau et al., 2012; Marusich et al., 2012; Motbey et al., 2012). Because bath salts cathinones interact with monoamine transporters, they would be expected to increase extracellular concentrations of monoamine neurotransmitters in the brain. Consistent with this notion, in vivo microdialysis studies from our laboratory demonstrate that i.v. injection of mephedrone or methylone (0.3 or 1.0 mg/kg) increases extracellular levels of dopamine and serotonin in rat nucleus accumbens (Baumann et al., 2012a). Kehr et al. (2011) and Wright et al. (2012) reported elevation of dialysate dopamine and serotonin in rat brain after subcutaneous (s.c.) mephedrone administration (3–10 mg/kg). Interestingly, the rise in extracellular serotonin is greater in magnitude than the rise in dopamine after mephedrone or methylone treatment, suggesting the neurochemical effects of both drugs are more akin to those produced by MDMA rather than methamphetamine (Baumann et al., 2012a; Kehr et al., 2011; Wright et al., 2012). No microdialysis studies have examined the effects of cathinones on extracellular norepinephrine in the brain, and this issue warrants investigation. Mephedrone has a much faster rate of clearance when compared to MDMA, and this kinetic feature may increase the propensity for repeated binge use of mephedrone (Kehr et al., 2011).
Several investigations have reported that mephedrone produces locomotor stimulant effects in rats (Baumann et al., 2012a; Kehr et al., 2011; Lisek et al., 2012; Motbey et al., 2012) and mice (Angoa-Perez et al., 2012; Lopez-Arnau et al., 2012; Marusich et al., 2012). Based on locomotor activity measures in rats undergoing microdialysis, mephedrone is similar in potency to MDMA but about three-fold less potent than amphetamine or methamphetamine (Baumann et al., 2012a; Kehr et al., 2011). Intraperitoneal (i.p.) administration of mephedrone to rats (3, 5, 10 or 30 mg/kg) stimulates locomotor activity which is reversed by the D1 receptor antagonist SCH23390 (Lisek et al., 2012). Lopez-Arnau et al. (2012) reported that administration of mephedrone, methylone, or the related compound butylone (5, 10 or 25 mg/kg, i.p.), elicits dose-dependent hyperactivity in mice, and these effects are antagonized by pretreatment with the serotonin-2 receptor blocker ketanserin or the dopamine-2 receptor blocker haloperidol.
The fact that synthetic cathinones stimulate dopamine transmission predicts the drugs possess high abuse liability (Howell and Kimmel, 2008; Wise, 2008). Administration of mephedrone to rats (15 or 30 mg/kg, i.p.) produces a robust increase in the expression of fos protein in reward-relevant brain regions such as the prefrontal cortex, ventral striatum, and ventral tegmental area (Motbey et al., 2012). In mice and rats, mephedrone (30 mg/kg, i.p.) engenders a positive place preference, which points to rewarding properties of the drug (Lisek et al., 2012). Robinson et al. (2012) showed that mephedrone administration (1, 3 or 10 mg/kg, i.p.) lowers brain stimulation reward thresholds in mice, and this effect is mimicked by identical doses of cocaine. Importantly, Hadlock et al. (2011) reported that i.v. mephedrone (0.24 mg/infusion) is self-administered by rats in a manner analogous to methamphetamine. Although less information is available about MDPV, one recent study demonstrated that i.v. MDPV (0.05, 0.1 or 0.2 mg/kg) is readily self-administered by rats, and when rats are allowed extended access to the drug, escalation of drug-taking behavior is observed (Watterson et al., 2012). The collective findings provide compelling evidence that mephedrone and MDPV have a substantial propensity for addiction.
4. Toxicity and adverse effects
Serotonin transporter substrates like MDMA can produce sustained deficits in brain serotonin neurons (Baumann et al., 2007; Fleckenstein et al., 2007), so mephedrone and methylone might be predicted to have similar actions. Binge administration of either drug to single-housed rats (3 or 10 mg/kg, s.c., 3 doses) has no long-lasting effects on brain tissue monoamines (Baumann et al., 2012a), while administration of higher doses of mephedrone to group-housed rats (10 or 25 mg/kg, s.c., 4 doses) produces persistent depletion of brain serotonin (Hadlock et al., 2011). The preclinical findings suggest that adverse effects of bath salts could be exacerbated in hot crowded conditions, such as those typical of rave dance parties where these drugs are sometimes ingested.
