Abstract
Vaccinia fetalis, the vertical transfer of vaccinia virus from mother to fetus, is a relatively rare but often fatal complication of primary vaccinia virus vaccination during pregnancy. To date there has been no attempt to develop an animal model to study the pathogenesis of this acute viral infection in vivo. Here we report that infection of gestating BALB/c mice by either intravenous or intraperitoneal routes with the Western Reserve strain of vaccinia virus results in the rapid colonization of the placenta and vertical transfer of virus to the developing fetus. Systemic maternal infections during gestation lead to the death of all offspring prior to or very shortly after birth. Using in situ hybridization for vaccinia virus mRNA to identify infected cells, we show that the virus initially colonizes cells lining maternal lacunae within the trophospongium layer of the placenta. The study of this model will significantly enhance our understanding of the pathogenesis of fetal vaccinia virus infections and aid in the development of effective treatments designed to reduce the risk of vaccinia virus-associated complications during pregnancy.
Vaccinia virus, the prototypical member of the Orthopoxvirus genus and a close relative of smallpox (variola) virus, was the live viral vaccine used in the global variola eradication campaign (8, 9). The recent decision to begin vaccinating civilian health care workers against smallpox and the outbreak of monkeypox in the American Midwest have renewed interest in the pathogenesis of acute poxvirus infections. According to present medical standards, vaccinia virus has a relatively poor safety profile and has the capacity to cause severe complications in pregnant women, very young children, and other immunocompromised individuals. An estimated 15 to 50 people/million who receive primary vaccinia virus vaccinations will experience potentially life-threatening side effects including allergic reactions at the site of vaccination, spread of the virus to other parts of the body, and infection of the central nervous system (6). The potential for vaccine-associated complications has led the U.S. advisory committee on immunization practices to recommend that infants under 1 year of age and adults with weakened immune systems or those with preexisting skin conditions such as eczema or atopic dermatitis not be routinely vaccinated in the absence of clear exposure to either smallpox or monkeypox (3). In addition, the advisory committee has also recommended against vaccinating pregnant women or women who are attempting to become pregnant due to the potential for vertical transmission of vaccinia virus from mother to fetus (3, 4, 30). Currently there have been fewer than 50 documented cases in the medical literature of vertical transfer of vaccinia virus during pregnancy, most of which resulted in the death of the affected offspring (11-13, 20, 21, 29). Unfortunately our knowledge of the pathological consequences of vaccinia virus infections during pregnancy has been limited by the lack of an appropriate small animal model with which to study the pathogenesis of the virus in pregnant hosts. Of particular interest to both the clinician and researcher would be the pathways that the virus uses to cross the placenta and the in vivo targets of viral replication within maternal reproductive tissue and in the fetus.
In this study we examined the ability of a common laboratory strain of vaccinia virus to successfully infect the murine placenta and spread vertically to the fetus prior to birth. We show that both intraperitoneal and intravenous delivery of the Western Reserve strain of vaccinia virus (VVWR) can lead first to placental infection and later to infection of the fetus. Surprisingly, compared to intraperitoneal infection, intravenous delivery of virus did not hasten the kinetics of transplacental transfer. In contrast, a moderate increase in the initial dose of virus did result in a more rapid transfer of virus across the placenta. The initial sites of virus replication in the placenta were in cells lining large maternal blood vessels in the placental trophospongium. At later time points discrete foci of infected cells could be observed throughout the placenta. Neonatal mortality studies carried out on infected dams revealed that vaccinia virus infection during midgestation was completely lethal to the developing fetus.
MATERIALS AND METHODS
Mice.
Female BALB/c mice were harem bred and examined each morning for evidence of a vaginal plug. The date on which a plug was observed was considered day 0.5, and females that were allowed to come to term invariably gave birth between days 19 and 21. All animals were housed in specific-pathogen-free environments and used in accordance with institutional and National Institutes of Health guidelines governing the humane care and use of laboratory animals.
Viral infections and plaque titrations.
Stocks of VVWR and a recombinant VVWR expressing the enhanced green fluorescent protein (eGFP) (a kind gift from J. Yewdell and B. Moss) were grown and plaque titrated at 37°C on BSC40 cells in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 50 U of penicillin G/liter, 50 μg of streptomycin/liter, and 20 mM l-glutamine (complete Dulbecco's modified Eagle's medium; all from Gibco BRL, Rockville, Md.) as previously described (5). The construction of the eGFP-expressing recombinant vaccinia virus has been previously described (23). Mice were infected with 0.5 ml of virus diluted to the concentrations specified in the figure legends in complete Dulbecco's modified Eagle's medium. At various times postinfection organs were removed and snap frozen in liquid nitrogen for later virus titration. Vaccinia virus titers were determined by plaque assay on BSC40 cell monolayers as previously described (5).
Histology and photomicroscopy.
