Abstract
Presenting episodes of intermittent viremia (EIV) under combination antiretroviral therapy (cART) is frequent, but there exists some controversy about their consequences. They have been described as inducing changes in immune responses potentially associated with a better control of HIV infection. Conversely, it has been suggested that EIV increases the risk of virological failure. A retrospective analysis of a prospective, randomized double-blinded placebo-controlled study was performed. Twenty-six successfully treated HIV-infected adults were randomized to receive an immunization schedule or placebo, and after 1 year of follow-up cART was discontinued. The influence of EIV on T cell subsets, HIV-1-specific T cell immune responses, and viral load rebound, and the risk of developing genotypic mutations were evaluated, taking into account the immunization received. Patients with EIV above 200 copies/ml under cART had a lower proportion of CD4+ and CD4+CD45RA+RO− T cells, a higher proportion of CD8+ and CD4+CD38+HLADR+ T cells, and higher HIV-specific CD8+ T cell responses compared to persistently undetectable patients. After cART interruption, patients with EIV presented a significantly higher viral rebound (p=0.007), associated with greater increases in HIV-specific lymphoproliferative responses and T cell populations with activation markers. When patients with EIV between 20 and 200 copies/ml were included, most of the differences disappeared. Patients who present EIV above 200 copies/ml showed a lower CD4+ T cell count and higher activation markers under cART. After treatment interruption, they showed greater specific immune responses against HIV, which did not prevent a higher virological rebound. EIV between 20 and 200 copies/ml did not have this deleterious effect.
Introduction
One objective of cART is to reach the highest viral suppression, assessed through the undetectability of plasmatic viral load (VL). However, 46% of patients present episodes of intermittent viremia (EIV), transient relapses, or “blips,” that is detectable VL that appear under combination antiretroviral therapy (cART) and that become undetectable a short time after.1–5
There exists some controversy about the real significance and consequences of EIV. It has been considered that it is both clinically irrelevant and could induce changes in immune responses, accelerate viral evolution, and jeopardize the long-term effectiveness of cART. On the one hand, patients with EIV present higher levels in both the magnitude and the breadth of total HIV-specific CD8+ and CD4+ T cell responses compared to persistently undetectable patients.6–9 These responses could help to control viral replication8 and they would be associated with higher CD4+ T cell counts4 without increasing the risk of virological failure.1,3,4,10–14 On the other hand, it has also been shown that EIV increases T cell activation and therefore facilitates the extension of HIV infection.13,15,16 Moreover, EIV could be associated with the development of mutations that confer resistance to cART10,14,15 and, therefore, to an increased risk of virological failure.5,15 Finally, one observational study evaluated the influence of EIV in the control of viral replication after cART interruption, and found a higher viral rebound among patients who had EIV (“blippers”).7
To explore the influence of EIV on T cell subsets, HIV-specific T cell responses, and viral rebound before and after discontinuing cART, we reanalyzed the data of a prospective study evaluating the influence of vaccination on VL rebound and immune responses, and where immunizations were not associated with an increase in detectable VL.
Materials and Methods
Study design
Data from a clinical trial evaluating the effect of a vaccination schedule in HIV-infected individuals was retrospectively analyzed.17 Briefly, the trial was a prospective, randomized, double-blinded, placebo-controlled study performed at the Hospital Clínic of Barcelona, Spain, from April 2003 to July 2006. Twenty-six HIV-infected patients successfully treated under cART were randomized to receive during 12 months either a vaccination program or placebo. The vaccination program included seven different usually recommended vaccines against 10 different agents: hepatitis B (Engerix B, Smithkline bF1 Beecham SA; months 0, 1, 2, and 6), hepatitis A (Havrix 1440, Smithkline Beecham SA; months 4 and 10), influenza (2003–2004 WHO recommended vaccine [A/New Caledonia/20/99 (H1N1), A/Moscow/10/99 (H3N2), and B/Hong Kong/330/2001]; month 1), pneumococcus (Pneumo 23, Aventis Pasteur MSD SA; month 2), varicella (Varilrix, Smithkline Beecham SA; months 4 and 6), measles-mumps-rubella (Priorix, Smithkline Beecham SA; month 8), and tetanus-diphtheria (Ditanrix Adult, Smithkline Beecham SA; month 10). The placebo group received the same doses of placebo (0.5 ml of saline solution) at the same months. On month 12, cART was interrupted in both groups. Blood samples were taken monthly during treatment and after interruption. All patients provided written informed consent. The study was approved by the institutional ethics review boards and was registered in the public clinical trials database of the NIH (number NCT00329251). In that previous study we concluded that immunizations were not associated with an increase in EIV. Once the study was finished we retrospectively reclassified patients into patients with EIV (those who present at least one detectable VL during the cART period) and patients without EIV (those whose VL was persistently under the level of detectability).
Virological evaluations
Plasma HIV-1 RNA levels were determined using the Amplicor HIV-1 Monitor Ultra Sensitive Specimen Preparation Protocol Ultra Direct Assay (Roche Molecular Systems, Inc., Somerville, NJ) with a limit of quantification of 200 copies/ml. Samples below the detection limits were retested with a lower limit of detection of 20 copies/ml. Population-based genotypic resistance testing was performed during treatment every time VL rose over 1,000 copies/ml and with the first VL over 1,000 copies/ml after cART interruption, with use of the TruGene Assay (Visible Genetics).
Immunological evaluations
Several immunological parameters were evaluated in order to explore the influence of EIV on the immunological system, especially over the CD4+ and CD8+ T cell subsets and their response against HIV.
Different T cell subsets were determined as previously described using three-color flow cytometry.18 Briefly, peripheral blood mononuclear cells (PBMCs) were obtained by separation on Ficoll Hypaque centrifugation gradient. Samples containing 105 cells were used for direct staining with different monoclonal antibodies (Becton Dickinson, Mountain View, CA). The stained cells were analyzed on a FacSCalibur (Becton Dickinson, San Jose, CA) flow cytometer. Data were analyzed using CellQuest software.
