Abstract
Drug-resistant HIV complicates management of HIV infection. Although an estimated 14% of all HIV-positive persons pass through a prison or jail in the United States each year, little is known about the overall prevalence of antiretroviral (ARV) resistance in incarcerated persons. All genotypic sequence data on HIV-positive prisoners in the North Carolina (NC) Department of Corrections (DOC) were obtained from LabCorp. Screening for major resistance mutations in protease (PI) and reverse transcriptase (NRTI and NNRTI) was done using Genosure and the Stanford HIV Database. For subjects with multiple genotype reports, each mutation was counted only once and considered present on all subsequent genotypes. Between October 2006 and February 2010, the NC DOC incarcerated 1,911 HIV+ individuals of whom 19.2% (n=367) had at least one genotype performed. The overall prevalence of a major resistance mutation was 28.3% (95% CI 23.7, 33.0). Among prisoners ever exposed to an ARV during incarceration (n=329) prevalence of a major resistance mutation was 29.8% (95% CI 24.9, 34.7); resistance by class was 20.4% (95% CI 16.0, 24.7) for NRTIs, 19.8% (95% CI 15.5, 24.1) for NNRTIs, and 8.8% (95% CI 5.8,11.9) for PIs. Single class drug resistance was most prevalent at 14.2% (10.2,17.7) followed by dual 12.5% (I8.9,16.0) and triple class 3.3% (1.4,5.3) resistance. The three most prevalent mutations were K103N 15.8% (12.0, 20.2), M184V 14.3% (10.7,18.5), and M41L 4.9% (2.8,7.8). In the NC DOC ARV resistance prevalence, dual and triple class drug resistance was moderate over the study period. Resistance to PIs was lower than NNRTIs and NRTIs, likely reflecting higher usage of these two classes or a lower barrier to resistance.
Introduction
In the United States, an estimated 14% of all HIV-positive persons pass through a prison or jail each year.1 While incarcerated, antiretroviral (ARV) adherence among U.S. prisoners is reported to be high.2,3 However, recidivism is common and cycling in and out of prison can be associated with treatment interruptions and reduced adherence to ARV medications.4–6 Suboptimal adherence promotes ARV resistance, reduces the availability of effective therapies, and results in more complex, expensive treatment options that may be less tolerable.
Determining the prevalence of ARV resistance among ARV therapy-exposed patients is challenging. Drug resistance testing is generally feasible clinically when plasma HIV RNA is >500 c/ml and resistance testing may not be performed in all cases of ARV failure or before wild-type virus overgrows resistant strains following ARV cessation. Comparing the prevalence estimates of HIV drug resistance across published studies is challenging as results are dependent on several variables including the population under study (chronically infected vs. treatment naive), the definition of resistance, the denominator used (all patients on treatment vs. only those with genotypes), and the time period under study. Reported prevalence estimates of HIV resistance among patients on ARV treatment can range from as little as 17% to an alarmingly high value of 76–81%.7–12
Although the development of drug-resistant virus has important implications for treatment of HIV infection, little is known about the overall prevalence of ARV resistance among incarcerated persons, a population that carries a disproportionate burden of HIV. To date, there have been only five studies examining ARV resistance among prisoners—two in the United States13,14 and three in Europe.15–17 Of the two studies conducted in the United States,13 one focused on 67 seroconverters during incarceration and restricted surveillance to only four major mutations.14 The second study surveyed only 25 ARV treatment-naive prisoners.13 The generalizability of the two studies conducted in the United States13 is limited by their small sample sizes and by their focus on HIV-infected ARV treatment-naive prisoners.
