Abstract
Human immunodeficiency virus (HIV) infection has altered both the epidemiology and outcome of enteric opportunistic parasitic infections. This study was done to determine the prevalence and species/genotypes of intestinal coccidian and microsporidial infections among HIV/AIDS patients with diarrhea and/or a history of diarrhea alternately with an asymptomatic interval, and their association with CD4 T cell count. This cross-sectional study was done from May 2010 to May 2011 in Shiraz University of Medical Sciences, South of Iran. A blood sample was obtained from HIV-positive patients for a CD4 T cell count upon enrollment. Sociodemographic data and a history of diarrhea were collected by interviewing 356 consecutive participants (273 males and 83 females). Whenever possible more than a fecal sample was collected from all the participants and examined for parasites using direct, physiological saline solution ethyl acetate, an acid-fast trichrome stain, nested polymerase chain reaction, and sequencing techniques for the detection, confirmation, and genotyping of Cryptosporidium spp., Cyclospora cayetanensis, Isospora belli, and intestinal microsporidia (Enterocytozoon bieneusi). The most common opportunistic and nonopportunistic pathogens were Cryptosporidium spp. (C. parvum and C. andersoni), E. bieneusi, Giardia lamblia, Sarcocystis spp., and Blastocystis homonis affecting 34, 8, 23, 1, and 14 patients, respectively. C. cayetanensis, I. belli, Enterobius vermicularis, and Hymenolepis nana were observed in few patients. A CD4 count <200 cells/μl was significantly associated with the presence of opportunistic parasites and diarrhea (p<0.05). Opportunistic intestinal parasites should be suspected in any HIV/AIDS patient with chronic diarrhea. Tropical epidemic nonopportunistic enteric parasitic infections among such patients should not be neglected in Iran.
Introduction
Enteric opportunistic microorganisms are one of the important etiological agents related to mortality in HIV/AIDS patients.1–2 Diarrhea is one of the most common presenting complaints in HIV-infected patients.1–2 Since the first acquired immunodeficiency syndrome (AIDS) cases were described, a high prevalence of gastrointestinal disturbance has been reported, especially diarrhea associated with parasitosis.2–3 The opportunistic parasites causing HIV/AIDS-associated diarrhea are coccidian parasites and microsporidia.3 Because of the delayed diagnosis of these pathogens in HIV-infected patients, the patients usually take over-the-counter drugs or local medications for symptomatic relief, so the underlying disease is left untreated. In developing countries, these parasites often result in weight loss and wasting syndrome leading to profound morbidity and mortality.4 Identification of these parasites will help the proper management of these patients, because effective drugs are available for the treatment of most of these opportunistic infections.4 To date, up to 14 species in eight genera of microsporidia and 13 Cryptosporidium species/genotypes have been reported to infect humans.5–6 C. hominis is the most prevalent species reported from developing countries, suggestive of anthroponotic transmission.5,7 In contrast, in some developed countries such as the United Kingdom, C. parvum was found to be the most frequent species, indicating the role of zoonotic transmission.5 Other species, C. muris, C. felis, C. canis, C. melagridis, C. andersoni, C. suis, and C. baileyi, have been infrequently reported in children and immunocompromised people.5–6 Among the microsporidian species infecting humans, Enterocytozoon bieneusi is the most increasingly recognized and commonly identified agent for chronic diarrhea in patients with AIDS.2,8 In spite of the current widespread HIV awareness programs, many patients either go undiagnosed or present late with diarrhea resulting from these opportunistic pathogens. The identification of the gut coccidian parasites and the kinds of microsporidia using polymerase chain reaction (PCR) in clinical samples is important in the assessment of treatment, the prognosis, and efforts to reduce dissemination risks among patients in Iran.9–12
Based upon the recent reports published by the Iranian Ministry of Health in its official web site (http://port.health.gov.ir/mfdc/hiv/default.aspx), the detected number of HIV/AIDS patients in Iran was 24,290 individuals as of March 2012. To the best of our knowledge, only a few reports regarding the prevalence of intestinal parasites, especially coccidian, and their association with diarrhea in patients with HIV infection are available from Iran.13–15 There is no information on the incidence of these pathogenic microorganisms causing diarrhea in immunocompromised and immunocompetent patients from Southern Iran, especially Fars province. We aimed to evaluate the prevalence and species/genotypes of the infectious agents causing diarrhea in HIV patients.
Materials and Methods
This study was performed at the laboratory of the Parasitology and Mycology Department of Shiraz University of Medical Sciences, from May 2010 to May 2011. The study's protocol was approved by the Ethics Committee of the university. HIV-infected children and adult patients suffering persistent and/or recurrent diarrhea were included in this study. A total of 356 consecutive patients were enrolled in the study. Of these, 103 patients presented with persistent/chronic diarrhea and 253 did not have diarrhea (asymptomatic interval).