Placental and fetal tissues from virus-infected and sham-infected pregnant female mice were fixed overnight in 10% neutral buffered formalin prior to cryosectioning. For visualization of virally encoded eGFP 15-μm sections were incubated for 5 min in a 300 nM solution of 4′,6′-diamidino-2-phenylindole dimethyl sulfoxide (Molecular Probes, Eugene, Oreg.) in phosphate-buffered saline. The slides were then rinsed and overlaid with phosphate-buffered saline and observed using a Nikon Optiphot fluorescence microscope. All images were acquired using a spot RT camera (Diagnostics Instruments Inc.).
In situ hybridization.
Placental tissues were removed and fixed overnight in 10% neutral buffered formalin. Paraffin-embedded cross sections (3 μm) were then processed for in situ hybridization as described previously (2). A 33P-labeled cRNA encoding a 316-nucleotide portion of the 5′ coding region of the vaccinia virus early gene C11R (kindly provided by R. C. Condit) was used as a probe. The construction and utilization of this probe have been previously described (14, 18). After hybridization and posthybridization washes sections were exposed for 96 h prior to fixation and development. All images were acquired using a spot RT camera (Diagnostics Instruments Inc.).
Statistical analysis.
Student's t test was performed to determine the statistical relatedness of data from groups of nonpregnant mice infected with vaccinia virus 3 or 9 days before by using Microsoft Excel 2000 for Windows (Microsoft Corp., Redmond, Wash.).
RESULTS
Clearance of VVWR is delayed in reproductive tissue.
VVWR exhibits a strong tropism for murine ovarian tissue, although the affinity of VVWR for other reproductive tissues has not been well described (16, 17). In order to develop a mouse model to study the pathological effects of vaccinia virus infections during pregnancy, we first wished to assess whether this strain could also replicate effectively in the uterus of nonpregnant mice. We hypothesized that the ability of the virus to grow well within the uterus might facilitate the infection of the placenta and subsequent transplacental transfer of virus to the developing fetus. Groups of three nonpregnant female BALB/c mice were infected intraperitoneally with 2 × 106 PFU of VVWR, and viral titrations were carried out on selected organs at 3, 9, and 15 days postinfection. At 3 days postinfection, virus was detectable in the spleens, livers, uterus, ovaries, heart, and lungs of most mice (Fig. 1, top panel). A single mouse also contained low but detectable levels of virus in the brain. In two out of three mice the highest levels of virus at 3 days postinfection were found in the ovaries and uterus, and the geometric mean viral titers in these tissues were 3 logs higher than that in the spleen. We conclude from these data that at early times after intraperitoneal infection VVWR does replicate well in both the ovaries and the uterus.
FIG. 1.
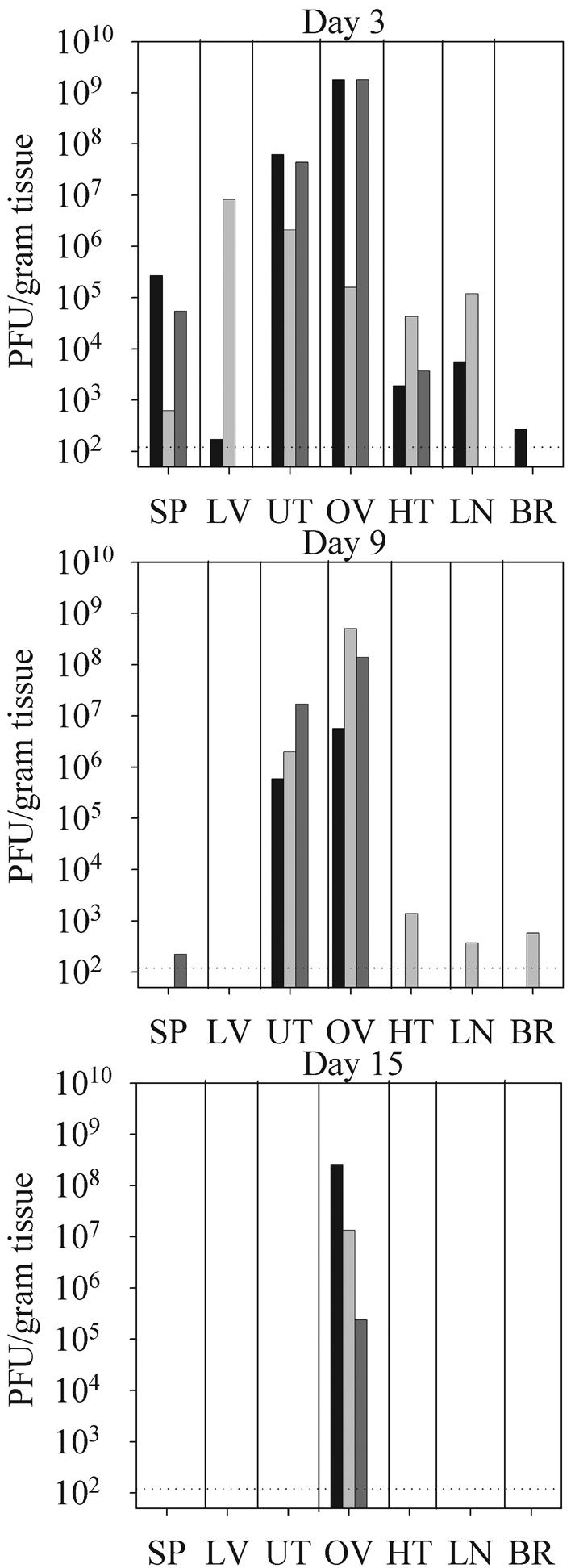
VVWR exhibits tropism for murine reproductive tissue. Groups of three nonpregnant female mice were infected intraperitoneally with 2 × 106 PFU of VVWR. Spleen (SP), liver (LV), uterus (UT), ovary (OV), heart (HT), lymph node (LN), and brain (BR) tissues were harvested 3, 9, or 15 days postinfection and assayed for infectious virus as described in Materials and Methods. Each bar represents vaccinia virus titers within a single organ. Similarly shaded bars represent viral titers in tissues from individual mice.
