Abstract
OBJECTIVES
To investigate the effects of estrogen, raloxifene, and levormeloxifene on the expression of Rho-kinase signaling molecules in urethral smooth muscle cells (USMCs).
METHODS
USMCs were isolated from female rats. Expression of calponin and estrogen receptors α (ERα was detected by immunofluorescence staining. Cells were treated with estrogen, raloxifene, or levormeloxifene at 0, 1, 10, and 100 nM for 48 h and then processed for western blotting with antibodies against RhoA, Rho kinase I and II (Rock-I and Rock-II), myosin light chain (MLC), phosphorylated MLC (p-MLC), and β-actin. Protein expression was quantitated by densitometry, followed by statistical analysis with β-actin as control.
RESULTS
USMCs expressed calponin and ERα. Treatment of USMCs with estrogen, raloxifene or levormeloxifene resulted in decreased expression of RhoA, Rock-I, Rock-II, and p-MLC in a dosage-dependent manner.
CONCLUSIONS
Estrogen, raloxifene, and levormeloxifene may affect urinary continence by inhibiting the expression of Rho-kinase signaling molecules.
Keywords: urethral smooth muscle cells, estrogen, raloxifene, levormeloxifene, SERMs, Rho-kinase signaling
INTRODUCTION
Urinary incontinence (UI) is a prevailing problem for middle-aged and older women 1. Urge UI (UUI), which accounts for approximately 45% of all UI cases, is defined as the involuntary loss of urine associated with a strong sensation to void. Stress UI (SUI), accounting for another 45% of all UI cases, is the involuntary loss of urine in the absence of a detrusor contraction. UUI is related to overactive detrusor function (motor urgency) and hypersensitivity (sensory urgency). SUI is thought to occur as a result of weakened muscles of the pelvic floor and the urethra, causing urine loss whenever there is an increased intra-abdominal pressure (e.g., coughing, sneezing, or laughing).
Hormonal changes due to aging and menopause are known to contribute to the development of UI. Earlier studies in which small-scale clinical trials were conducted support the commonly held notion that supplementation with exogenous estrogen may improve urinary function and decrease incontinence rate 2-4. However, more recently published long-term studies involving thousands of women indicated that estrogen supplementation (with or without progestin) actually increased the frequency of UI 5-7. Presently, the prescription of oral estrogen for UI treatment is not recommended, but the mechanism through which estrogen affects continence remains poorly understood.
In addition to UI, osteoporosis is another important health problem for postmenopausal women. Clinical trials have shown that estrogen supplementation reduced hip fracture risk by 39%; however, this beneficial effect was offset by adverse side effects such as breast cancer 8. Thus, synthetic compounds termed “selective estrogen receptor modulators” or “SERMs” were developed. Ideally, SERMs should have the beneficial effects of estrogen but with no or reduced risks. In 1997 and 1999, the Food and Drug Administration (FDA) approved raloxifene, a SERM, for the prevention and treatment of postmenopausal osteoporosis, respectively 9. Another SERM, levormeloxifene, was also developed for the prevention and treatment of osteoporosis. However, its phase III clinical trial was aborted due to the high incidence of adverse events, including an increased rate of UI 10.
In various types of smooth muscle cells, the Rho-kinase signaling pathway plays key roles in regulating contractility 11. Binding of agonist, such as epinephrine, to its receptor triggers the conversion of RhoA-GDP to RhoA-GTP. RhoA-GTP migrates to the cytoplasmic membrane, where it binds to and activates Rho-kinase (Rock-I and Rock-II). Rock phosphorylates and inactivates myosin light chain phosphatase (MLCP), allowing MLC to stay phosphorylated and consequently actin contracted. This Rho-kinase signaling pathway apparently also operates in the urethra because inhibition of Rock abolishes urethra smooth muscle tone 12.
Although numerous studies have examined the effect of estrogen and SERMs on blood vessels, vagina and uterus, there are few studies on the urinary bladder and none on the urethra. In addition, there appears to be a lack of information regarding the effect of SERMs on Rho-kinase signaling. Therefore the present study was designed to investigate the effect of estrogen and SERMs (raloxifene and levormeloxifene) on the expression of Rho-kinase signaling molecules in cultured rat urethra smooth muscle cells (USMCs).