Demographic and clinical data were collected using a structured questionnaire. A fecal sample was collected from each patient and was concentrated by the physiological saline solution ethyl acetate technique for the detection of parasitic ova, cysts, and larvae. Direct smear was also used for detecting trophozoites and cysts. Staining by a modified acid fast trichrome was performed for the determination of coccidian oocysts and microsporidia spores.16 Polymerase chain reaction (PCR) was done using specific PCR primers based on the region coding for the small subunit ribosomal RNA (SSU rRNA) for diagnostic confirmation of Cryptosporidium spp., I. belli, C. cayetanensis, and E. bieneusi on all the stool samples.17–19 Because the internal transcribed spacer (ITS) region of the rRNA has a high degree of diversity among the isolates, it has been useful in many studies for the detection and identification of E. bieneusi genotypes.20 Therefore, DNA extraction was done for all the samples as previously described.21
Further genomic analyses were carried out for amplifications of all fecal samples followed by sequencing. Extracted DNA from fecal specimens was subjected to PCR using primers (designed to ITS2) from those described elsewhere in the I. belli ribosomal gene cluster (GenBank accession no. DQ060680) as follows: forward primer, 5′-CCGAACGTCATCCGAAATAG-3′ and reverse primer, 5′-ACTAGGAGCTGACGATACAC-3′. The presence of a 175-base pair amplicon was considered to indicate I. belli infection.18 A two-step nested PCR protocol was used to amplify the SSU rRNA gene using primers, which amplified sequences unique to all species of the Cryptosporidium genus. A PCR product of 1,300 bp was amplified using forward (5′-′TTCTAGAGCTAATACATGCG-3′) and reverse (5′-CCATTTCCTTCGAAACAGGA-3′) primers. For the secondary nested PCR step, a PCR product that was 836–849 bp long (depending on the species) was amplified using primers (5′-GGAAGGGTTGTATTTATTAGATAAAG-3′ and 5′-CTCATAAGGTGCTGAAGGAGTA-3′).17 A two-step nested PCR was used to amplify the ITS region as well as a portion of the flanking large and small subunit ribosomal RNA genes (400 bp) for the detection of E. bieneusi. The outer primers were EBITS3 (5′-GGTCATAGGGATGAAGAG-3′) and EBITS4 (5′-TTCGAGTTCTTTCGCGCTC-3′) and the inner primers were EBITS1 (5′-GCTCTGAATATCTATGGCT-3′) and EBITS2.4 (5′-ATCGCCGACGGATCCAAGTG-3′). Finally, these reactions produced a fragment of 390 bp.17 Nested PCR for the detection of Cyclospora and Eimeria species was performed using the primer pairs F1E/R2B (5′-TACCCAATGAAAACAGTTT-3′/5′-CAGGAGAAGCCAAGGTAGG-3′) and F3E-R4B (5′-CCTTCCGCGCTTCGCTGCGT-3′/5′-CGTCTTCAAACCCCCTACTG-3′) to generate an amplified fragment of 292 bp.19
All PCR-positive samples were directly sequenced with the inner set of primers used for the secondary PCR. For this purpose, PCR products of the positive samples were then treated with a QIAquick Gel Extraction kit (QIAGEN Gmbdh, D-40724 Hilden, Germany) and sent to the Kowsar Tech Exploration Company (Iran) for sequencing. Sequence chromatograms from each strand were edited and aligned using Gene Runner software (version 3.05). The sequences were compared with sequences in the GenBank database by BLAST analysis. Data were analyzed using SPSS (version 12).
Results
Of the 356 patients with an age range of 10–69 years, 273 (76.68%) were males and 83 (23.31%) females. The mean age of the male and female patients was 38.68 and 35.69 years, respectively. The study population consisted of 51 patients with CD4 count >500 cells/μl, 117 patients with CD4 count 200–500 cells/μl, and 188 patients with CD4 count <200 cells/μl. Among the 188 patients with a CD4 count <200 cells/μl, 53 patients were infected with intestinal parasites and opportunistic parasites were detected in 34 cases. Cryptosporidium (23 patients) was the most common opportunistic pathogen followed by E. bieneusi (eight patients), I. belli (two patients), and C. cayetanensis (one patient) in this group (Table 1) (Figs. 1–5). Of the 117 patients with CD4 count 200–500 cells/μl, pathogenic parasites were identified in 26 patients and opportunistic parasites in eight patients. Pathogenic parasites were detected in only 14 patients with CD4 count >500 cells/μl (Table 1). E. histolytica/E. dispar were found in seven patients (two samples from patients with CD4 count <200 cells/μl, three samples from patients with CD4 count 200–500 cells/μl, and two samples from patients with CD4 count >500 cells/μl) (Table 1).
Table 1.
Enteric Parasites Detected from HIV Patients
| Parasites | CD4<200 cells/μl (n=188) | CD4 200–500 cells/μl (n=117) | CD4>500 cells/μl (n=51) | Total (356) |
|---|---|---|---|---|
| Cryptosporidiuma | 23 | 8 | 3 | 34 |
| Isospora belli | 2 | 0 | 0 | 2 |
| Cyclospora cayetanensis | 1 | 0 | 0 | 1 |
| Microsporidiab | 8 | 0 | 0 | 8 |
| Entameoba histolytica/E. dispar | 2 | 3 | 2 | 7 |
| Giardia lamblia | 9 | 9 | 5 | 23 |
| Blastocystis hominis | 8 | 5 | 1 | 14 |
| Enterobius vermicularis | 0 | 1 | 2 | 3 |
| Hymenolepis nana | 0 | 0 | 1 | 1 |
| Total | 53/356 (14.890%) | 26/356 (7.30%) | 14/356 (3.93%) | 93/356 (26.12%) |
Confirmed as C. parvum and C. andersoni by PCR sequencing using specific primers of the SSU-rRNA gene.
Confirmed as Enterocytozoon bieneusi (genotypes D and K) by PCR sequencing using primers of the ITS region of the rRNA gene.
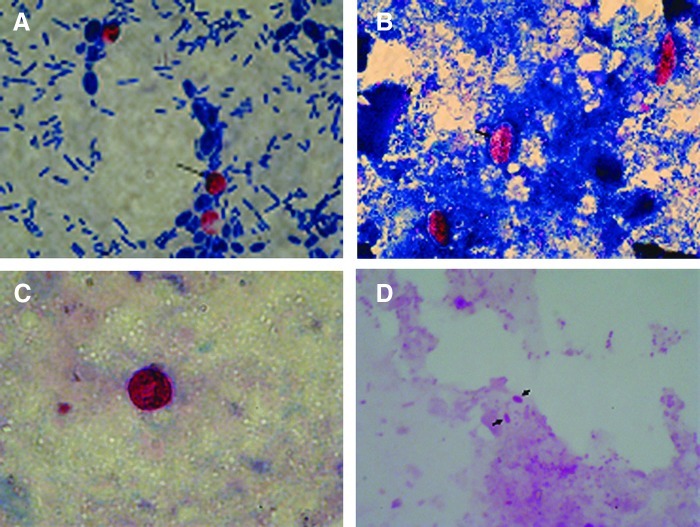
FIG. 1.