By 9 days postinfection virus had been largely cleared from most internal organs except the reproductive tissues (Fig. 1, middle panel). There were no statistically significant differences in the geometric mean titers of virus present at 3 compared to 9 days postinfection in either the uterus (1.8 × 107 versus 2.0 × 106 PFU/g, P = 0.09) or the ovaries (8.0 × 107 versus 7.4 × 107 PFU/g, P = 0.19). Thus, following a low-dose intraperitoneal infection nonpregnant, immunocompetent female hosts successfully control viral replication in most organs within 9 days of infection. However, at this time point virus continues to persist in both the ovaries and the uterus. By 15 days postinfection virus had been cleared from the uterus but was still present in the ovaries of all three mice analyzed (Fig. 1, bottom panel). The inability of the host to rapidly eliminate vaccinia virus within reproductive tissues may increase the likelihood that the placenta, and eventually the fetus, will be infected.
Low-dose intraperitoneal or intravenous infections in midgestation result in rapid placental infection but delayed vertical transfer of virus from mother to fetus.
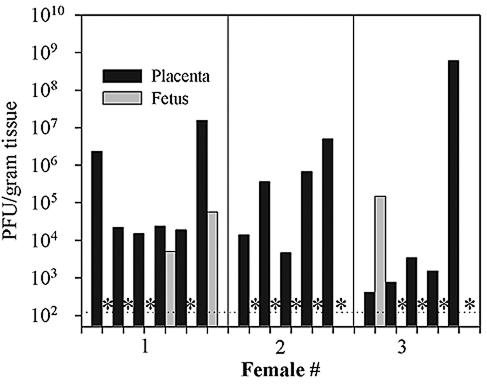
To evaluate if maternal infection during pregnancy would result in successful placental colonization and rapid vertical transfer of virus to the fetus, three female mice were infected intraperitoneally on the 11th day of gestation with 2 × 106 PFU of vaccinia virus. Viral titers in maternal placental-fetal pairs were then measured 4 days later on the 15th day of pregnancy. In total, 21 placentas were analyzed, 16 of which were successfully infected by vaccinia virus. The titers of these placentas and the corresponding fetuses are shown in Fig. 2. By this dose and route, only 19% of fetuses (3 of 16) were infected by 4 days postinoculation. Interestingly, there appeared to be no direct relationship between the titer of virus in the placenta and successful vertical transfer of virus to the fetus. Similar experiments using an intravenous dose of 2 × 106 PFU of vaccinia virus during midgestation also resulted in the colonization of the placenta but little vertical transfer to the fetus within the first 4 days of the infection (data not shown).
FIG. 2.
Low-dose intraperitoneal infection of pregnant mice with vaccinia virus leads to efficient placental infection but limited vertical transfer within the first 4 days of infection. Pregnant mice were infected by the intraperitoneal route with 2 × 106 PFU of VVWR on the 11th day of pregnancy, and placenta and fetal pairs were harvested 4 days later and plaque titrated on BSC40 cells as described in Materials and Methods. The dotted horizontal line indicates the level of detectability of the plaque assay (120 PFU/g of tissue). Asterisks indicate samples in which no virus was detected.