MATERIALS AND METHODS
Rat urethral smooth muscle cells (USMCs) were isolated and cultured as previously described 13. Briefly, urethras were excised from 2-month-old female Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), de-epithelialized, washed 3 times in sterile phosphate-buffered saline (PBS), and cut into 2-3 mm3 segments. The segments were placed evenly onto a 100-mm cell culture dish (Falcon-Becton Dickinson Labware, Franklin Lakes, NJ). Ten minutes later, 10 ml of Dulbecco’s Modified Eagle Medium (DMEM) containing penicillin (100 units/ml), streptomycin (100 μg/ml), and 10% fetal bovine serum (FBS) was added to the dish, which was then kept undisturbed in a humidified 37°C incubator with 5% CO2. Five days later, tissue segments that had detached from the dish were removed, and the culture medium was replaced. This process was repeated after another five days of culture. When small islands of cells were noticeable, the cells were treated with trypsin and transferred to a fresh dish. Expansion of each cell strain was continued with change of medium every 3 days and passages approximately every 10 days. All cells used in the following experiments were from passages 3 or 4.
The smooth muscle identity of USMCs and ERα expression were verified by immunofluorescence staining as previously described 13. Briefly, USMCs (8×104 cells/well) were cultured on coverslips placed in 6-well plates with 3 ml/well of DMEM and 10% FBS. After 24 h, the cells were fixed in cold methanol for 5 min at 4 °C, permeabilized with 0.05% Triton X-100 for 5 min, and blocked with 5% normal horse serum in PBS for 1 h at room temperature. The cells were then incubated with anti-calponin or anti-ERα antibody (Abcam Inc., Cambridge, MA) for 1 h at room temperature. After washing with PBS three times, the cells were incubated with Texas red-conjugated anti-IgG antibody for another h at room temperature. After three washes with PBS, the cells were further stained with 4’,6-diamidino-2-phenylindole (DAPI, for nuclear staining) for 5 min and viewed under a fluorescence microscope (Zeiss, Thornwood, NY). Negative controls were prepared as above but without the primary antibodies.
For drug effect analysis, USMCs (6.5×105 cells) were seeded in 10-cm dishes with DMEM and 10% FBS. When the cells reached 80% confluence, the medium was changed to DMEM with 10% charcoal/dextran-treated FBS (Hyclone Inc., Logan, UT). After another 12 h of incubation, the cells were treated with estrogen, raloxifene (Sigma-Aldrich, St. Louis, MO), or levormeloxifene (Novo Nordisk, Bagsvaerd, Denmark) at the indicated concentrations for additional 24 h. The cells were then subjected to Western blot analysis as previously described 13. Briefly, the cells were homogenized in lysis buffer containing 1% IGEPAL CA-630, 0.5% sodium deoxycholate, 0.1% SDS, aprotinin (10 μg/ml), leupeptin (10 μg/ml), and phosphate-buffered saline. Cell lysates containing 20 μg of protein were electrophoresed in SDS–PAGE and then transferred to PVDF membrane (Millipore Corp., Bedford, MA). The membrane was incubated with a primary antibody overnight at 4°C, and then incubated with HRP-conjugated secondary antibody (Pierce Chemical Company, Rockford, IL) for 1 h at room temperature. Detection of reactive antigens was performed with ECL kit (Amersham Life Sciences Inc., Arlington Heights, IL), followed by exposure of the membrane to X-ray film. The resulting images were analyzed with ChemiImager 4000 (Alpha Innotech Corporation, San Leandro, CA) to determine the integrated density value (IDV) of each protein band normalized to the IDV of β-Actin. Re-probing of the cellular proteins with different antibodies was done by stripping the membrane in 62.5 mM Tris-HCl, pH 6.7, 2% SDS, and 10 mM 2-mercaptoethanol at 55 °C for 30 min. The stripped membrane was then incubated with the primary antibody and further processed as described above. Primary antibodies were anti-RhoA, anti-Rock-I, anti-Rock-II (BD Biosciences, CA), anti-MLC (Santa Cruz Biotechnology, Santa Cruz, CA), anti-p-MLC (phosphorylated Ser19, Cell Signaling Technology, Danvers, MA), and anti-β-actin (Sigma-Aldrich). All experiments were done in triplicates with cells isolated from 6 rats. All data are presented as the average of three independent experiments. Statistical analysis was performed with the Prism 4 program (GraphPad Software, Inc., San Diego, CA). The continuous data was compared using one-way analysis of variance. The Tukey-Kramer test was used for post-hoc comparisons. Statistical significance was set at P<0.05.
