Abstract
Human cytomegalovirus (HCMV) exploits the host transcription factor NF-κB to enhance its own replication, dissemination, and reactivation from latency. Here we report that HCMV infection activates the upstream IκB kinase (IKK) complex and that its catalytic IKK2 subunit is required for HCMV-induced NF-κB activation, as well as the replication of different HCMV strains. These results indicate that IKK2 is essential for HCMV replication and emphasize the feasibility of blocking NF-κB activation as a way of inhibiting infection.
Human cytomegalovirus (HCMV) is a ubiquitous betaherpesvirus that generally causes benign or asymptomatic infections. However, it is the leading cause of congenital viral infection in humans and a primary cause of morbidity in immunocompromised hosts (5, 12, 16, 18, 20).
During HCMV infection, a coordinated cascade of events must occur. The activity of viral immediate-early (IE) proteins is essential for HCMV replication, since they regulate the subsequent expression of early (E) and late (L) genes. Expression of IE genes is closely associated with cellular activation pathways and involves several transcription factors whose activities are stimulated by infection (5, 6, 16, 18). Of these, the NF-κB pathway plays a crucial role by transactivating the major IE enhancer-promoter that regulates the expression of the major IE gene products during both replication and reactivation from latency (14, 18). NF-κB translocations into the nucleus and DNA binding, in fact, are hallmarks of CMV infection (10, 11, 15, 24, 28, 29).
Several stimuli leading to NF-κB activation converge onto a multiprotein kinase complex designated IKK (IκB kinase) or signalosome (9). This consists of two catalytic subunits, IKK1 (IKKα) and IKK2 (IKKβ), and the regulatory subunit IKKγ (NF-κB essential modulator). Activated IKK phosphorylates the cytoplasmic NF-κB inhibitors, IκBs, and tags them for proteasomal degradation. It thus allows NF-κB proteins to translocate into the nucleus and activate the transcription of cellular and viral responsive genes.
Viral protein products that activate NF-κB appear to act through several distinct mechanisms involving either alteration of the normal cellular signal transduction pathways acting upstream from the IKK complex (as in the case of Epstein-Barr virus LMP1 protein), or promoting a persistent degradation of IκBs (as for hepatitis B virus), or by direct association with the IKK complex (as for the HTLV-1 tax protein) (13, 26). However, it is not yet known which mechanism is exploited by HCMV.
Since elucidation of the molecular mechanisms of virus-mediated regulation of the host biochemical pathway may identify new targets suitable for the design of molecules with antiviral activity, we investigated the effects of HCMV infection on IKK activity. The results showed that HCMV infection indeed stimulates IKK2 activity and that it is required for HCMV replication.
HCMV infection stimulates cellular IKK activity.
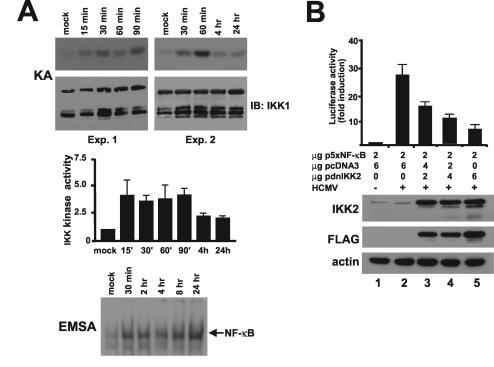
To determine whether HCMV regulates the activity of the upstream IKK complex, quiescent HELF cells were infected with HCMV AD169 at a multiplicity of infection (MOI) of 10 PFU/cell, and whole-cell extracts were prepared at the indicated times. The complex was immunoprecipitated with an anti-IKK1 monoclonal antibody (MAb), and IKK activity was measured with the specific glutathione S-transferase (GST)-IκBα substrate, as previously described (17), and compared with that in mock-infected cells. Preliminary experiments confirmed the coimmunoprecipitation of IKK1 and IKK2. As shown in Fig. 1A (upper panel), HCMV induced a significant increase in IKK activity after only 15 min. This lasted until 90 min and then declined. Quantitation of band intensity from four independent experiments demonstrated that the increase was about fourfold between 15 and 90 min and twofold at 4 and 24 h (Fig. 1A, middle panel). These results demonstrate that HCMV infection activates IKK during the same time frame as induction of NF-κB DNA binding activity (Fig. 1A, lower panel).