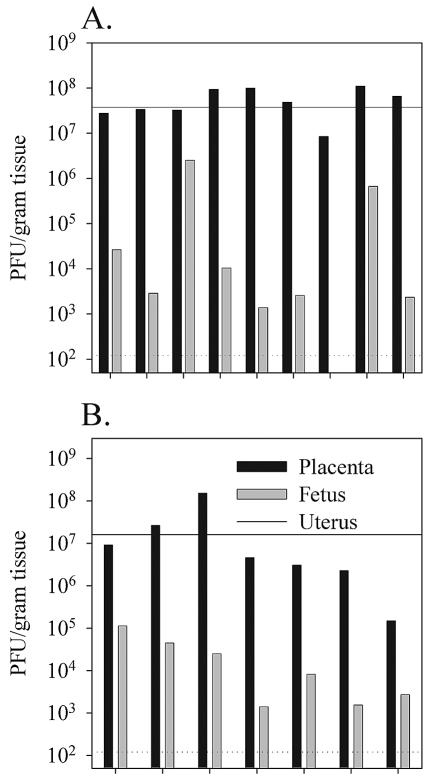
The above infection conditions clearly led to efficient placental colonization but failed to result in detectable transplacental transfer of virus to the developing fetus within the first 4 days of infection. To determine if vaccinia virus is able to successfully cross the murine placenta at later time points, we infected groups of gestating female mice either intraperitoneally on day 12 (Fig. 3A) or intravenously on day 9 (Fig. 3B) postconception with 2 × 106 PFU of VVWR. Viral titers were then measured in the uterus, placenta, and fetuses 7 days later on the 19th and 16th days of pregnancy, respectively. By 1 week postinfection, virus was readily detectable in 15 out of the 16 fetuses tested although in most cases fetal titers were much lower than titers in the corresponding placenta. By 7 days postinfection the route of virus administration had no discernible affect on the overall efficiency of vertical transfer.
FIG. 3.
Low-dose intraperitoneal or intravenous infections can result in efficient vertical transfer of virus to the fetus by 1 week postinfection. Pregnant female mice were infected intraperitoneally (A) or intravenously (B) 9 or 12 days postconception, respectively, with 2 × 106 PFU of VVWR. Viral titers in the uterus, placenta, and fetuses were determined 7 days postinfection by plaque titration as described in Materials and Methods. The dotted horizontal line indicates the limit of detectability of the plaque assay (120 PFU/g of tissue). The maternal virus titer in the uterus is shown as a solid horizontal line.
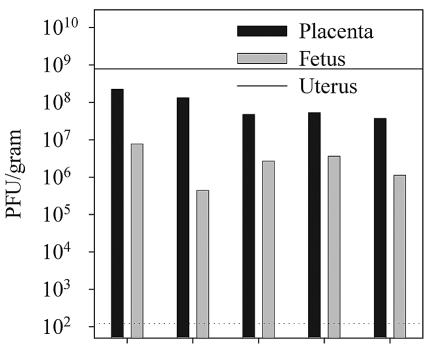
High-dose intravenous infections result in substantial placental infection and rapid vertical transfer of vaccinia virus to the fetus.
Our initial attempts to study the effects of vaccinia virus infection during gestation were encouraging. However, to examine the effects of fetal vaccinia virus infections at discrete gestational ages, we wished to develop a more reproducible infection protocol, one which would lead to the successful infection of all placentas and the rapid vertical transfer of virus to the fetus. To do this, we increased the dose of virus threefold to 6 × 106 PFU and infected groups of female BALB/c mice intravenously on days 13 to 14 of gestation. Maternal tissues as well as placental-fetal pairs were again harvested 4 days later and plaque titrated. By use of this infection regimen, vaccinia virus was detectable by plaque assay in the maternal uteri and all placental-fetal pairs. Data from one representative pregnant female of three tested are shown in Fig. 4. Placental titers ranged from 1.5 × 105 to 1.5 × 108 PFU/g with a mean geometric titer among all placentas of 5.6 × 106 PFU/g. The titer of virus within the fetuses, which ranged from 1.4 × 103 to 1.1 × 105 PFU/g (mean geometric titer of 9.3 × 103), was again substantially lower than that observed in the corresponding placentas. No attempt was made to determine the titers in individual fetal organs; therefore, in this experiment, as in the experiment shown in Fig. 3, we cannot compare the relative affinities of VVWR for placental and for fetal tissue. However, these data do demonstrate that a relatively moderate increase in the initial dose of vaccinia virus delivered intravenously during midgestation can result in the rapid vertical transfer of virus from the placenta to the fetus.
FIG. 4.
The ability of vaccinia virus to rapidly cross the placenta is dependent upon the initial dose of infection. Pregnant female mice were infected intraperitoneally 14 days postconception with 6 × 106 PFU of VVWR. Viral titers in the uterus, placenta, and fetuses were determined 4 days later by plaque titration as described in Materials and Methods. The dotted horizontal line indicates the limit of detectability of the plaque assay (120 PFU/g of tissue). The maternal virus titer in the uterus is shown as a solid horizontal line.
Maternal vaccinia virus infections during pregnancy result in complete fetal mortality.
