Abstract
In 1998 a collaboration between Duke University and the University of North Carolina, Chapel Hill (UNC) was founded to enhance identification of persons with acute HIV-1 infection (AHI). The Duke-UNC AHI Research Consortium Cohort consists of patients ≥18 years old with a positive nucleic acid amplification test (NAAT) and either a negative enzyme immunoassay (EIA) test or a positive EIA with a negative/indeterminate Western blot. Patients were referred to the cohort from acute care settings and state-funded HIV testing sites that use NAAT testing on pooled HIV-1 antibody-negative samples. Between 1998 and 2010, 155 patients with AHI were enrolled: 81 (52%) African-Americans, 63 (41%) white, non-Hispanics, 137 (88%) males, 108 (70%) men who have sex with men (MSM), and 18 (12%) females. The median age was 27 years (IQR 22–38). Most (n=138/155) reported symptoms with a median duration of 17.5 days. The median nadir CD4 count was 408 cells/mm3 (IQR 289–563); the median observed peak HIV-1 level was 726,859 copies/ml (IQR 167,585–3,565,728). The emergency department was the most frequent site of initial presentation (n=55/152; 3 missing data). AHI diagnosis was made at time of first contact in 62/137 (45%; 18 missing data) patients. This prospectively enrolled cohort is the largest group of patients with AHI reported from the Southeastern United States. The demographics reflect the epidemic of this geographic area with a high proportion of African-Americans, including young black MSM. Highlighting the challenges of diagnosing AHI, less than half of the patients were diagnosed at the first healthcare visit. Women made up a small proportion despite increasing numbers in our clinics.
Introduction
Acute HIV-1 infection (AHI) can be defined as the period between HIV-1 acquisition and the development of HIV-1-specific antibodies. Although screening tests have markedly improved in terms of earlier detection and diagnostic accuracy, HIV-1 antibodies are usually not detected during the earliest phase of infection when patients often have the highest levels of viremia. Since routine HIV-1 antibody tests are typically negative during the first few weeks of infection, the diagnosis of AHI during this period requires a high index of suspicion and use of specific tests for viral nucleic acid or HIV-1 antigens. Unfortunately, symptoms of AHI are common, nonspecific,1,2 and often mistaken for other illnesses. The failure to identify AHI is especially significant as acutely infected patients are often highly infectious due to high levels of viremia.3,4 After diagnosis, HIV-1-infected patients have been observed to reduce high-risk behaviors, thus diminishing risk of transmission.5–7 Initiation of antiretroviral therapy (ART) also reduces transmission by decreasing viral replication and shedding in infectious fluids.8–11 Thus, timely diagnosis of AHI has become an important public health measure.12,13
To enhance identification and management of persons with AHI, the Duke-UNC Acute HIV Infection Research Consortium was founded as a collaboration between Duke University and the University of North Carolina, Chapel Hill (UNC). Patients identified during AHI, either clinically or by pooled screening strategies, have been enrolled since 1998. We describe our cohort of AHI patients diagnosed in Southeastern United States. To better understand and characterize this population, we analyzed the cohort by a variety of factors including site of initial presentation and HIV-1 antibody status at time of diagnosis—enzyme immunoassay (EIA) seronegative or EIA seropositive with a negative or indeterminate Western blot (WB).
Materials and Methods
The Duke-UNC AHI Cohort consists of patients ≥18 years old diagnosed with AHI between January 1, 1998 and December 31, 2010. Enrolled patients had a positive nucleic acid amplification test (NAAT) and either a negative EIA test or a positive EIA with a negative or indeterminate WB. An indeterminate WB was defined as detection of fewer than two of the following bands: gp120/160, gp41, or p24.
Patients with AHI were referred to the cohort from acute care settings [emergency departments (ED), urgent care centers, student health centers, or primary care clinics] and from the North Carolina State Screening and Tracing for Active HIV-1 Transmission (STAT) program. In 2002 the North Carolina Department of Health and Human Services and the North Carolina State Laboratory of Public Health initiated the STAT program to diagnose AHI using NAAT testing of pooled HIV-1 antibody-negative samples.14,15 This program tests all persons presenting to publicly funded testing sites in NC for AHI (if the initial HIV-1 antibody test is negative or indeterminate).15 Specimens with detectable HIV-1 RNA that are either EIA negative or EIA positive with a negative/indeterminate WB are considered acute infections and are confirmed by subsequent HIV-1 antibody testing
Patients enrolled in the cohort had demographic information recorded including age, gender, race/ethnicity, and self-reported sexual orientation. At the time of study enrollment (typically within 2 weeks of diagnosis), patients were asked by a research study team member about symptoms consistent with AHI, including start and stop dates. The site and date of initial presentation and the number of visits to healthcare settings for similar symptoms prior to diagnosis were also collected. CD4 counts and HIV-1 levels were measured and recorded at time points as close as possible to the date of AHI diagnosis (defined as the collection date of the first positive HIV-1 RNA test); there were no specific criteria set for the duration of chart review for either the CD4 nadir or peak HIV-1 level. Follow-up EIA results were used to estimate the date of seroconversion; the estimated date of infection was defined as occurring 14 days prior to onset of symptoms.1,16,17 For 10/17 patients without symptoms, BEAST (Bayesian Evolutionary Analysis by Sampling Trees v.1.5.3) analysis performed in an earlier study on this cohort was used to estimate the date of infection.17 The protocol was approved by the Institutional Review Boards at each institution; written informed consent was obtained from all study participants.
