Abstract
Dendritic cell (DC) migration from the site of infection to the site of T-cell priming is a crucial event in the generation of antiviral T-cell responses. Here we present to our knowledge the first functional evidence that human cytomegalovirus (HCMV) blocks the migration of infected monocyte-derived DCs toward lymphoid chemokines CCL19 and CCL21. DC migration is blocked by viral impairment of the chemokine receptor switch at the level of the expression of CCR7 molecules. The inhibition occurs with immediate-early-early kinetics, and viral interference with NF-κB signaling is likely to be at least partially responsible for the lack of CCR7 expression. DCs which migrate from the infected cultures are HCMV antigen negative, and consequently they do not stimulate HCMV-specific CD8+ T cells, while CD4+-T-cell activation is not impaired. Although CD8+ T cells can also be activated by alternative antigen presentation mechanisms, the spatial segregation of naive T cells and infected DCs seems a potent mechanism of delaying the generation of primary CD8+-T-cell responses and aiding early viral spread.
Dendritic cells (DCs), the most potent professional antigen (Ag)-presenting cells, survey peripheral tissues where they take up Ags. Ag uptake and activation signals induce DCs to migrate to secondary lymphoid organs and interact with Ag-specific naive T cells (5). This migration is an essential part of the initiation of an immune response. Activation of virus-specific cytotoxic T lymphocytes in the draining lymph nodes in a localized peripheral viral infection occurs soon after infection (6 to 8 h) and is due to the swift recruitment of DC into the lymph nodes (26).
DC migration is regulated by changes in the expression of chemokine receptors on the surface of DCs, a process often referred to as the chemokine receptor switch. The molecular events of this chemokine receptor switch involve the downregulation of the proinflammatory-type chemokine receptors, such as CCR5, and the increase of the expression of the lymphoid-type chemokine receptors, such as CCR7. Lymph nodes of CCR7 knockout mice are devoid of T cells, and DCs from these mice are unable to mount primary T-cell responses in vivo (14). CCR7 expression during DC maturation enables DCs to respond to the lymphoid chemokines, CCL19 (ELC or MIP-3β) and CCL21 (SLC, 6Ckine, or TCA-4), which are constitutively produced by secondary lymphoid tissues. CCL19 and CCL21 are extremely potent and selective chemoattractants of mature DCs (9, 12, 20) with overlapping functions (3). The expression of CCR7 can be induced on DCs by treatment with alpha/beta interferon (IFN-α/β), interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α) (30), or bacterial cell wall proteins (19) or upon interaction with apoptotic tumor cells (17). Augmented expression of CCR7 results in enhanced DC migratory responses to CCL21 both in vitro and in vivo (38). The common feature of all these signals is the activation of the transcription factor NF-κB, which is a key event in DC maturation (4). The most studied pathway enabling DC to migrate is the stimulation via TNF receptors (TNFRs). DC migration is suppressed in mice lacking TNFR-p75 (33, 34). Migration is also impaired in TNF-α-deficient or anti-TNF-α antibody-treated mice (11).
Human cytomegalovirus (HCMV) is a significant cause of morbidity and mortality in immunosuppressed hosts. HCMV has the capacity to persist asymptomatically in healthy individuals, indicating that it has developed various strategies to avoid elimination by the immune system. We have previously shown that HCMV can infect human DCs and inhibits the expression of major histocompatibility complex class I and class II and costimulatory molecules (CD80, CD86, CD40) in infected, but not in bystander, DCs (25). Infected DCs maintain an immature-like phenotype even upon stimulation with maturation agents (25).
Under certain circumstances CCR7 can be upregulated on immature DCs, and these DCs are able to migrate into lymph nodes (15, 24), where, due to their phenotype, they play a crucial role in the induction or maintenance of peripheral tolerance. This study was initiated to investigate whether HCMV-infected DCs are able to migrate toward gradients of lymphoid chemokines upon infection or after further stimulation. Our findings indicate that HCMV-infected DCs in fact do not respond to CCL19 or CCL21 gradients even when stimulated with lipopolysaccharide (LPS). Moreover, migrated DCs, which represent uninfected, bystander DCs, fail to stimulate HCMV-specific CD8+ T cells.
MATERIALS AND METHODS
Cells.
Peripheral blood mononuclear cells (PBMC) were isolated from HCMV-seropositive and -seronegative healthy laboratory volunteers. Ethical approval was granted for the study by the Bro Taf Local Ethical Committee. Informed consent was obtained from the volunteers according to the Declaration of Helsinki. DCs were generated as previously described (25, 28). In brief, plastic-adherent or CD14+ cells, the latter isolated by a MACS monocyte isolation kit to obtain untouched monocytes (Miltenyi Biotech) according to the manufacturer's instructions, were grown in the presence of granulocyte-macrophage colony-stimulating factors (50 ng/ml; Leucomax, Novartis Pharmaceuticals, East Hanover, N.J.) and IL-4 (500 U/ml; BD Pharmingen, San Diego, Calif.) for 5 to 7 days. Nonadherent and loosely adherent DCs were collected. More than 90% of these cells were of DC phenotype (CD1a+ HLA-DR+ CD14− CD80+) following gating on forward and side scatter parameters to exclude lymphocytes. DC maturation was induced by adding LPS (1 μg/ml; Sigma, St. Louis, Mo.), TNF-α (50 ng/ml; R&D Systems, Minneapolis, Minn.), and IFN-γ (100 ng/ml; PeproTech, Rocky Hill, N.J.) to the culture medium. Human foreskin fibroblasts (HFFs) were grown as described previously (25, 31).
Virus.
DCs were infected with an endothelial-cell-grown HCMV strain, TB40/E (kindly provided by Christian Sinzger, Tuebingen, Germany) (27), at a multiplicity of infection (MOI) of 3 to 10. The virus stock was prepared by propagating HCMV in HFFs followed by enrichment by ultracentrifugation (80,000 × g for 1 h in a Beckman L8-M ultracentrifuge), which removed soluble factors present in the supernatant of infected fibroblasts. Mock-infected cells were given the same volume of RPMI medium without the virus.
Flow cytometry.