To determine what effect a maternal vaccinia virus infection has on the survival of the fetus, groups of four pregnant mice were injected intraperitoneally with either 2 × 106 PFU of vaccinia virus or medium alone on the 12th day of pregnancy. Females were then allowed to come to term, and the number of births for each individual mother was recorded. Neonatal survival was also assessed in the same experiment by comparing the number of births to the number of pups that survived until 3 weeks of age. The results of this experiment are shown in Table 1. A total of 22 mice were born to sham-infected mothers, 18 of which (83%) survived till weaning at 3 weeks of age. In contrast, no live births were observed among any of the vaccinia virus-infected mice. Unlike higher mammals in which the prenatal death of the fetus results in abortion, the premature termination of pregnancy in mice is often followed by reabsorption of fetal and placental tissues by the mother. The lack of any evidence of births in most of the infected females is consistent with the idea that most fetuses were reabsorbed prior to birth. We therefore conclude from these data that maternal infections during midpregnancy lead to complete fetal mortality in utero, a phenomenon most likely due to the active infection of the fetus by vaccinia virus.
TABLE 1.
Fetal mortality rates among vaccinia virus-infected pregnant mice
| Female | Infected
|
Uninfected
|
||
|---|---|---|---|---|
| No. of births | % Survivors | No. of births | % Survivors | |
| 1 | 0 | 0 | 6 | 67 |
| 2 | 3a | 0 | 6 | 83 |
| 3 | 0 | 0 | 4 | 100 |
| 4 | 0 | 0 | 6 | 83 |
Pups born dead.
Vaccinia virus initially infects placental cells lining maternal vessels in the trophospongium.
The above data clearly indicate that vaccinia virus infections initiate first within the placenta and later spread to the fetus. To determine what tissues within the placenta are the initial targets of the virus, mice were either infected intravenously with 2 × 106 PFU of VVWR or sham injected with medium alone on the 12th day of pregnancy. Placentas were harvested 4 days postinfection and subjected to in situ hybridization with a probe specific for a viral mRNA corresponding to the vaccinia virus early gene C11R. At 4 days postinfection vaccinia virus transcripts were detected exclusively in placental cells that line, or are adjacent to, large maternal blood vessels within the trophospongium layer of the placenta (Fig. 5B and C). The presence of virus on the maternal side of the placenta and the lack of any detectable viral RNA within labyrinthine trophoblasts at 4 days postinfection are entirely consistent with the observations made in Fig. 2, namely, that at this time point the virus is confined to the maternal side of the placenta and has not yet had time to spread appreciably to the fetus.
FIG. 5.
The initial sites of vaccinia virus replication in the placenta are in cells lining maternal blood spaces in the trophospongium. Females were sham infected or infected intravenously with 2 × 106 PFU of VVWR on the 12th day of pregnancy. Placentas were harvested 4 days later, embedded in paraffin, subjected to in situ hybridization with a vaccinia virus-specific probe for the viral early gene C11R, and counterstained with hematoxylin and eosin as described in Materials and Methods. (A) Representative uninfected placenta (magnification, ×5). (B) Infected placenta showing viral mRNA in cells lining maternal blood spaces within the trophospongium layer of the placenta (magnification, ×5). (C) Higher magnification (×20) of the area within the box outlined in panel B. Arrows indicate infected cells.
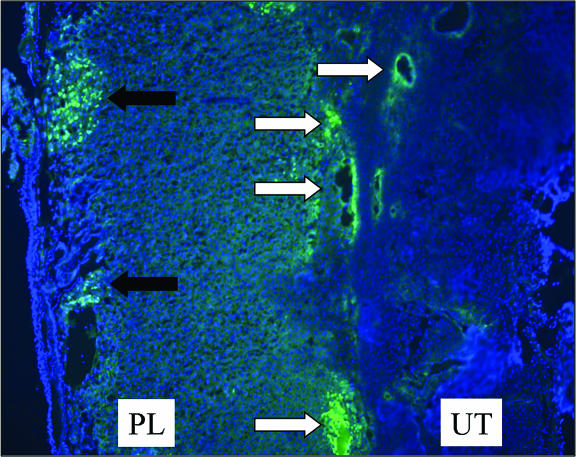
To identify sites of virus replication within the placenta at later time points, a vaccinia virus recombinant expressing the eGFP was used. This virus (eGFPVV) contains the eGFP gene, under the transcriptional control of a vaccinia virus 7.5K early-late promoter, inserted into the viral thymidine kinase locus. Thymidine kinase-negative vaccinia viruses are somewhat attenuated in vivo (1). To ensure that the placenta was adequately infected by eGFPVV, we increased the initial dose of virus accordingly and inoculated a pregnant female (day 12 postconception) intravenously with 107 PFU of eGFPVV. Placentas were then examined 7 days later on the 19th day of pregnancy (Fig. 6). At this time point, virally encoded eGFP was detected in cells lining blood vessels within the uterine decidua and the placental trophospongium as well as in labyrinthine cells on the fetal side of the placenta directly adjacent to the chorionic plate. Thus, by 7 days postinfection vaccinia virus has spread from the maternal blood spaces within the trophospongium throughout the placenta.