FIG. 1.
HCMV infection activates cellular IKK. (A) HCMV infection rapidly increases IKK activity. HELF cells were growth arrested in low-serum medium for 48 h and then infected with HCMV AD169 (MOI of 10 PFU/cell) or mock infected. Whole-cell protein extracts were then prepared at the indicated times and assayed for IKK activity using GST-IκBα as the substrate (17). Endogenous IKK recovery after immunoprecipitation was determined by immunoblotting for IKK1 (IB: IKK1). The autoradiograms of two independent experiments are shown as representative (upper panel). Following autoradiography, band intensity was determined with a densitometer, and its quantitation is shown. The data are the means of four experiments ± the standard errors (error bars) (middle panel). EMSA analysis was performed with nuclear extracts prepared from HELF cells infected with HCMV AD169 (MOI of 5 PFU/cell) or mock infected (lower panel). (B) IKK2 is functionally required for HCMV-induced NF-κB-dependent gene expression. HELF cells were transiently cotransfected with 2 μg of the 5xNF-κB LUC indicator plasmid and increasing amounts of the expression vector for the dnIKK2 protein. After 18 h they were washed and then maintained in low-serum medium for 48 h. Thereafter, transfectants were infected with HCMV AD169 (MOI of 5 PFU/cell) or mock infected. Total cytoplasmic extracts were isolated at 18 h p.i. and assayed for luciferase activity. Reporter gene activity was normalized to the amount of plasmid DNA introduced into recipient cells by DNA dot blot analysis (1). Luciferase activity is expressed as induction relative to the basal level in cells transfected with the 5xNF-κB LUC, which was set at 1. The data are the means of three experiments ± the standard errors (error bars). The extracts from transfected cells were also subjected to immunoblotting analysis with the anti-IKK2, the anti-FLAG, or the anti-actin MAb. Extracts were from cells cotransfected with the p5xNB-κB and the empty pcDNA3 vector and then mock infected (lane1), cells cotransfected with p5xNB-κB and the empty vector and then infected with HCMV (lane 2), or cells cotransfected with p5xNB-κB and increasing concentrations (2 μg [lane 3], 4 μg [lane 4], or 6 μg [lane 5]) of the dnIKK2 vector and then infected with HCMV.
IKK2 is required for HCMV-mediated NF-κB activation.
IKK2 is the predominant IKK activated by all known proinflammatory stimuli (4, 9). To evaluate its contribution to HCMV-induced IKK activity, a FLAG-tagged IKK2 protein bearing a substitution of Lys 44 to Ala was transiently transfected in HELF cells with the indicator plasmid 5xNF-κB LUC. Mutagenesis of the critical Lys residue abolished the protein's kinase activity and gave it a dominant-negative (dn) phenotype (19). The cells were then infected with HCMV at an MOI of 5 PFU/cell, and the reporter gene activity was measured 18 h later. As shown in Fig. 1B, overexpression of increasing amounts of dnIKK2 significantly reduced HCMV-induced NF-κB transactivation of the reporter gene. A fourfold reduction was measured in extracts from cells that received the highest amount of dnIKK2, suggesting that endogenous IKK2 is required for virus-stimulated activation of the NF-κB pathway. Immunoblotting analysis of extracts from these transfected cells with anti-IKK2 or anti-FLAG MAbs confirmed the expression of exogenous IKK2 (Fig. 1B).
Effect of an IKK2 inhibitor on HCMV-mediated NF-κB activation.