DCs were surface labeled with antibodies specific for HLA-DR (CyChrome conjugated; BD Pharmingen), CCR5 (phycoerythrin [PE] conjugated; Serotec, Oxford, United Kingdom), CCR7 (BD Pharmingen), and TNFR-p55 and TNFR-p75 (both PE conjugated; Caltag Laboratories, Burlingame, Calif.) and with a PE-conjugated anti-mouse immunoglobulin G (Serotec). T cells were surface labeled with antibodies specific for CD8 or CD4 (both CyChrome conjugated; BD Pharmingen). Intracellular staining for IFN-γ or for HCMV pp52 was carried out following fixing either DCs or T cells with 4% paraformaldehyde and permeabilizing them with 0.025% Triton X-100 (20 min each step at room temperature). Nonspecific binding was blocked with 2% mouse serum for 10 min followed by adding fluorescein isothiocyanate (FITC)-conjugated HCMV pp52-specific (delayed early DNA-binding protein, clone CCH2; Dako, Carpinteria, Calif.) or IFN-γ-specific (BD Pharmingen) antibodies for 1 h at 37°C. The cells were analyzed on a FACScalibur (BD Biosciences) flow cytometer using CellQuest software (version 3.1).
DC migration assay.
DC migration was performed in 24-well polycarbonate transwell culture chambers (Costar, Corning, N.Y.) with 6.5-mm diameter and 5-μm pore size. DCs (0.5 × 106 to 2 × 106) were placed in 100 μl of RPMI medium supplemented with 10% human AB serum into the inserts of transwells. The bottom chamber contained 600 μl of medium supplemented as described above and also containing 100 to 330 ng of CCL19/ml, except for the control wells. The wells were incubated for 3 to 5 h at 37°C, when cells were collected from the lower and upper chambers and used in the experiments as indicated.
T-cell stimulation and IFN-γ production assay.
Nonadherent PBMC of seropositive donors were stimulated with a mixture of the following HCMV peptides at 5 μg/ml each for 7 days: HLA-A2-restricted peptides IE1 (YVLEETSVML) and pp65 (ARNLVPMVATVQGON) and HLA-B7-restricted peptides IE1 (FCRVLCCYVL) and pp65 (TPRVTGGGAM). T cells and peptides were placed at 2 × 106 PBMC/ml in RPMI medium plus 10% human AB serum in 12-well trays (2 ml/well) or in upright tissue culture flasks (10 ml). T-cell stimulation was carried out 7 days later by mixing DC with the prestimulated T cells at a 1:10 ratio, at 1 × 105 to 5 × 105 T cells per group overnight. Golgi-Plug (1 μl/ml; Pharmingen) was added to each group 1 h after the initiation of DC-T-cell cultures. Negative controls contained no DCs or mock-infected DCs, while positive controls were stimulated with phorbol myristate acetate (10 ng/ml) and ionomycin (500 ng/ml). IFN-γ production by CD3+ CD8+ and CD3+ CD8− cells was measured by intracellular cytokine staining as described earlier. In the control experiments, allogeneic PBMC were added to DCs for 5 days at a 10:1 ratio in 96-well tissue culture plates at 105 PBMC/well (not shown).
Preparation of nuclear extracts and electrophoretic mobility shift assay.
Nuclear extracts were generated as described previously (8). Cell treatments were terminated by adding 5 ml of ice-cold phosphate-buffered saline. Each sample was centrifuged at 163 × g in a swing-out rotor and then resuspended in 1 ml of buffer A (10 mM HEPES [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 10 mM phenylmethylsulfonyl fluoride [PMSF]) and centrifuged at 12,000 × g for 10 min in a benchtop microcentrifuge at 4°C. Cells were then lysed by addition to 20 μl of buffer A containing 0.1% Nonidet P-40 and left on ice for 10 min, after which time they were centrifuged at 12,000 × g for 10 min. The nuclear extract was prepared by adding 15 μl of buffer C (20 mM HEPES [pH 7.9], 420 mM NaCl, 1.5 mM MgCl2, 25% glycerol, 0.5 mM PMSF) to the supernatant. The mixture was left on ice for 15 min. It was then centrifuged at 12,000 × g for 10 min, and the supernatant was added to 75 μl of buffer D (10 mM HEPES [pH 7.9], 50 mM KCl, 0.2 mM EDTA, 20% glycerol, 0.5 mM PMSF). The protein in these crude extracts was determined by the Bradford method. The extracts were assayed immediately for NF-κB activity or stored at −70°C until further use. Equivalent amounts of protein from nuclear extracts were incubated with approximately 10,000 cpm of a 32P-labeled oligonucleotide containing the consensus sequence for NF-κB in binding buffer (40% glycerol, 1 mM EDTA, 10 mM Tris [pH 7.5], 100 mM NaCl, nuclease-free bovine serum albumin [0.1 mg/ml], 50 mM dithiothreitol) and 2 μg of poly(dI-dC) at room temperature for 30 min, as described previously (8). T4 polynucleotide kinase and the 22-bp oligonucleotide containing the NF-κB consensus sequence (underlined) (5′-AGTTGAGGGGACTTTCCCAGGC-3′) were from Promega (Madison, Wis.). [γ-32P]ATP was from Amersham International (Amersham, Buckinghamshire, United Kingdom). Incubated proteins were subjected to electrophoresis on native 4% polyacrylamide gels that were subsequently dried and autoradiographed at −70°C overnight.
RESULTS
HCMV infection inhibits DC migration toward CCL19 and CCL21.