FIG. 6.
At late times postinfection virus is present in both the trophospongium and the placental labyrinth. A pregnant female was infected intravenously on day 12 postconception with 107 PFU of eGFPVV. Placentas were harvested 7 days later, fixed in neutral buffered formalin overnight, embedded, cryosectioned, and observed by fluorescence microscopy (×15) as described in Materials and Methods. White arrows indicate virus-infected cells within the uterine decidua and placental trophospongium. The black arrows identify clusters of infected cells within the placental labyrinth. UT, uterus; PL, placenta.
DISCUSSION
The ability of vaccinia virus to be transmitted vertically to the fetus makes pregnancy one the major contraindications for primary vaccination with this virus (3). Although in utero transmission of vaccinia virus in humans is relatively rare, the related orthopoxvirus variola virus, the causative agent of smallpox, is a significant fetal pathogen. Rao, who has collected the most extensive epidemiological data on the effects of smallpox during pregnancy, has reported that 60% of women infected after the fetus has become viable (between the 25th and 36th week of gestation) terminated their pregnancies prematurely. Over half of the infants born alive to infected mothers died within the first 2 weeks of life, although less than 10% exhibited clinical evidence of infection, i.e., dermal pox lesions on the face and trunk. Because most infant deaths occurred within 72 h of birth, these children may actually have been infected in utero but succumbed before exhibiting clear signs of smallpox (25). Although the obstetrical complications associated with both maternal smallpox and vaccinia virus vaccination in pregnant humans have been well known for over 3 decades, there have been very few attempts to examine the pathogenesis of either virus in pregnant hosts. The purpose of this study was to develop a small animal model with which to investigate the pathogenesis of poxvirus infections contracted during pregnancy. We chose VVWR because it has been previously reported to exhibit a strong tropism for murine ovaries, and we hypothesized that this tropism may also extend to other tissues within the female reproductive tract. Indeed, our results confirm that immunocompetent, nonpregnant mice readily clear VVWR from most internal organs with the exception of the ovaries and the uterus. The inability of the host's immune system to rapidly control virus replication within the reproductive tract may be a crucial factor in enabling vaccinia virus to infect the placenta and spread to the fetus.
The results in pregnant mice suggest that systemic infections with VVWR during mid- to late gestation can lead to the efficient colonization of the murine placenta and the eventual transfer of the virus to the fetus. These data complement earlier studies that have documented fetal infections in laboratory mice infected with ectromelia (mousepox) and in vaccinia virus-infected baboons and humans and a cowpox-infected African elephant (15, 22, 26, 31). One of the issues raised by the present data is that if VVWR is so efficient in colonizing the murine placenta and spreading to the fetus, why is the incidence of fetal infection among vaccinated pregnant humans so low? During the smallpox era most women would most likely have been vaccinated during childhood, prior to reaching sexual maturity. Revaccination during pregnancy would therefore occur in a host with some level of preexisting anti-vaccinia virus immunity. This immunity could very well limit the ability of the virus to effectively spread systemically within the host and colonize the placenta. In support of this hypothesis, previous studies failed to find any evidence of either placental pathology or fetal infection among women who were revaccinated with a vaccine strain of vaccinia virus during various stages of pregnancy (27, 28). If immunity to vaccinia virus is a crucial factor in protecting the fetus from infection, then, in today's society where few women of reproductive age have been previously vaccinated, one could expect the incidence of in utero infection to be significantly higher than previously reported. Other possibilities that may account for the low incidence of congenital vaccinia virus infections are that the vaccine strains used in the eradication program have a reduced tropism for the placenta or that the scarification process used to inoculate vaccinees fails to provoke a viremia of sufficient strength to allow the virus to effectively colonize the placenta. All of these possibilities are now testable by using this murine model and will undoubtedly be the focus of much-needed research in the future.