Photomicrographs of fecal smears. (A) Oocysts of Cryptosporidium spp. in acid-fast stain (1000×). (B) Oocysts of Isospora belli in acid-fast stain (1000×). (C) Oocysts of Cyclospora cayetanensis in acid-fast stain (1000×). (D) Spores of Enterocytozoon bienusei in acid-fast trichrome (1000×). Color images available online at www.liebertpub.com/aid
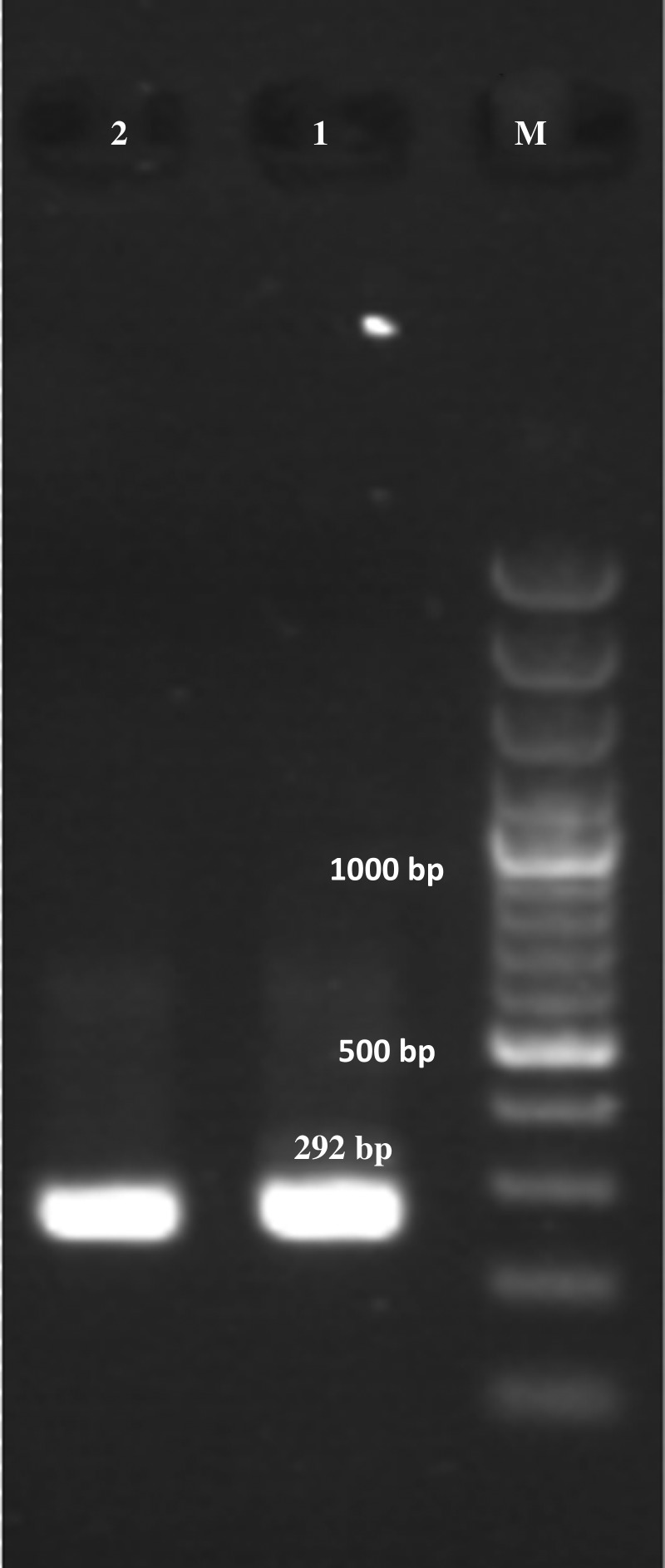
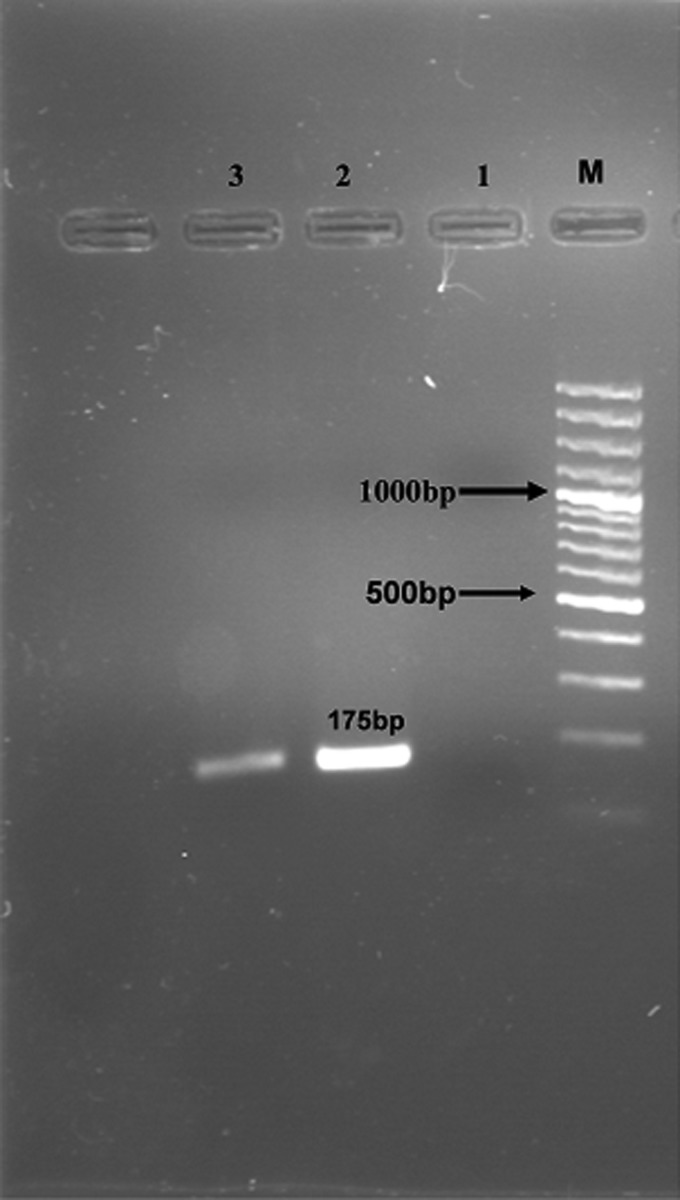
FIG. 5.