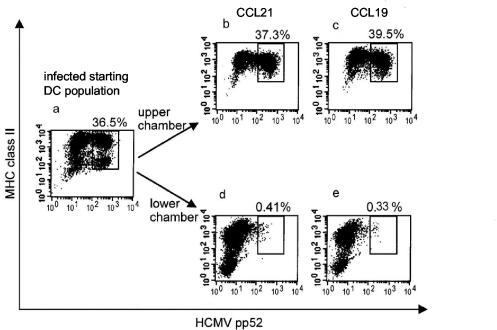
The ability of maturing DCs, which carry processed Ag, to migrate to secondary lymphoid organs where naive T cells reside is a crucial step in the generation of primary T-cell responses. Migration of maturing DCs is controlled by lymphoid chemokines CCL19 and CCL21, which are produced by secondary lymphoid organs. Viruses that infect DCs may interfere with the normal pattern of DC migration. We studied whether this is the case when HCMV infects DCs. Transwell experiments were carried out with CCL19 or CCL21, as chemoattractants, placed in the lower parts of the transwells. DCs, either infected with an endothelial-cell-grown strain of HCMV (TB40/E) (27) for 48 h or mock infected, were placed in the upper parts of migration chambers. The TB40/E strain of HCMV infected 30 to 70% of DCs in these experiments, as assessed by intracellular staining with an antibody specific for the HCMV pp52 Ag. Thus, by detecting the presence of HCMV-infected cells in the premigrated (Fig. 1a) and postmigrated (Fig. 1b to e) populations of DCs following infection with TB40/E strain at an MOI of 10, we were able to compare the migratory capacity of infected and uninfected DCs by flow cytometry. As, regardless of infection, only a few cells migrated under these conditions (data not shown), additional stimulation was required. Day 1 infected DCs were treated with LPS in the presence of IFN-γ and TNF-α for 24 h. We found that HCMV Ag-positive DCs were selectively prevented from migrating toward CCL19 or CCL21. Infected DCs remained in the upper chambers of the migration wells (Fig. 1b and c), and those which migrated toward CCL21 (Fig. 1d) or CCL19 (Fig. 1e) were uninfected (i.e., HCMV Ag negative [<0.41%]). DCs before migration (Fig. 1a) seemed to express HLA class II at a high or medium level, respectively, while HLA class II expression on cells collected from the migration chambers was more homogenous (Fig. 1b and c or d and e) and lower than that before migration. The presence of four subgroups of DCs before migration was not observed from all donors. The reasons for the changes in HLA class II expression on DCs in the migration chambers are not known. The level of HLA class II expression on migrated DCs was similar to or higher than that on nonmigrated DCs, as seen when comparing Fig. 1d and b (mean fluorescence intensity [MFI], 1,210 versus 1,272) and Fig. 1e and c (MFI, 1,961 versus 1,761); furthermore, HLA class II expression on HCMV Ag-positive cells was lower than on Ag-negative ones (Fig. 1b [MFI, 905 versus 1,272] and c [MFI, 1,180 versus 1,761]), confirming previous observations.
FIG. 1.
HCMV Ag-positive DCs do not migrate toward lymphoid chemokines. DCs, infected with HCMV TB40/E at an MOI of 10 for 24 h and then matured with LPS/IFN-γ/TNF-α for a further 24 h, were placed into transwell migration chambers which contained a 200-ng/ml concentration of either CCL21 (b and d) or CCL19 (c and e) in the lower chambers. The experiment was run for 4 h, and samples of the input DCs (a), DCs which did not migrate (b and c), and those which migrated into the lower chambers (d and e) were analyzed for the rate of HCMV infection by immunofluorescent staining as described in Materials and Methods. The dot plots show the intensity of binding of HCMV pp52-FITC antibody on the x axes and of the HLA-DR-CyChrome antibody on the y axes by fixed and permeabilized cells. The numbers express the frequency of HCMV pp52 Ag-positive cells, showing that migration was selective for DCs that remained uninfected (HCMV pp52 Ag-negative DCs). The results are representative of five experiments.
The proportion of HCMV-infected DCs was about 100-fold lower in the migrated population of DCs than that in the starting population of DCs. The few DCs which were HCMV pp52 positive in the migrated pool had only very low levels of pp52 expression, indicating that successful inhibition of DC migration correlates with a high level of viral replication in DCs.
Migrated DCs do not stimulate HCMV-specific T-cell responses.
To determine whether migrated DCs, containing only a low proportion (<0.41%) of HCMV Ag-positive DCs (Fig. 1d and e), are able to stimulate HCMV-specific memory CD8+-T-cell responses, DCs from the upper or lower chambers of transwells were mixed with autologous T cells from seropositive donors. HCMV-specific T cells were enriched in the responder population of this experiment by stimulating nonadherent, HLA-A2, B7 PBMCs with HLA-A2- and B7-restricted HCMV IE1 and pp65 peptides for 7 days. T-cell activation was measured by flow cytometric detection of IFN-γ-producing CD8+ T cells (Fig. 2a to d, upper-right quadrants). The background level of CD8+-T-cell activation in the absence of DCs was low (0.6% [Fig. 2a]), while mock-infected DCs nonspecifically activated a small proportion of CD8+ T cells (2.7% [Fig. 2b]). Following HCMV infection of DCs, migrated cells contained only a low proportion of DCs positive for HCMV pp52 (<0.4%; not shown), and migrated DCs were only able to stimulate CD8+ T cells at levels (3.1% [Fig. 2c]) comparable to those with mock-infected DCs (2.7% [Fig. 2b]). In contrast to CD8+ T cells, an ∼20-fold increase was observed in the proportion of CD8− T cells, representing mainly CD4+ T cells (>90% of CD3+ CD8− cells are CD3+ CD4+; not shown), which became activated by the migrated DCs (compare Fig. 2c and b, upper left quadrants [2.0 versus 0.1%]). This result indicates that uptake of viral Ag in the absence of viral replication by DCs does not impair the migratory and Ag-presenting capacity of these DCs, and consequently, the generation of CD4+-T-cell responses is not impaired. When mixed directly with T cells, infected DCs (from the upper chambers; 41.4% positive for HCMV pp52) supported strong IFN-γ production by both CD8− and CD8+ T cells from an HCMV-seropositive individual (Fig. 2d). Statistical analysis of the results from triplicate samples (Fig. 2e and f) reveals that the slight increase in the proportion of activated CD8+ T cells when the migrated population of HCMV-encountered DCs were stimulators (Fig. 2e, bar C) did not reach statistical significance compared to the proportion of activated CD8+ T cells stimulated with mock-infected DCs (Fig. 2e, bar B). Coculture of HCMV-specific T cells with infected DCs from the upper chambers resulted in high levels of T-cell activation (bars D of Fig. 2e and f). In contrast to CD8+ T cells, a significant proportion of CD8− T cells was activated by migrated, HCMV-encountered DCs (Fig. 2f, bar C versus bar B). The impaired ability of migrated DCs, after being exposed to HCMV, to stimulate HCMV-specific CD8+ T cells above background level was Ag specific, as DCs that migrated from either HCMV-infected or mock-infected DC populations were equally able to stimulate allogeneic T-cell responses (not shown).
FIG. 2.