PBMC proliferation assays [lymphoproliferative responses (LPR)] were performed essentially as previously described.19,20 Briefly, cells were cultured in the absence or presence of phytohemagluttinin (PHA) 0.5 and 1% 90 μg/ml (Murex, Biotech Ltd, England), OKT3 10 ng/ml (Ortho Biotech Inc., Raritan, NJ), anti-CD28 100 μg/ml, pokeweed mitogen 10 μg/ml (Sigma, St Louis, MO), Tetanus toxoid 2750 U, cytomegalovirus (CMV) antigen 10 μg/ml, and 5 μg/ml of HIV-1 antigens gp160 and p24 (Protein Sciences, Meriden, CT). Incorporation of tritium-labeled thymidine was assessed for the last 18 h of culture (Betaplate LKB Wallac, Sweden). Results were expressed as mean counts per minute (cpm). The stimulation index (SI) was calculated for each sample as cpm for cells with stimulus/cpm for cells without stimulus. A positive response to polyclonal stimulation was considered when the SI was greater than 15. Positive antigen-specific responses were defined as more than 3,000 cpm and an SI greater than 3.
An ELISpot assay (enzyme-linked immunospot assay) was used to measure HIV epitope-specific CD8+ T cell interferon-γ release from cryopreserved PBMC samples, as previously described.21–23 Briefly, PBMCs were plated in the presence of different HLA class I-restricted synthetic peptides from gag, pol, env, and nef proteins. Spot-forming cells (SFC) were counted using an AID ELISpot reader (Autoimmun Diagnostica GmHb, Germany). After subtracting background counts obtained with PBMCs and medium alone, results were normalized to SFC/106 PBMCs. A positive response was considered when counts were >40 SFC/106 PBMCs.
Statistical analysis
The analysis was done for two different limits of detectability: 200 and 20 copies/ml. Virological and immunological data were analyzed comparing both groups (patients with EIV vs. patients without EIV) taking into account the immunizations received. Continuous variables are reported as medians and interquartile ranges. Comparisons between groups were made by using the Mann–Whitney U test for continuous data and the χ2 or Fisher's exact test for qualitative data. Correlations were studied by using Spearman's rank correlation. Changes and durability in quantitative variables were analyzed by an area-under-the curve (AUC) measurement that incorporated the baseline value. Two-sided tests were considered significant when p<0.05.
Results
Clinical characteristics
The 26 patients included had a median age of 39 years (interquartile range 25–58). There were 21 men and 5 women, and their main risk factor for HIV infection was sexual contact (92%). With the assay with a limit of quantification of 200 copies/ml there were 10 patients with some detectable VL and 16 persistently undetectable. There were no significant differences between the detectable and undetectable group in clinical baseline characteristics (Table 1). Even the proportion of vaccinated and placebo patients was balanced (50% in each group). However, the undetectable group included all the women in the study and presented a higher proportion of patients treated with inhibitors or protease (IP).
Table 1.
Baseline Characteristics of Patients Included in the Study
| |
Limit of detectability≥200 copies/ml |
Limit of detectability≥20 copies/ml |
||||
|---|---|---|---|---|---|---|
| Variablea | Undetectable group (n=16) | Detectable group (n=10) | p | Undetectable group (n=12) | Detectable group (n=14) | p |
| Age (years) | 38.86 (34.33–49.79) | 38.58 (29.93–41.49) | 0.55 | 39.35 (33.58–49.79) | 38.46 (32.27–41.49) | 0.59 |
| Gender (men:women) | 11:5 | 10:0 | 0.12b | 7:5 | 14:0 | 0.012b |
| Hepatitis C virus, n (%) | 2 (12.5) | 2 (20) | 0.62b | 1 (8.33) | 3 (21.43) | 0.6b |
| Risk factor, n (%) | ||||||
| Homosexual | 9 (56.25) | 5 (50) | 1b | 6 (50) | 8 (57.14) | 0.97 |
| Heterosexual | 6 (37.5) | 3 (30) | 1b | 5 (41.67) | 4 (28.57) | 0.68b |
| Intravenous drug user | 1 (6.25) | 2 (20) | 0.54b | 1 (8.33) | 2 (14.28) | 1b |
| Months of known HIV infection | 69.7 (26.08–109.26) | 40.15 (21.93–172.94) | 0.98 | 79.85 (35–118.23) | 40.15 (21.93–125.8) | 0.5 |
| Months on cART | 45.50 (14.55–73.42) | 17.71 (16.21–67.68) | 0.64 | 52.40 (15.29–75.84) | 17.71 (15.18–67.68) | 0.29 |
| ≥3 previous strategies of treatment, n (%) | 7 (43.75) | 3 (30) | 0.69b | 5 (41.67) | 5 (35.71) | 1b |
| Previous mono or bitherapy, n (%) | 3 (18.75) | 1 (10) | 1b | 3 (25) | 1 (7.14) | 0.31 |
| cART includes inhibitor of protease, n (%) | 7 (43.75) | 7 (70) | 0.25b | 4 (33.33) | 10 (71.42) | 0.12 |
| Peak viral load (log10 copies/ml) | 4.97 (3.94–5.5) | 4.85 (4.71–5.25) | 0.62 | 4.9 (3.8–5.5) | 4.87 (4.71–5.25) | 0.9 |
| Nadir CD4+ T cells | ||||||
| Absolute (cells/mm3) | 434 (384–522) | 411 (336.5–551.5) | 0.64 | 434 (388.25–522) | 411 (340.5–551.5) | 0.69 |
| Percentage | 24.5 (20.65–30.82) | 22 (15.3–26.5) | 0.28 | 27.75 (23.7–32.97) | 22 (15.3–25.5) | 0.02 |
| Received vaccination during study, n (%) | 8 (50) | 5 (50) | 1b | 6 (50) | 7 (50) | 1 |
These are expressed as the median and interquartile range for quantitative variables and the number of patients and percentage for categorical variables.
Fisher's exact test.
cART, combination antiretroviral therapy.