Nested PCR amplification of Cyclospora cayetanensis DNA. Lane M: molecular size markers; lanes 1 and 2: positive control and study specimen.
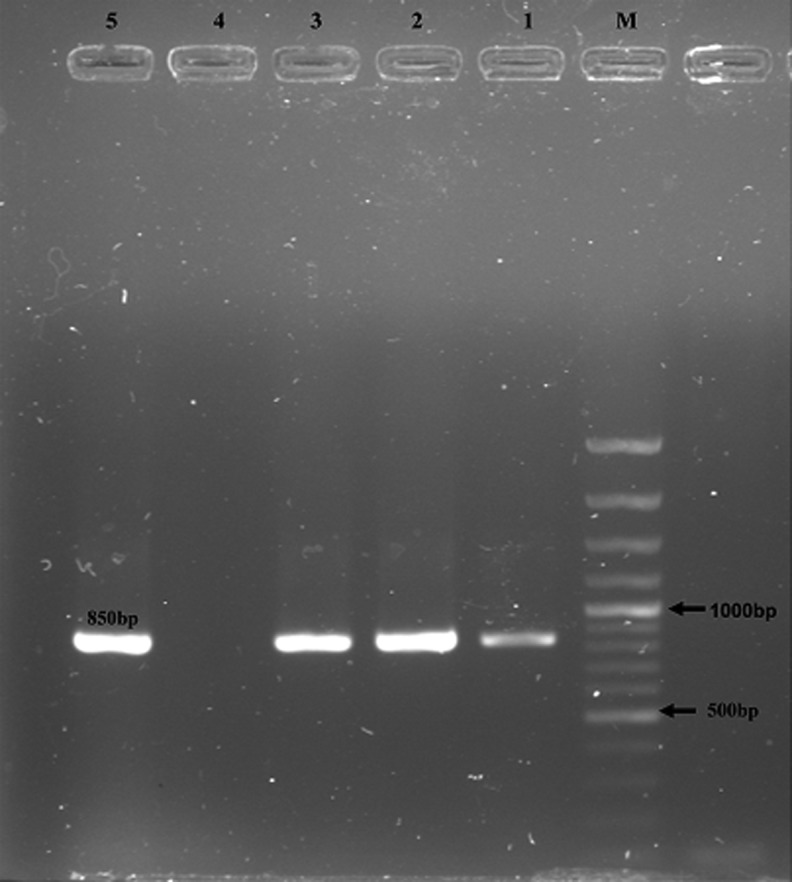
FIG. 3.
Detection of Cryptosporidium-specific fragments by nested PCR. Lanes: M, molecular marker; 1–3, positive samples; 4–5, negative and positive control samples, respectively. The molecular weight marker was a 100-bp ladder.
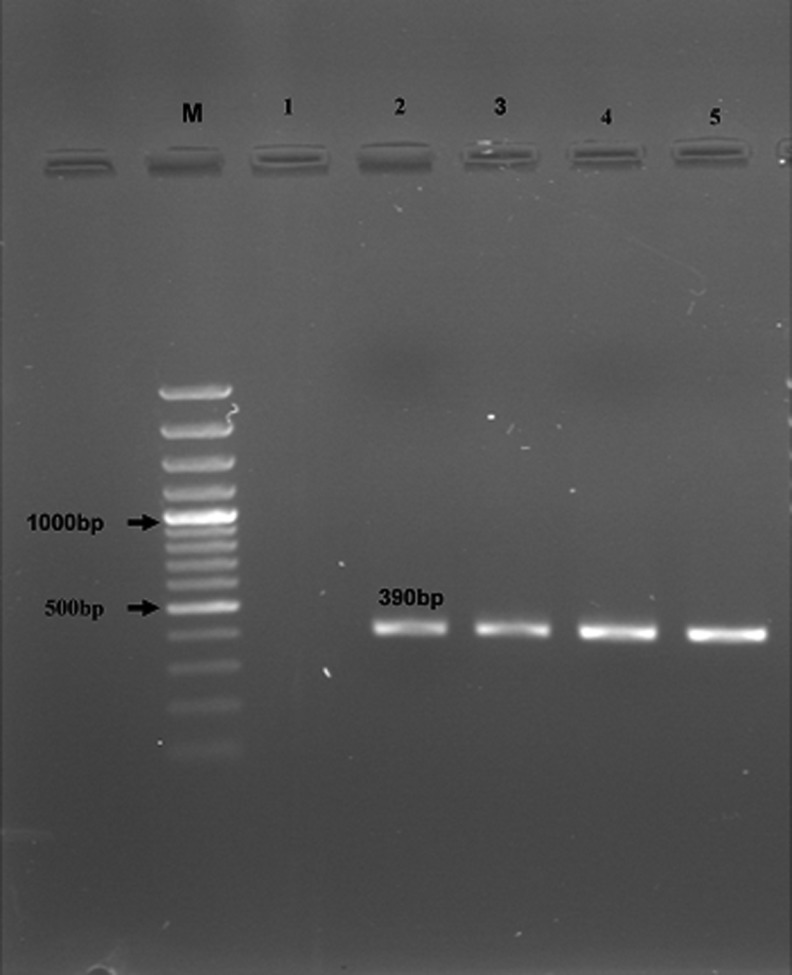
In microscopy-positive stool samples, E. bieneusi spores were found to be oval measuring 1–1.5 μm in size (Fig. 1). PCR amplification of E. bieneusi DNA was done in all samples of 356 patients by nested PCR using the species-specific primers (Fig. 4). Of the 356 HIV-positive patients evaluated in this study, eight with persistent chronic diarrhea were found to be positive for E. bieneusi using microscopic examination and the nested PCR technique. E. bieneusi (genotypes D and K) were detected in all eight positive cases without coinfection with other enteric parasites. No microscopic detection or PCR amplification was found from HIV-positive patients with asymptomatic intervals who were negative for E. bieneusi (n=253). It must be emphasized that infections with intestinal nonpathogenic parasites are not shown here. The dominant species of Cryptosporidium was C. parvum (33 out of 34 patients), and only one patient was infected with C. andersoni. G. lamblia (23 patients) and B. hominis (14 patients) were the more prevalent nonopportunistic pathogens in our study (Table 1). Genotyping of E. bieneusi from patients with diarrhea by sequence analysis of the ITS region showed genotypes D and K. The great proportion of opportunistic pathogens in patients with CD4 count <200 cells/μl was significantly higher than that in the other two groups of patients with CD4 count >200 cells/μl (p<0.05).
FIG. 4.
Nested PCR-amplified products of Enterocytozoon bieneusi from stool samples (M: 100-bp MW; lanes 1 and 2: negative and positive control, respectively; lanes 3–5: positive samples).
In this particular group, the most common opportunistic and nonopportunistic pathogens were Cryptosporidium spp., E. bieneusi, G. lamblia, Sarcocystis spp., and B. homonis, observed in 34, 8, 23, 1, and 14 patients, respectively. The few helminthes, E. vermicularis and H. nana, were observed in three patients and one patient, respectively (Table 1). Overall, out of 356 HIV-infected patients, enteric pathogenic parasites were detected in 93 (26.12%) stool samples, of which 45 patients had opportunistic and 48 had nonopportunistic pathogenic parasites.
Discussion
Numerous opportunistic infections occur in HIV-infected patients, because of the down-regulation of the immune system.22 Gastrointestinal opportunistic parasitic infections are a universally recognized problem in such patients. These infections mostly present with diarrhea, leading to life-threatening complications.11,23 Unfortunately, the diarrhea caused by these parasites cannot be differentiated from that caused by other enteric infectious agents such as G. lamblia, noninvasive bacterial infections (e.g., enterotoxigenic Escherichia coli), and viral infections (e.g., Norwalk and Rotavirus).1,4 Diarrhea is a significant problem in immunocompromised patients worldwide, especially patients with HIV/AIDS with CD4 counts <200 cells.24