Descriptive statistics, including frequencies for categorical variables and medians with interquartile ranges (IQR) for continuous variables are presented in this analysis. Additionally, we assessed trends in yearly enrollment by race, age, and sexual risk group. Finally, we examined differences by site of initial presentation and compared patients who initially presented to a health department or related publicly funded facility vs. those presenting to an acute care setting (ED, urgent care center, student health center, or primary care clinic). An AHI diagnosis was not always made at the site of first presentation. Comparisons were also made between patients who were EIA negative vs. EIA positive with a negative/indeterminate WB at the time of diagnosis, both of which served as criteria for enrollment.
Quantitative variables were described using means and standard deviations (SD) in cases where the underlying distribution was normal; medians with ranges were used for variables without normal distributions. Differences in continuous variables between the groups were analyzed using the Student's t test (mean) or Wilcoxon rank-sum testing (median). To compare categorical variables, we used Pearson's χ2 with Fisher's exact test to account for small numbers. Statistical significance was defined as α<0.05 for all analyses. All statistical analyses were performed using SAS v.9.2 (Cary, NC).
Results
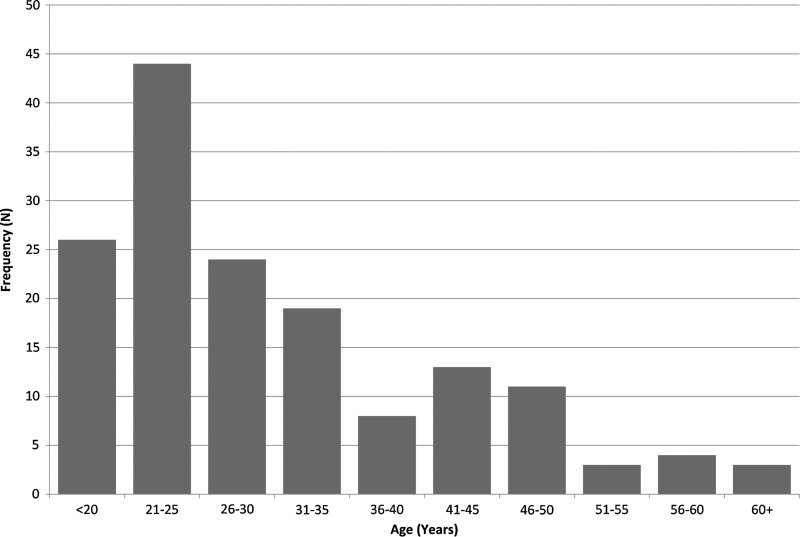
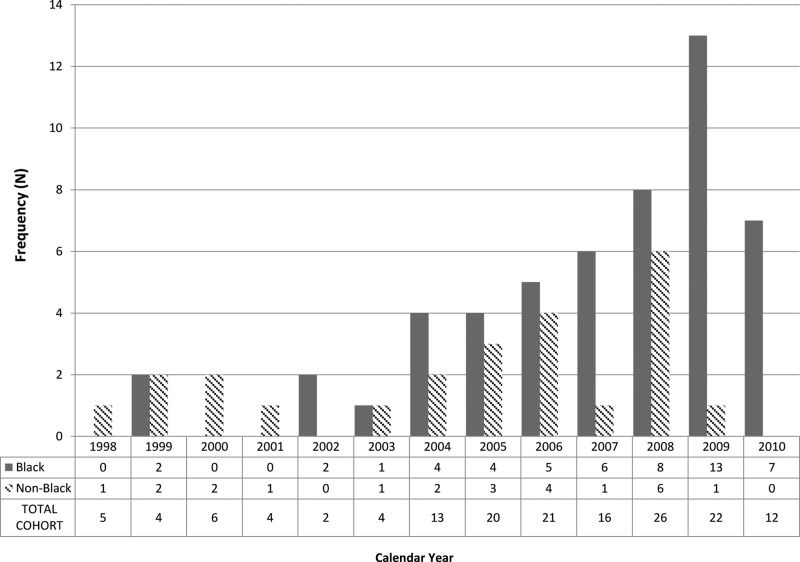
Between January 1, 1998 and December 31, 2010, 155 patients with AHI were enrolled; of these 137 (88%) were male (Table 1). Annual enrollment increased in 2003 with the inception of the STAT program. The median age at diagnosis was 27 years (IQR 22–38), with a range of 17–66 years (Fig. 1). Most patients were African-American (n=81; 52%) or white, non-Hispanic (n=63; 41%). Men who have sex with men (MSM) were the most common risk group (n=108; 70%), followed by heterosexual men (n=24; 15%) and women (n=18; 12%). No patients endorsed injection drug use. Acutely infected males were younger than females with a median age of 26 (IQR 22–36) vs. 33 years (IQR 22–43). Notably, the number of patients who were black MSM≤30 years old increased over time (Fig. 2). From 1998 to 2001, 10.5% of newly diagnosed patients were young, black MSM, which increased to 26.7% from 2002–2006 and 44.8% from 2007–2010 (p=0.02).