DCs, which migrate toward CCL19, do not stimulate HCMV-specific T cells. HCMV-specific T cells were enriched by incubating the nonadherent fraction of PBMC from an HCMV-seropositive donor with A2- and B7-restricted pp65 and IE1 peptides for 7 days. These T cells were then restimulated with the following: medium only (a); mock-infected, nonmigrated DC from the upper chambers of transwells (b); migrated DC from the lower chambers of HCMV-infected input DC (c); and nonmigrated DC from the upper chambers of HCMV-infected input DC (d). DC:T ratio was 1:10. T-cell activation following stimulation with phorbol myristate acetate and CaI is not shown (87.9% ± 1.4% for CD8+ T cells and 33.4% ± 3.2% for CD8− T cells). The proportion of IFN-γ-producing CD8+ (upper right quadrants) and CD8− (upper left quadrants) T cells from a representative experiment of three repeated ones are shown, as detected by flow cytometry. (e and f) Summary of results and statistical analysis of CD8+-T-cell (e) and CD8−-T-cell (f) stimulation from triplicate wells. T cells were stimulated as described above with medium only (bars A), mock-infected nonmigrated DC (bars B), migrated DCs from HCMV-infected input cells (bars C), and nonmigrated DCs of HCMV-infected input cells (bars D). Statistical analysis was carried out by applying Student's t test. Significant differences (P < 0.01) are indicated (*). NS, not significant; error bars, standard deviations.
HCMV-infected DCs do not express CCR7.
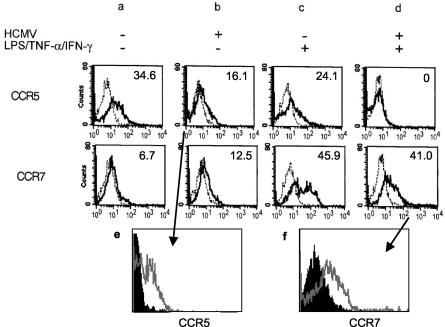
Migration of DCs to lymph nodes requires the expression of CCR7. CCR7 is the receptor for lymphoid chemokines CCL19 and CCL21, which are produced constitutively by secondary lymphoid organs. Upon receiving stimulatory signals, DCs downregulate inflammatory chemokine receptors such as CCR5 and upregulate CCR7, the process known as chemokine receptor switch. As this is a crucial step in enabling DCs to migrate to lymph nodes, we analyzed the effect of HCMV infection on DC chemokine receptor switch (Fig. 3). CCR5 expression on immature DCs, infected with HCMV, was compared to that on mock-infected DCs (Fig. 3, first row). We found a medium level of CCR5 surface expression on immature DCs (34.6% [Fig. 3a]), which was downregulated upon HCMV infection (16.1% [Fig. 3b]) and also upon stimulation with LPS/TNF-α/IFN-γ (24.1% [Fig. 3c]). LPS/TNF-α/IFN-γ and HCMV together acted synergistically, resulting in the complete downregulation of CCR5 (0% [Fig. 3d]). Thus, HCMV infection seems to provide a signal for DCs to undergo the first part of the chemokine receptor switch, which would enable infected DCs in vivo to leave the site of infection. The second step of the receptor switch, the upregulation of CCR7 molecules, was studied in a similar experiment (Fig. 3, second row). CCR7 expression was low on immature DCs (6.7% [Fig. 3a]), while LPS/TNF-α/IFN-γ treatment was highly efficient in inducing CCR7 expression (45.9% [Fig. 3c]). However, CCR7 molecules were only slightly upregulated by HCMV (12.5% [Fig. 3b]). When HCMV-infected DCs were stimulated with LPS/TNF-α/IFN-γ, CCR7 expression was lower on HCMV-infected (41%; MFI, 57 [Fig. 3d]) than on mock-infected, stimulated (45.9%; MFI, 111 [Fig. 3c]) DCs. As not all DCs become infected with HCMV, two-color flow cytometric analysis was carried out to analyze the level of CCR5 and CCR7 expression separately on pp52 Ag-positive and Ag-negative DCs (Fig. 3e and f) from the HCMV-infected DC cultures. Following infection, CCR5 expression was low on both HCMV Ag-positive and Ag-negative cells even without LPS treatment (Fig. 3e), although the downregulation was more efficient on Ag-positive DCs (black histogram). More importantly, CCR7 upregulation following LPS treatment seemed to be the property of bystander, HCMV Ag-negative DCs (Fig. 3f, gray line). These experiments reveal that the second part of the chemokine receptor switch, the upregulation of surface CCR7 molecules, is inhibited in HCMV-infected, Ag-positive DCs. This inhibition is complete in DCs expressing the HCMV early Ag pp52; thus, it is likely to be an immediate-early or early direct viral effect.
FIG. 3.
CCR5 and CCR7 expression following HCMV infection of DCs. DCs were either mock infected (a and c) or infected with HCMV for 24 h (b and d). DCs then were either left untreated (a and b) (immature DCs) or treated with LPS/TNF-α/IFN-γ for a further 24 h (c and d) (mature DCs). Surface expression of CCR5 or CCR7 was tested by flow cytometry. The broken lines represent the binding of an irrelevant antibody, while the continuous lines represent the binding of CCR5 or CCR7 antibodies, respectively. The numbers represent the percentage of chemokine receptor-expressing cells above background (irrelevant antibody). The results are representative of three experiments. (e and f) CCR5 and CCR7 expression in HCMV-infected cultures on HCMV Ag-positive DCs (black histograms), compared to Ag-negative DCs (gray lines). Two-color flow cytometric analysis of cell surface expression of chemokine receptors and intracellular detection of HCMV pp52 Ag are shown. (e) Expression of CCR5 following HCMV infection of immature DCs. (f) Upregulation of CCR7 by LPS/TNF-α/IFN-γ on TB40/E-infected DCs.
DCs upregulate CCR7 upon encountering infected cells.