The North Carolina (NC) state prison system incarcerates approximately 40,000 prisoners annually18 with an estimated HIV prevalence of 2.1%, the fifth highest prevalence of HIV among all U.S. state prisons.19 Although resistance testing is conducted in the NC prison system as a component of routine HIV care, as in other state prison systems, the prevalence of ARV-resistant variants among the population of HIV-positive prisoners has not been well characterized. To broaden our current understanding of HIV resistance among prisoners, we sought to determine the ARV resistance burden among a cohort of HIV-positive incarcerated men and women receiving HIV care in the North Carolina Department of Correction, a large prison system in the Southeast United States.13
Materials and Methods
Study population
For the period October 2006 to February 2010, we obtained the following data for all HIV-positive inmates from all 87 prison facilities within the NC Department of Correction (NC DOC) prison system: demographic characteristics (age, race, gender), dates of incarceration, recidivism status, an indicator of any ARV use in prison (based on electronic prescription data), and among prisoners who received a genotype test for drug resistance to HIV-1, the nucleoside sequencing result and corresponding plasma viral load within 120 days of genotype testing.
Within the NC DOC, four Infectious Disease (ID) clinics provide HIV care to all HIV-positive inmates housed in the 87 prison facilities. Because inmates may be moved between different prison facilities, they might start HIV care in one ID clinic but later be seen in another of the ID clinics. As all four clinics are staffed by Infectious Disease clinicians, and there is some overlap of clinicians between clinics, HIV care is very centralized and standardized and there is little in the way of unequal access to genotypes. Only the ID trained clinicians providing HIV care at the four clinics order genotype tests for routine clinical care.
ARV resistance testing
Genotype tests were obtained as part of routine clinical care and were performed by LabCorp (Research Triangle Park, NC) using HIV GenoSure. Nucleotide sequences were submitted to the Stanford University HIV Drug Resistance Database (HIVdb) via Sierra, the Stanford Algorithm web service.20 Mutations were categorized as major mutations if identified in the HIVdb as “associated with higher levels of phenotypic resistance or clinical evidence for reduced virologic response.”21
Antiretroviral treatment history
Data were not available describing prisoners' use of ARVs prior to their incarceration and for recidivists between periods of incarceration. Data describing in-prison use of ARVs were limited to an indicator variable differentiating prisoners never and ever prescribed an ARV medication during their incarceration. For simplicity, we refer to these groups as ever prescribed an ARV during incarceration (EPADI) and never prescribed an ARV during incarceration (NPADI).
Statistical analysis
Prisoners with genotypes vs. without genotypes
In bivariate analyses, we compared sex, race, recidivism, and ARV experience (EPADI vs. NPADI) by genotype testing status using tests of chi-square.
Analyses of resistance prevalence
For prisoners with multiple genotype reports, resistant mutations were considered present for all subsequent genotype reports; mutations appearing repeatedly across multiple genotype reports for the same individual were counted only once in our prevalence estimates. All tests for statistical significance were conducted at the level of α=0.05, and 95% confidence intervals were calculated for all prevalence point estimates.
Prisoners ever prescribed an ARV during incarceration (EPADI) with ≥1 genotype
Among prisoners with an indication of an ARV prescription in prison, we compared the proportion of prisoners across categories within the variables sex, race, and recidivism status. We then obtained prevalence estimates for individual resistance mutations over four time periods (October 2006–February 2010, October 2006–December 2007, January 2008–December 2008, and January 2009–February 2010) and by number of ARV-resistant medication classes (no-, single-, dual-, and triple-ARV class resistance) across the entire study period.
Prisoners never prescribed an ARV during incarceration (NPADI) with ≥1 genotype
Among prisoners with no indication of an ARV prescription during their incarceration, we estimated the prevalence of each detected mutation for the entire study period, October 2006–February 2010.
This study was approved by the Biomedical Institutional Review Board of the University of North Carolina at Chapel Hill and by the Human Subjects Review Committee of the North Carolina Department of Corrections
Results
Sample characteristics
Between October 2006 to February 2010, the NC DOC incarcerated 1,911 known HIV-positive persons of whom 19.2% (n=367) had at least one genotype performed. Of the 367 prisoners with genotypes most were male (85.8%), black (79.3%), experienced with ARV treatment in prison (89.4%), and recidivists (68.4%) at the time of their first incarceration during the study period. Twenty-eight percent had multiple incarcerations during the study period (Table 1). The median plasma HIV RNA level obtained within 120 days prior to genotype testing was 4.41 log10 (IQR: 3.78, 4.87). A small number (n=36) of prisoners had multiple genotypes; 28 inmates had two, seven inmates had three, and one inmate had four genotypes.