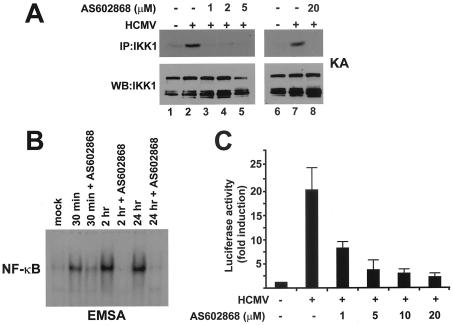
AS602868 is an anilino derivative selected for its ability to block IKK2. It is a competitive ATP binding inhibitor and is highly specific for IKK2 (50% inhibitory concentration [IC50] = 62 nM), since it has no effect on IKK1 (IC50 > 30 μM), nor on a large panel of recombinant kinases, and prevents NF-κB activation in different cell lines (7). To determine its effects on HCMV-induced IKK activity, endogenous IKK was immunoprecipitated from whole extracts of HCMV-infected HELF cells for 1 h and IKK activity was determined in the presence of increasing concentrations of AS602868. As shown in Fig. 2A, it blocked the ability of immunopurified IKK to phosphorylate in vitro the GST-IκBα substrate at a concentration as low as 1 μM (lanes 3 to 5). Moreover, treatment of the infected cells with AS602868 (20 μM for 1 h before HCMV infection) completely inhibited virus-mediated IKK activation at 1 h postinfection (p.i.) (Fig. 2A, lanes 7 and 8). We also looked to see whether the subsequent steps in the NF-κB activation pathway were altered by IKK2 inhibition. First, the effect of inhibition on NF-κB DNA binding activity triggered by HCMV infection was assessed by electrophoretic mobility shift assay (EMSA). Quiescent HELF cells pretreated for 1 h with AS602868 were infected with HCMV, and nuclear extracts were prepared at the indicated time points p.i. and evaluated for NF-κB binding activity. As shown in Fig. 2B, IKK2 inhibition prevented NF-κB activation for up to 24 h p.i. Then, to determine the consequences of IKK2 inhibition on HCMV-stimulated NF-κB-dependent gene expression, we analyzed the effects of AS602868 on expression of transiently transfected 5xNF-κB LUC. Transfected HELF cells were serum starved and then infected with HCMV at an MOI of 5 PFU/cell in the absence or presence of increasing AS602868 concentrations. At 18 h p.i., cell extracts were prepared and assayed for luciferase activity. As shown in Fig. 2C, the HCMV-induced NF-κB transactivation of the reporter gene was inhibited in a dose-dependent manner.
FIG. 2.
Inhibition of HCMV-induced NF-κB activation by the IKK2 inhibitor AS602868. (A) Inhibition of HCMV-induced IKK activation by AS602868. Quiescent HELF cells were infected with HCMV AD169 (MOI of 10 PFU/cell) or mock infected. Whole-cell protein extracts were then prepared at 1 h p.i. and assayed for IKK activity. Where indicated, immunopurified IKK complex was incubated with increasing concentrations of AS602868 during the assay (lanes 3 to 5). Extracts analyzed for IKK activity in lane 8 were from cells treated with 20 μM AS602868 1 h prior to and during infection. Endogenous IKK recovery after immunoprecipitation was determined by immunoblotting for IKK1 (IB: IKK1). The autoradiogram of a representative experiment is shown. (B) AS602868 inhibits the induction of NF-κB DNA binding activity following HCMV infection. Quiescent HELF cells were infected with HCMV AD169 (MOI of 5 PFU/cell) or mock infected. Nuclear extracts were then prepared at the indicated times and assayed for NF-κB activation by EMSA. Where indicated, cells were treated with 20 μM AS602868 1 h prior to and during infection. This experiment was repeated three times, and a representative autoradiogram is shown. (C) Inhibition of IKK2 activity inhibits HCMV-induced NF-κB-dependent transactivation. HELF cells were transiently transfected with 2 μg of p5xNF-κB LUC and 10 μg of carrier DNA (pBluescript SK). After 18 h, cells were washed and growth arrested in low-serum medium for 48 h. Thereafter, transfectants were infected with HCMV AD169 (MOI of 5 PFU/cell) or mock infected. Where indicated, cells were treated with different concentrations of AS602868 1 h prior to and during infection. Luciferase activity was measured at 18 h p.i. and is expressed as induction (n-fold) relative to the basal level measured in cells transfected with p5xNF-κB LUC and then mock infected, which was set at 1. The data shown are the means of three experiments ± the standard errors (error bars).
