Abstract
A host of new and reliable low-cost medical devices specially adapted to needs of low- and middle-income countries is on the way, driven by increased demand for cost-effective health care. Ajanthy Arasaratnam and Gary Humphreys report.
“What’s important is equipment durability – the cost of ownership over time.”
John Anner
How do you do blood transfusions when you have no medical equipment, no donors and no blood? This is a question that comes up all too frequently in sub-Saharan Africa, where blood donations in many countries fail to meet the demand for transfusions and where clinics, particularly those in remote rural areas, are underequipped. In the absence of blood and equipment, patients in need of transfusion – such as women haemorrhaging as a result of a ruptured ectopic pregnancy – are forced to fall back on a crude form of auto-transfusion using kitchen equipment.
Dr Kathleen Sienko, an assistant professor of Biomedical Engineering at the University of Michigan in the United States of America (USA), explains: “Blood pools into incisions in the woman’s abdomen, before a sterilized gallipot or soup ladle scoops it into a basin of anticoagulants. From there, it filters through a gauze-lined funnel, feeds into a blood bag and drips back into her body.”
Such crude measures, while doubtless saving some lives, underscore a failure or inability on the part of health authorities in the countries concerned to provide adequate health care. But these measures also reflect the global medical device industry’s traditional focus on the needs of wealthier countries. According to Espicom, a United Kingdom-based company that gathers data and does market analyses of the pharmaceutical and medical device industry, global spending on medical devices in 2010 is estimated to have been around US$ 260 billion, the bulk of those sales generated by a handful of manufacturers based in high-income countries.
Medical device use is similarly concentrated in high-income countries, with just 13% of the global population accounting for 76% of global medical device use.
In the past, when medical device companies did address the needs of low- and middle-income countries, they tended to remove the features from high-tech products that were designed for more developed countries to market them in poorer countries, an approach known as “glocalization”.
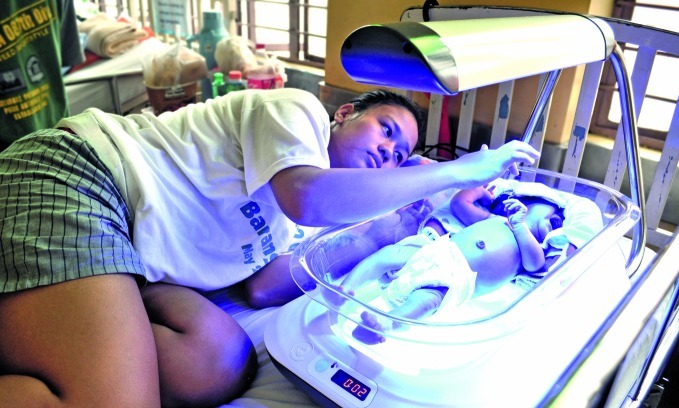
A phototherapy device to treat neonatal jaundice. The device, called “Firefly”, was designed by non-profit group Design that Matters. It is being evaluated in a clinical trial in the Philippines and Viet Nam.
Will Harris/Design that Matters
According to Denise Kruzikas, Director of General Electric’s (GE) Healthymagination, a GE initiative committed to promoting health-care innovation, the industry is increasingly recognizing that stripping out the bells and whistles is seldom sufficient to adapt medical devices for health workers, who may lack training and technical skills and who are working in environments that aren’t always equipped to handle the technology.
For example, power-hungry devices – conceived to function in high-income countries with electrical power grids – can leave hospitals and clinics in poorer countries struggling to find generators or scavenging for batteries. Moreover, devices that are not designed to cope with heat, humidity and dust or to be used intensively are unlikely to last very long.
These kinds of issues have contributed to a situation in which a large proportion of medical equipment in developing countries may be partly or totally unusable. In sub-Saharan Africa up to 70% of medical equipment stands idle, according to WHO’s Guidelines for health care equipment donations.
As companies begin to recognize the market potential in low- and middle-income countries, their approach looks set to change. Radha Basu, director of Santa Clara University’s Frugal Innovation Laboratory in the USA state of California, believes that one of the big drivers of change is the increased demand for effective and robust low-cost medical devices from emerging economies, notably China and India.
China’s current five-year plan (2011–15) allocates US$ 41 billion to the development of new hospitals and the upgrading of the country’s grassroots health service system, while India’s latest five-year plan includes a commitment to increasing the government’s share of total health-care expenditure by 2017. “The development of emerging markets brings new reasons to create appropriate, affordable and accessible medical innovations for low-income markets. It is no longer just something that ‘well intentioned’ people do to reduce health-care disparities. It is now a profitable activity,” Basu says.