Differences in virological and immunological parameters during the cART period in patients with EIV above 200 copies/ml
Virological changes
There were 314 determinations of VL. Twenty-two of them were detectable above 200 copies/ml [incidence of 0.07 detectable VL/determination (95% CI 0.044–0.104)]. Of them, six were isolated episodes and the remaining 18 appeared in four clusters of consecutive determinations (from 2 to 6). The range of magnitude of detectable VL was 210–51,200 copies/ml, median 544.5 copies/ml (Fig. 1A). Seven of these determinations (31.8%) were related either to a discontinuation or a decrease in adherence to cART. We did not find other potential causes for the remaining EIV.
FIG. 1.
(A) Samples of plasma viral load during treatment (months 0 to 12) with an assay with a limit of detection of 200 copies/ml. There were 22 detectable determinations. (B) Evolution of viral load (VL) in the detectable and undetectable group. After combined antiretroviral therapy (cART) interruption (month 12), detectable patients presented a higher peak VL (5.33 vs. 4.68 log10, p=0.022), a higher VL in month 14 (4.85 vs. 4.08 log10, p=0.005), and a higher AUC of VL after the interruption (13.51 vs. 9.57, p=0.007) (■ and black line, detectable group; Δ and gray line, undetectable group). Median and interquartile range are shown.
We did not find clinical relevant mutations associated with EIV. Only one patient presented an M184V mutation during treatment. It was associated with a high increase in VL (51,200 copies/ml), which the patient reported was due to a decrease in cART adherence to 75%. He was taking lamivudine, didanosine, and nelfinavir, and lamivudine was changed to tenofovir and the VL returned to undetectable levels after 1 month.
Changes in T cell subsets, LPR, and HIV-specific CD8+ T cell responses
At baseline and/or during treatment, patients with EIV above 200 copies/ml had a lower proportion of CD4+(p=0.047, 0.053, 0.012, and 0.055 in months 3, 6, 9, and 12, respectively) and CD4+CD45RA+RO− T cells and a higher proportion of CD8+ and CD4+CD38+HLADR+ T cells compared to persistently undetectable patients (Table 2). There were no differences in other T cell subsets, although at baseline a trend to lower CD8+CD38+ corrected after it was found.
Table 2.
Changes in Immune Parameters in the Detectable (≥200 Copies/ml) and Undetectable Group in Month 0 (Baseline), 12, and 18 (After 6 Months of Combination Antiretroviral Therapy Interruption)
| |
Detectable group (≥200 copies/ml) (n=16) |
Undetectable group (<200 copies/ml) (n=10) |
||||
|---|---|---|---|---|---|---|
| Parametersa | Month 0 | Month 12 | Month 18 | Month 0 | Month 12 | Month 18 |
| T cell subsets (%) | ||||||
| CD4+ | 33.63 (30.78–37.88) | 33.62 (25.31–39.55) | 21.08 (17.02–33.65) | 40.36 (34.42–48.17) | 41.12 (36.91–44.06) | 29.01 (24.52–35.63) |
| CD4+CD28+ | 95.21 (90.16–98.80) | 96.34 (91.00–98.57) | 96.73 (92.01–98.54) | 93.26 (83.57–98.62) | 93.7 (88.26–99.55) | 95.33 (88.56–98.03) |
| CD4+CD38+ | 41.67 (28.67–52.60) | 35.77 (30.28–59.73) | 52.01 (42.11–62.73) | 45.61 (41.69–54.32) | 52.46 (43.75–60.28) | 51.83 (44.41–64.57) |
| CD4+CD38+HLADR+ | 2.15 (1.20–2.41) | 1.87 (1.14–3.59) | 3.30 (2.30–7.46) | 2.03 (1.43–3.09) | 1.91 (1.42–3.88) | 4.31 (2.76–4.92) |
| CD4+CD45RA+CD45RO− | 28.57 (21.50–35.70) | 30.50 (20.50–38.71) | 36.60 (24.69–38.34) | 39.59 (27.92–45.68) | 39.71 (32.39–49.05) | 42.47 (35.11–45.81) |
| CD4+CD45RA−CD45RO+ | 50.85 (39.93–63.09) | 63.66 (47.14–74.77) | 58.13 (54.53–68.06) | 48.45 (40.77–56.70) | 52.94 (42.49–64.40) | 54.66 (42.44–62.12) |
| CD8+ | 40.81 (36.46–46.48) | 34.52 (31.40–39.37) | 46.75 (40.62–56.66) | 37.03 (31.88–43.17) | 31.86 (26.13–38.96) | 39.76 (33.28–50.19) |
| CD8+CD28+ | 51.18 (45.36–57.75) | 52.82 (49.16–57.29) | 42.57 (38.13–54.98) | 48.26 (41.51–59.65) | 52.39 (46.45–67.80) | 50.76 (43.10–52.57) |
| CD8+CD38+ | 22.55 (16.70–31.36) | 35.09 (20.98–42.17) | 54.21 (50.95–62.41) | 34.78 (23.99–39.07)* | 34.91 (30.77–48.41) | 59.77 (45.77–67.46) |