Altogether, these results demonstrate that inhibition of the upstream IKK2 activity blocked the subsequent steps of the NF-κB signaling pathway stimulated by HCMV infection.
IKK2 activity is required for completion of the HCMV lytic cycle.
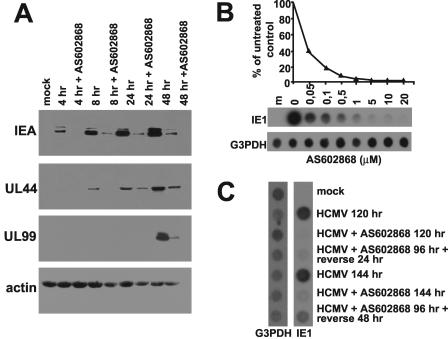
To analyze the dependence of HCMV gene expression on induction of IKK2 activity, the effects of its inhibition on viral protein expression were examined by immunoblotting. Figure 3A shows that AS602868 significantly reduced the expression of the two major IE proteins throughout infection, since their content was strongly decreased at both early (4 and 8 h) and late (24 and 48 h) times p.i. compared to cells infected in the absence of the inhibitor. AS602868 also reduced the expression of both an early gene, UL44 (DNA polymerase processivity factor), and a true late gene (UL99) (Fig. 3A). High levels of both UL44 and UL99 were detected at 24 and 48 h p.i., respectively, in infected controls that did not receive AS602868. These results demonstrate that HCMV gene expression is blocked even at the IE phase and that all subsequent events in the cascade are inhibited.
FIG. 3.
Effects of AS602868 on HCMV AD169 protein and DNA synthesis. (A) Expression of HCMV proteins is decreased by IKK2 inhibition. Growth-arrested HELF cells were infected with HCMV AD169 (MOI of 5 PFU/cell) or mock infected. Where indicated, they were treated with 20 μM AS602868 1 h prior to and during infection. Total cell extracts were prepared at the indicated times after infection, fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (50 μg of protein per lane), and analyzed by immunoblotting with the anti-IEA, anti-UL44, or anti-UL99 MAbs. Actin immunodetection was performed as an internal control. (B) Effect of AS602868 on viral DNA replication. Quiescent HELF cells were infected with HCMV AD169 (MOI of 1 PFU/cell) or mock infected. Where indicated, cells were treated with increasing concentrations of AS602868 1 h prior to and during infection. At 96 h p.i., total genomic DNA was purified and twofold dilutions were immobilized on a hybridization membrane. The same filter was sequentially hybridized with 32P-labeled HCMV IE1 and glyceraldehyde-3-phosphate dehydrogenase (G3PDH) probes. Hybridization signals were quantitated with a densitometer. (C) Reversibility of AS602868 treatment. Quiescent HELF cells were infected with HCMV AD169 (MOI of 1 PFU/cell) or mock infected. Where indicated, cells were treated with 10 μM AS602868 1 h prior to and during infection. At 96 h p.i., where indicated, AS602868 was removed from the culture medium and the infection was allowed to continue for another 24 or 48 h. At 120 or 144 h p.i., total genomic DNA was purified and analyzed by dot blot hybridization.