Previous studies have demonstrated that C. parvum is frequently associated with diarrhea in patients with AIDS with CD4 counts <200 cells in developed countries where the prevalence of infection ranges from 17% to 62%.25–26 In our study, we used the genus/species-specific PCR primers for the detection of the enteric coccidia and E. bieneusi in stool samples (Figs. 2–5). The DNA extraction method in combination with a modified physiological saline solution ethyl acetate sedimentation procedure may result in increasing sensitivity, raising the number of oocysts and spores. False-positive results and cross-amplification were not observed. An increase in oocysts and spore concentration by sedimentation method has also been reported in another study.
FIG. 2.
PCR products of Isospora belli on electrophoresis on 1.5% agarose gel. Lane M: 100 bp DNA size marker. Lanes 1–2: negative and positive control, respectively. Lane 3: positive sample.
The interpretation of genotyping results with sufficient concern is highly recommended and is the basis for the detection of the sources of infection. For example, in two studies in India, the Cryptosporidium mouse genotype was identified in a child and an HIV-positive adult based on the results of RFLP analysis of the SSU rRNA PCR products.27–28 The true identity of the parasite was probably C. meleagridis, which has the same RFLP pattern as the mouse genotype in the genotyping technique used. The detection of C. andersoni in a Malawian child was also largely based on RFLP analysis,29 which needs to be confirmed by DNA sequencing because DraI and AseI RFLP is mainly used in species differentiation and C. muris may also share the same pattern. Although the microscopic examination of feces still remains the mainstay of laboratory diagnosis of cryptosporidiosis, the results indicate that PCR is more effective than microscopic examination for the detection of coccidiosis.
To determine if there was a correlation between the prevalence of coccidian and microsporidia parasites and CD4 counts, we calculated the mean CD4+ T cell counts in these patients and we found that CD4 counts <200 cells, especially CD4 counts less than 100 cells, were significantly associated with the presence of opportunistic parasites and diarrhea. In the present study, microscopic stool examination and PCR techniques showed that 45 (12.64%) patients were infected with enteric opportunistic parasites. Among the opportunistic parasites, C. parvum is the predominant pathogen in some countries, similar to what was found in our study.5 The prevalence of opportunistic parasites in patients with CD4 counts <200 cells/μl was 18.08% (34/188 patients). In our study, C. parvum infection was detected in 34/356 (9.55%) patients using microscopic examination and a molecular method.