Table 1.
Genotype Testing Among HIV-Positive Prisoners in the North Carolina Department of Corrections, October 2006–February 2010
| |
Study population (N=1911) |
Population with genotype (N=367) |
|
||
|---|---|---|---|---|---|
| N | % | N | % | p value | |
| Sex | |||||
| Female | 305 | 16.0 | 52 | 14.2 | 0.30 |
| Male | 1606 | 315 | |||
| Race | |||||
| Black | 1527 | 79.9 | 291 | 79.3 | 0.74 |
| Nonblack | 384 | 76 | |||
| Incarceration | |||||
| First incarcerationa | 494 | 25.9 | 93 | 25.3 | 0.97 |
| Reincarceration | 1296 | 67.8 | 251 | 68.4 | |
| Otherb | 121 | 23 | |||
| ARV treatment in prison | |||||
| Ever | 1445 | 75.6 | 329 | 89.6 | <0.0001 |
| Never | 466 | 38 | |||
First incarceration during study period.
Other includes safekeeper, returned from parole, or awaiting sentencing.
ARV, antiretroviral.
Comparing the characteristics of prisoners with genotypes to those without a genotype, we found no statistically significant differences by race, gender, and recidivism rate (Table 1). However, prisoners with genotypes were modestly younger than those without genotypes (mean age 38.4 years vs. 40.0 years, p<0.05) and were more likely to have been prescribed an ARV in prison (Table 1) (p<0.05).
The characteristics of the prisoners ever prescribed an antiretroviral during incarceration with genotypes (n=329) were similar to the overall HIV-infected prison population, with most being male (85.4%), black (80.2%), and recidivists (69.3%) (Table 2).
Table 2.
Demographics of HIV-Positive Prisoners ever Exposed to an ARV During Incarceration (EPADI) with Genotype Testing in North Carolina Department of Corrections, October 2006 - February 2010
| Study population | N 329 | % 100 | p value |
|---|---|---|---|
| Sex | p<0.05 | ||
| Female | 48 | 14.6 | |
| Male | 281 | 85.4 | |
| Race | p<0.05 | ||
| Black | 264 | 80.2 | |
| Nonblacka | 65 | 19.8 | |
| Incarceration | p<0.05 | ||
| First Incarecrationa | 79 | 24.0 | |
| Reincarecration | 228 | 69.3 | |
| Otherb | 22 | 6.7 | |
| Age | 329 | 37.9 | IQR (31.5–44.2) |
| Viral load (log10) | 292 | 4.45 | IQR (3.75–4.88) |
First incarceration during study period.
Other includes safekeeper, returned from parole, or awaiting sentencing.
ARV resistance prevalence
All prisoners with genotypes
The overall prevalence of a major resistance mutation was 28.3%, (95% CI 23.7, 33.0). Resistance by class was 18.8% (95% CI 14.8, 22.8) for nucleoside reverse transcriptase inhibitors (NRTIs), 19.1% (95% CI 15.1, 23.1) for nonnucleoside reverse transcriptase inhibitors (NNRTIs), and 7.9% (95% CI 5.1, 10.7) for protease inhibitors (PIs).