Although it is clear that infection of the placenta is a prerequisite step prior to the congenital transmission of cytomegalovirus, human immunodeficiency virus, and now vaccinia virus, we know very little about how these and other viruses actually cross the placenta (reviewed in reference 19). The placenta, which is composed of the decidua, the trophospongium, and the labyrinth, is a dynamic organ whose structure evolves considerably throughout the course of pregnancy. Embedded within the wall of the uterus are fetal and maternal cells that make up the decidua. The decidua is connected to the placental disk by the trophospongium, a multicell thick layer of fetal trophoblasts that separate the decidua from the labyrinth. The labyrinth makes up the bulk of the placenta and consists of a fine network of fetal capillaries, each of which is surrounded by a single layer of labyrinthine trophoblast cells. Most nutrient and gas exchange between the mother and fetus occurs within the labyrinth. The trophoblast cells that comprise the placental labyrinth are bathed directly in maternal blood and serve as the final barrier between the fetal and maternal blood supplies (7, 10). Our initial results showed that following a low-dose infection virus is not detectable by plaque assay in most fetuses within the first 4 days; therefore, it is unlikely that the virus has penetrated to the labyrinth trophoblasts at this stage, as infection of these cells would put the virus in close contact with the fetal blood supply. A histological examination of infected placentas by in situ hybridization for a vaccinia virus early mRNA confirmed that at these early time points vaccinia virus is confined to cells surrounding maternal blood spaces located between the outer surface of the uterine decidua and the placental trophospongium. However, later in infection we were able to detect evidence of a virally encoded protein (eGFP) within the placental labyrinth. Somewhat surprisingly, at 7 days postinfection we did not detect more substantial involvement of the internal layers of the trophospongium; virus-infected cells still seemed to be largely confined to areas surrounding maternal blood spaces. This may suggest that vaccinia virus reaches the labyrinth by the sequential infection of cells surrounding the maternal lacunae rather than through productive infection of trophospongium trophoblasts. Although primary human trophoblast cell lines derived from the labyrinth of term placenta can be productively infected with vaccinia virus in vitro, it is unclear if trophospongium trophoblasts are also susceptible to infection (24). A more thorough examination of the cellular pathways that vaccinia virus utilizes to gain access to the fetus from the murine placenta may very well provide insight into how this and other viruses are congenitally transmitted.
The successful elimination of congenitally transmitted viral infections will require the development of both vaccines and antiviral drugs that can be safely applied during pregnancy. In addition to providing us with a wealth of new data on the biology of poxvirus infections during pregnancy, the model that we have described here will also be very useful both in determining if any of the new, more attenuated smallpox vaccines currently under development have retained the ability to cause congenital infections and in the preclinical testing of antiviral chemotherapies.
Acknowledgments
We thank Annette Lord for excellent secretarial support and S. Harkins for help with the in situ hybridizations.
This work was supported by NIH grant AI-37186 to D.E.H.
Footnotes
Paper no. 5937-NP from the Scripps Research Institute.
REFERENCES
- 1.Buller, R. M., G. L. Smith, K. Cremer, A. L. Notkins, and B. Moss. 1985. Decreased virulence of recombinant vaccinia virus expression vectors is associated with a thymidine kinase-negative phenotype. Nature 317:813-815. [DOI] [PubMed] [Google Scholar]
- 2.Campbell, I. L., M. V. Hobbs, P. Kemper, and M. B. Oldstone. 1994. Cerebral expression of multiple cytokine genes in mice with lymphocytic choriomeningitis. J. Immunol. 152:716-723. [PubMed] [Google Scholar]
- 3.Centers for Disease Control. 1991. Vaccinia (smallpox) vaccine. Recommendations of the Immunization Practices Advisory Committee (ACIP). Morb. Mortal. Wkly. Rep. Recomm. Rep. 40:1-10. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2003. Women with smallpox vaccine exposure during pregnancy reported to the National Smallpox Vaccine in Pregnancy Registry-United States, 2003. Morb. Mortal. Wkly. Rep. 52:386-388. [PubMed] [Google Scholar]
- 5.Condit, R. C., and A. Motyczka. 1981. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology 113:224-241. [DOI] [PubMed] [Google Scholar]
- 6.Cono, J., C. G. Casey, and D. M. Bell. 2003. Smallpox vaccination and adverse reactions. Guidance for clinicians. Morb. Mortal. Wkly. Rep. Recomm. Rep. 52:1-28. [PubMed] [Google Scholar]
- 7.Cross, J. C. 2000. Genetic insights into trophoblast differentiation and placental morphogenesis. Semin. Cell Dev. Biol. 11:105-113. [DOI] [PubMed] [Google Scholar]
- 8.Fenner, F. 1988. Smallpox and its eradication. World Health Organization, Geneva, Switzerland.
- 9.Fenner, F., R. Wittek, and K. R. Dumbell. 1988. The orthopoxviruses. Academic Press, Inc., San Diego, Calif.