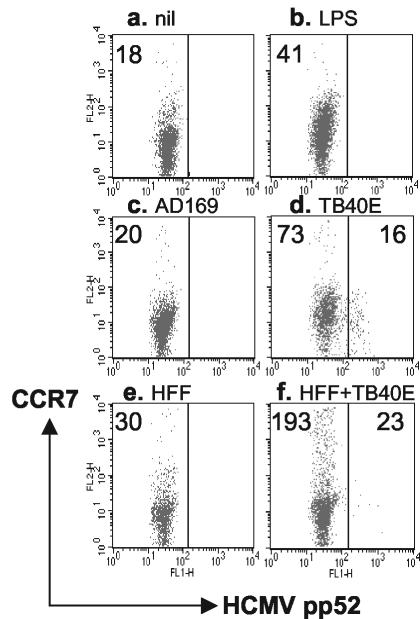
We have shown previously in a cross-presentation model that on DCs, following coculture with infected fibroblasts, the expression of major histocompatibility complex class II and CD83 molecules is upregulated (31), indicating bystander maturation induced by interaction with infected cells. We have now studied whether DCs, cocultured with infected fibroblasts, are induced to express CCR7 molecules. Compared with immature DCs (Fig. 4a), maturation agents such as LPS/IFN-γ/TNF-α (Fig. 4b) or coculture with TB40/E-infected fibroblasts (Fig. 4f) indeed increased the expression rate of CCR7 on DCs, while infection with TB40/E (without further treatment) (Fig. 4d) also caused bystander DC maturation, although to a lesser extent. In this case, DCs which became infected with TB40/E (pp52 Ag positive) remained negative for CCR7, again demonstrating the viral inhibition of CCR7 expression (Fig. 4d, right panel). The difference in bystander DC activation seems to depend upon the type of infected cells in the cocultures, as infected HFFs were more efficient than infected DCs in inducing CCR7 upregulation. Laboratory HCMV strain AD169, which does not infect DCs (Fig. 4c), and coculture of DCs with uninfected fibroblasts (Fig. 4e) were relatively inefficient in upregulating CCR7. This experiment indicates that although directly infected DCs do not express receptors for lymphoid chemokines, DCs which encountered infected HFFs express lymphoid chemokine receptors and are thus likely to be able to migrate into the lymph nodes. Further work is in progress to identify whether migrated bystander DCs are involved and are efficient in HCMV Ag cross-presentation for CD8+ T cells.
FIG. 4.
Coculture of HCMV-infected fibroblasts with DC upregulates CCR7 expression. Immature DCs were either left untreated for 24 h (a), treated with LPS/TNF-α/IFN-γ for a further 24 h (b), infected with 5 MOI of HCMV AD169 (c) or TB40/E (d) for 24 h, or mixed at a 1:1 ratio with HFFs which were either uninfected (e) or infected with 5 MOI of TB40/E for 24 h (f), and the cells were cultured for a further 24 h together. Fluorescence-activated cell sorter analysis was carried out following three-color staining for surface class II and CCR7 and intracellular HCMV pp52 Ag expression. DCs were gated upon forward-scatter and FL3 (class II expression) parameters. The numbers represent the MFI of CCR7 expression in HCMV pp52 Ag-negative (left panels) or -positive (right panels) DCs. Coculture of DCs with HCMV-infected fibroblasts (f) resulted in a marked upregulation of CCR7 on uninfected DCs. The results are representative of two experiments.
TNFR-p75 expression is not impaired following HCMV infection of DCs.
TNF-α is necessary for optimal DC migration in vivo (11, 38), and CCR7 mRNA is induced upon treating DCs with TNF-α in vitro (13, 32, 38). Thus, to reveal why HCMV-infected DCs were unable to migrate toward lymphoid chemokines, we analyzed the ability of HCMV-infected DCs to produce TNF-α and to express TNFRs. We showed earlier that HCMV-infected DCs produce low but significant levels of TNF-α upon infection and upon further stimulation with LPS (25). Furthermore, in the experiments described here, the exogenous concentration of TNF-α was identical for both Ag-positive and Ag-negative DCs as they were in the same culture wells. Thus, lack of TNF-α is unlikely to be responsible for the selective inhibition of the migration of HCMV-infected DCs.
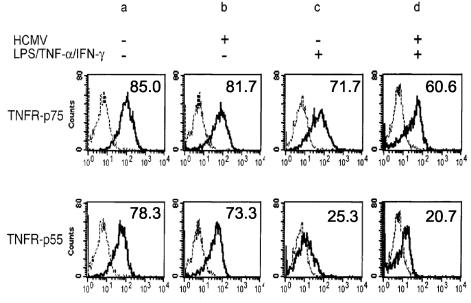
Next we studied the expression of TNFRs on DCs following HCMV infection (Fig. 5). TNFR-p75 and TNFR-p55 were expressed at medium levels on both mock-infected (Fig. 5a) and HCMV-infected (Fig. 5b) DCs. Lower levels of both TNF receptors, but especially that of TNFR-p55, were observed on DCs following stimulation with LPS/TNF-α/IFN-γ, compared to untreated DCs (Fig. 5c versus a). This downregulation was also observed when DCs were infected with HCMV before stimulation (Fig. 5d versus a). Two-color flow cytometric analysis to detect TNF receptor expression on HCMV-infected DCs confirmed that HCMV Ag-positive DCs expressed levels of TNFR-p75 similar to those expressed by HCMV Ag-negative DCs, while expression of TNFR-p55 was slightly lower in Ag-positive DCs (not shown). Thus, as the expression of TNFR-p75 remained at an intermediate level on DCs following HCMV infection, it is unlikely that viral inhibition of DC migration involves altered TNFR expression.
FIG. 5.
TNFR-p75 expression following HCMV infection is not impaired on DCs. DCs were either mock infected (a and c) or infected with HCMV for 24 h (b and d). DCs were then either left untreated (a and b) (immature DCs) or were treated with LPS/TNF-α/IFN-γ for a further 24 h (c and d) (mature DCs). Surface TNFR-p75 or TNFR-p55 expression was tested by flow cytometry. The broken lines represent the binding of an irrelevant antibody while the continuous lines represent the binding of TNFR-p75 (first row) or TNFR-p55 (second row) antibodies. The numbers represent the percentage of positive cells after deducting the percentage of positive cells in the control groups. The results are representative of three experiments.
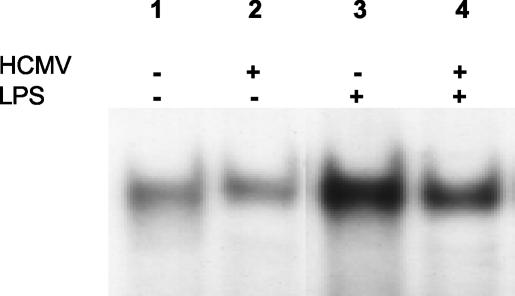
Inhibition of NF-κB DNA binding in HCMV-infected DCs.