RESULTS
Smooth Muscle Identity and Estrogen Receptor Expression
The smooth muscle identity of rat USMCs was verified by immunofluorescence staining with anti-calponin antibody. The results showed that greater than 95% of cells stained positive for calponin (Fig. 1). Because estrogen receptor expression is required for cell response to estrogen and SERMs, ERα expression was in rat USMCs was also examined, and the results showed greater than 95% positivity (Fig. 1).
FIG. 1.
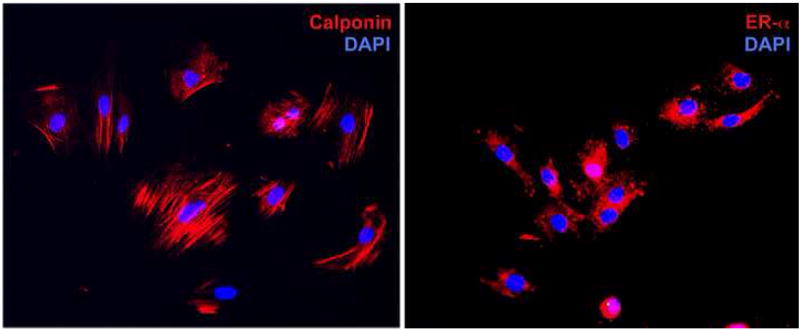
Localization of estrogen receptor and calponin in rat urethra smooth muscle cells (USMCs). UMSC at passage 4 were processed for immunofluorescence staining as described in Materials and Methods. Red fluorescence indicates expression of calponin or ERα. Blue fluorescence indicates cell nuclei (DAPI).
Effects of Estrogen
Estrogen at 1 nM had significantly (P<0.05) negative effects on the expression of RhoA and Rock-II when compared to control (0 nM) treatment (Fig. 2A & 2B). These effects were accompanied with a significantly (P<0.05) downregulated p-MLC expression when compared to control treatment (Fig. 2C). At 10 nM, the negative effects of estrogen on the expression of RhoA, Rock-I, and Rock-II were more pronounced (Fig. 2A & 2B), and these effects were accompanied by a further reduction of MLC phosphorylation (Fig. 2A & 2C). At 100 nM, the negative regulatory effects of estrogen were generally even stronger (Fig. 2).
FIG. 2.
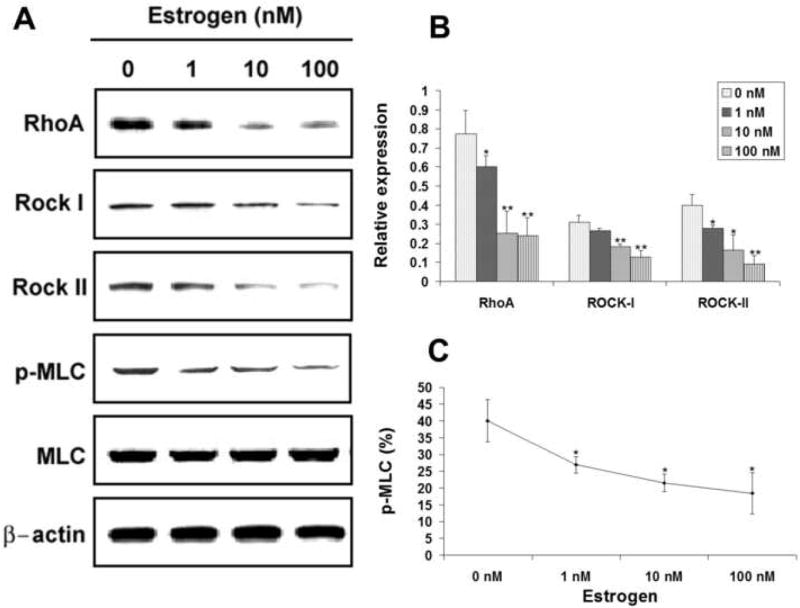
Effects of estrogen on the expression of RhoA, Rock-I, Rock-II, MLC, and p-MLC. USMCs were treated with estrogen at 0, 1, 10, and 100 nM and analyzed by western blotting for RhoA, Rock-I, Rock-II, MLC, and p-MLC. Three independent experiments were conducted for each drug. One representative graph of the three experiments is shown in panel A for each drug. The expression levels of RhoA, Rock-I, and Rock-II are displayed as relative expression against β-Actin expression (panel B). MLC phosphorylation level is the ratio (in percentage) between p-MLC and MLC expression levels (panel C). Asterisks denote significant difference in comparison with control (0 nM). *, P<0.05; **, P<0.01.