ARV resistance among prisoners EPADI
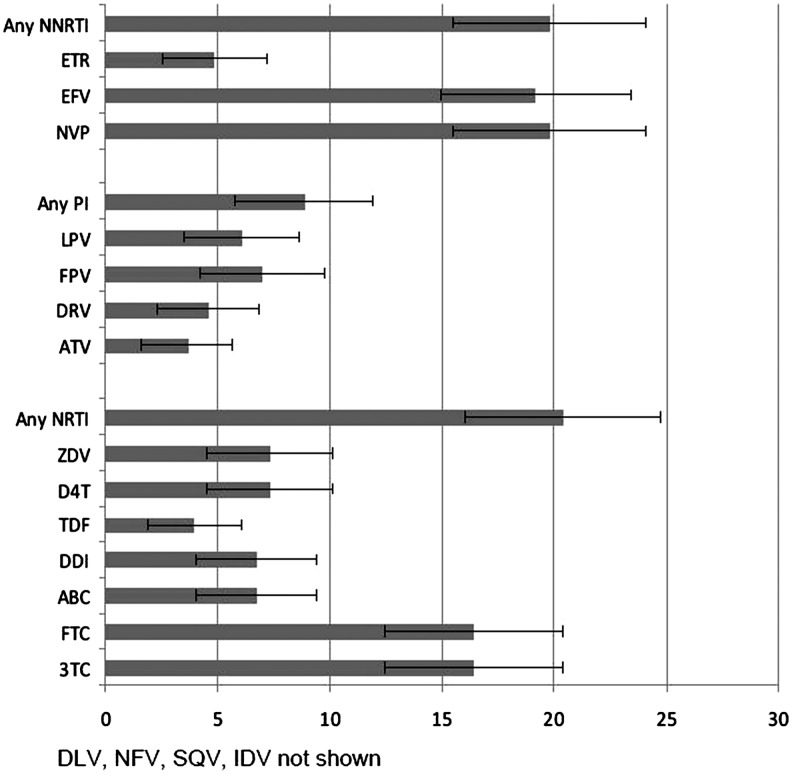
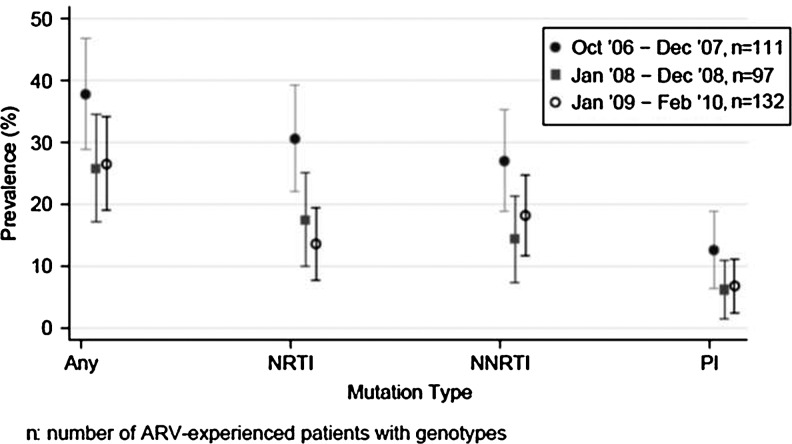
Overall prevalence of a major resistance mutation was 29.8% (95% CI 24.9, 34.7). Resistance by class was 20.4% (95% CI 16.0, 24.7) for NRTIs, 19.8% (95% CI 15.5, 24.1) for NNRTIs, and 8.8% (95% CI 5.8,11.9) for PIs (Fig. 1). Resistance to specific ARV drugs is shown in Fig. 1. Comparing 2007 to 2009, prevalent and class specific resistance declined (Fig. 2). The three most prevalent mutations were K103N 15.8% (95% CI 12.0, 20.2), M184V 14.3% (95% CI 10.7,18.5), and M41L 4.9% (95% CI 2.8,7.8) (Table 3). K103N and M184V remained the two most prevalent mutations for each time period studied. Nineteen patients had a single mutation with K103N and eight patients had a single mutation with M184V.
FIG. 1.
Proportion of HIV-positive prisoners ever prescribed an ARV during incarceration with genotype testing and ARV medication resistance in the North Carolina Department of Corrections, October 2006 - February 2010.
FIG. 2.
Prevalent and antiretroviral class-specific resistance among HIV-infected persons in the North Carolina prison system (95% CI).
Table 3.