Table 1.
Demographics of Acutely Infected Persons in North Carolina
| |
|
Initial site of presentationa |
|
|
|
||
|---|---|---|---|---|---|---|---|
| Total cohort | Health Department | Acute care settings | pb | EIA− | EIA+c | pb | |
| Total | 155 | 46 | 106 | NA | 103 | 50 | NA |
| Median age [interquartile range (IQR)] | 27 (22–38) | 24 (22–32) | 29 (22–41) | 0.09 | 27 (22–36) | 26 (21–39) | 0.8 |
| Race/ethnicity (%) | |||||||
| Black | 81 (52) | 32 (70) | 48 (45) | 55 (53) | 26 (52) | ||
| White | 63 (41) | 12 (26) | 49 (46) | 0.09 | 41 (40) | 20 (40) | 0.3 |
| Hispanic | 7 (5) | 2 (4) | 5 (5) | 5 (5) | 2 (4) | ||
| Other | 4 (3) | 0 (0) | 4 (5) | 2 (2) | 2 (4) | ||
| Sexual risk group (%) | |||||||
| MSM | 108 (70) | 36 (72) | 72 (67) | 76 (74) | 33 (60) | ||
| Heterosexual men | 24 (15) | 3 (10) | 21 (22) | 14 (14) | 10 (18) | ||
| Female | 18 (12) | 6 (13) | 12 (11) | 0.2 | 12 (13) | 6 (11) | 0.7 |
| Unknown male | 5 (3) | 1 (4) | 1 (3) | 1 (1) | 1 (1) | ||
| Median initial CD4 (cells/mm3; IQR) | 484 (340–675) | 555 (393–722) | 416 (324–644) | 0.01 | 461 (313–675) | 496 (392–701) | 0.2 |
| Median nadir CD4d (cells/mm3; IQR) | 408 (289–563) | 493 (333–601) | 368 (247–521) | 0.005 | 369 (272–563) | 427 (311–541) | 0.2 |
| Median initial VL (copies/ml; IQR) | 569,399 (91,980–2,260,000) | 173,308 (27,152–1,404,655) | 614,898 (117,648–2,553,350) | 0.07 | 572,904 (84,193–2,251,415) | 294,373 (91,980–1,730,000) | 0.9 |
| Median peak VLd (copies/ml; IQR) | 726,859 (167,585–3,565,728) | 643,084 (123,928–2,267,808) | 801,677 (310,525–3,893,310) | 0.07 | 840,933 (310,525–4,163,742) | 476,788 (132,021–2,089,534) | 0.03 |
Three patients missing data on initial site of presentation.
Wilcoxon p-value.
Two patients missing data on first EIA result.
Prior to antiretroviral therapy; lowest CD4 count from time of diagnosis.
EIA, enzyme immunoassay; MSM, men who have sex with men; VL, viral load.
FIG. 1.
Age distribution at diagnosis of acute HIV-1 infection (AHI) patients in North Carolina.
FIG. 2.
Frequency of men who have sex with men (MSM) ≤30 years of total AHI cohort (N=155) by race and calendar year.
Most patients reported symptoms at presentation suggestive of acute retroviral syndrome (138/155; 89%). The median duration of symptoms was 17.5 days (IQR 7–33). The three most frequently reported symptoms were fever (66%), fatigue (57%), and body ache (54%) (Table 2). Females were more apt to complain of fatigue (56%), followed by fever (50%), body ache (50%), and nausea (50%). Although more women than men reported symptoms [17/18 women (94%) vs. 121/137 (88.3%)], the median duration of symptoms was shorter among women than men (9 vs. 18 days), and women reported fewer symptoms overall (median number of symptoms: 4.5 vs. 6).
Table 2.