Effects of Raloxifene
Raloxifene at 1 nM had insignificant effects on the expression of RhoA, Rock-I and Rock-II (Fig. 3A & 3B). These negligible effects were accompanied with little change in the level of MLC phosphorylation (Fig. 3A & 3C). At 10 nM, raloxifene had significantly negative effects on RhoA, Rock-I and Rock-II expression (Fig. 3A & 3B), and these were accompanied by a reduction of MLC phosphorylation (Fig. 3A & 3C). Similar effects were observed with 100 nM of raloxifene (Fig. 3).
FIG. 3.
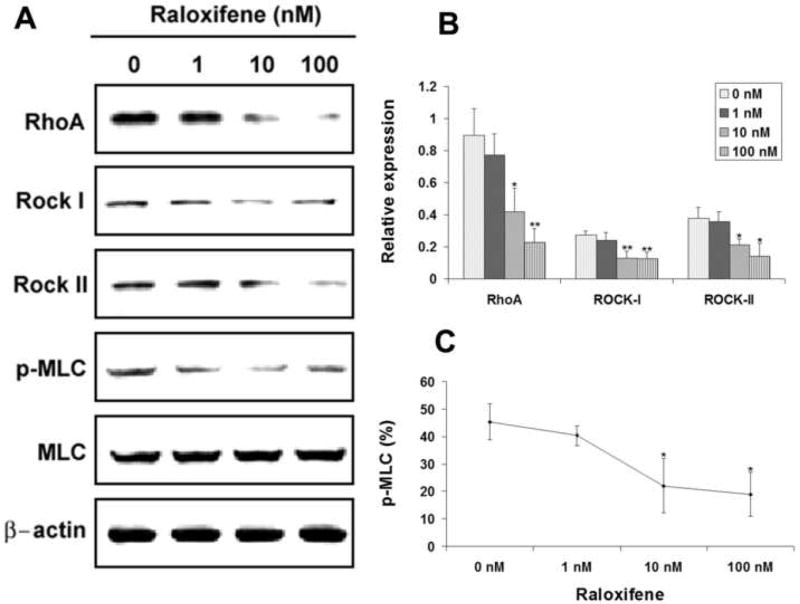
Effects of raloxifene on the expression of RhoA, Rock-I, Rock-II, MLC, and p-MLC. USMCs were treated with raloxifene at 0, 1, 10, and 100 nM and analyzed by western blotting for RhoA, Rock-I, Rock-II, MLC, and p-MLC. Three independent experiments were conducted for each drug. One representative graph of the three experiments is shown in panel A for each drug. The expression levels of RhoA, Rock-I, and Rock-II are displayed as relative expression against β-Actin expression (panel B). MLC phosphorylation level is the ratio (in percentage) between p-MLC and MLC expression levels (panel C). Asterisks denote significant difference in comparison with control (0 nM). *, P<0.05; **, P<0.01.
Effects of Levormeloxifene
All three Rho-kinase signaling molecules, RhoA, Rock-I, and Rock-II, were expressed at lower levels in USMCs treated with 1, 10 and 100 nM of levormeloxifene when compared to control (0 nM) treatment (Fig. 4A & 4B). Downregulation of these Rho-kinase signaling molecules was accompanied by a decrease of phosphorylation of their target protein MLC (Fig. 4A & 4C).