Frequency of Major ARV Mutations Among HIV-Positive Prisoners ever Exposed to an ARV During Incarceration (EPADI) with Genotype Testing in North Carolina Department of Corrections, October 2006 - February 2010 (n=329)
| NNRTI | N | % (95% CI) | NRTI | N | % (95% CI) | PI | N | % (95% CI) |
|---|---|---|---|---|---|---|---|---|
| K103N | 52 | 15.81 (12.0,20.2) | M184V | 47 | 14.29 (10.7,18.5) | I50V | 12 | 3.65 (1.9,6.3) |
| Y181C | 9 | 2.74 (1.3,5.1) | M41L | 16 | 4.86 (2.8,7.8) | L90M | 7 | 2.13 (0.9,4.3) |
| G190S | 4 | 1.22 (0.3,3.1) | K65R | 8 | 2.43 (1.1,4.7) | I54V | 6 | 1.82 (0.7,3.9) |
| K101E | 4 | 1.22 (0.3,3.1) | M184I | 7 | 2.13 (0.9,4.3) | M46I | 6 | 1.82 (0.7,3.9) |
| K103S | 4 | 1.22 (0.3,3.1) | L74V | 6 | 1.82 (0.8,3.9) | I84V | 5 | 1.52 (0.5,3.5) |
| G190A | 3 | 0.91 (0.2,2.6) | L74I | 5 | 1.52 (0.5,3.5) | V82A | 5 | 1.52 (0.5,3.5) |
| K101P | 3 | 0.91 (0.2,2.6) | T215F | 5 | 1.52 (0.5,3.5) | N88D | 4 | 1.22 (0.3,3.1) |
| K238T | 3 | 0.91 (0.2,2.6) | T215Y | 5 | 1.52 (0.5,3.5) | D30N | 3 | 0.91 (0.2,2.6) |
| L100I | 3 | 0.91 (0.2,2.6) | T69ADNT | 3 | 0.91 (0.2,2.6) | I54L | 2 | 0.61 (0.1,2.2) |
| Y188L | 3 | 0.91 (0.2,2.6) | T69NT | 2 | 0.61 (0.1,2.2) | V32I | 2 | 0.61 (0.1,2.2) |
| V106M | 2 | 0.61 (0.1,2.2) |
Mutations with only a single occurrence were M230L, V106A, T69AT, V75T, Y115F, F53L, G48V, G73S, G73T, I47V, I50L, L23I, M46L, and V82T.
NNRTI, nonnucleoside reverse transcriptase inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; PI, protease inhibitors.
Single class drug resistance was most prevalent at 14.0% (95% CI 10.2,17.7) followed by dual 12.5% (95% CI 8.9,16.0) and triple class 3.3% (95% CI 1.4,5.3) resistance (Table 3).
ARV resistance among prisoners NPADI
Among the 367 patients with genotypes, 38 had no known history of ARV treatment in prison. Of these, 15.8% (6/38) had a major mutation (one patient: K103N and M184V; four patients: K103N only; and one patient: M41L only) (Table 4).
Table 4.
Number of ARV Classes with a Major Mutation Among HIV-Positive Prisoners ever Exposed to an Antiretroviral During Incarceration with Genotype Testing in North Carolina Department of Corrections, October 2006 - February 2010
| No mutations | NRTI | NNRTI | PI | NRTI/NNRTI | NRTI/PI | NNRTI/PI | Three classes | |
|---|---|---|---|---|---|---|---|---|
| N | 231 | 16 | 22 | 8 | 31 | 9 | 1 | 11 |
| % | 70.2 | 4.9 | 6.7 | 2.4 | 9.4 | 2.7 | 0.3 | 3.3 |
| 95% CI | 65.3, 75.2 | 2.5, 7.2 | 4.0, 9.4 | 0.8, 4.1 | 6.3, 12.6 | 1.0, 4.5 | 0, 0.9 | 1.4, 5.3 |
NNRTI, nonnucleoside reverse transcriptase inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; PI, protease inhibitors.
Discussion
This is the first study to characterize the prevalence of ARV resistance among prisoners with chronic HIV infection in the United States.13 Our analysis included all prison facilities in NC, includes a contemporary time period, and is the largest study of ARV resistance among prisoners in the United States.13 Between 2006 and 2010, ARV resistance prevalence, including dual and triple class drug resistance, was found to be low among HIV-positive persons incarcerated in the North Carolina Department of Corrections.