Symptoms of Acute HIV Infection
| All N (%) | |
|---|---|
| N | 155 |
| Any symptom | 138 (89) |
| Median number of symptoms (IQR) | 6 (4–8) |
| Fever | 103 (66) |
| Fatigue | 88 (57) |
| Body ache | 83 (54) |
| Nausea | 68 (44) |
| Headache | 56 (36) |
| Diarrhea | 66 (43) |
| Loss of appetite | 69 (45) |
| Sore throat | 57 (37) |
| Night sweats | 56 (36) |
| Weight loss | 54 (35) |
| Swollen lymph nodes | 51 (33) |
| Rash | 41 (26) |
| Stomach pain | 34 (22) |
| Joint pain | 24 (15) |
| Cough | 26 (17) |
| Thrush | 22 (14) |
| Sores/ulcers mouth | 14 (9) |
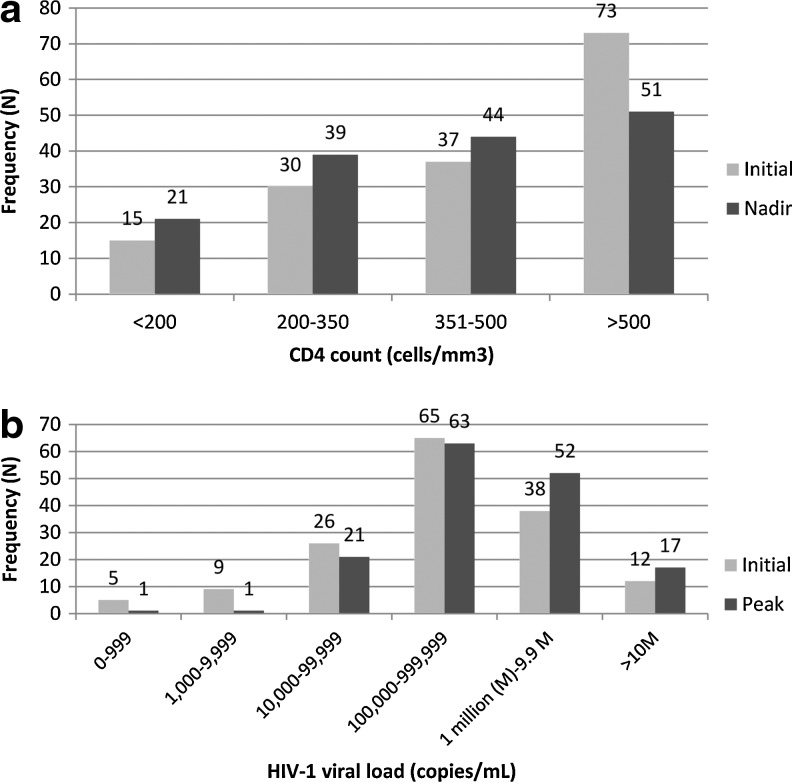
For the overall cohort the median initial CD4 count was 484 cells/mm3 (IQR 340–675) and the median nadir CD4 count was 408 cells/mm3 (IQR 289–563). However, 39% (n=60) had a nadir≤350 cells/mm3, 14% (n=21) had a nadir <200 cells/mm3, and 5% (n=7) had a nadir <50 cells/mm3 (Fig. 3a). The median time from diagnosis to nadir CD4 count collection was 25 days (IQR 14–181).
FIG. 3.
(a) Initial and nadir CD4 counts in acutely infected patients. (b) Initial and peak HIV-1 levels in acutely infected patients.
The median initial HIV-1 level was 569,399 copies/ml (IQR 91,980–2,260,000) and the median observed peak HIV-1 level was 726,859 copies/ml (IQR 167,585–3,565,728). Thirty-two percent (n=50) had an initial HIV-1 level≥1,000,000 copies/ml at diagnosis, while 9% (n=14) had an initial HIV-1 level <10,000 copies/ml (Fig. 3b). In 10 patients (8 of whom were EIA negative at diagnosis), serial HIV-1 levels prior to seroconversion did not exceed 10,000 copies/ml; in 29 patients (23 were EIA negative at diagnosis) preseroconversion HIV-1 levels did not exceed 100,000 copies/ml. Conversely, 17 (11%) patients had peak HIV-1 levels≥10,000,000 copies/ml with the highest HIV-1 level observed at 160,000,000 copies/ml. Patients with initial HIV-1 levels <100,000 copies/ml had higher median CD4 count nadirs compared to those with initial HIV-1 levels >100,000 copies/ml (502 vs. 375 cells/mm3; p=0.0009), in addition to fewer symptoms (median number of symptoms 4.5 vs. 6; p=0.02). Women had lower initial HIV-1 levels than men (median 64,263 vs. 596,000 copies/ml) and higher initial CD4 counts (median 629 vs. 457 cells/mm3). Fifty-two (33.3%) patients had an increase in HIV-1 level from the first measurement to the second, suggesting early diagnosis during the viral expansion phase and prior to peak HIV-1 levels.