Patients coming to medical attention with bath salts intoxication can display agitation, combative behavior, psychosis, tachycardia, and hyperthermia (Borek and Holstege, 2012; Kyle et al., 2011; Prosser and Nelson, 2012; Spiller et al., 2011). Health care workers should be cognizant that patients presenting with this constellation of symptoms may have taken bath salts. Because synthetic cathinones are not detected by routine toxicology screens, definitive proof of bath salts exposure is often difficult to confirm. Treatment is primarily supportive, with benzodiazepines such as lorazepam for agitation and excessive sympathetic stimulation, and aggressive cooling for severe hyperthermia (Ross et al., 2011; Spiller et al., 2011). In some instances, risperidone has been used effectively to manage psychotic behaviors, and in a single reported case, etomidate and succinyl choline were administered in addition to midazolam to sedate the patient (Kasick et al., 2012, Antonowicz et al., 2011, Penders et al., 2011, Borek et al., 2012).
MDPV and mephedrone have been directly implicated in a number of fatalities reported in the medical literature (Lusthof et al., 2011; Maskell et al., 2011; Murray et al., 2012). In one case involving MDPV (Murray et al., 2012), the cause of death was consistent with excited delirium syndrome, a condition that is associated with cocaine overdose and attributable to excessive dopaminergic stimulation (Mash et al., 2009; Ruttenber et al., 1997). Symptoms of excited delirium include agitation, delirium, acidosis, sustained hyperthermia and autonomic dysfunction. Excited delirium has also been observed in bath salts overdose patients with analytical confirmation of mephedrone consumption (Kasick et al., 2012; Lusthof et al., 2011). In a notable example, Kasick et al. (2012) described a patient who had taken bath salts and was hallucinating, agitated, and hyperthermic; urinalysis revealed a presumptive positive for phencyclidine (PCP) as well as mephedrone (Kasick et al., 2012). Interestingly, it was recently shown that MDPV cross-reacts with the PCP immunoassay used in hospitals (Macher and Penders, 2012), suggesting the possibility that the patient described by Kasick et al. ingested MDPV in combination with mephedrone. Penders and colleagues (Penders et al., 2012) have concluded that MDPV is the most likely culprit responsible for causing excited delirium in patients who abuse bath salts products in the US. Because symptoms of excited delirium can be life-threatening, proper patient care is paramount. Physical restraints should be avoided and pharmacological treatment of agitation or cardiovascular symptoms should be administered prudently and monitored closely.
5. Summary
Psychoactive “bath salts” contain one or more synthetic cathinones which target plasma membrane monoamine transporters. In vitro data have identified a mechanistic dichotomy among common bath salts constituents: ring-substituted cathinones like mephedrone act as non-selective transporter substrates, whereas pyrrolidinophenones like MDPV act as potent catecholamine-selective transporter blockers (Baumann et al., 2012b; Nagai et al., 2007; Simmler et al., 2012). Recent in vivo findings show that bath salts cathinones produce locomotor activation in rats and mice (Huang et al., 2012; Lisek et al., 2012; Lopez-Arnau et al., 2012; Marusich et al., 2012), but few studies have examined pharmacokinetics and metabolism of synthetic cathinones (Kamata et al., 2006; Meyer et al., 2010), and the consequences of chronic drug dosing are unknown. The stimulation of dopamine transmission by bath salts cathinones is likely to mediate their abuse potential (Hadlock et al., 2011; Watterson et al., 2012) and certain clinical side-effects (Murray et al., 2012; Penders et al., 2012). Given the emergence of new “replacement” cathinones with unknown pharmacology (Brandt et al., 2010; Shanks et al., 2012), it seems that emergency departments will continue to encounter patients suffering from the complications caused by synthetic stimulants. More research is urgently needed to characterize the pharmacology and toxicology of the growing list of synthetic cathinones. The data derived from these investigations will inform public health policy and improve strategies for treating the medical consequences of bath salts abuse.