| CD8+CD38+HLADR+ | 5.28 (3.39–7.03) | 8.89 (6.36–11.64) | 26.43 (18.55–36.16) | 7.03 (3.66–9.05) | 9.92 (5.25–11.71) | 26.65 (15.20–35.65) |
| CD8+CD45RA+CD45RO− | 55.08 (42.09–65.96) | 48.52 (44.61–63.71) | 37.03 (32.05–56.43) | 56.45 (42.49–60.69) | 55.09 (42.10–64.89) | 37.18 (31.07–48.59) |
| CD8+CD45RA−CD45RO+ | 26.82 (22.78–33.46) | 31.34 (24.20–41.73) | 50.63 (30.07–56.80) | 28.29 (25.64–44.58) | 33.47 (25.89–44.71) | 45.77 (36.13–58.96) |
| LPR againstb | ||||||
| PHA 1% | 10.82 (1.32–16.16) | 15.26 (6.09–38.06) | 23.23 (13.91–33.37) | 3.75 (0.67–12.55) | 8.42 (0.84–26.71) | 26.20 (6.54–37.29) |
| CMV antigens | 1.8 (1.02–1.99) | 1.22 (0.54–3.96) | 2.44 (1.64–3.36) | 2.59 (1.56–3.49)* | 1.62 (1.02–2.87) | 2.64 (1.30–4.23) |
| gp160 HIV | 1.41 (1.04–3.40) | 1.47 (0.91–2.49) | 1.16 (0.82–1.88) | 1.23 (0.93–2.04) | 1.27 (0.92–1.89) | 1.45 (0.88–1.81) |
| p24 HIV | 1.52 (1.09–10.86) | 1.52 (1.10–3.26) | 1.99 (0.95–2.84) | 1.91 (1.06–4.35) | 1.22 (0.82–1.96) | 1.72 (1.49–2.18) |
| HIV-specific CD8+ responsesc | ||||||
| Total | 742 (208–1,990.5) | 787 (206.5–1,235) | 1244 (436–2,250) | 306 (151–775.25) | 427 (250–1,023) | 440 (158–2,220) |
| Number of peptides | 2 (1–4) | 3 (1–3.5) | 2 (2–6) | 3 (1.75–4) | 3 (2–5) | 3 (1–5) |
| p24 | 506 (142.5–1,844) | 275 (45.5–956.5) | 926 (80–3,113) | 244 (30–542.75) | 382 (77–642) | 320 (80–954) |
| Small | 114 (89.5–560.5) | 168 (0–374) | 49 (0–1,035) | 0 (0–112)* | 61 (0–217) | 0 (0–421.5) |
| p17 | 283 (57–1,073) | 150 (0–544) | 382 (60–1,863) | 66.5 (0–208.25) | 46 (0–300) | 83 (24–526.5) |
| gag | 687 (610–3,433) | 820 (236–1,470) | 1711 (300–5,023) | 518 (93.25–728.75) | 515 (313–826) | 606 (108–2,263.5) |
These are expressed as the median and interquartile range.
Measured by stimulation index.
Measured by spot-forming cells/106 peripheral blood mononuclear cells (SFC/106 PBMCs).
p<0.05 for the comparison between groups in that month.
LPR, lymphoproliferative responses; PHA, phytohemagluttinin; CMV, cytomegalovirus.
There were no differences between groups in LPR to polyclonal mitogens or to recall and HIV antigens (Table 2).
The detectable group showed higher HIV-specific CD8+ T cell responses at baseline both in the magnitude of the responses [against gag peptides p1, p2, p6, and p7 (small pool) p=0.014, total gag peptides pool p=0.059; p17 p=0.085, total specific CD8+ T cell responses p=0.25] and in the breadth of responses (p=0.032 for the quotient between total specific CD8+ T cell responses and number of peptides). These differences were maintained and in some cases increased during treatment (Table 2).
Differences in virological and immunological parameters after cART interruption
Virological changes
Undetectable and detectable patients presented a significantly different evolution. Although viral rebound was present in both groups, it was higher in the detectable patients as defined by a higher peak VL (5.33 vs. 4.68 log10, p=0.022), a higher VL at month 14 (2 months after interruption) (4.85 vs. 4.08 log10, p=0.005), and a higher AUC of VL after interruption (13.51 vs. 9.57, p=0.007) (Fig. 1B). We did not find clinical relevant mutations.
Changes in T cell subsets, LPR, and HIV-specific CD8+ T cell responses
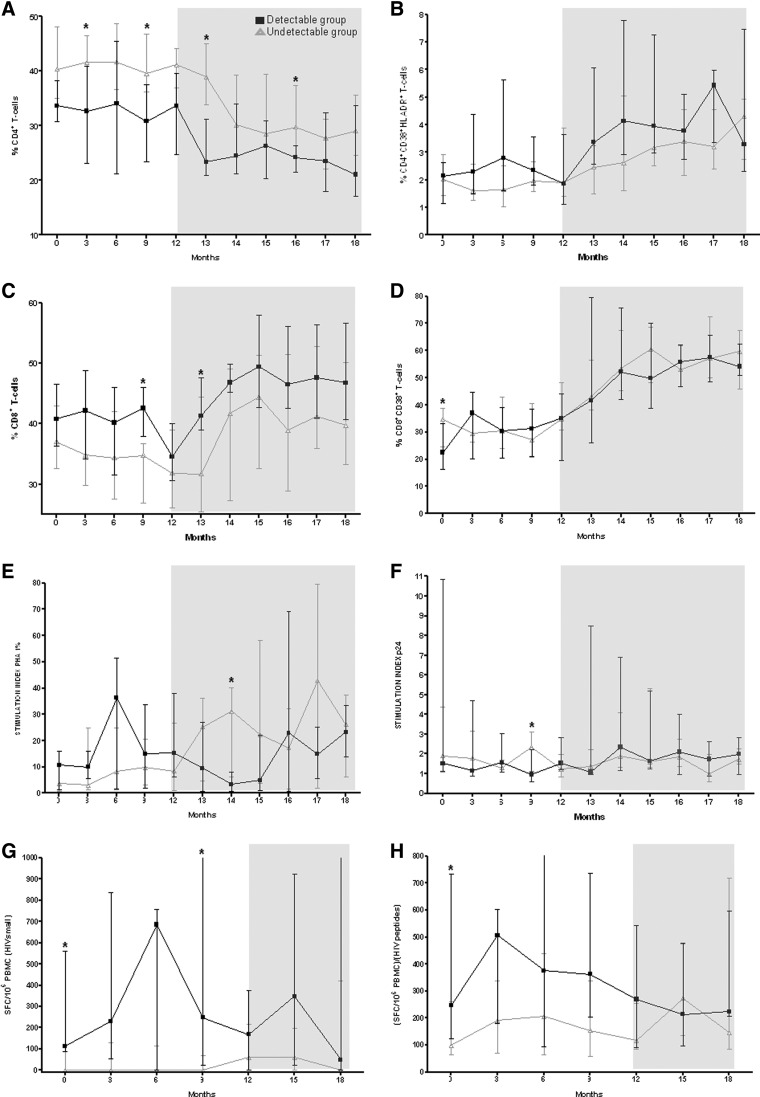
Some T cell subsets had a different evolution in the detectable and undetectable group, mainly in the initial period of interruption (months 12 to 14). CD4+ T cells dropped significantly more and sooner in the detectable group (p=0.026 for comparison of change between groups); meanwhile T cells with activation markers such as CD4+CD38+ and CD4+CD38+HLADR+ increased significantly more and sooner during the early phase of this period in these patients (p=0.038 and 0.007, respectively, for the comparison of change between groups), as well as CD8+CD38+ and CD8+CD38+HLADR+ (but not significantly). However, all these differences tended to disappear at month 18 (6 months after cART interruption) (Fig. 2 and Table 2).