The use of parasitological combination with molecular methods in our study suggests that the prevalence of Cryptosporidium spp. infections in HIV-infected patients may be significantly greater than reported previously. DNA sequencing analysis of SSU rRNA of Cryptosporidium spp. showed that except for one case, all isolates were C. parvum. The occurrence of zoonotic genotypes of Cryptosporidium in all of the cases in the present study indicates that zoonotic transmission is of considerable significance in the epidemiology of cryptosporidiosis in the studied region. The presence of cryptosporidial infections in patients with HIV/AIDS in our study is probably related to an increased risk of acquiring infection from contact with infected animals and prolonged excretion, which in turn increases the risk of subsequent transmission. In these patients, the risk of acquiring cryptosporidiosis is associated with the degree of immune suppression as measured by CD4 cell counts. Other risk factors for cryptosporidiosis in our patients with HIV/AIDS include gender, age, personal health, occupation, and level of education.
Studies from Nairobi and London demonstrate that cryptosporidiosis is a common opportunistic enteric protozoan disease encountered in HIV/AIDS-infected individuals.2,30,31 This is supported by a recent prospective, comparative study comparing the prevalence of enteric protozoa among HIV-positive and HIV-negative men in Australia.2,32 A total of 1,868 inpatients submitted stool specimens over a 36-month period to determine the presence of enteric parasites.2 In this study C. parvum cases occurred exclusively in HIV-positive patients. In our survey I. belli, C. cayetanensis, and E. bieneusi were detected in two, one, and eight patients, respectively. I. belli is found worldwide, but occurs predominantly in tropical and subtropical climates and is endemic in South America, Africa, and Southern Asia, and immunodeficiency was shown to increase the susceptibility to infection with I. belli.33–35 Since the use of trimethoprim-sulfamethoxazole to prevent Pneumocystis jirovecii in developed countries, there has been a marked reduction in the occurrence and clinical course of isosporiasis.
The lower prevalence of isosporiasis in developed countries and Iran and especially in this area may be ascribed to the secondary prophylaxis for pneumocystosis through the administration of sulfamethoxazole-trimethoprim during the course of AIDS, since I. belli and C. cayetanensis are sensitive to this treatment. In contrast, in developing countries, I. belli infections are frequently accompanied by chronic diarrhea in patients with HIV/AIDS, occurring in 5–26% of these individuals.33–34 Also, since the introduction of highly active antiretroviral therapy (HAART), there has been evidence of improvement in chronic diarrhea caused by enteric coccidian.36 Although these changes have been attributed to the restoration of cell-mediated immunity, it has been suggested that some antiretroviral compounds used in HAART may have a direct inhibitory effect on Cryptosporidium and I. belli.37 E. bieneusi is the most common microsporidian causing diarrhea in humans and the second most prevalent in immunocompromised patients, especially those with HIV/AIDS, after Cryptosporidium.2,38
In countries with access to HAART, the prevalence of microsporidial infections has declined. In a study from Australia, the total incidence of intestinal microsporidiosis in HIV-infected patients decreased from 11% in 1995 to 0% in 2004.39 In contrast, in developing countries because of limited access to HAART, the incidence of coccidiosis and microsporidiosis still remains high. Therefore, because of the use of HAART in most of our patients with HIV/AIDS, a low prevalence of microsporidiosis, isosporiasis, and cyclosporiasis was seen. To the best of our knowledge, this study is the first report of genotypes of E. bieneusi using sequencing from our country. A few studies from Iran have reported a prevalence ranging from 1.5% to 9.4% of enteric coccidiosis in patients with HIV/AIDS.13,15 Both C. parvum and C. hominis have been reported from HIV-positive adult patients using genetic tools.40 Thus, the dramatic decline of enteric microsporidiosis, isosporiasis, and cyclosporiasis in our study confirms the importance of effective HAART in preventing advanced immunodeficiency, opportunistic parasitic infections, and associated AIDS-related deaths.
A few studies from other regions of Iran have reported a prevalence of enteric parasites in patients with HIV/AIDS ranging from 17.2% to 18.4%.13–15 In our study, nonopportunistic parasites such as G. lamblia and B. hominis were detected in 10.39% (23 and 14 patients, respectively) of all HIV-positive patients across different CD4 groups, which emphasizes the need for early detection and treatment of such infections among HIV-infected patients to reduce the morbidity. The results of our study highlight the importance of the evaluation of intestinal parasitic infections, especially enteric coccidian and microsporidia, in HIV-infected individuals with diarrhea for intestinal parasitic infections, which may help to improve the management of these patients. The etiology of diarrhea could not be determined in most patients, suggesting a need for comprehensive etiological studies covering bacterial, fungal, viral, and parasitic causes of diarrhea among HIV-infected patients in Iran. Also, the importance of tropical and subtropical endemic nonopportunistic intestinal parasitic infections among HIV-infected patients should not be neglected. Therefore, our study emphasizes the need for routine screening of enteric coccidiosis as well as education about practicing personal hygiene and taking timely and appropriate measures.
In conclusion, understanding the species and genotypes of intestinal coccidia and microsporidia can also be useful in establishing the identity of the parasites infecting humans, assessment of the public health significance of these pathogens from animals and the environment, and tracking of infection and contamination sources. All these will promote understanding of the transmission and epidemiology of human coccidiosis and the development of preventive measures to minimize exposures to infections. It could finally lead to strengthening the assessment of accurate risk in high-risk groups.