Acknowledgments
The research described herein was generously supported by the Intramural Research Program at NIDA, NIH. The authors wish to thank Dr. Amy H. Newman for thoughtful comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angoa-Perez M, Kane MJ, Francescutti DM, Sykes KE, Shah MM, Mohammed AM, Thomas DM, Kuhn DM. Mephedrone, an abused psychoactive component of ‘bath salts’ and methamphetamine congener, does not cause neurotoxicity to dopamine nerve endings of the striatum. Journal of neurochemistry. 2012;120:1097–1107. doi: 10.1111/j.1471-4159.2011.07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniwicz JL, Metzger AK, Ramanujam SL. Paranoid psychosis induced by consumption of methylenedioxypyrovalerone: two cases. General hospital psychiatry. 2011;33:640.e5–640.e6. doi: 10.1016/j.genhosppsych.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012a;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive “bath salts” products. Neuropsychopharmacology. 2012b doi: 10.1038/npp.2012.204. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Wang X, Rothman RB. 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology. 2007;189:407–424. doi: 10.1007/s00213-006-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4-methylenedioxypyrovalerone. Annals of emergency medicine. 2012;60:103–105. doi: 10.1016/j.annemergmed.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Brandt SD, Sumnall HR, Measham F, Cole J. Analyses of second-generation ‘legal highs’ in the UK: initial findings. Drug testing and analysis. 2010;2:377–382. doi: 10.1002/dta.155. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control Prevention (CDC) Emergency department visits after use of a drug sold as “bath salts”--Michigan, November 13, 2010-March 31, 2011. MMWR Morbidity and mortality weekly report. 2011;60:624–627. [PubMed] [Google Scholar]
- Coppola M, Mondola R. Synthetic cathinones: chemistry, pharmacology and toxicology of a new class of designer drugs of abuse marketed as “bath salts” or “plant food”. Toxicology letters. 2012;211:144–149. doi: 10.1016/j.toxlet.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Cozzi NV, Sievert MK, Shulgin AT, Jacob P, 3rd, Ruoho AE. Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines. European journal of pharmacology. 1999;381:63–69. doi: 10.1016/s0014-2999(99)00538-5. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration (DEA) Schedules of controlled substances: temporary placement of three synthetic cathinones in Schedule I. Final Order. Fed Regist. 2011;76:65371–65375. [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annual review of pharmacology and toxicology. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Yousif M, Naiman N, Kalix P. Methcathinone: a new and potent amphetamine-like agent. Pharmacol biochem behav. 1987;26:547–551. doi: 10.1016/0091-3057(87)90164-x. [DOI] [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, Hoonakker AJ, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. J Pharmacol Exp Ther. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron C, Costentin J, Bonnet JJ. Evidence that pure uptake inhibitors including cocaine interact slowly with the dopamine neuronal carrier. European journal of pharmacology. 1994;264:391–398. doi: 10.1016/0014-2999(94)00502-8. [DOI] [PubMed] [Google Scholar]
- Howell LL, Kimmel HL. Monoamine transporters and psychostimulant addiction. Biochemical pharmacology. 2008;75:196–217. doi: 10.1016/j.bcp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Huang PK, Aarde SM, Angrish D, Houseknecht KL, Dickerson TJ, Taffe MA. Contrasting effects of d-methamphetamine, 3,4-methylenedioxymethamphetamine, 3,4-methylenedioxypyrovalerone, and 4-methylmethcathinone on wheel activity in rats. Drug and alcohol dependence. 2012;126:168–175. doi: 10.1016/j.drugalcdep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D, Adams RD, Spears R, Cooper G, Lupton DJ, Thompson JP, Thomas SH. Clinical characteristics of mephedrone toxicity reported to the U.K. National Poisons Information Service. Emergency medicine journal: EMJ. 2011;28:686–689. doi: 10.1136/emj.2010.096636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalix P. Cathinone, a natural amphetamine. Pharmacology and toxicology. 1992;70:77–86. doi: 10.1111/j.1600-0773.1992.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Kamata HT, Shima N, Zaitsu K, Kamata T, Miki A, Nishikawa M, Katagi M, Tsuchihashi H. Metabolism of the recently encountered designer drug, methylone, in humans and rats. Xenobiotica; the fate of foreign compounds in biological systems. 2006;36:709–723. doi: 10.1080/00498250600780191. [DOI] [PubMed] [Google Scholar]