FIG. 2.
Evolution of immunological parameters during the study period. Months 0 to 12, patients were on cART. At month 12 cART was discontinued (shady area) [■ and black line, detectable (≥200 copies/ml) group; Δ and gray line, undetectable group]. (A–D) T cell subsets (CD4+, CD4+CD38+HLADR+, CD8+, and CD8+CD38+). (E–F) Lymphoproliferative responses against phytohemagluttinin (PHA) 1% and HIV p24. (G–H) HIV-specific CD8+ T cell responses against gag peptides p1, p2, p6, and p7 (small pool) and quotient between whole CD8+ T cell responses (SFC/106 PBMCs) and number of positive peptides detected. Median and interquartile range are shown.
LPR presented some differences between groups, mainly in the earlier period. The detectable group tended to present a greater increase in specific anti-HIV LPR responses, whereas LPR against polyclonal mitogens tended to increase more among the undetectable group. However, the differences were not significant (Fig. 2 and Table 2).
Finally, HIV-specific CD8+ T cell responses in undetectable patients presented a greater and earlier increase, tending to reach the level of response in the detectable group (p=0.045 for the differences among months 12 and 14 in the quotient of total responses and number of peptides) (Fig. 2 and Table 2).
Substudy with patients with EIV above 20 copies/ml
When samples with VL below 200 copies/ml (293 determinations) were reanalyzed with an assay with a limit of detection of 20 copies/ml, the total number of detectable VL increased to 49 determinations (incidence of 0.156, 95% CI 0.12–0.2), with a range of 21–51,200 copies/ml and a median of 210 copies/ml. Four new patients were then considered detectable (n=14).
All previous variables were reassessed in this new scenario. All women enrolled in the study (n=5) were persistently undetectable and 50% of each group had received the vaccination program. In addition to that, the nadir CD4+ T cell count was higher in the undetectable group (Table 1).
However, most of the differences that had been found previously between the detectable and undetectable group decreased and lost significance. Virological rebound, changes in T cell subsets, LPR responses, and HIV-specific CD8+ T cell responses, both during treatment and after its interruption, were more similar between groups (data not shown).
Discussion
Some studies have suggested that EIV did not have any influence on the virological and immunological outcome.2,4,10,24 Conversely, other authors report that EIV could improve HIV-specific responses, positively influencing the clinical outcome in HIV-infected patients.1,3,11–14 We have analyzed the data from a comparative double-blinded placebo-controlled study from a schedule of different usually recommended vaccines in HIV-infected patients who had monthly blood sampling during 18 months (including 12 months on cART and 6 months of cART interruption) to further address this issue.17 We have observed that patients with EIV above 200 copies/ml presented important virological and immunological differences (both during and after the interruption of cART) compared to persistently undetectable patients (<200 copies/ml). Patients with EIV have a worse CD4+ T cell count recovery, in parallel with an increased level of activation markers. These findings likely explain the higher rebound of viral load after cART interruption despite presenting a higher level of HIV-specific responses. Finally, it seems that EIV between 20 and 200 copies/ml did not have this deleterious effect.
The baseline clinical characteristics were similar between groups and we did not find differences in some aspects previously reported to be associated with the presence of EIV, such as age,15 current cART regimen,4,5,11–13,24 and previous cART regimen.1,2,5,11,12,15,25 We did not find a clear cause of the EIV in 68.2% of the cases, as opposed to other previous publications in which 75.8% of patients did present a detectable cause.15 In fact, although EIV had previously been identified as markers of low adherence, it has been demonstrated that this statement was wrong,11,13,24 except for greater increases in VL.11 It is noteworthy that 50% of patients in each group received vaccinations, and that three cases of EIV (13.6%) were preceded by a vaccination, but not the remaining 22.
Detectable patients showed immunological differences under cART compared to previously described undetectable patients. They had less CD4+ T cells13,15,16 and an increase in CD4+CD38+HLADR+ T cells.16 After cART discontinuation, we also found that the detectable group presented a higher viral rebound, similar to the only previous work that explored this issue.7 These data support the hypothesis that a high level of immune activation (and not the absolute number of CD4+ T cells) is the force that fuels viral replication.
These findings could have two possible explanations (or a combination of them). On the one hand, patients who present EIV can shelter a more aggressive virus, with a higher replication capacity. This would induce EIV, and then, due to higher replication and activation, a CD4+ T cells loss, an increase in cells with activation markers, and a higher viral rebound. Previous findings of an association between EIV and pre-cART VL or the time needed to reach undetectability, as well as the reduction in number of EIV with intensification strategies, support this hypothesis.1,15,26–28 On the other hand, detectable patients could have a less efficient immune system in some of the antiviral mechanisms (cellular or humoral, specific or not) due to either a genetic predisposition or to greater devastation induced by HIV previous to the initiation of cART. Therefore, immunological findings will be the cause and not the consequence of EIV. Studies that have demonstrated an association between a higher incidence of EIV and a lower CD4+ T cell count before initiating cART support this idea.12,26,28
Detectable patients showed higher HIV-specific CD8+ T cell responses under cART, as previously described.6,7,9 Although it has been hypothesized that these responses would help to control viral replication,8,29–31 we have demonstrated in one previous work7 that these patients presented a higher viral rebound after cART discontinuation. This apparent paradox could be explained by the existence of parallel dysfunctions in innate immune responses,32,33 clonal depletion,34 or impaired CD8+ T cell function.35 Another possible explanation is the absence of differences in LPR responses specific against HIV that we have found (which have also been described as being increased in detectable patients6,7,9 in other works). It is probable that the increase in HIV-specific responses seen in detectable patients, both CD4+ and CD8+, was more a consequence of more antigen availability than a strategy of the immune system to improve viral control.