Acknowledgments
This article is based in part on the Ph.D. thesis of Mahmoud Agholi, which was financially supported by the office of the Vice-Chancellor for Research of Shiraz University of Medical Sciences (Grant 4859). The authors would also like to acknowledge the office of the Vice-Chancellor of Health for its kind support.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bhaijee F. Subramony C. Tang SJ. Pepper DJ. Human immunodeficiency virus-associated gastrointestinal disease: Common endoscopic biopsy diagnoses. Pathol Res Int. 2011;26:247. doi: 10.4061/2011/247923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stark D. Barratt JL. van Hal S. Marriott D. Harkness J. Ellis JT. Clinical significance of enteric protozoa in the immunosuppressed human population. Clin Microbiol Rev. 2009;22:634–650. doi: 10.1128/CMR.00017-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dwivedi KK. Prasad G. Saini S. Mahajan S. Lal S. Baveja UK. Enteric opportunistic parasites among HIV infected individuals: Associated risk factors and immune status. Jpn J Infect Dis. 2007;60:76–81. [PubMed] [Google Scholar]
- 4.Pawlowski SW. Warren CA. Guerrant R. Diagnosis and treatment of acute or persistent diarrhea. Gastroenterology. 2009;136:1874–1886. doi: 10.1053/j.gastro.2009.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao L. Molecular epidemiology of cryptosporidiosis: An update. Exp Parasitol. 2010;124:80–89. doi: 10.1016/j.exppara.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Xiao L. Ryan UM. Cryptosporidiosis: An update in molecular epidemiology. Curr Opin Infect Dis. 2004;17:483–490. doi: 10.1097/00001432-200410000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Hunter PR. Nichols G. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clin Microbiol Rev. 2002;15:145–154. doi: 10.1128/CMR.15.1.145-154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santín M. Fayer R. Microsporidiosis: Enterocytozoon bieneusi in domesticated and wild animals. Res Vet Sci. 2011;90:63–71. doi: 10.1016/j.rvsc.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Colford JM., JR Tager IB. Hirozawa AM. Lemp GF. Aragon T. Petersen C. Cryptosporidiosis among patients infected with human immunodeficiency virus. Factors related to symptomatic infection and survival. Am J Epidemiol. 1996;144:807–816. doi: 10.1093/oxfordjournals.aje.a009015. [DOI] [PubMed] [Google Scholar]
- 10.Sorvillo F. Beall G. Turner PA. Beer VL. Kovacs AA. Kraus P, et al. Seasonality and factors associated with cryptosporidiosis among individuals with HIV infection. Epidemiol Infect. 1998;121:197–204. doi: 10.1017/s0950268898001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khalakdina A. Tabnak F. Sun RK. Colford JM:, Jr Race/ethnicity and other risk of factors associated with cryptosporidiosis as an initial AIDS-defining condition in California 1980–1999. Epidemiol Infect. 2001;127:535–543. doi: 10.1017/s0950268801006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inungu JN. Morse AA. Gordon C. Risk factors, seasonality, and trends of cryptosporidiosis among patients infected with human immunodeficiency virus. Am J Trop Med Hyg. 2000;62:384–387. doi: 10.4269/ajtmh.2000.62.384. [DOI] [PubMed] [Google Scholar]
- 13.Daryani A. Sharif M. Meigouni M. Mahmoudi FB. Rafiei A. Gholami Sh, et al. Prevalence of intestinal parasites and profile of CD4+ counts in HIV+/AIDS people in north of Iran, 2007–2008. Pak J Biol Sci. 2009;15:1277–1281. doi: 10.3923/pjbs.2009.1277.1281. [DOI] [PubMed] [Google Scholar]
- 14.Nahrevanian H. Assmar M. Cryptosporidiosis in immunocompromised patients in the Islamic Republic of Iran. J Microbiol Immunol Infect. 2008;41:74–77. [PubMed] [Google Scholar]
- 15.Zali MR. Mehr AJ. Rezaian M. Meamar AR. Vaziri S. Mahraz M. Prevalence of intestinal parasitic pathogens among HIV-positive individuals in Iran. Jpn J Infect Dis. 2004;57:268–270. [PubMed] [Google Scholar]
- 16.Reisner BS. Spring J. Evaluation of a combined acid-fast-trichrome stain for detection of microsporidia and Cryptosporidium parvum. Arch Pathol Lab Med. 2000;124:777–779. doi: 10.5858/2000-124-0777-EOACAF. [DOI] [PubMed] [Google Scholar]
- 17.Santín M. Trout JM. Vecino JA. Dubey JP. Fayer R. Cryptosporidium, Giardia and Enterocytozoon bieneusi in cats from Bogota (Colombia) and genotyping of isolates. Vet Parasitol. 2006;141:334–339. doi: 10.1016/j.vetpar.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Walther Z. Topazian MD. Isospora cholangiopathy: Case study with histologic characterization and molecular confirmation. Hum Pathol. 2009;40:1342–1346. doi: 10.1016/j.humpath.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Orlandi PA. Lampel KA. Extraction-free, filter-based template preparation for rapid and sensitive PCR detection of pathogenic parasitic protozoa. J Clin Microbiol. 2000;38:2271–2277. doi: 10.1128/jcm.38.6.2271-2277.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santín M. Fayer R. Enterocytozoon bieneusi genotype nomenclature based on the internal transcribed spacer sequence: A consensus. J Eukaryot Microbiol. 2009;56:34–38. doi: 10.1111/j.1550-7408.2008.00380.x. [DOI] [PubMed] [Google Scholar]