- 10.Georgiades, P., A. C. Ferguson-Smith, and G. J. Burton. 2002. Comparative developmental anatomy of the murine and human definitive placentae. Placenta 23:3-19. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein, J. A., J. M. Neff, J. M. Lane, and J. P. Koplan. 1975. Smallpox vaccination reactions, prophylaxis, and therapy of complications. Pediatrics 55:342-347. [PubMed] [Google Scholar]
- 12.Green, D. M., S. M. Reid, and K. Rhaney. 1966. Generalised vaccinia in the human foetus. Lancet i:1296-1298. [DOI] [PubMed] [Google Scholar]
- 13.Harley, J. D., and A. M. Gillespie. 1972. A complicated case of congenital vaccinia. Pediatrics 50:150-153. [PubMed] [Google Scholar]
- 14.Hassett, D. E., J. I. Lewis, X. Xing, L. DeLange, and R. C. Condit. 1997. Analysis of a temperature-sensitive vaccinia virus mutant in the viral mRNA capping enzyme isolated by clustered charge-to-alanine mutagenesis and transient dominant selection. Virology 238:391-409. [DOI] [PubMed] [Google Scholar]
- 15.Kalter, S. S., R. L. Heberling, M. Panigel, M. Brack, and P. J. Felsburg. 1973. Fetal infection of the baboon (Papio cynocephalus) with vaccinia virus. Proc. Soc. Exp. Biol. Med. 143:1022-1024. [DOI] [PubMed] [Google Scholar]
- 16.Karupiah, G., and R. V. Blanden. 1990. Anti-asialo-GM1 inhibits vaccinia virus infection of murine ovaries: asialo-GM1 as an additional virus receptor? Immunol. Cell Biol. 68:343-346. [DOI] [PubMed] [Google Scholar]
- 17.Karupiah, G., B. Coupar, I. Ramshaw, D. Boyle, R. Blanden, and M. Andrew. 1990. Vaccinia virus-mediated damage of murine ovaries and protection by virus-expressed interleukin-2. Immunol. Cell Biol. 68:325-333. [DOI] [PubMed] [Google Scholar]
- 18.Keck, J. G., C. J. Baldick, Jr., and B. Moss. 1990. Role of DNA replication in vaccinia virus gene expression: a naked template is required for transcription of three late trans-activator genes. Cell 61:801-809. [DOI] [PubMed] [Google Scholar]
- 19.Koi, H., J. Zhang, and S. Parry. 2001. The mechanisms of placental viral infection. Ann. N. Y. Acad. Sci. 943:148-156. [DOI] [PubMed] [Google Scholar]
- 20.Levine, M. M. 1974. Live-virus vaccines in pregnancy. Risks and recommendations. Lancet ii:34-38. [DOI] [PubMed] [Google Scholar]
- 21.Lynch, F. W. 1932. Dermatologic conditions of the fetus with particular reference to variola and vaccinia. Arch. Dermatol. Syphilol. 26:997-1019. [Google Scholar]
- 22.Mims, C. A. 1969. Effect on the fetus of maternal infection with lymphocytic choriomeningitis (LCM) virus. J. Infect. Dis. 120:582-597. [DOI] [PubMed] [Google Scholar]
- 23.Norbury, C. C., D. Malide, J. S. Gibbs, J. R. Bennink, and J. W. Yewdell. 2002. Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo. Nat. Immunol. 3:265-271. [DOI] [PubMed] [Google Scholar]
- 24.Norskov-Lauritsen, N., V. Zachar, P. M. Petersen, H. Hager, G. Aboagye-Mathiesen, and P. Ebbesen. 1992. In vitro infection of human placental trophoblast by wild-type vaccinia virus and recombinant virus expressing HIV envelope glycoprotein. Res. Virol. 143:321-328. [DOI] [PubMed] [Google Scholar]
- 25.Rao, A. R. 1972. Smallpox, 1st ed., vol. 1. The Kothari Book Depot, Bombay, India.
- 26.Ricken, K. H. 1975. Fetal vaccinia. Infection 3:209-212. [DOI] [PubMed] [Google Scholar]
- 27.Teodorescu, M., V. Topciu, L. Plavosin, N. Tudose, and E. Moldovan. 1975. The influence of smallpox revaccination upon the foetus. Virologie 26:137-139. [PubMed] [Google Scholar]
- 28.Topciu, V., V. Braga, L. Plavosin, S. Schiopu, E. Moldovan, and E. Lazar. 1976. The action of the vaccinia virus upon placenta and fetus in revaccinated pregnants. Zentbl. Bakteriol. Orig. B 161:551-556. [PubMed] [Google Scholar]
- 29.Vorst, E. J., and J. L. Gaillard. 1983. Vaccinial osteomyelitis in a case of generalized intrauterine virus infection. Pediatr. Pathol. 1:221-228. [DOI] [PubMed] [Google Scholar]
- 30.Wharton, M., R. A. Strikas, R. Harpaz, L. D. Rotz, B. Schwartz, C. G. Casey, M. L. Pearson, and L. J. Anderson. 2003. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). Morb. Mortal. Wkly. Rep. Recomm. Rep. 52:1-16. [PubMed] [Google Scholar]
- 31.Wisser, J., J. Pilaski, G. Strauss, H. Meyer, G. Burck, U. Truyen, M. Rudolph, and K. Frolich. 2001. Cowpox virus infection causing stillbirth in an Asian elephant (Elephas maximus). Vet. Rec. 149:244-246. [DOI] [PubMed] [Google Scholar]