Our findings could have important clinical applications. If a patient presents with an EIV over 200 copies/ml despite having a good adherence to cART, this could be a marker of “worse outcome” and a predictor of failure. Although there is controversy about the association between EIV and virological failure,1,3,4,10–14,36,37 this is probably because virological suppression induced by current treatments is potent enough to limit the consequences of these EIV in the short-term. But it is possible that in longer time periods detectable patients finally presented some differences in outcome, as we have found that their immunological responses are different. Larger prospective studies are needed to confirm this issue, and to determine if there is a higher risk of clinical events in patients with EIV (i.e., non-AIDS-associated events such as a higher risk of cancer, cardiovascular events, or neurological disorders); optimization or cART in order to reduce EIV or complementary treatments to control its consequences (higher level of immune activation) would be required.38
Interestingly, when patients were reclassified with a lower limit of detectability (20 copies/ml), we found that most immunological and virological differences between detectable and undetectable patients decreased and disappeared. Two interpretations of this finding are possible. On the one hand, it is possible that some of the EIV over 20, but not over 200 copies/ml, are the result of technique or biological variability and therefore they do not reflect a “real” increase in VL. Some studies have demonstrated that repeated analysis of the same sample can show variations to 40%,24,39–41 in particular when VL is low or depending on the assay used.42,43 On the other hand, some patients presented several low level EIV, which is contrary to a random variation.44 Thus, another possible explanation is that patients who present with very low-level EIV are the ones who will really benefit from a controlled virus exposition, large enough to increase specific responses, but small enough to avoid the deleterious effects of activation and increase of replication. In any case, there were only four patients, which limits every hypothesis that could be made about this.
Therefore different levels of EIV could have different implications, depending mainly on their magnitude and number of consecutive determinations. Those around the current limit of detection of 50 copies/ml and that occur only once may not be predictors of future failure45 and could even have some positive effect. Conversely, a higher EIV, especially over 200 copies/ml, will be deleterious. A previous study suggested a significant increase in risk of virological failure in patients whose EIV is over 120 copies/ml.37
This study has several limitations. First, the most important is the sample size, which could be too small to find differences in some variables. Second, patients included in our study were in a “preserved” immune status because of inclusion criteria. This could influence the incidence of EIV and therefore the changes they induce. Also, this is a retrospective analysis of data from a clinical trial designed to evaluate the effect of a vaccination schedule on HIV immunological responses and EIV. However, we previously demonstrated that EIV was not associated with such vaccinations, vaccinated and placebo patients were balanced between detectable and undetectable patients, and the immunizations received are those currently prescribed for the HIV population. We employed an assay with a limit of quantification of 200 copies/ml because it was the one used in the clinical routine when the trial was done. Viral load targets have evolved over the years, dropping to the current goal of <50 copies/ml.46 However, we continue to see patients with EIV>200 copies/ml in clinical practice, and the results found in this study are also valid for them. Finally, the follow-up time is short as we have analyzed EIV only during the 12 months under cART.
In conclusion, patients who present with an EIV above 200 copies/ml had a lower CD4+ T cell count and higher activation markers under cART. After treatment interruption, they showed higher specific immune responses against HIV that did not prevent a higher virological rebound. It seems that an EIV level between 20 and 200 copies/ml does not have this deleterious effect.
Acknowledgments
This study was supported in part by grants ISCIII-RETIC RD06/006, FIPSE 36536/05, SAF 05/05566, FIS PI050058, FIT 090100-2005-9, FIS PI050265, FIS 04/0503, and FIS 07/0291. Dr. Felipe García was a recipient of a research grant from IDIBAPS, Barcelona, Spain. Dr. Montserrat Plana is a researcher from the Institut d'Investigacions Biomèdiques August Pi I Sunyer (IDIBAPS) and is supported by the ISCIII (Instituto de Salud Carlos III) and the Health Department of the Catalan Government (Generalitat de Catalunya).
FIPSE is a nonprofit foundation including the Spanish Ministry of Health, Abbott Laboratories, Boehringer Ingelheim, Bristol Myers Squibb, GlaxoSmithKline, Merck Sharp and Dohme, and Roche.
FIS is the Red Temática Cooperativa de Grupos de Investigación en Sida del Fondo de Investigación Sanitaria.