- 21.Amar C. Pedraza-Díaz S. McLauchlin J. Extraction and genotyping of Cryptosporidium parvum DNA from fecal smears on glass slides stained conventionally for direct microscope examination. J Clin Microbiol. 2001;39:401–403. doi: 10.1128/JCM.39.1.401-403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva CV. Ferreira MS. Borges AS. Costa-Cruz JM. Intestinal parasitic infections in HIV/AIDS patients: Experience at a teaching hospital in central Brazil. Scand J Infect Dis. 2005;37:211–215. doi: 10.1080/00365540410020875. [DOI] [PubMed] [Google Scholar]
- 23.Chui DW. Owen RL. AIDS and the gut. J Gastroenterol Hepatol. 1994;9:291–303. doi: 10.1111/j.1440-1746.1994.tb01729.x. [DOI] [PubMed] [Google Scholar]
- 24.Kulkarni SV. Kairon R. Sane SS. Padmawar PS. Kale VA. Thakar MR, et al. Opportunistic parasitic infections in HIV/AIDS patients presenting with diarrhea by the level of immune suppression. Indian J Med Res. 2009;130:63–66. [PubMed] [Google Scholar]
- 25.Kurniawan A. Karyadi T. Dwintasari SW. Sari IP. Yunihastuti E. Djauzi S, et al. Intestinal parasitic infections in HIV/AIDS patients presenting with diarrhea in Jakarta, Indonesia. Trans R Soc Trop Med Hyg. 2009;103:892–898. doi: 10.1016/j.trstmh.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Lee JK. Song HJ. Yu JR. Prevalence of diarrhea caused by Cryptosporidium parvum in non HIV patients in Jeollanam-do, Korea. Korean J Parasitol. 2005;43:111–114. doi: 10.3347/kjp.2005.43.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muthusamy D. Rao SS. Ramani S. Monica B. Banerjee I. Abraham OC, et al. Multilocus genotyping of Cryptosporidium sp. isolates from HIV infected individuals in South India. J Clin Microbiol. 2006;44:632–634. doi: 10.1128/JCM.44.2.632-634.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ajjampur SS. Gladstone BP. Selvapandian D. Muliyil JP. Ward H. Kang G. Molecular and spatial epidemiology of cryptosporidiosis in children in a semi urban community in South India. J Clin Microbiol. 2007;45:915–920. doi: 10.1128/JCM.01590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morse TD. Nichols RA. Grimason AM. Campbell BM. Tembo KC. Smith HV. Incidence of cryptosporidiosis species in pediatric patients in Malawi. Epidemiol Infect. 2007;135:1307–1315. doi: 10.1017/S0950268806007758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dryden MS. Shanson DC. The microbial causes of diarrhea in patients infected with the HIV. J Infect. 1988;17:107–114. doi: 10.1016/s0163-4453(88)91515-0. [DOI] [PubMed] [Google Scholar]
- 31.Mwachari CW. Meier AS. Muyodi J. Gatei W. Waiyaki P. Cohen CR. Chronic diarrhea in HIV-1-infected adults in Nairobi, Kenya: Evaluation of risk factors and the WHO treatment algorithm. AIDS. 2003;26:2124–2126. doi: 10.1097/01.aids.0000088182.01779.09. [DOI] [PubMed] [Google Scholar]
- 32.Stark D. Fotedar R. Van Hal S. Beebe N. Marriott D. Ellis JT, et al. Prevalence of Enteric protozoa in HIV positive and HIV-negative men who have sex with men from Sydney, Australia. Am J Trop Med Hyg. 2007;76:549–552. [PubMed] [Google Scholar]
- 33.Waywa D. Kongkriengdaj S. Chaidatch S. Tiengrim S. Kowadisaiburana B. Chaikachonpat S, et al. Protozoan enteric infection in AIDS related diarrhea in Thailand. Southeast Asian J Trop Med Public Health. 2001;32:151–155. [PubMed] [Google Scholar]
- 34.Lebbad M. Norrgren H. Nauclér A. Dias F. Andersson S. Linder E. Intestinal parasites in HIV-2 associated AIDS cases with chronic diarrhea in Guinea-Bissau. Acta Trop. 2001;80:45–49. doi: 10.1016/s0001-706x(01)00142-5. [DOI] [PubMed] [Google Scholar]
- 35.Vignesh R. Balakrishnan P. Shankar EM. Murugavel KG. Hanas S. Cecelia AJ, et al. High proportion of isosporiasis among HIV-infected patients with diarrhea in Southern India. Am J Trop Med Hyg. 2007;77:823–824. [PubMed] [Google Scholar]
- 36.Bachur TP. Vale JM. Coêlho IC. Queiroz TR. Chaves Cde S. Enteric parasitic infections in HIV/AIDS patients before and after the highly active antiretroviral therapy. Braz J Infect Dis. 2008;12:115–122. doi: 10.1590/s1413-86702008000200004. [DOI] [PubMed] [Google Scholar]
- 37.Pozio E. Morales MA. The impact of HIV-protease inhibitors on opportunistic parasites. Trends Parasitol. 2005;21:58–63. doi: 10.1016/j.pt.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Raccurt CP. Fouché B. Agnamey P. Menotti J. Chouaki T. Totet A, et al. Presence of Enterocytozoon bieneusi associated with intestinal coccidia in patients with chronic diarrhea visiting an HIV center in Haiti. Am J Trop Med Hyg. 2008;79:579–580. [PubMed] [Google Scholar]
- 39.van Hal SJ. Muthiah K. Matthews G. Harkens J. Stark D. Cooper D, et al. Declining incidence of intestinal microsporidiosis and reduction in AIDS-related mortality following introduction of HAART in Sydney, Australia. Trans R Soc Trop Med Hyg. 2007;101:1096–1100. doi: 10.1016/j.trstmh.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Meamar AR. Guyot K. Certad G. Dei-Cas E. Mohraz M. Mohebali M, et al. Molecular characterization of Cryptosporidium isolates from humans and animals in Iran. Appl Environ Microbiol. 2007;73:1033–1035. doi: 10.1128/AEM.00964-06. [DOI] [PMC free article] [PubMed] [Google Scholar]