- Kasick DP, McKnight CA, Klisovic E. “Bath salt” ingestion leading to severe intoxication delirium: two cases and a brief review of the emergence of mephedrone use. The American journal of drug and alcohol abuse. 2012;38:176–180. doi: 10.3109/00952990.2011.643999. [DOI] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. British journal of pharmacology. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle PB, Iverson RB, Gajagowni RG, Spencer L. Illicit bath salts: not for bathing. Journal of the Mississippi State Medical Association. 2011;52:375–377. [PubMed] [Google Scholar]
- Lisek R, Xu W, Yuvasheva E, Chiu YT, Reitz AB, Liu-Chen LY, Rawls SM. Mephedrone (‘bath salt’) elicits conditioned place preference and dopamine-sensitive motor activation. Drug and alcohol dependence. 2012;126:257–262. doi: 10.1016/j.drugalcdep.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Arnau R, Martinez-Clemente J, Pubill D, Escubedo E, Camarasa J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methylone. British journal of pharmacology. 2012;167:407–420. doi: 10.1111/j.1476-5381.2012.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusthof KJ, Oosting R, Maes A, Verschraagen M, Dijkhuizen A, Sprong AG. A case of extreme agitation and death after the use of mephedrone in The Netherlands. Forensic science international. 2011;206:e93–95. doi: 10.1016/j.forsciint.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Macher AM, Penders TM. False-positive phencyclidine immunoassay results caused by 3,4-methylenedioxypyrovalerone (MDPV) Drug testing and analysis. 2012 doi: 10.1002/dta.1371. in press. [DOI] [PubMed] [Google Scholar]
- Martinez-Clemente J, Escubedo E, Pubill D, Camarasa J. Interaction of mephedrone with dopamine and serotonin targets in rats. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2012;22:231–236. doi: 10.1016/j.euroneuro.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Grant KR, Blough BE, Wiley JL. Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observation battery in mice. Neurotoxicology. 2012;33:1305–1313. doi: 10.1016/j.neuro.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash DC, Duque L, Pablo J, Qin Y, Adi N, Hearn WL, Hyma BA, Karch SB, Druid H, Wetli CV. Brain biomarkers for identifying excited delirium as a cause of sudden death. Forensic science international. 2009;190:e13–19. doi: 10.1016/j.forsciint.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Maskell PD, De Paoli G, Seneviratne C, Pounder DJ. Mephedrone (4- methylmethcathinone)-related deaths. J Anal Toxicol. 2011;35:188–191. doi: 10.1093/anatox/35.3.188. [DOI] [PubMed] [Google Scholar]
- Meltzer PC, Butler D, Deschamps JR, Madras BK. 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogues: a promising class of monoamine uptake inhibitors. Journal of medicinal chemistry. 2006;49:1420–1432. doi: 10.1021/jm050797a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Du P, Schuster F, Maurer HH. Studies on the metabolism of the alpha-pyrrolidinophenone designer drug methylenedioxypyrovalerone (MDPV) in rat and human urine and human liver microsomes using GC-MS and LC-high-resolution MS and its detectability in urine by GC-MS. Journal of mass spectrometry: JMS. 2010;45:1426–1442. doi: 10.1002/jms.1859. [DOI] [PubMed] [Google Scholar]
- Motbey CP, Hunt GE, Bowen MT, Artiss S, McGregor IS. Mephedrone (4-methylmethcathinone, ‘meow’): acute behavioural effects and distribution of Fos expression in adolescent rats. Addiction biology. 2012;17:409–422. doi: 10.1111/j.1369-1600.2011.00384.x. [DOI] [PubMed] [Google Scholar]