Part of the information contained in this article has been previously presented at the 13th Conference on Retroviruses and Opportunistic Infections (February 2006, Denver, Colorado).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Havlir DV. Bassett R. Levitan D, et al. Prevalence and predictive value of intermittent viremia with combination HIV therapy. JAMA. 2001;286:171–179. doi: 10.1001/jama.286.2.171. [DOI] [PubMed] [Google Scholar]
- 2.Raboud JM. Rae S. Woods R. Harris M. Montaner JS. Consecutive rebounds in plasma viral load are associated with virological failure at 52 weeks among HIV-infected patients. AIDS. 2002;16:1627–1632. doi: 10.1097/00002030-200208160-00008. [DOI] [PubMed] [Google Scholar]
- 3.Mira JA. Macias J. Nogales C, et al. Transient rebounds of low-level viraemia among HIV-infected patients under HAART are not associated with virological or immunological failure. Antivir Ther. 2002;7:251–256. doi: 10.1177/135965350200700404. [DOI] [PubMed] [Google Scholar]
- 4.Sklar PA. Ward DJ. Baker RK, et al. Prevalence and clinical correlates of HIV viremia ('blips') in patients with previous suppression below the limits of quantification. AIDS. 2002;16:2035–2041. doi: 10.1097/00002030-200210180-00008. [DOI] [PubMed] [Google Scholar]
- 5.Greub G. Cozzi-Lepri A. Ledergerber B, et al. Intermittent and sustained low-level HIV viral rebound in patients receiving potent antiretroviral therapy. AIDS. 2002;16:1967–1969. doi: 10.1097/00002030-200209270-00017. [DOI] [PubMed] [Google Scholar]
- 6.Karlsson AC. Younger SR. Martin JN, et al. Immunologic and virologic evolution during periods of intermittent and persistent low-level viremia. AIDS. 2004;18:981–989. doi: 10.1097/00002030-200404300-00005. [DOI] [PubMed] [Google Scholar]
- 7.Papasavvas E. Kostman JR. Thiel B, et al. HIV-1-specific CD4+ T cell responses in chronically HIV-1 infected blippers on antiretroviral therapy in relation to viral replication following treatment interruption. J Clin Immunol. 2006;26:40–54. doi: 10.1007/s10875-006-7518-8. [DOI] [PubMed] [Google Scholar]
- 8.Ortiz GM. Hu J. Goldwitz JA, et al. Residual viral replication during antiretroviral therapy boosts human immunodeficiency virus type 1-specific CD8+ T-cell responses in subjects treated early after infection. J Virol. 2002;76:411–415. doi: 10.1128/JVI.76.1.411-415.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alatrakchi N. Duvivier C. Costagliola D, et al. Persistent low viral load on antiretroviral therapy is associated with T cell-mediated control of HIV replication. AIDS. 2005;19:25–33. doi: 10.1097/00002030-200501030-00003. [DOI] [PubMed] [Google Scholar]
- 10.Cohen Stuart JW. Wensing AM. Kovacs C, et al. Transient relapses (“blips”) of plasma HIV RNA levels during HAART are associated with drug resistance. J Acquir Immune Defic Syndr. 2001;28:105–113. doi: 10.1097/00042560-200110010-00001. [DOI] [PubMed] [Google Scholar]
- 11.Miller LG. Golin CE. Liu H, et al. No evidence of an association between transient HIV viremia (“blips”) and lower adherence to the antiretroviral medication regimen. J Infect Dis. 2004;189:1487–1496. doi: 10.1086/382895. [DOI] [PubMed] [Google Scholar]
- 12.Sungkanuparph S. Overton ET. Seyfried W, et al. Intermittent episodes of detectable HIV viremia in patients receiving nonnucleoside reverse-transcriptase inhibitor-based or protease inhibitor-based highly active antiretroviral therapy regimens are equivalent in incidence and prognosis. Clin Infect Dis. 2005;41:1326–1332. doi: 10.1086/496985. [DOI] [PubMed] [Google Scholar]
- 13.Martinez V. Marcelin AG. Morini JP, et al. HIV-1 intermittent viraemia in patients treated by non-nucleoside reverse transcriptase inhibitor-based regimen. AIDS. 2005;19:1065–1069. doi: 10.1097/01.aids.0000174453.55627.de. [DOI] [PubMed] [Google Scholar]
- 14.Macias J. Palomares JC. Mira JA, et al. Transient rebounds of HIV plasma viremia are associated with the emergence of drug resistance mutations in patients on highly active antiretroviral therapy. J Infect. 2005;51:195–200. doi: 10.1016/j.jinf.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Easterbrook PJ. Ives N. Waters A, et al. The natural history and clinical significance of intermittent viraemia in patients with initial viral suppression to <400 copies/ml. AIDS. 2002;16:1521–1527. doi: 10.1097/00002030-200207260-00009. [DOI] [PubMed] [Google Scholar]
- 16.Ostrowski SR. Katzenstein TL. Thim PT, et al. Low-level viremia and proviral DNA impede immune reconstitution in HIV-1-infected patients receiving highly active antiretroviral therapy. J Infect Dis. 2005;191:348–357. doi: 10.1086/427340. [DOI] [PubMed] [Google Scholar]
- 17.Castro P. Plana M. Gonzalez R, et al. Influence of a vaccination schedule on viral load rebound and immune responses in successfully treated HIV-infected patients. AIDS Res Hum Retroviruses. 2009;25(12):1249–1259. doi: 10.1089/aid.2009.0015. [DOI] [PubMed] [Google Scholar]
- 18.Libois A. Lopez A. Garcia F, et al. Dynamics of T cells subsets and lymphoproliferative responses during structured treatment interruption cycles and after definitive interruption of HAART in early chronic HIV Type-1-infected patients. AIDS Res Hum Retroviruses. 2006;22:657–666. doi: 10.1089/aid.2006.22.657. [DOI] [PubMed] [Google Scholar]
- 19.Garcia F. Plana M. Ortiz GM, et al. The virological and immunological consequences of structured treatment interruptions in chronic HIV-1 infection. AIDS. 2001;15:F29–F40. doi: 10.1097/00002030-200106150-00002. [DOI] [PubMed] [Google Scholar]
- 20.Plana M. Garcia F. Gallart T, et al. Lack of T-cell proliferative response to HIV-1 antigens after 1 year of highly active antiretroviral treatment in early HIV-1 disease. Immunology Study Group of Spanish EARTH-1 Study. Lancet. 1998;352:1194–1195. doi: 10.1016/s0140-6736(05)60532-6. [DOI] [PubMed] [Google Scholar]