The prevalence of at least one ARV resistance mutation (29.8%) among prisoners ever prescribed an antiretroviral during incarceration (EPADI) in NC is slightly lower but in keeping with the reported prevalence in prisons in Spain (38.6%).15 This prevalence estimate is also lower than the reported prevalence estimates (37–88%) from studies among the nonincarcerated HIV-positive population receiving ARV therapy.7–12 However, we emphasize that comparing prevalence estimates across studies is complex due to differences in resistance definitions, time periods under study, analytic methods, and study populations. In our cohort, the dates of ARV treatment exposure were not obtainable and sufficient time may have elapsed between treatment discontinuation and obtaining prisoners' genotypes to allow for the emergence of wild-type virus. We are further limited by lack of data on medication adherence, and poor or nonadherence could have influenced our prevalence estimates. In keeping with other studies, ARV resistance prevalence declined from the earliest to the most recent time period.8,10,22 This could be due to the availability of more effective, potent, tolerable therapy and the availability of potent combination therapy with activity against resistant variants including a new agent in a novel class (raltegravir).
Among prisoners ever prescribed an antiretroviral during incarceration, ARV class-specific resistance was highest for the NNRTIs and NRTIs. In line with the class-specific resistance, the most prevalent detected mutations were M184V and K103N. PI resistance was the lowest. These results are in agreement with the study conducted in Spanish prisoners15 and might be explained by the lower genetic barrier to resistance of NRTIs and NNRTIs. Our results could also be a reflection of increased use of NRTIs and NNRTIs, due to their tolerability and, with fixed dose combinations, greater ease of administration. Reassuringly, dual and triple class drug resistance was low. The prevalence estimates for triple class resistance (defined as at least one mutation to the NRTI, NNRTI, and PI drug class) among ARV treatment-experienced nonincarcerated North Carolinians has been reported as 8% contrasted with the 3.3% observed in our study.23
Among prisoners with no indication of ARV treatment experience during incarceration, we detected a higher (13.2%) prevalence of the K103 N mutation in comparison to prevalence estimates among treatment-naive populations from both correctional and noncorrectional settings.13,15,16,24 However, the relatively high prevalence of K103 mutations in our study as compared to others may simply reflect unmeasured ARV exposure immediately prior to incarceration; the estimate should also be interpreted with caution due to the small sample size. Although small, our sample of prisoners with no indication of ARV treatment in prison is similar in size to the naive subjects in other prison studies (Spanish n=43; Italian n=16; Massachusetts n=25).13,15,16 Furthermore, our overall prevalence estimate for ARV resistance in this group of prisoners is similar to that of the Massachusetts cohort.13
Centralization of HIV care in the NC DOC, limited to only four ID clinics for the entire state and staffed by a dedicated group of ID trained clinicians, is a study strength as it likely results in similar access to genotype testing for all HIV-positive inmates. Therefore, our study population likely is a good representation of all HIV-positive NC state prisoners in the study period.
In addition to the limitations we have discussed so far, our results are limited by lack of information on specific ARV treatment regimens at the time of genotype testing, prior ARV treatment history, and adherence to prescribed ARV treatments. Second, use of clinical genotype data may not fully capture the prevalence of resistance mutations in the population. We note that the denominator for our prevalence estimates was based on the number of prisoners with genotypes—a population representing less than 20% of all known HIV-infected NC prisoners incarcerated during the study period. However, the proportion of prisoners with genotypes in our study is similar to that of other published reports.16 Because resistant testing can be conducted only when viral load is >500 HIV RNA c/ml, it is possible that our results underestimate the true prevalence of resistance in the NC prison population because prisoners with viral loads between 40 and 500 HIV RNA c/ml may not have been genotyped.
This study provides important surveillance data on a population that can be difficult to access (both in prison and in the community) and one that may have high rates of treatment interruptions given the often chaotic lives of people who cycle in out of the criminal justice system. The low estimates of ARV resistance prevalence is very encouraging. However, given the low number of resistance tests performed during the study period we believe there is an urgent need to more rapidly and rigorously implement current guidelines for genotype testing in this group.25 In addition, we recommend that all correctional facilities be included in antiretroviral resistance surveillance programs at the state and national levels.
Acknowledgments
This study was supported in part by the University of North Carolina at Chapel Hill, Center for AIDS Research, National Institutes of Health funded program P30 AI50410 and by Gilead Sciences Inc., Foster City, CA. The funders have had no influence on the study design, data collection, data analysis, interpretation, reporting of results, and manuscript preparation.