Next, HCMV DNA synthesis in the presence of increasing concentrations of AS602868 was measured by dot blot DNA hybridization. DNA samples from HCMV-infected HELF cells were isolated at 96 h p.i. and sequentially hybridized with 32P-labeled HCMV IE1 and glyceraldehyde-3-phosphate dehydrogenase probes. Figure 3B shows that AS602868 significantly inhibited synthesis in a dose-dependent manner with an IC50 lower than 0.05 μM. Since late gene expression cannot start before the beginning of viral DNA replication, these results corroborate the impairment of UL99 expression shown in Fig. 3A. Since NF-κB regulates a wide variety of cell processes, inhibition of IKK2 activity may alter the overall ability of infected cells to support HCMV DNA synthesis. To demonstrate that cells treated with AS602868 can support viral DNA replication, HELF cells were infected in the presence of 10 μM AS602868 for 96 h. The inhibitor was then removed, and the infection was allowed to proceed for another 24 or 48 h. Viral DNA synthesis was analyzed by dot blot hybridization. As shown in Fig. 3C, DNA synthesis resumed after the removal of AS602868. Inhibition of viral DNA replication by AS602868 is thus reversible.
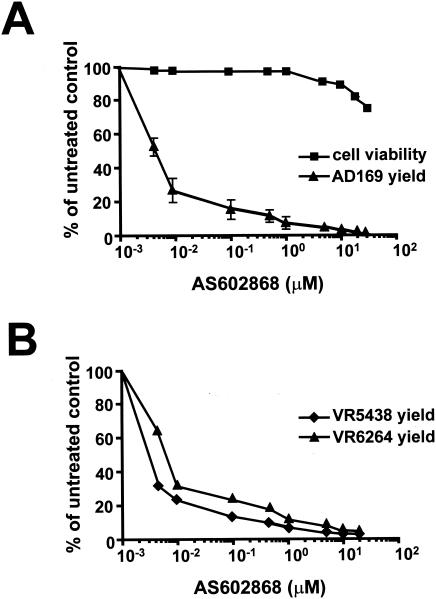
Finally, to determine whether IKK2 activity is required for progression and completion of HCMV replication, virus yield along with cell viability were measured in HELF cells infected with HCMV AD169 in the presence of increasing concentrations of AS602868. As shown in Fig. 4A, inhibition of IKK2 activity produced a significant dose-dependent inhibition of HCMV yield at 6 days p.i. The calculated IC50 and IC90 values were less than 0.01 and 1 μM, respectively. Moreover, AS602868 did not affect the viability of HELF cells at concentrations up to 30 μM for up to 4 days of exposure. The 50% cytotoxic concentration was >30 μM, demonstrating that AS602868's antiviral activity is indeed specific and not due to generalized cytotoxicity.
FIG. 4.
Antiviral effects of the IKK2 inhibitor AS602868. (A) Inhibitory effect of AS602868 on HCMV AD169 replication in HELF cells. Growth-arrested HELF cells were infected with HCMV AD169 (MOI of 1 PFU/cell) or mock infected. Where indicated, cells were treated with increasing concentrations of AS602868 1 h prior to and during infection until an extensive viral cytopathic effect was observed in the untreated control. Supernatants of cell suspension were then assayed for infectivity by standard plaque reduction assay on HELF cells. The number of plaques was plotted as a function of drug concentration, and the IC50 and IC90 were determined. Values are the means of two independent determinations. To determine cell viability, HELF cells were growth arrested in low-serum medium and then exposed to increasing concentrations of AS602868. After four days, the number of viable cells was determined by the MTT method, as previously described (21). (B) Inhibitory effect of AS602868 on GCV-resistant HCMV VR5438 and VR6264 strains. Quiescent HELF cells were infected with HCMV VR5438 or HCMV VR6264 (MOI of 1 PFU/cell) or mock infected. Where indicated, cells were treated with increasing concentrations of AS602868 1 h prior to and during infection until an extensive viral cytopathic effect was observed in the untreated control. The extent of VR5438 or VR6264 replication was then assessed by titrating the infectivity of supernatants of cell suspensions by the IE antigen indirect immunoperoxidase staining technique (8). The number of plaques was plotted as a function of drug concentration, and the IC50 and IC90 values were determined.