- 21.Oxenius A. Price DA. Easterbrook PJ, et al. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc Natl Acad Sci USA. 2000;97:3382–3387. doi: 10.1073/pnas.97.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plana M. Garcia F. Oxenius A, et al. Relevance of HIV-1-specific CD4+ helper T-cell responses during structured treatment interruptions in patients with CD4+ T-cell nadir above 400/mm3. J Acquir Immune Defic Syndr. 2004;36:791–799. doi: 10.1097/00126334-200407010-00005. [DOI] [PubMed] [Google Scholar]
- 23.Garcia F. Lejeune M. Climent N, et al. Therapeutic immunization with dendritic cells loaded with heat-inactivated autologous HIV-1 in patients with chronic HIV-1 infection. J Infect Dis. 2005;191:1680–1685. doi: 10.1086/429340. [DOI] [PubMed] [Google Scholar]
- 24.Nettles RE. Kieffer TL. Kwon P, et al. Intermittent HIV-1 viremia (blips) and drug resistance in patients receiving HAART. JAMA. 2005;293:817–829. doi: 10.1001/jama.293.7.817. [DOI] [PubMed] [Google Scholar]
- 25.Moore AL. Youle M. Lipman M, et al. Raised viral load in patients with viral suppression on highly active antiretroviral therapy: Transient increase or treatment failure? AIDS. 2002;16:615–618. doi: 10.1097/00002030-200203080-00013. [DOI] [PubMed] [Google Scholar]
- 26.Di Mascio M. Markowitz M. Louie M, et al. Viral blip dynamics during highly active antiretroviral therapy. J Virol. 2003;77:12165–12172. doi: 10.1128/JVI.77.22.12165-12172.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramratnam B. Ribeiro R. He T, et al. Intensification of antiretroviral therapy accelerates the decay of the HIV-1 latent reservoir and decreases, but does not eliminate, ongoing virus replication. J Acquir Immune Defic Syndr. 2004;35:33–37. doi: 10.1097/00126334-200401010-00004. [DOI] [PubMed] [Google Scholar]
- 28.De Rosa SC. Lu FX. Yu J, et al. Vaccination in humans generates broad T cell cytokine responses. J Immunol. 2004;173:5372–5380. doi: 10.4049/jimmunol.173.9.5372. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz JE. Kuroda MJ. Santra S, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 30.Pontesilli O. Kerkhof-Garde S. Notermans DW, et al. Functional T cell reconstitution and human immunodeficiency virus-1-specific cell-mediated immunity during highly active antiretroviral therapy. J Infect Dis. 1999;180:76–86. doi: 10.1086/314837. [DOI] [PubMed] [Google Scholar]
- 31.Papasavvas E. Grant RM. Sun J, et al. Lack of persistent drug-resistant mutations evaluated within and between treatment interruptions in chronically HIV-1-infected patients. AIDS. 2003;17:2337–2343. doi: 10.1097/00002030-200311070-00008. [DOI] [PubMed] [Google Scholar]
- 32.Chehimi J. Campbell DE. Azzoni L, et al. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J Immunol. 2002;168:4796–4801. doi: 10.4049/jimmunol.168.9.4796. [DOI] [PubMed] [Google Scholar]
- 33.Azzoni L. Papasavvas E. Chehimi J, et al. Sustained impairment of IFN-gamma secretion in suppressed HIV-infected patients despite mature NK cell recovery: Evidence for a defective reconstitution of innate immunity. J Immunol. 2002;168:5764–5770. doi: 10.4049/jimmunol.168.11.5764. [DOI] [PubMed] [Google Scholar]
- 34.Pantaleo G. Soudeyns H. Demarest JF, et al. Evidence for rapid disappearance of initially expanded HIV-specific CD8+ T cell clones during primary HIV infection. Proc Natl Acad Sci USA. 1997;94:9848–9853. doi: 10.1073/pnas.94.18.9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Appay V. Nixon DF. Donahoe SM, et al. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Napravnik S. Edwards D. Stewart P, et al. HIV-1 drug resistance evolution among patients on potent combination antiretroviral therapy with detectable viremia. J Acquir Immune Defic Syndr. 2005;40(1):34–40. doi: 10.1097/01.qai.0000174929.87015.d6. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Gasco P. Maida I. Blanco F, et al. Episodes of low-level viral rebound in HIV-infected patients on antiretroviral therapy: Frequency, predictors and outcome. J Antimicrob Chemother. 2008;61(3):699–704. doi: 10.1093/jac/dkm516. [DOI] [PubMed] [Google Scholar]
- 38.Buzon MJ. Massanella M. Llibre JM, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16(4):460–465. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 39.Jackson JB. Piwowar-Manning E. Johnson-Lewis L, et al. Comparison of versions 1.0 and 1.5 of the UltraSensitive AMPLICOR HIV-1 MONITOR test for subjects with low viral load. J Clin Microbiol. 2004;42:2774–2776. doi: 10.1128/JCM.42.6.2774-2776.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brambilla D. Reichelderfer PS. Bremer JW, et al. The contribution of assay variation and biological variation to the total variability of plasma HIV-1 RNA measurements. The Women Infant Transmission Study Clinics. Virology Quality Assurance Program. AIDS. 1999;13:2269–2279. doi: 10.1097/00002030-199911120-00009. [DOI] [PubMed] [Google Scholar]
- 41.Schockmel GA. Yerly S. Perrin L. Detection of low HIV-1 RNA levels in plasma. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:179–183. doi: 10.1097/00042560-199702010-00013. [DOI] [PubMed] [Google Scholar]
- 42.Smit E. Bhattacharya S. Osman H. Taylor S. Increased frequency of HIV-1 viral load blip rate observed after switching from Roche Cobas Amplicor to Cobas Taqman assay. J Acquir Immune Defic Syndr. 2009;51(3):364–365. doi: 10.1097/QAI.0b013e3181aa13b3. [DOI] [PubMed] [Google Scholar]
- 43.Verhofstede C. Van WF. Reynaerts J, et al. Viral load assay sensitivity and low level viremia in HAART treated HIV patients. J Clin Virol. 2010;47(4):335–339. doi: 10.1016/j.jcv.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 44.Percus JK. Percus OE. Markowitz M, et al. The distribution of viral blips observed in HIV-1 infected patients treated with combination antiretroviral therapy. Bull Math Biol. 2003;65:263–277. doi: 10.1016/S0092-8240(02)00095-2. [DOI] [PubMed] [Google Scholar]
- 45.Aldous JL. Haubrich RH. Defining treatment failure in resource-rich settings. Curr Opin HIV AIDS. 2009;4(6):459–466. doi: 10.1097/COH.0b013e328331dea5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson MA. Aberg JA. Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010;304(3):321–333. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]