Abstract
Indirect evidence suggests that amniotic fluid (AF) may play a role in the pathogenesis of in utero HIV-1 transmission. The purpose of this study was to evaluate the potential innate inhibitory role of AF on HIV replication, which may contribute to protection of the fetus against intrauterine transmission. AF was collected from term HIV-1-negative women undergoing scheduled cesarean section. The inhibitory effect of AF against HIV-1BA-L replication was tested in vitro with or without the addition of protease inhibitor cocktail (PIC) in PHA-stimulated PBMC cultures. Quantitative measurement of human neutrophil peptides 1-3 (HNP1-3) was performed on all AF samples, using an ELISA assay. AF exhibited a dose-dependent inhibitory activity against HIV-1BA-L replication, with all samples (n=12) reaching significant inhibitory effect using 50% AF. In vitro, this activity decreased over time, but was able to be sustained with the addition of PIC. The HNP1-3 concentration in AF samples (n=12) ranged from undetectable (<41 pg/ml, n=3) to >250,000 pg/ml with a median of 5,146 pg/ml. AF exhibited a significant and dose-dependent innate inhibitory activity against HIV-1 replication, which was present in all AF samples tested. This effect was prolonged in the presence of PIC, suggesting that the inhibitory factor was in the cell-free protein fraction. The HNP1-3 concentration in AF was in the subinhibitory range for HIV with no correlation between its concentration and the HIV-1 inhibitory activity. These data show the presence of a significant innate inhibitory activity against HIV in AF.
Introduction
Pediatric HIV infection is a global public health challenge with an estimated 1,000 children newly infected every day.1 Mother-to-child transmission (MTCT) can be effectively reduced to less than 2% with appropriate and timely maternal treatment. In utero transmission of HIV2 occurs in 7–10% of untreated pregnant women3 and in the absence of breastfeeding can contribute from 30% to 50% of perinatal HIV infections. To date, little is known about the exact mechanisms of in utero transmission and potential targets for prevention.
Amniotic fluid (AF) has been implicated in in utero transmission of some perinatal infections including human cytomegalovirus.4 Direct evidence for the role of AF in the pathogenesis of in utero HIV transmission is limited to an early case report of an HIV-positive woman undergoing amniocentesis. Viral culture and p24 antigen assay performed on AF were positive, although the authors were unable to rule out contamination.5 Follow-up studies are scant and limited to indirect evidence as amniocentesis is relatively contraindicated in HIV-positive women based on concerns for increased risk of transmission.6 In the animal model, nontraumatic inoculation of AF with animal equivalent of the virus (SIV) can result in transmission to the fetus in the absence of maternal infection.7 HIV-1 has been isolated from the immediate postdelivery gastric aspirate of infants with in utero transmission of HIV8 suggesting introduction of the virus via fetal swallowing during gestation.
Prevention of in utero transmission of HIV and other pathogens presents a difficult challenge, partly because of the limited knowledge of events that transpire in utero. Improved understanding of host defense mechanisms that contribute to HIV protection may provide knowledge on additional methods for prevention of intrauterine transmission. Amniotic fluid contains components of both the innate and adaptive immune systems. It is possible that immune factors in AF contribute to the paradoxically low rates of in utero transmission of HIV-1. Immunoglobulin G (IgG) is transported actively across the placenta beginning around 32 weeks of gestation.9 Amniotic fluid has been shown to contain pathogen-specific antibodies, at higher portions relative to the total IgG concentration as compared to the maternal serum.10 In the developing fetus, the innate immune system is the first line of defense in warding off bacterial and viral infections. The nonspecific antimicrobial role of AF has been of interest for several decades, with reports of antibacterial activity as early as 1949,11 followed by evidence for innate antiviral activity against herpes simplex virus-1 (HSV-1) and poliovirus-1 reported in 1977.12 The potentially protective role of AF in in utero transmission of HIV-1 has not been investigated. This study was undertaken to investigate the baseline innate activity of AF from HIV-negative women against HIV-1 in vitro.
Materials and Methods
Population, collection, and processing of amniotic fluid
Approval from University of California, Los Angeles Institutional Review Board (IRB) was obtained. Following informed verbal and written consent, AF was collected from HIV-negative women at the time of scheduled cesarean delivery and prior to the onset of labor. All women were HIV seronegative and had no other known infections. After incision of the myometrium, the bulging amniotic bag was irrigated with sterile normal saline. Amniotic fluid was collected by inserting a needle while the fetus was visualized. The sample was placed on ice to inhibit protease activity and processed within 6 h. Amniotic fluid was spun at 200 g×10 min to remove cells and debris. Supernatant was aliquoted and frozen at −70°C until batch analysis.