Comparison by site of initial presentation
The ED was the most frequent site of first clinical presentation (n=55/152; 36%; three missing data) followed by the health department (n=46/152; 30%) and primary care clinic (n=27/152; 18%) (Table 3). Patients initially presenting to a health department tended to be younger than those presenting to acute care settings (median age 24 vs. 29 years; p=0.09) (Table 1). There was also a trend toward African-Americans presenting first to a health department rather than acute care setting (70% vs. 45%; p=0.09). There were no significant differences in gender or sexual risk groups when comparing sites of presentation. Patients presenting to acute care sites were more often symptomatic than those presenting to a health department (93% vs. 83%; p=0.04) and reported more symptoms (median number of symptoms 6 vs. 5; p=0.08). Patients presenting to a health department had significantly higher median nadir and initial CD4 counts and lower median initial and peak viral loads compared to those presenting to acute care settings (Table 1).
Table 3.
Sites of Initial Presentation to Care and HIV Diagnosis Among Acute HIV Infection Patients
| Site of initial presentation to carea(N=152) | N | % |
|---|---|---|
| Health Department | 46 | 30 |
| Acute Care settings | ||
| Emergency Department | 55 | 52 |
| Primary Care Clinic | 27 | 25 |
| Urgent Care Center | 14 | 13 |
| Student Health | 7 | 7 |
| Hospital | 0 | 0 |
| Otherb | 3 | 3 |
| 106 | 70 | |
| Site of HIV diagnosisc (N=127) | N | % |
|---|---|---|
| Health Department | 65 | 51 |
| Acute Care settings | ||
| Emergency Department | 13 | 21 |
| Primary Care Clinic | 42 | 68 |
| Urgent Care Center | 0 | 0 |
| Student Health | 2 | 3 |
| Hospital | 5 | 8 |
| Other | 0 | 0 |
| 62 | 49 |
Three patients missing data on initial site of presentation.
Other includes prenatal clinic, prison blood draw, and community outreach.
Twenty-eight patients missing site of HIV diagnosis (defined as date of collection of first positive HIV-1 RNA test).
Comparison by initial EIA
One hundred and three patients (67%) had an initial negative EIA test; 50/153 (33%; two missing data) presented with a positive EIA with a negative/indeterminate Western blot. There were no significant differences in age, race/ethnicity, or gender/sexual risk category between these two groups (Table 1). Symptomatic AHI was more common in persons with negative EIA results compared to patients with positive EIA results (94% vs. 84%; p=0.1), but the median number of symptoms was identical (n=6). Median nadir CD4 counts and initial CD4 counts were slightly lower among the EIA-negative group. Both median initial viral loads and peak viral loads were higher in the EIA-negative group (Table 1).
Timeline
Overall, less than half of the cohort (n=62/137; 45%; 18 missing data) was diagnosed with AHI at their first healthcare visit. Seventy-five patients (55%) required one or more healthcare encounters before AHI diagnosis was made, and 29 patients (21%) were diagnosed after two or more visits. Among 46 patients with initial presentation to a health department, the majority (n=36/37; 97%; 9 missing data) were diagnosed at first visit. Conversely, of the 100 patients presenting initially to acute care settings with data on the number of healthcare visits before diagnosis, 26 (26%) were diagnosed at first visit, and 74 (74%) experienced one or more healthcare visits prior to their AHI diagnosis (6 missing data).
Overall, the median time from initial presentation to diagnosis was 2 days, but ranged from 0 to 90 days (IQR 0–8 days), with a mean time of 6 days. The median time from estimated date of infection to presentation was 18 days (IQR 15–25 days), but ranged from 4 to 68 days. The median time from estimated date of infection to diagnosis was 23 days (IQR 18–31).
The median time from initial presentation to diagnosis was significantly shorter for patients who presented to a health department vs. acute care setting (0 vs. 4 days; p<0.001). The median time from estimated date of infection to presentation was significantly longer for patients presenting to a health department vs. acute care setting (23 vs. 17 days; p=0.001). The median time from estimated date of infection to diagnosis did not differ by site of presentation.
The median time from initial presentation to diagnosis was shorter for EIA negative vs. EIA positive patients (1 vs. 5 days; p=0.06), as was the median time from estimated date of infection to diagnosis (21 vs. 26 days; p=0.01). Notably, the median time from estimated date of infection to presentation for these two groups was quite similar (17 vs. 19 days; p=0.2).