In view of AS602868's potent inhibition of the AD169 laboratory strain, the sensitivity of HCMV isolated from clinical specimens was assessed. Moreover, since ganciclovir (GCV)-resistant strains have been noted during prolonged treatment, inhibition in this context was evaluated by measuring the effect of AS602868 on two GCV-resistant strains deficient in drug phosphorylation due to mutations in the UL97 gene (8). Figure 4B shows the effect of different AS602868 concentrations on viral titers determined by measuring the viral yield of virus-infected HELF at 10 days p.i. The sensitivities of the GCV-resistant strains were comparable to that of the AD169 strain.
This study provides the first illustration of the ability of HCMV to stimulate IKK activity. It also showed that IKK2 is functionally required for HCMV-induced NF-κB activation and that IKK2 activity is essential for the completion of productive viral replication, since its inhibition suppressed viral DNA synthesis and production of viral progeny.
Increasing knowledge of the molecular mechanisms that regulate the NF-κB pathway provides a further rationale for the development of new inhibitors (2, 27). Since NF-κB is important for ensuring adequate levels of expression of both viral and cellular proteins critical for completion of the viral replicative cycle, blocking HCMV-induced NF-κB activation may be a way of inhibiting viral replication. Specific inhibition of NF-κB signaling by overexpression of a degradation-resistant IκBα protein resulting in suppression of stimulation of HCMV major IE enhancer-promoter activity by tumor necrosis factor alpha is relevant in this respect (22). This finding suggests that inhibitors of NF-κB activation may be an alternative way of interfering with HCMV reactivation from latency, since this event is partly regulated by NF-κB activation and is tumor necrosis factor alpha dependent in individuals with a high risk of inflammation-related HCMV reactivation (14, 18, 25).
Since it is now clear that IKK2 is the kinase primarily responsible for regulating NF-κB activation, the search for specific inhibitors of its activity may provide a new class of agents with multiple therapeutic benefits as anti-inflammatory, anticancer, and antimicrobial drugs. Here we have reported the anti-HCMV activity of the specific IKK2 inhibitor AS602868. In vivo studies have demonstrated its efficacy in preventing NF-κB activation as well as its disease-modifying effects in animals with chronic inflammatory diseases whose pathogenesis involves NF-κB activation, such as rat adjuvant arthritis and mouse collagen-induced arthritis (3, 23).
We observed that different HCMV strains are highly sensitive to AS602868 at concentrations that did not significantly alter cell viability. This specificity may depend on the low levels of NF-κB activity in uninfected cells. Selective induction of this pathway by HCMV infection could thus provide a specific target for anti-CMV molecules. Inhibition of NF-κB activation by AS602868 may thus be deleterious to viral replication only. The overall decrease in the concentration of essential IE1 and IE2 (Fig. 3A) is likely to be sufficient to inhibit progression of viral replication by affecting the initiation of early gene expression and viral DNA synthesis. However, inhibition of the expression of other HCMV genes may contribute to AS602868's antiviral effect. A database search of potential NF-κB sites within the HCMV AD169 genome, in fact, revealed that several binding sites for NF-κB factors are dispersed throughout the viral genome in addition to those already characterized within the enhancers controlling the expression of ie1/ie2 (UL122 and -123) and the immunomodulatory US3 protein (18). Five sites are contained within the 5′-flanking region of genes encoding putative viral glycoproteins (TRL10/IRL10, UL11, UL13, and UL40), suggesting that expression of these viral genes may also be affected by IKK2 inhibition.