Our results so far indicate that, while TNFR-p75 expression is not inhibited by HCMV infection, the induction of CCR7 expression is prevented. This suggests that the signals that induce CCR7 may be inhibited. TNF-α has been shown to cause an increase in CCR7 at the level of mRNA (13, 32, 38), suggesting that it regulates CCR7 transcriptionally. TNF signals through a protein complex that is associated with the TNF receptors. These proteins include TNFR-associated death domain and TNFR-associated factors. They in turn link to at least three distinct nuclear signals, including NF-κB, AP1, and JNK. Of these three transcription factors, the best characterized is the transcription factor NF-κB, which has been implicated as an important transcription factor for DC (35) mediating CCR7 upregulation in lymphoid cells (18). To characterize a mechanism for the inhibition of CCR7, we investigated whether HCMV infection affects NF-κB binding to cellular DNA. Day 5 DCs were mock infected or infected with HCMV for 24 h and were either left untreated or treated with LPS/TNF-α/IFN-γ for a further 24 h. Nuclear extracts from these cells were prepared, and NF-κB DNA binding was measured using an electrophoretic mobility shift assay. In this assay 32P-radiolabeled DNA containing a specific NF-κB binding site was incubated with DC nuclear extracts. The results in Fig. 6 show that a substantial increase in NF-κB is observed upon treatment with LPS/TNF-α/IFN-γ (compare lanes 1 and 3). HCMV infection alone did not increase the level of NF-κB binding (compare lanes 2 and 1). However, an inhibition of NF-κB binding is seen in nuclear extracts from cells infected with HCMV and then treated with LPS/TNF-α/IFN-γ compared to that of cells receiving treatment alone (lane 4 versus 3). These data agree with observations in the U373MG astrocytoma cell line where NF-κB DNA binding was not induced by HCMV, and the infection resulted in a general loss of NF-κB binding by 96 h postinfection (data not shown). The findings indicate that HCMV can prevent NF-κB-DNA interaction in DCs and that this inhibition is likely to contribute to the inability of infected DCs to express CCR7.
FIG. 6.
NF-κB activity in HCMV-infected DCs. DCs were infected with HCMV TB40/E (lanes 2 and 4) or were mock-infected (lanes 1 and 3) for 48 h. Lanes 1 and 2 represent immature DC while the cells in lanes 3 and 4 were treated with LPS/TNF-α/IFN-γ for the last 24 h before the NF-κB assay, and thus they represent mature DC. Nuclear extracts were analyzed for consensus binding sites of NF-κB by incubation with 32P-labeled oligonucleotides containing the binding site of NF-κB. The results are representative of three experiments.
DISCUSSION
This study describes, for the first time, an important effect of HCMV infection on DC function, namely, the inhibition of DC migration. Characterization of the molecular mechanism behind this viral effect reveals that the inhibition of DC migration is due to an inability of infected cells to express CCR7, the gene of which is regulated by NF-κB via TNFR signaling. We found that the induction of NF-κB DNA binding is inhibited in infected DC. The possible physiological consequences of this inhibition are also discussed.
DC migration depends upon the downregulation of inflammatory cytokine receptors, such as CCR5, and upregulation of lymphoid chemokine receptors, such as CCR7. HCMV very efficiently triggers the downregulation of CCR5 but does not induce the expression of CCR7 in infected DCs. Moreover, even following stimulation with LPS/TNF-α/IFN-γ, a normally potent stimulus for CCR7 induction, infected DCs remain negative for CCR7.
DCs survey the periphery and deliver Ag to the draining lymph nodes to generate an immune response. Their importance as key regulators of the immune system makes their manipulation a good target for altering immune responsiveness. This report, our previous data (25) and work with murine cytomegalovirus (1) demonstrate that infection of DC with cytomegalovirus is an important element of the virus-host interaction. Despite viral alteration of DC mobility and function, frequencies of T cells specific for HCMV Ags are among the highest in PBMC of healthy HCMV-seropositive subjects. This suggests the presence of other mechanisms by which HCMV-specific T-cell responses are generated. One possibility would be that the effect of HCMV infection differs on different subsets of DCs and that HCMV-mediated inhibition of migration of monocyte-derived DCs (MDDCs) is not representative. However, a recent study on CD34+ progenitor cell-derived Langerhans cells (16) revealed that in HCMV TB40/E (100 MOI)-infected DCs which have been matured with granulocyte-macrophage colony-stimulating factors and CD40L, downregulation of class I, class II, and costimulatory molecules is similar to that observed in MDDCs. Furthermore, our preliminary results for blood DCs, isolated from the peripheral blood of healthy donors with the Miltenyi magnetic separation kit, also indicate that in HCMV-infected blood DCs, class I molecules become downregulated and CCR7 surface expression is not induced upon infection (M. Moutaftsi, unpublished data). Another mechanism of efficient T-cell stimulation during HCMV infection may be cross-presentation of HCMV Ags by uninfected DCs as proposed earlier (2, 31) and observed for other herpesvirus Ags (6). Thus, while infection of DCs makes recognition of HCMV Ags by naive CD8+ T cells on the surface of infected DCs unlikely, due to spatial segregation of DCs and T cells, the cross-presentation mechanism would make CD8+-T-cell priming, via the alternative Ag presentation pathway, possible. In this work we studied secondary CD8+-T-cell responses induced by infected DCs and found that migrated DCs did not stimulate HCMV-specific memory CD8+ T cells. This was likely to be due to a very low proportion of DCs carrying endogenous viral Ags, which alone would be sufficient to prevent optimal T-cell activation. But, prevention of DC maturation (25) and production of inhibitory cytokines such as viral IL-10 (21) should also be considered as factors seriously hampering T-cell priming. We also found that HCMV pp52 negative bystander DCs, which migrated toward CCL19, were very efficient stimulators of HCMV-specific CD4+ T cells. This observation suggests that effective presentation of viral Ags, probably derived from the inoculum (23) in the absence of viral replication, is undisturbed, which may explain the high in vivo frequencies of HCMV Ag-specific CD4+ T cells (29).