Author Disclosure Statement
Dr. Menezes received grant support for this study from Gilead Sciences. Dr. Wohl receives grant support from GSK and Merck and has attended advisory boards for Tibotec and Gilead. Dr. Eron receives grant support from GSK, Merck, Tobira, and BMS and is a consultant for BMS, Merck, GSK, Tobira, Tibotec, and Gilead. Dr. White is a speaker for Gilead Sciences, attended advisory boards for Tibotec and Gilead, and has received grant support from Gilead. Dr. Kiziah is an employee of and holds stock in Gilead Sciences.
References
- 1.Spaulding AC. Seals RM. Page MJ. Brzozowski AK. Rhodes W. Hammett TM. HIV/AIDS among inmates of and releasees from US correctional facilities, 2006: Declining share of epidemic but persistent public health opportunity. PLoS One. 2009;4(11):e7558. doi: 10.1371/journal.pone.0007558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altice FL. Mostashari F. Friedland GH. Trust and the acceptance of and adherence to antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;28(1):47–58. doi: 10.1097/00042560-200109010-00008. [DOI] [PubMed] [Google Scholar]
- 3.Wohl DA. Stephenson BL. Golin CE. Kiziah CN. Rosen D. Ngo B. Liu H. Kaplan AH. Adherence to directly observed antiretroviral therapy among human immunodeficiency virus-infected prison inmates. Clin Infect Dis. 2003;36(12):1572–1576. doi: 10.1086/375076. [DOI] [PubMed] [Google Scholar]
- 4.Baillargeon JG. Giordano TP. Harzke AJ. Baillargeon G. Rich JD. Paar DP. Enrollment in outpatient care among newly released prison inmates with HIV infection. Public Health Rep. 2010;125(Suppl 1):64–71. doi: 10.1177/00333549101250S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milloy MJ. Kerr T. Buxton J. Rhodes T. Guillemi S. Hogg R. Montaner J. Wood E. Dose-response effect of incarceration events on nonadherence to HIV antiretroviral therapy among injection drug users. J Infect Dis. 2011;203(9):1215–1221. doi: 10.1093/infdis/jir032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pew Center on the States. State of Recidivism: The Revolving Door of America's Prisons. The Pew Charitable Trusts; Washington, DC: Apr, 2011. [Google Scholar]
- 7.Pillay D. Green H. Matthias R the UK Collaborative Group on HIV Drug Resistance. Estimating HIV-1 drug resistance in antiretroviral-treated individuals in the United Kingdom. J Infect Dis. 2005;192:967–973. doi: 10.1086/432763. [DOI] [PubMed] [Google Scholar]
- 8.Richman DD. Bozzette S. Morton S, et al. The prevalence of antiretroviral drug resistance in the United States. AIDS. 2004;18:1393–1401. doi: 10.1097/01.aids.0000131310.52526.c7. [DOI] [PubMed] [Google Scholar]
- 9.Sista P. Rinehart AR. Wasikowski B, et al. Nine year trends in clinically relevant reduced susceptibility of HIV-1 to antiretrovirals. J Clin Virol. 2009;44:190–194. doi: 10.1016/j.jcv.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Di Giambenedettos Zazzi M. Corsi P, et al. Evolution and predictors of HIV type-1 drug resistance in patients failing combination antiretroviral therapy in Italy. Antivir Ther. 2009;14:359–369. [PubMed] [Google Scholar]
- 11.Von Wyl V. Yerly S. Burgisser P, et al. Long term trends of HIV type 1 drug resistance prevalence among treatment-experienced patients in Switzerland. Clin Infect. Dis. 2009;48:979–87. doi: 10.1086/597352. [DOI] [PubMed] [Google Scholar]