- Murray BL, Murphy CM, Beuhler MC. Death following recreational use of designer drug “bath salts” containing 3,4-methylenedioxypyrovalerone (MDPV) J Med Toxicol. 2012;8:69–75. doi: 10.1007/s13181-011-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai F, Nonaka R, Satoh Hisashi Kamimura K. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. European journal of pharmacology. 2007;559:132–137. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- Penders TM, Gestring RE, Vilensky DA. Intoxication delirium following use of synthetic cathinone derivatives. The American journal of drug and alcohol abuse. 2012;38:616–617. doi: 10.3109/00952990.2012.694535. [DOI] [PubMed] [Google Scholar]
- Prosser JM, Nelson LS. The toxicology of bath salts: a review of synthetic cathinones. J Med Toxicol. 2012;8:33–42. doi: 10.1007/s13181-011-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum CD, Carreiro SP, Babu KV. Here today, gone tomorrow… and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (Bath Salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8:15–32. doi: 10.1007/s13181-011-0202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross EA, Watson M, Goldberger B. “Bath salts” intoxication. The New England journal of medicine. 2011;365:967–968. doi: 10.1056/NEJMc1107097. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. European journal of pharmacology. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Vu N, Partilla JS, Roth BL, Hufeisen SJ, Compton-Toth BA, Birkes J, Young R, Glennon RA. In vitro characterization of ephedrine-related stereoisomers at biogenic amine transporters and the receptorome reveals selective actions as norepinephrine transporter substrates. J Pharmacol Exp Ther. 2003;307:138–145. doi: 10.1124/jpet.103.053975. [DOI] [PubMed] [Google Scholar]
- Ruttenber AJ, Lawler-Heavner J, Yin M, Wetli CV, Hearn WL, Mash DC. Fatal excited delirium following cocaine use: epidemiologic findings provide new evidence for mechanisms of cocaine toxicity. Journal of forensic sciences. 1997;42:25–31. [PubMed] [Google Scholar]
- Schifano F, Albanese A, Fergus S, Stair JL, Deluca P, Corazza O, Davey Z, Corkery J, Siemann H, Scherbaum N, Farre M, Torrens M, Demetrovics Z, Ghodse AH Psychonaut Web, M., Re, D.R.G. Mephedrone (4-methylmethcathinone; ‘meow meow’): chemical, pharmacological and clinical issues. Psychopharmacology. 2011;214:593–602. doi: 10.1007/s00213-010-2070-x. [DOI] [PubMed] [Google Scholar]
- Shanks KG, Dahn T, Behonick G, Terrell A. Analysis of first and second generation legal highs for synthetic cannabinoids and synthetic stimulants by ultra-performance liquid chromatography and time of flight mass spectrometry. J Anal Toxicol. 2012;36:360–371. doi: 10.1093/jat/bks047. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. Pharmacological characterization of designer cathinones in vitro. British journal of pharmacology. 2012 doi: 10.1111/j.1476-5381.2012.02145.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitte HH, Freissmuth M. The reverse operation of Na(+)/Cl(−)-coupled neurotransmitter transporters--why amphetamines take two to tango. Journal of neurochemistry. 2010;112:340–355. doi: 10.1111/j.1471-4159.2009.06474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogawa C, Sogawa N, Ohyama K, Kikura-Hanajiri R, Goda Y, Sora I, Kitayama S. Methylone and monoamine transporters: correlation with toxicity. Current neuropharmacology. 2011;9:58–62. doi: 10.2174/157015911795017425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol (Phila) 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addiction biology. 2012 doi: 10.1111/j.1369-1600.2012.00474.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotoxicity research. 2008;14:169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Angrish D, Aarde SM, Barlow DJ, Buczynski MW, Creehan KM, Vandewater SA, Parsons LH, Houseknecht KL, Dickerson TJ, Taffe MA. PLos one. 2012;7:e44652. doi: 10.1371/journal.pone.0044652. [DOI] [PMC free article] [PubMed] [Google Scholar]