Discussion
For the past 13 years, the Duke-UNC AHI Research Consortium has enrolled AHI patients, including those recognized during clinical illness characteristic of AHI and those identified from a state-wide screening program for antibody-negative specimens collected from publically funded testing centers. This prospectively enrolled cohort is the largest population of AHI reported to date from the Southeastern United States. Demographic characteristics of the population are broad and generally reflect the epidemic of this geographic area with a high proportion of African-Americans. In our cohort, racial preponderance among AHI cases has evolved; approximately half (50%) were white in 2003, with an increase in African-Americans from 56% in 2007 to 67% of the total cohort in 2010. These characteristics distinguish the present cohort from other AHI studies, where African-Americans are often underrepresented (constituting only 7–18% of the population1,18,19), despite the fact that this racial group accounts for 44% of all new HIV-1 infections.20 Recent incidence data indicate that young black MSM have become the driver of new HIV-1 infections nationwide.21 This trend is reflected in our cohort wherein black MSM≤age 30 represent 34% and comprise a significantly increasing percentage over recent years. The composition of our cohort may reflect the demographics of HIV-1 in the Southeast United States and/or the pooled screening procedure that ultimately established the diagnosis in 53% of our patients (n=82). This population-based approach likely accesses a more representative group of persons at risk for AHI than do more focused diagnostic strategies.
As in other published AHI cohorts,1,22 the majority of our patients (89%) were symptomatic. Of concern, only 45% were diagnosed at their first healthcare encounter, highlighting the challenges of diagnosing AHI, including insufficient assessment of patient risk factors, failure of healthcare providers to consider AHI as a potential diagnosis, limited knowledge regarding proper diagnostic testing, and lack of easy access to diagnostic tools. The general absence of a defined infrastructure for HIV-1 testing with follow-up of results and the lack of awareness among providers of the potential benefits of early diagnosis and treatment may also play a role.
Patients presenting to acute care settings were predictably more symptomatic than those presenting to the health department. At acute care settings, however, 74% of patients were seen more than once prior to being diagnosed with AHI, compared to only one person presenting to the health department who was seen more than once. These data may be biased in that patients who presented to the health department were typically requesting sexually transmitted disease (STD) screening in addition to HIV-1 testing. In a subset analysis of our cohort evaluating missed opportunities for AHI diagnosis in acute care settings from 1998 to 2001, 10.5% were diagnosed at time of first visit.23 Some improvement has been seen with 29.6% being diagnosed in acute care settings at time of first visit in years 2002–2010. Our results suggest that these sites need ongoing educational efforts regarding AHI with better access to diagnostic tools and support recent measures to facilitate HIV-1 testing in such settings.24
A widely accepted belief is that patients presenting with AHI invariably have very high viral loads. In this cohort, 10 patients with confirmed AHI (including 8 with negative EIA tests at diagnosis) never had HIV-1 levels exceeding 10,000 copies/ml. Overall, however, patients had lower initial CD4 counts and higher HIV-1 levels than other reported groups.1,22,25,26 Interestingly, the observed range of results included a substantial proportion with very low CD4 cell counts during AHI. Eight patients had CD4 counts <50 cells/mm3 at time of diagnosis, 6 of whom had negative EIA assays. Patients with these levels of HIV-1 RNA and CD4 counts may have been excluded from other studies based on the belief that such extremes are not likely among persons with AHI. Our data illustrate the frequency with which such substantial variability occurs among individuals with AHI and represent important observations for providers who may encounter these patients.
The median time from presentation to diagnosis in our cohort was only two days, a finding largely attributable to the substantial proportion of patients diagnosed at publically funded testing sites in which HIV-1 antibody-negative samples are screened for AHI in a pooling strategy.15 When considering only patients presenting initially to acute care settings, the median time from presentation to diagnosis was 4 days, an improvement from previously published data in which the median time from presentation to diagnosis was 14 days.23
Despite the short interval from presentation to diagnosis, the time from estimated infection to presentation was actually longer for those diagnosed at the health department vs. acute care sites, perhaps due to the fact that patients at the health department were less symptomatic with higher CD4 counts and lower HIV-1 levels. In our analysis we defined estimated date of infection as 14 days prior to the onset of symptoms, primarily based on prior work from our own cohort,27 which is dependent on recall of symptoms. Notably, patients presenting to health departments were younger and more likely to be African-American. Reasons for these demographic differences may be due to limited access to healthcare among younger persons and African-Americans, resulting in greater use of publically funded health services.
We observed, as others have, that individuals who were EIA negative had higher HIV-1 levels than those who were EIA positive. One common interpretation is that these individuals are “earlier” in their course of acute infection. However, we found no significant difference in time from estimated infection to presentation between patients who were EIA negative vs. EIA positive. An alternative explanation is that many individuals with AHI captured with a negative EIA are not diagnosed earlier but instead take longer to seroconvert due to less robust innate or adaptive immune responses. Consistent with this hypothesis is our observation that EIA-negative patients were more apt to be symptomatic and had lower nadir CD4 counts (5/7 patients with CD4 counts <50 cells/mm3 were EIA negative). For scientists studying AHI, preferentially studying subjects who are EIA negative may result in a bias toward individuals with less robust immune responses.