FIG. 4.
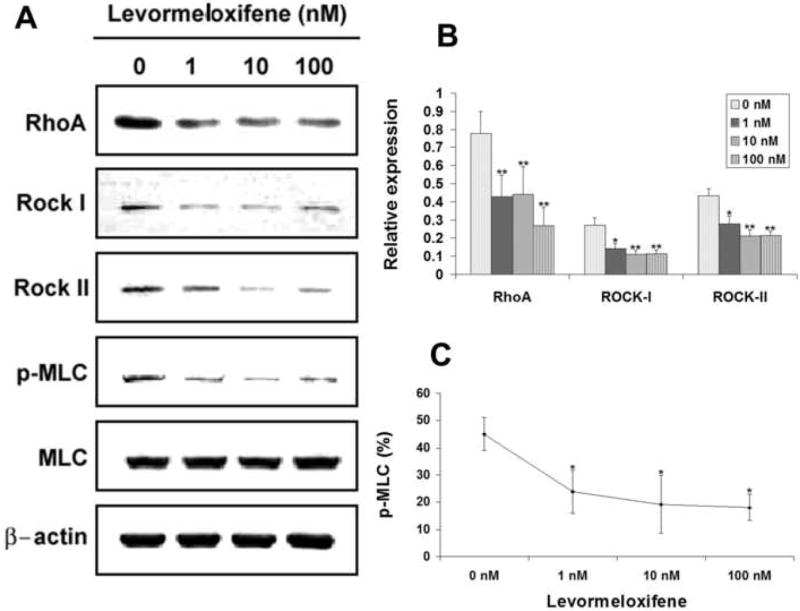
Effects of levormeloxifene on the expression of RhoA, Rock-I, Rock-II, MLC, and p-MLC. USMCs were treated with levormeloxifene at 0, 1, 10, and 100 nM and analyzed by western blotting for RhoA, Rock-I, Rock-II, MLC, and p-MLC. Three independent experiments were conducted for each drug. One representative graph of the three experiments is shown in panel A for each drug. The expression levels of RhoA, Rock-I, and Rock-II are displayed as relative expression against β-Actin expression (panel B). MLC phosphorylation level is the ratio (in percentage) between p-MLC and MLC expression levels (panel C). Asterisks denote significant difference in comparison with control (0 nM). *, P<0.05; **, P<0.01.
DISCUSSION
Hormonal changes due to aging and menopause are known to contribute to the development of UI. While it is generally agreed that physiologically produced estrogen is important for maintaining continence, it has been controversial whether exogenously applied estrogen is beneficial. From recent studies that involved larger numbers of patients and better controls than earlier studies, it is now generally agreed that estrogen supplements increase UI rate 5-7. In addition, certain SERMs, particularly levormeloxifene, have also been found to increase UI rate 5,14.
By using a postpartum ovariectomized rat model we have previously shown that estrogen and SERMs negatively impact urinary continence 15,16. However, how estrogen and SERMs influence the continence mechanism remains poorly understood. In the present study, we hypothesized that they might affect the expression of Rho-kinase signaling molecules in USMCs. In addition, because smooth muscle contraction is mainly regulated by the level of MLC phosphorylation 11, we also investigated the effect of each drug on the expression of p-MLC (as a ratio between p-MLC and MLC expression). In these experiments we chose concentrations of 1, 10, and 100 nM for each drug because this dosage range is most pharmacologically relevant and most frequently used in published studies. We also chose 24 h as the most optimal time point for drug treatment based on past studies 13,17. Cell lysates were then analyzed by western blotting to determine the expression and phosphorylation levels of above-mentioned Rho-kinase signaling molecules. It should be noted that RhoA is membrane bound when activated 11; thus, ideally RhoA expression should be separately examined with a cytosolic and membrane preparation. However, due to the need to probe all of the above-mentioned signaling molecules simultaneously, we chose to prepare cell lysates that contained both the cytosolic and membrane fractions.