The viral inhibition of the induction of CCR7 expression by DCs is the principal element in the impaired DC migration. Following infection with HCMV, DCs produce significant levels of TNF-α (25) and express unchanged levels of both TNFR-p75 and -p55, indicating that inhibition of CCR7 induction is not dependent on these factors. LPS-induced CCR7 upregulation is prevented in the presence of NF-κB inhibitors (7), and the gene encoding CCR7 is described as a direct target of NF-κB (18). Although HCMV infection leads to the activation of NF-κB in fibroblasts (22, 37) and to its nuclear translocation in monocytes (36), the effect may be cell type dependent, as in HCMV-infected retinal pigment epithelial cells where NF-κB activation was not observed (10). The effect of HCMV infection on NF-κB DNA binding in DCs has not been investigated previously. Our findings show that HCMV fails to activate and inhibits LPS/TNF-α/IFN-γ-mediated induction of NF-κB DNA binding in MDDCs. This effect also correlates with the observed inhibitory effect of the virus on DC maturation. As for the exact nature of viral product(s) responsible for the inhibitory effect, although we know that the inhibition of DC migration occurs as an immediate-early-early effect of HCMV infection, further experiments are needed to reveal the fine mechanism.
In summary, we have shown here a hitherto-undescribed viral evasion mechanism, the prevention of DC migration by HCMV. This effect of HCMV has the potential of avoiding or delaying the activation of HCMV-specific primary T-cell responses by infected DCs in vivo. The interactions between HCMV and DCs and the effects on the chemokine receptor switch suggest a complex host-virus interplay. The findings also emphasize the importance of alternative Ag presentation mechanisms in the generation of HCMV-specific CD8+-T-cell responses.
Acknowledgments
This work was supported by a University of Wales College of Medicine Ph.D. scholarship to M.M., by the Leukemia Research Fund (P.B.), and by the Wellcome Trust (Z.T.).
We thank S. Man, M. Rowe, and A. Clayton for critical reading of the manuscript and C. Sinzger for providing the TB40/E HCMV strain.
REFERENCES
- 1.Andrews, D. M., C. E. Andoniou, F. Granucci, P. Ricciardi-Castagnoli, and M. A. Degli-Esposti. 2001. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat. Immunol. 2:1077-1084. [DOI] [PubMed] [Google Scholar]
- 2.Arrode, G., C. Boccaccio, J. Lule, S. Allart, N. Moinard, J. P. Abastado, A. Alam, and C. Davrinche. 2000. Incoming human cytomegalovirus pp65 (UL83) contained in apoptotic infected fibroblasts is cross-presented to CD8+ T cells by dendritic cells. J. Virol. 74:10018-10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baekkevold, E. S., T. Yamanaka, R. T. Palframan, H. S. Carlsen, F. P. Reinholt, U. H. von Andrian, P. Brandtzaeg, and G. Haraldsen. 2001. The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J. Exp. Med. 193:1105-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baltathakis, I., O. Alcantara, and D. H. Boldt. 2001. Expression of different NF-κB pathway genes in dendritic cells (DCs) or macrophages assessed by gene expression profiling. J. Cell. Biochem. 83:281-290. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 6.Blake, N., T. Haigh, G. Shaka'a, D. Croom-Carter, and A. Rickinson. 2000. The importance of exogeneous antigen in priming the human CD8+ T cell response: lessons from the EBV nuclear antigen EBNA1. J. Immunol. 165:7078-7087. [DOI] [PubMed] [Google Scholar]
- 7.Bouchon, A., C. Hernandez-Munain, M. Cella, and M. Colonna. 2001. A DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. J. Exp. Med. 194:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan, P., and L. A. O'Neill. 1995. Effects of oxidants and antioxidants on nuclear factor kappa B activation in three different cell lines: evidence against a universal hypothesis involving oxygen radicals. Biochim. Biophys. Acta 1260:167-175. [DOI] [PubMed] [Google Scholar]
- 9.Caux, C., S. Ait-Yahia, K. Chemin, O. de Bouteiller, M. C. Dieu-Nosjean, B. Homey, C. Massacrier, B. Vanbervliet, A. Zlotnik, and A. Vicari. 2000. Dendritic cell biology and regulation of dendritic cell trafficking by chemokines. Springer Semin. Immunopathol. 22:345-369. [DOI] [PubMed] [Google Scholar]
- 10.Cinatl, J., S. Margraf, J. U. Vogel, M. Scholz, and H. W. Doerr. 2001. Human cytomegalovirus circumvents NF-kappa B dependence in retinal pigment epithelial cells. J. Immunol. 167:1900-1908. [DOI] [PubMed] [Google Scholar]
- 11.Cumberbatch, M., and I. Kimber. 1995. Tumour necrosis factor-alpha is required for accumulation of dendritic cells in draining lymph nodes and for optimal contact sensitization. Immunology 84:31-35. [PMC free article] [PubMed] [Google Scholar]
- 12.Cyster, J. G. 2000. Leukocyte migration: scent of the T zone. Curr. Biol. 10:R30-R33. [DOI] [PubMed] [Google Scholar]
- 13.Dieu, M. C., B. Vanbervliet, A. Vicari, J. M. Bridon, E. Oldham, S. Ait-Yahia, F. Briere, A. Zlotnik, S. Lebecque, and C. Caux. 1998. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 188:373-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forster, R., A. Schubel, D. Breitfeld, E. Kremmer, I. Renner-Muller, E. Wolf, and M. Lipp. 1999. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99:23-33. [DOI] [PubMed] [Google Scholar]
- 15.Geissmann, F., M. C. Dieu-Nosjean, C. Dezutter, J. Valladeau, S. Kayal, M. Leborgne, N. Brousse, S. Saeland, and J. Davoust. 2002. Accumulation of immature Langerhans cells in human lymph nodes draining chronically inflamed skin. J. Exp. Med. 196:417-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hertel, L., V. G. Lacaille, H. Strobl, E. D. Mellins, and E. S. Mocarski. 2003. Susceptibility of immature and mature Langerhans cell-type dendritic cells to infection and immunomodulation by human cytomegalovirus. J. Virol. 77:7563-7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirao, M., N. Onai, K. Hiroishi, S. C. Watkins, K. Matsushima, P. D. Robbins, M. T. Lotze, and H. Tahara. 2000. CC chemokine receptor-7 on dendritic cells is induced after interaction with apoptotic tumor cells: critical role in migration from the tumor site to draining lymph nodes. Cancer Res. 60:2209-2217. [PubMed] [Google Scholar]