IKK2 is thus an attractive anticytomegaloviral target and may be a good candidate for a new class of antiviral drugs for use in combination with conventional anticytomegaloviral chemotherapy.
Acknowledgments
We thank Rainer de Martin for providing the dnIKK2 plasmid, Giuseppe Gerna for the GCV-resistant HCMV strains, and Ed Mocarski for constructive suggestions.
This work was supported by grants from MIUR (40 and 60%), from the AIDS Research Project, from FIRB, and from the Ricerca Sanitaria Finalizzata (Regione Piemonte) and by a research grant from Serono International (Geneva, Switzerland).
Footnotes
This paper is dedicated to the memory of Giorgio Cavallo.
REFERENCES
- 1.Abken, H., and B. Reifenrath. 1992. A procedure to standardize CAT reporter gene assay. Nucleic Acids Res. 20:3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin, A. S., Jr. 2001. The transcription factor NF-κB and human disease. J. Clin. Investig. 107:3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhagwat, S., I. B. Brydon, I. Satoh, E. C. O′Leary, J. Leisten, G. S. Firestein, D. L. Boyle, M. Dreano, D. W. Anderson, and C. E. Grimshaw. 2001. The small molecule IKK2 inhibitor SPC839 is efficacious in an animal model of arthritis. Arthritis Rheum. 44:S213. [Google Scholar]
- 4.Dixit, V., and T. W. Mak. 2002. NF-κB signaling: many roads lead to Madrid. Cell 111:615-619. [DOI] [PubMed] [Google Scholar]
- 5.Fortunato, E. A., and D. H. Spector. 1999. Regulation of human cytomegalovirus gene expression. Adv. Virus Res. 54:1-128. [DOI] [PubMed] [Google Scholar]
- 6.Fortunato, E. A., A. K. McElroy, V. Sanchez, and D. H. Spector. 2000. Exploitation of cellular signaling and regulatory pathways by human cytomegalovirus. Trends Microbiol. 8:111-119. [DOI] [PubMed] [Google Scholar]
- 7.Frelin, C., V. Imbert, E. Griessinger, A. Loubat, M. Dreano, and J.-F. Peyron. 2003. AS602868, a pharmacological inhibitor of IKK2, reveals the apoptotic potential of TNF-α in Jurkat leukemic cells. Oncogene 22:8187-8194. [DOI] [PubMed] [Google Scholar]
- 8.Gerna, G., F. Baldanti, M. Zavattoni, A. Sarasini, E. Percivalle, and M. G. Revello. 1992. Monitoring of ganciclovir sensitivity of multiple human cytomegalovirus strains coinfecting blood of an AIDS patient by an immediate-early antigen plaque assay. Antivir. Res. 19:333-345. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-κB puzzle. Cell 109:81-96. [DOI] [PubMed] [Google Scholar]
- 10.Gribaudo, G., S. Ravaglia, M. Gaboli, M. Gariglio, R. Cavallo, and S. Landolfo. 1995. Interferon-α inhibits the murine cytomegalovirus immediate-early gene expression by down-regulating NF-κB activity. Virology 211:251-260. [DOI] [PubMed] [Google Scholar]
- 11.Gribaudo, G., S. Ravaglia, L. Guandalini, R. Cavallo, M. Gariglio, and S. Landolfo. 1996. The murine cytomegalovirus immediate-early 1 protein stimulates NF-kappa B activity by transactivating the NF-kappa B p105/p50 promoter. Virus Res. 45:15-27. [DOI] [PubMed] [Google Scholar]
- 12.Griffith, P. D. 2000. Cytomegalovirus, p. 79-115. In A. J. Zuckerman, J. E. Banatvala, and J. R. Pattison (ed.), Principles and practice of clinical virology, 4th ed. Wiley, New York, N.Y.