The results show that different concentrations of each drug had different effects on the expression of Rho-kinase signaling molecules and p-MLC. In general, the effects were increasingly more negative as USMCs were treated with higher concentrations of each drug. These data are generally consistent with previously published studies. In the case with estrogen, it has been shown that estrogen concentration-dependently inhibits Rho-kinase mRNA expression in coronary VSMC 17. More recently estrogen has also been found to attenuate vascular smooth muscle contraction by inhibiting Rho-kinase pathway in a concentration-dependent manner 18. In addition, this study also showed that p-MLC expression was concentration-dependently inhibited by estrogen while MLC expression was unaffected. Thus, these two studies with vascular smooth muscle cells are in agreement with our study with USMCs. Furthermore, in another study Lin et al 19 showed that Rock-II was significantly increased in the bladder of ovariectomized rabbits and such increased expression was downregulated by estrogen supplementation. Thus, these findings in the bladder are consistent with our study in regard to the effect of estrogen on Rock-II expression.
We also found that higher concentrations (10 and 100 nM) of raloxifene had negative effects on the expression of Rho-signaling molecules and p-MLC in USMCs. This seems to contradict clinical findings as raloxifene has not been found to increase UI rate. However, it should be noted that short-term treatment (20 weeks) with raloxifene increased pelvic organ prolapse 20 and change of treatment from estrogen to raloxifene resulted in an increase of vaginal atrophy 21. In addition, short-term (3 weeks) treatment with raloxifene has been shown to increase voiding dysfunction rate in a SUI rat model16. Thus, species difference is a probable explanation for the disagreement between clinical and basic science data (obtained with animal models, tissues, or cells).
In regard to levormeloxifene, our data are in line with expectations. That is, its negative effects on the expression of Rho-kinase signaling molecules and p-MLC are consistent with its negative effects on urinary continence. Thus, our overall data show that estrogen and SERMs may affect urinary continence by downregulating Rho-kinase signaling molecules and p-MLC. At present it is not known how estrogen and SERMs downregulate Rho-kinase expression. Based on the study of Hiroki et al that showed estrogen-induced downregulation of Rho kinase mRNA expression 17, it appears that estrogen and SERMs may target Rho-kinase gene promoter via estrogen receptor binding sites.
While the present study provides data suggesting that estrogen and SERMs may affect urinary continence by downregulating the Rho-kinase signaling pathway, it lacks direct evidence linking such downregulation with urethral smooth muscle contractility. It should also be pointed out that estrogen and SERMs can affect urinary continence by downregulating other signaling pathways in urethral smooth muscle, for example, adrenergic signaling 13. Thus, the present study is only one of many steps that could lead us to a better understanding of how estrogen and SERMs affect urinary continence.
CONCLUSIONS
Estrogen, raloxifene, and levormeloxifene may attenuate urinary continence by inhibiting the expression of RhoA, Rock-I, Rock-II, and p-MLC.
Acknowledgments
This research was funded by NIH/NIDDK grants P50-DK64538 and R01-DK069655.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gibbs CF, Johnson TM, 2nd, Ouslander JG. Office management of geriatric urinary incontinence. Am J Med. 2007;120:211–220. doi: 10.1016/j.amjmed.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 2.Faber P, Heidenreich J. Treatment of stress incontinence with estrogen in postmenopausal women. Urol Int. 1977;32:221–223. doi: 10.1159/000280134. [DOI] [PubMed] [Google Scholar]
- 3.Judge TG. The use of quinestradol in elderly incontinent women, a preliminary report. Gerontol Clin (Basel) 1969;11:159–164. doi: 10.1159/000245230. [DOI] [PubMed] [Google Scholar]