- 18.Hopken, U. E., H. D. Foss, D. Meyer, M. Hinz, K. Leder, H. Stein, and M. Lipp. 2002. Up-regulation of the chemokine receptor CCR7 in classical but not in lymphocyte-predominant Hodgkin disease correlates with distinct dissemination of neoplastic cells in lymphoid organs. Blood 99:1109-1116. [DOI] [PubMed] [Google Scholar]
- 19.Jeannin, P., G. Magistrelli, N. Herbault, L. Goetsch, S. Godefroy, P. Charbonnier, A. Gonzalez, and Y. Delneste. 2003. Outer membrane protein A renders dendritic cells and macrophages responsive to CCL21 and triggers dendritic cell migration to secondary lymphoid organs. Eur. J. Immunol. 33:326-333. [DOI] [PubMed] [Google Scholar]
- 20.Kellermann, S. A., S. Hudak, E. R. Oldham, Y. J. Liu, and L. M. McEvoy. 1999. The CC chemokine receptor-7 ligands 6Ckine and macrophage inflammatory protein-3 beta are potent chemoattractants for in vitro- and in vivo-derived dendritic cells. J. Immunol. 162:3859-3864. [PubMed] [Google Scholar]
- 21.Kotenko, S. V., S. Saccani, L. S. Izotova, O. V. Mirochnitchenko, and S. Pestka. 2000. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10). Proc. Natl. Acad. Sci. USA 97:1695-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowalik, T. F., B. Wing, J. S. Haskill, J. C. Azizkhan, A. S. Baldwin, Jr., and E.-S. Huang. 1993. Multiple mechanisms are implicated in the regulation of NF-κB activity during human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 90:1107-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le-Roy, E., M. Baron, W. Faigle, D. Clement, D. M. Lewinsohn, D. N. Streblow, J. A. Nelson, S. Amigorena, and J. L. Davignon. 2002. Infection of APC by human cytomegalovirus controlled through recognition of endogenous nuclear immediate early protein 1 by specific CD4+ T lymphocytes. J. Immunol. 169:1293-1301. [DOI] [PubMed] [Google Scholar]
- 24.Lutz, M. B., and G. Schuler. 2002. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 23:445-449. [DOI] [PubMed] [Google Scholar]
- 25.Moutaftsi, M., A. M. Mehl, L. K. Borysiewicz, and Z. Tabi. 2002. Human cytomegalovirus inhibits maturation and impairs function of monocyte-derived dendritic cells. Blood 99:2913-2921. [DOI] [PubMed] [Google Scholar]
- 26.Mueller, S. N., C. M. Jones, C. M. Smith, W. R. Heath, and F. R. Carbone. 2002. Rapid cytotoxic T lymphocyte activation occurs in the draining lymph nodes after cutaneous herpes simplex virus infection as a result of early antigen presentation and not the presence of virus. J. Exp. Med. 195:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riegler, S., H. Hebart, H. Einsele, P. Brossart, G. Jahn, and C. Sinzger. 2000. Monocyte-derived dendritic cells are permissive to the complete replicative cycle of human cytomegalovirus. J. Gen. Virol. 81:393-399. [DOI] [PubMed] [Google Scholar]
- 28.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sester, M., U. Sester, B. Gartner, B. Kubuschok, M. Girndt, A. Meyerhans, and H. Kohler. 2002. Sustained high frequencies of specific CD4 T cells restricted to a single persistent virus. J. Virol. 76:3748-3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sozzani, S., P. Allavena, G. D'Amico, W. Luini, G. Bianchi, M. Kataura, T. Imai, O. Yoshie, R. Bonecchi, and A. Mantovani. 1998. Differential regulation of chemokine receptors during dendritic cell maturation: a model for their trafficking properties. J. Immunol. 161:1083-1086. [PubMed] [Google Scholar]
- 31.Tabi, Z., M. Moutaftsi, and L. K. Borysiewicz. 2001. Human cytomegalovirus pp65- and immediate early 1 antigen-specific HLA class I-restricted cytotoxic T cell responses induced by cross-presentation of viral antigens. J. Immunol. 166:5695-5703. [DOI] [PubMed] [Google Scholar]
- 32.Vecchi, A., L. Massimiliano, S. Ramponi, W. Luini, S. Bernasconi, R. Bonecchi, P. Allavena, M. Parmentier, A. Mantovani, and S. Sozzani. 1999. Differential responsiveness to constitutive vs. inducible chemokines of immature and mature mouse dendritic cells. J. Leukoc. Biol. 66:489-494. [DOI] [PubMed] [Google Scholar]
- 33.Wang, B., H. Fujisawa, L. Zhuang, S. Kondo, G. M. Shivji, C. S. Kim, T. W. Mak, and D. N. Sauder. 1997. Depressed Langerhans cell migration and reduced contact hypersensitivity response in mice lacking TNF receptor p75. J. Immunol. 159:6148-6155. [PubMed] [Google Scholar]
- 34.Wang, B., S. Kondo, G. M. Shivji, H. Fujisawa, T. W. Mak, and D. N. Sauder. 1996. Tumour necrosis factor receptor II (p75) signalling is required for the migration of Langerhans' cells. Immunology 88:284-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshimura, S., J. Bondeson, F. M. Brennan, B. M. Foxwell, and M. Feldmann. 2001. Role of NFkappaB in antigen presentation and development of regulatory T cells elucidated by treatment of dendritic cells with the proteasome inhibitor PSI. Eur. J. Immunol. 31:1883-1893. [DOI] [PubMed] [Google Scholar]
- 36.Yurochko, A. D., and E. S. Huang. 1999. Hum. cytomegalovirus binding to human monocytes induces immunoregulatory gene expression. J. Immunol. 162:4806-4816. [PubMed] [Google Scholar]
- 37.Yurochko, A. D., T. F. Kowalik, S. M. Huong, and E. S. Huang. 1995. Human cytomegalovirus upregulates NF-κB activity by transactivating the NF-κB p105/p50 and p65 promoters. J. Virol. 69:5391-5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang, W., Z. Chen, F. Li, H. Kamencic, B. Juurlink, J. R. Gordon, and J. Xiang. 2003. Tumour necrosis factor-alpha (TNF-alpha) transgene-expressing dendritic cells (DCs) undergo augmented cellular maturation and induce more robust T-cell activation and antitumour immunity than DCs generated in recombinant TNF-alpha. Immunology 108:177-188. [DOI] [PMC free article] [PubMed] [Google Scholar]