- 13.Hiscott, J., H. Kwon, and P. Génin. 2001. Hostile takeovers: viral appropriation of the NF-κB pathway. J. Clin. Investig. 107:143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hummel, M., and M. M. Abecassis. 2002. A model for reactivation of CMV from latency. J. Clin. Virol. 25:S123-S139. [DOI] [PubMed] [Google Scholar]
- 15.Kowalik, T. M., B. Wing, J. S. Haskill, J. C. Azizkhan, A. S. Baldwin, and E. S. Huang. 1993. Multiple mechanisms are implicated in the regulation of NF-κB activity during human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 90:1107-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landolfo, S., M. Gariglio, G. Gribaudo, and D. Lembo. 2003. The human cytomegalovirus. Pharmacol. Ther. 98:269-297. [DOI] [PubMed] [Google Scholar]
- 17.Mercurio, F., H. Zhu, B. W. Murray, A. Shevchenko, B. L. Bennet, J. W. Li, D. B. Young, M. Barbosa, M. Mann, A. Manning, and A. Rao. 1997. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science 278:860-866. [DOI] [PubMed] [Google Scholar]
- 18.Mocarski, E. S., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2673. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott-Raven, New York, N.Y.
- 19.Oitzinger, W., R. Hofer-Warbinek, J. A. Schmid, Y. Koshelnick, B. R. Binder, and R. de Martin. 2001. Adenovirus-mediated expression of a mutant IκB kinase 2 inhibits the response of endothelial cells to inflammatory stimuli. Blood 97:1611-1617. [DOI] [PubMed] [Google Scholar]
- 20.Pass, R. F. 2001. Cytomegalovirus, p. 2675−2705. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott-Raven, New York, N.Y.
- 21.Pauwels, R., J. Balzarini, M. Baba, R. Snoeck, D. Schols, P. Hederwijin, J. Desmyter, and E. De Clerq. 1988. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods 20:309-321. [DOI] [PubMed] [Google Scholar]
- 22.Prosch, S., R. Wuttke, D. H. Kruger, and H. D. Volk. 2002. NF-κB—a potential therapeutic target for inhibition of human cytomegalovirus (re)activation? Biol. Chem. 383:1601-1609. [DOI] [PubMed] [Google Scholar]
- 23.Sagot, Y., P. Sattonet-Roche, S. Bhagwat, C. E. Grimshaw, M. Dreano, and C. Plater-Zyberk. 2001. Two IKK2 inhibitors are orally active small molecules decreasing severity of collagen induced arthritis in DBA/1 mice. Arthritis Rheum. 44:S368. [Google Scholar]
- 24.Sambucetti, L. C., J. M. Cherrington, G. W. G. Wilkinson, and E. S. Mocarski. 1989. NF-κB activation of the cytomegalovirus enhancer is mediated by a viral transactivator and T cell stimulation. EMBO J. 8:4251-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Streblow, D. N., and J. A. Nelson. 2003. Models of HCMV latency and reactivation. Trends Microbiol. 11:293-295. [DOI] [PubMed] [Google Scholar]
- 26.Tato, C. M., and C. A. Hunter. 2002. Host-pathogen interactions: subversion and utilization of the NF-κB pathway during infection. Infect. Immun. 70:3311-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto, Y., and R. B. Gaynor. 2001. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J. Clin. Investig. 107:135-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yurochko, A. D., T. F. Kowalik, S. M. Huong, and E. S. Huang. 1995. Human cytomegalovirus upregulates NF-kappa B activity by transactivating the NF-kappa B p105/p50 and p65 promoters. J. Virol. 69:5391-5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yurochko, A. D., E. S. Hwang, L. Rasmussen, S. Keay, L. Pereira, and E. S. Huang. 1997b. The human cytomegalovirus UL55 (gB) and UL75 (gH) glycoprotein ligands initiate the rapid activation of Sp1 and NF-κB during infection. J. Virol. 71:5051-5059. [DOI] [PMC free article] [PubMed] [Google Scholar]