A striking finding is the low number of women enrolled in our cohort, a trend observed in other AHI studies.22,28 This is an important observation as women now represent 25% of all new HIV-1 diagnoses in the United States.29 In the Duke and UNC HIV Clinics, women constitute one-third of all active patients. The reasons why women are underrepresented in this cohort are not entirely clear. Although women <65 years old in the United States were more likely than men to have ever had an HIV test,30 providers may not consider or recognize HIV infection, including AHI, as frequently in women. Since women reported fewer symptoms overall than men, perhaps the diagnosis was less often considered. Women in our cohort had lower initial HIV-1 levels and higher median initial CD4 counts than men, a finding also noted by others.22
Increases in the numbers of HIV-1 infections in the Southeastern United States make the potential for transmission of HIV-1 during AHI a particular concern. Screening for AHI is an important target for public health intervention since early treatment during AHI may alter the immunological decline31,32 and may dramatically reduce the risk of HIV-1 transmission.13 It is notable that 81% of AHI patients in this cohort elected to be started on antiretroviral therapy within 45 days of diagnosis.33 These data emphasize the increasingly diverse nature of AHI and provide impetus for improved approaches to diagnosis going forward.
Contributor Information
Collaborators: the Duke-UNC Acute HIV Infection Consortium
Acknowledgments
We would like to acknowledge and thank Lynn McNeil, Sabrina Zadrozny, Ashley Mayo, Jeff Anderson, Alyssa Sugarbaker, and Peter Leone for their valuable contributions on this study. We are indebted to the staff at the North Carolina Department of Health and Human Services and to the Disease Intervention Specialists who trace and counsel individuals with acute HIV infection. We greatly appreciate the support of all study staff members, HIV care providers, and particularly the individuals who participated in this study.
This research was supported by the following NIH-funded programs: the Duke Center for AIDS Research (1P30 A1 64518), the University of North Carolina, Chapel Hill, Center for AIDS Research (1P30 A1 50410), and a K24 award (NIAID 1K24 AI01608). Antiretrovirals were generously provided by Bristol-Myers Squibb and Gilead Sciences, Inc. These companies and all other funders had no role in the design and conduct of the study: collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Schacker T. Collier AC. Hughes J, et al. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125(4):257–264. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 2.Kahn JO. Walker BD. Acute human immunodeficiency virus type 1 infection. N Engl J Med. 1998;339(1):33–39. doi: 10.1056/NEJM199807023390107. [DOI] [PubMed] [Google Scholar]
- 3.Quinn TC. Wawer MJ. Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 4.Baeten JM. Kahle E. Lingappa JR, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med. 2011;3(77):77ra29. doi: 10.1126/scitranslmed.3001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks G. Crepaz N. Senterfitt JW. Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: Implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39(4):446–453. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 6.Fox J. White PJ. Macdonald N, et al. Reductions in HIV transmission risk behaviour following diagnosis of primary HIV infection: A cohort of high-risk men who have sex with men. HIV Med. 2009;10(7):432–438. doi: 10.1111/j.1468-1293.2009.00708.x. [DOI] [PubMed] [Google Scholar]
- 7.Metsch LR. Pereyra M. Messinger S, et al. HIV transmission risk behaviors among HIV-infected persons who are successfully linked to care. Clin Infect Dis. 2008;47(4):577–584. doi: 10.1086/590153. [DOI] [PubMed] [Google Scholar]
- 8.Graham SM. Holte SE. Peshu NM, et al. Initiation of antiretroviral therapy leads to a rapid decline in cervical and vaginal HIV-1 shedding. AIDS. 2007;21(4):501–507. doi: 10.1097/QAD.0b013e32801424bd. [DOI] [PubMed] [Google Scholar]
- 9.Vernazza PL. Troiani L. Flepp MJ, et al. Potent antiretroviral treatment of HIV-infection results in suppression of the seminal shedding of HIV. The Swiss HIV Cohort Study. AIDS. 2000;14(2):117–121. doi: 10.1097/00002030-200001280-00006. [DOI] [PubMed] [Google Scholar]
- 10.Powers KA. Poole C. Pettifor AE. Cohen MS. Rethinking the heterosexual infectivity of HIV-1: A systematic review and meta-analysis. Lancet Infect Dis. 2008;8(9):553–563. doi: 10.1016/S1473-3099(08)70156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson DP. Law MG. Grulich AE, et al. Relation between HIV viral load and infectiousness: A model-based analysis. Lancet. 2008;372(9635):314–320. doi: 10.1016/S0140-6736(08)61115-0. [DOI] [PubMed] [Google Scholar]