GE is a prime example of a company that sees the commercial potential of what has become known as “frugal innovation”. Recently GE revamped its operations in India to tap into the country’s growing demand for medical devices. GE isn’t just marketing low-cost medical devices in India. The company is also taking account of local conditions when it develops and tests new products, conditions which include power outages, voltage fluctuations, high levels of dust and pollution, and intensive equipment use. One of the most successful products to come out of GE’s efforts in the field of “frugal innovation” is the Lullaby baby warmer, which provides direct heat in an open cradle and is used to help new-born babies adjust to room temperature.
At US$ 3000 per unit in India, the Lullaby warmer is cheap compared to the baby warmer GE sells in the USA, that starts at US$ 12 000 and which, on top of the basic warming function, performs other functions such as monitoring a baby’s pulse and weight. The Lullaby warmer was launched in India in May 2009 and is now sold in 62 countries, including Belgium, Brazil, Dubai, Egypt, Italy, the Russian Federation and Switzerland.
“The Lullaby was intended for rural markets in India and Indonesia, but has sold in many other countries,” says Kruzikas. Following its launch, GE sold about 1500 units in the first 12 months: half of those in India including in the smaller, rural towns.
While private-sector companies, such as GE, are making headway in the development and deployment of low-cost medical devices, Radha Basu’s “well-intentioned” people continue to make a significant contribution to this field of innovation and are, in many ways, blazing a trail, setting out the ground rules for what makes a good frugal medical device.
A good example of such efforts is the work being carried out at Rice University in Texas, USA, where biomedical engineering students and their faculty have teamed up with physicians from the University of Malawi and the Texas Children’s Hospital. Together with Californian-based industrial design firm 3rd Stone Design, they have developed an extremely low-cost bubble continuous positive airway pressure (bCPAP) machine to help babies, especially when they are premature, to breathe.
Bubble CPAP devices are a common sight in paediatric wards in the developed world, where they are used to treat infants that have difficulty breathing. However, as Dr Rebecca Richards-Kortum, Director of Rice 360: Institute for Global Health Technology at Rice University, points out: bCPAP devices can cost as much as US$ 6000 per unit and are thus often too expensive for hospitals in low- and middle-income countries.
Using basic off-the-shelf components, the Rice bCPAP can be built for US$ 160 and, according to Richards-Kortum, delivers the same therapeutic pressure as devices used in hospitals in the developed world. Preliminary data from a recent clinical trial at Queen Elizabeth Central Hospital in Blantyre, Malawi, suggest that the device can significantly improve survival for infants struggling with respiratory distress. In July 2012, the bCPAP project was one of three nominated to win a grant of up to US$ 2 million from the Saving Lives at Birth: a Grand Challenge for Development initiative to support deployment of the technology in Malawi.
For John Anner, president of East Meets West, a nongovernmental organization focused on health and education in Asia, making medical devices accessible in low-income settings is not just about getting the initial unit-cost down. “What’s important is equipment durability – the cost of ownership over time. That’s the key metric,” Anner says. This is a point easily understood by anyone who has ever had to pay US$ 50 for a printer cartridge. With regard to bCPAPs, Anner argues that even if a clinic or hospital has managed to procure a cheap unit, it still has to pay for consumables.
“A replacement breathing tube can cost US$ 300 per week,” he says. It is for this reason that East Meets West engineered its bubble CPAP to be extremely durable and not to require any consumable parts. The East Meets West Breath of Life programme also provides clinical and technical training for hospital staff, and monitors every one of the 4500 pieces of equipment it has in use in more than 300 hospitals in eight countries.
Staff at a children’s hospital in Myanmar learn how to use an alcohol metre to mix chlorhexidine gel concentrate and alcohol for disinfection.
East Meets West/Luciano Moccia
Like Kruzikas, Anner also stresses the importance of durability and simplicity of use, but argues that these objectives should not be pursued at the expense of appearance. “Front-end aesthetics are in fact important,” Anner says. “Devices need to look modern, so that hospitals are proud to use them and patients feel that they are receiving the proper treatment.” The question of first impressions also arose in the development of the Rice University bCPAP, where early versions were criticized for looking too improvised.
Of course, at the end of the day, good looks mean nothing if the device in question turns out to be unreliable. Coordinator of the Medical Devices Unit at the World Health Organization in Geneva, Dr Adriana Velazquez Berumen, explains: “A surgeon who needs to use an anaesthesia machine can use one that is simple, but it must be safe and effective, apart from being affordable and appropriate for the local hospital setting.”
Moreover, the emphasis placed on locally contextualized development and design, clinical efficacy and robustness that seem to characterize low-cost medical device endeavours, suggest that such devices could turn out to be more reliable, on the whole, than their more expensive counterparts. And, beyond being more reliable, they may also be more accessible.
Says Basu: “Low-cost medical devices have enormous potential to reduce the gap between the developed and developing worlds, and to reach the ‘last mile’ of people in the most innovative ways.”