- 12.Bannister WP. Cozzi-Lepri A. Kjaer J, et al. Estimating prevalence of accumulated HIV-1 drug resistance in a cohort of patients on antiretroviral therapy. J Antimicrob Chemother. 2011;66:901–911. doi: 10.1093/jac/dkr006. [DOI] [PubMed] [Google Scholar]
- 13.Stone DR. Corcoran C. Wurcel A. McGovern B. Quirk J. Brewer A. Sutton L. D'Aquila RT. Antiretroviral drug resistance mutations in antiretroviral-naive prisoners. Clin Infect Dis. 2002;35(7):883–886. doi: 10.1086/342697. [DOI] [PubMed] [Google Scholar]
- 14.Jafa K. McElroy P. Fitzpatrick L. Borkowf CB. Macgowan R. Margolis A. Robbins K. Youngpairoj AS. Stratford D. Greenberg A. Taussig J. Shouse RL. Lamarre M. McLellan-Lemal E. Heneine W. Sullivan PS. HIV transmission in a state prison system, 1988–2005. PLoS One. 2009;4(5):e5416. doi: 10.1371/journal.pone.0005416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.García-Guerrero J. Sáiz de la Hoya P. Portilla J. Marco A. Sánchez-Payá J. Moreno S Estudio de Resistencias en Prisiones Group. Prevalence of HIV-1 drug resistance mutations among Spanish prison inmates. Eur J Clin Microbiol Infect Dis. 2006;25(11):695–701. doi: 10.1007/s10096-006-0206-z. [DOI] [PubMed] [Google Scholar]
- 16.Pontali E. Ventura A. Bruzzone B. Icardi G. Ferrari F. Unexpected high rate of wild-type HIV-1 genotype among inmates failing antiretroviral therapy. HIV Clin Trials. 2008;9(5):341–347. doi: 10.1310/hct0905-341. [DOI] [PubMed] [Google Scholar]
- 17.García-Guerrero J. Herrero A. Bedia M. Araújo R. Castellano JC Grupo de Estudio REPRICOVA. [Primary HIV drug resistance in a prison population. REPRICOVA-2 Study]. [Article in Spanish.] Enferm Infecc Microbiol Clin. 2004;22(1):29–31. doi: 10.1016/s0213-005x(04)73027-x. [DOI] [PubMed] [Google Scholar]
- 18.Rosu R. Research Bulletin. North Carolina Department of Correction. Issue No. 52. Mar 4, 2009. http://randp.doc.state.nc.us/scripts/broker.exe?_SERVICE=default&_PROGRAM=sasjobs.Research.sas&_DEBUG=0 http://randp.doc.state.nc.us/scripts/broker.exe?_SERVICE=default&_PROGRAM=sasjobs.Research.sas&_DEBUG=0
- 19.Maruschak LM. Beavers R. HIV in Prisons, 2007–08. Bureau of Justice Statistics. Dec, 2009. bjs.ojp.usdoj.gov/content/pub/pdf/rpr94.pdf bjs.ojp.usdoj.gov/content/pub/pdf/rpr94.pdf NCJ 228307.
- 20.http://hivdb.stanford.edu/pages/webservices/ http://hivdb.stanford.edu/pages/webservices/
- 21.HIV Drug Resistance Mutations by Drug Class (November 6, 2009) http://hivdb.stanford.edu/pages/download/resistanceMutations. http://hivdb.stanford.edu/pages/download/resistanceMutations handout.pdf.
- 22.Aldous J. Jain S. Sun S, et al. Decreasing prevalence of drug resistance mutations over a 7 year period in the CFAR network of integrated clinical systems. 17th Conference on Retroviruses and Opportunistic Infections; Feb;2010 ; Paper #585. [Google Scholar]
- 23.Napravnik S. Keys JR. Quinlivan EB. Triple class antiretroviral drug resistance: Risk and predictors among HIV-1 infected patients. AIDS. 2007;21:825–834. doi: 10.1097/QAD.0b013e32805e8764. [DOI] [PubMed] [Google Scholar]
- 24.Hurt CB. McCoy SI. Kuruc J, et al. Transmitted drug resistance among acute and recent HIV infections in North Carolina, 1998 to 2007. Anitviral Ther. 2009;14(5):673–678. [PMC free article] [PubMed] [Google Scholar]
- 25.Department of Health and Human Services; Jan 10, 2011. [Jun 24;2011 ]. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents; pp. 1–166. [Google Scholar]