- 12.Powers K. Ghani A. Miller W. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: A modelling study. Lancet. 2011;378:256–268. doi: 10.1016/S0140-6736(11)60842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen MS. Chen YQ. McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilcher CD. McPherson JT. Leone PA, et al. Real-time, universal screening for acute HIV infection in a routine HIV counseling and testing population. JAMA. 2002;288(2):216–221. doi: 10.1001/jama.288.2.216. [DOI] [PubMed] [Google Scholar]
- 15.Pilcher CD. Fiscus SA. Nguyen TQ, et al. Detection of acute infections during HIV testing in North Carolina. N Engl J Med. 2005;352(18):1873–1883. doi: 10.1056/NEJMoa042291. [DOI] [PubMed] [Google Scholar]
- 16.Lindback S. Thorstensson R. Karlsson AC, et al. Diagnosis of primary HIV-1 infection and duration of follow-up after HIV exposure. Karolinska Institute Primary HIV Infection Study Group. AIDS. 2000;14(15):2333–2339. doi: 10.1097/00002030-200010200-00014. [DOI] [PubMed] [Google Scholar]
- 17.Gay C. Dibben O. Anderson JA, et al. Cross-sectional detection of acute HIV Infection: Timing of transmission, inflammation and antiretroviral therapy. PLoS One. 2011;6(5):e19617. doi: 10.1371/journal.pone.0019617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meditz AL. MaWhinney S. Allshouse A, et al. Sex, race, and geographic region influence clinical outcomes following primary HIV-1 infection. J Infect Dis. 2011;203(4):442–451. doi: 10.1093/infdis/jiq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daar ES. Little S. Pitt J, et al. Diagnosis of primary HIV-1 infection. Los Angeles County Primary HIV Infection Recruitment Network. Ann Intern Med. 2001;134(1):25–29. doi: 10.7326/0003-4819-134-1-200101020-00010. [DOI] [PubMed] [Google Scholar]
- 20.CDC. HIV among African Americans. 2011.
- 21.Prejean J. Song R. Hernandez A. Estimated HIV incidence in the United States, 2006–2009. PLoS One. 2011;6(8):e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meditz AL. MaWhinney S. Allshouse A, et al. Sex, race, and geographic region influence clinical outcomes following primary HIV-1 infection. J Infect Dis 15. 203(4):442–451. doi: 10.1093/infdis/jiq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weintrob AC. Giner J. Menezes P, et al. Infrequent diagnosis of primary human immunodeficiency virus infection: Missed opportunities in acute care settings. Arch Intern Med. 2003;163(17):2097–2100. doi: 10.1001/archinte.163.17.2097. [DOI] [PubMed] [Google Scholar]
- 24.Rothman RE. Hsieh YH. Harvey L, et al. 2009 US emergency department HIV testing practices. Ann Emerg Med. 2011;58(1 Suppl 1):S3–9. doi: 10.1016/j.annemergmed.2011.03.016. e1–4. [DOI] [PubMed] [Google Scholar]
- 25.Markowitz N. O'Connor M. Brar I. Initial CD4 cell count after recent infection–Michigan; International AIDS Conference; Capetown. 2009. [Google Scholar]
- 26.Volberding P. Demeter L. Bosch RJ, et al. Antiretroviral therapy in acute and recent HIV infection: A prospective multicenter stratified trial of intentionally interrupted treatment. AIDS. 2009;23(15):1987–1995. doi: 10.1097/QAD.0b013e32832eb285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gay C. Dibben O. Anderson JA, et al. Cross-sectional detection of acute HIV infection: Timing of transmission, inflammation and antiretroviral therapy. PLoS One. 2011;6(5):e19617. doi: 10.1371/journal.pone.0019617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hightow LB. Golin CE. Green K, et al. Identifying people with acute HIV infection: Demographic features, risk factors, and use of health care among individuals with AHI in North Carolina. AIDS Behavior. 2009;13(6):1075–1083. doi: 10.1007/s10461-008-9519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.CDC. HIV/AIDS among Women. Atlanta: Aug, 2008. [Google Scholar]
- 30.Cohen SS. Li C. Ding L, et al. Pronounced acute immunosuppression in vivo mediated by HIV Tat challenge. Proc Natl Acad Sci USA. 1999;96(19):10842–10847. doi: 10.1073/pnas.96.19.10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmid A. Gianella S. von Wyl V, et al. Profound depletion of HIV-1 transcription in patients initiating antiretroviral therapy during acute infection. PLoS One. 2010;5(10):e13310. doi: 10.1371/journal.pone.0013310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hocqueloux L. Prazuck T. Avettand-Fenoel V, et al. Long-term immunovirologic control following antiretroviral therapy interruption in patients treated at the time of primary HIV-1 infection. AIDS. 2010;24(10):1598–1601. doi: 10.1097/qad.0b013e32833b61ba. [DOI] [PubMed] [Google Scholar]
- 33.Gay CL. Mayo AJ. Mfalila CK, et al. Efficacy of NNRTI-based antiretroviral therapy initiated during acute HIV infection. AIDS. 2011;25(7):941–949. doi: 10.1097/QAD.0b013e3283463c07. [DOI] [PMC free article] [PubMed] [Google Scholar]