- 4.Siegel I, Zelinger BB, Kanter AE. Estrogen therapy for urogenital conditions in the aged. Am J Obstet Gynecol. 1962;84:505–507. doi: 10.1016/s0002-9378(16)35699-x. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein SR, Johnson S, Watts NB, et al. Incidence of urinary incontinence in postmenopausal women treated with raloxifene or estrogen. Menopause. 2005;12:160–164. doi: 10.1097/00042192-200512020-00010. [DOI] [PubMed] [Google Scholar]
- 6.Hendrix SL, Cochrane BB, Nygaard IE, et al. Effects of estrogen with and without progestin on urinary incontinence. Jama. 2005;293:935–948. doi: 10.1001/jama.293.8.935. [DOI] [PubMed] [Google Scholar]
- 7.Steinauer JE, Waetjen LE, Vittinghoff E, et al. Postmenopausal hormone therapy: does it cause incontinence? Obstet Gynecol. 2005;106:940–945. doi: 10.1097/01.AOG.0000180394.08406.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulder JE, Kolatkar NS, LeBoff MS. Drug insight: Existing and emerging therapies for osteoporosis. Nat Clin Pract Endocrinol Metab. 2006;2:670–680. doi: 10.1038/ncpendmet0325. [DOI] [PubMed] [Google Scholar]
- 9.Draper MW. An update on raloxifene. Int J Gynecol Cancer. 2006;16(Suppl 2):502–503. doi: 10.1111/j.1525-1438.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein SR, Nanavati N. Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol. 2002;187:521–527. doi: 10.1067/mob.2002.123938. [DOI] [PubMed] [Google Scholar]
- 11.Peters SL, Schmidt M, Michel MC. Rho kinase: a target for treating urinary bladder dysfunction? Trends Pharmacol Sci. 2006;27:492–497. doi: 10.1016/j.tips.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Malmqvist U, Hedlund P, Sward K, et al. Female pig urethral tone is dependent on Rho guanosine triphosphatases and Rho-associated kinase. J Urol. 2004;171:1955–1958. doi: 10.1097/01.ju.0000121601.95857.5a. [DOI] [PubMed] [Google Scholar]
- 13.Banie L, Lin G, Ning H, et al. Effects of estrogen, raloxifene and levormeloxifene on alpha1A-adrenergic receptor expression. J Urol. 2008;180:2241–2246. doi: 10.1016/j.juro.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Waetjen LE, Brown JS, Modelska K, et al. Effect of raloxifene on urinary incontinence: a randomized controlled trial. Obstet Gynecol. 2004;103:261–266. doi: 10.1097/01.AOG.0000109429.67671.d1. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi N, Bella AJ, Wang G, et al. Effect of extended-term estrogen on voiding in a postpartum ovariectomized rat model. Can Urol Assoc J. 2007;1:256–263. doi: 10.5489/cuaj.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tantiwongse K, Fandel TM, Wang G, et al. The potential of hormones and selective oestrogen receptor modulators in preventing voiding dysfunction in rats. BJU Int. 2008;102:242–246. doi: 10.1111/j.1464-410X.2008.07582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiroki J, Shimokawa H, Mukai Y, et al. Divergent effects of estrogen and nicotine on Rho-kinase expression in human coronary vascular smooth muscle cells. Biochem Biophys Res Commun. 2005;326:154–159. doi: 10.1016/j.bbrc.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Yang E, Jeon SB, Baek I, et al. 17beta-estradiol attenuates vascular contraction through inhibition of RhoA/Rho kinase pathway. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:35–44. doi: 10.1007/s00210-009-0408-x. [DOI] [PubMed] [Google Scholar]
- 19.Lin AD, Levin RM, Kogan BA, et al. Alteration of contractile and regulatory proteins in estrogen-induced hypertrophy of female rabbit bladder. Urology. 2006;68:1139–1143. doi: 10.1016/j.urology.2006.08.1094. [DOI] [PubMed] [Google Scholar]
- 20.Vardy MD, Lindsay R, Scotti RJ, et al. Short-term urogenital effects of raloxifene, tamoxifen, and estrogen. Am J Obstet Gynecol. 2003;189:81–88. doi: 10.1067/mob.2003.374. [DOI] [PubMed] [Google Scholar]
- 21.Checa MA, Garrido A, Prat M, et al. A comparison of raloxifene and calcium plus vitamin D on vaginal atrophy after discontinuation of long-standing postmenopausal hormone therapy in osteoporotic women. A randomized, masked-evaluator, one-year, prospective study. Maturitas. 2005;52:70–77. doi: 10.1016/j.maturitas.2004.12.006. [DOI] [PubMed] [Google Scholar]