Reagents, cell lines, and viruses
Tissue culture medium (TCM) containing 20% fetal bovine serum, penicillin, streptomycin, glutamine, and interleukin (IL)-2 was used. Peripheral blood mononuclear cells (PBMCs) were collected from healthy donors using Ficoll-Hypaque density gradient centrifugation. PBMCs were stimulated in tissue culture medium containing phytohemagglutinin (PHA). Macrophage-tropic (R5) HIV-1BA-L was obtained from the NIH AIDS Research and Reference Reagent Program (catalog number 510). Virus stocks were generated by infecting PHA-stimulated normal donor PBMCs and supernatant stored at −70°C in aliquots until use. Protease inhibitory cocktail (PIC) p2714 containing AEBSF, E-64, bestatin, leupeptin, aprotinin, and sodium EDTA was obtained from Sigma-Aldrich (St. Louis, MO). None of these components is known to exhibit an inhibitory effect on HIV-1 protease activity. A commercial human neutrophil defensin (HNP1-3) enzyme-linked immunosorbent assay (ELISA) kit was obtained from Hycult biotechnology (Uden, the Netherlands). This kit has been developed for quantitative measurement of human HNP1-3 in biological fluid and has previously been used for measurement of HNP1-3 in AF. The Perkin Elmer p24 assay was used for quantitative measurement of p24 antigen from cell culture supernatants.
Peripheral blood mononuclear cell viability
PHA-stimulated PBMCs were seeded into a 96-well culture dish. Cell viability using the Trypan Blue exclusion test was assessed in the presence of 10%, 20%, or 50% AF, with or without addition of PIC. PBMCs in TCM, and in some cases with the addition of PIC, served as a control. Cell viability was confirmed upon completion of the experiment via gross examination of well contents and microscopic visualization to assess cell condition and distribution. Some well contents were reexamined using a Trypan Blue exclusion test.
In vitro HIV-1 inhibitory effect of amniotic fluid
PHA-stimulated normal PBMCs were infected with HIV-1BA-L in TCM at a multiplicity of infection of 0.01 or 0.001 viruses/cell. As the TCID50 may differ between PBMC donors, two viral dilutions were used in some assays. After 90 min, cells were washed to remove excess virus and were placed in the 96-well plate to provide 100,000 cells per well. AF at a concentration of 10–60%, PIC, or both were added for a total volume of 220 μl per well. Plates were incubated at 37°C containing 5% CO2. PIC was added every 48 h to the appropriate wells to prevent in vitro degradation of proteins and peptides. PIC was prepared by reconstituting the lyophilized powder in 10 ml of sterile normal saline and was stored in aliquots at −20°C [1×PIC contains 2 mM 4-(2-aminoethyl)benzenesulfonyl fluoride, 1 mM EDTA, 130 μM bestatin, 1.4 μM E-64, 1 μM leupeptin, and 0.3 μM aprotinin]. PIC was added to wells at a concentration of 1:800 at the beginning of the assay, yielding a final concentration of 1:200 after serial addition of PIC. The supernatant was collected on day 4 of the assay (unless indicated otherwise) and frozen until batch analysis of p24 antigen.
Quantitative ELISA measurement of HNP1-3 in amniotic fluid
HNP1-3 was quantified in AF supernatant using the ELISA kit according to the manufacturer's protocol. The kit has a measurable concentration range of 41–10,000 pg/ml. Samples were serially diluted to as much as 1:200,000 to increase detection range as necessary.
Statistics and calculation methods
All p24 antigen experiments including controls were performed in triplicate. Percent inhibition was calculated as [1 – (mean p24 antigen in wells containing AF divided by the mean p24 antigen of the corresponding control wells)×100]. Significant HIV-1 inhibition was defined as at least 50% inhibition. All analysis was performed with STATA version 11.1.
Results
Patient population
Amniotic fluid samples were collected from 12 HIV-negative women undergoing scheduled cesarean section at term gestation. There was no history of labor, clinical chorioamnionitis, or rupture of membranes prior to cesarean delivery. Clinical indications for scheduled cesarean section in the order of frequency included a history of previous cesarean delivery, macrosomic fetus, breach presentation, and at patient request. All women were considered healthy by the obstetrician and underwent a standard HIV test during the current pregnancy, confirming their seronegative status. All pregnancies were singleton with median gestational age at delivery of 38.8 weeks (36.9–40.7 weeks). The median age at delivery was 37.9 years (21.6–44.2 years). Women were of diverse ethnic background with 50% white, 42% Asian/Pacific Islander, and 8% African-American. Past medical history of long-term systemic illness and infectious disease included diabetes mellitus, asthma, and polycystic ovarian disease. All placentas were examined by the obstetrician in the operating room and did not exhibit gross evidence of chorioamnionitis. One patient had AF collected also at the time of amniocentesis, which was performed to assess fetal lung maturity prior to delivery. All collected AF samples were clear without evidence of gross blood contamination.
PBMC viability
Viability of PHA-stimulated PBMCs was assessed in the presence of AF at various concentrations. At 72 h, there was a cell viability of ≥90% in the presence of 10%, 20%, and 50% AF, comparable to the control. Addition of PIC up to a 1:200 dilution was also comparable to control at 92%. Cells were monitored throughout the experiment, with the gross and microscopic appearance of the cell confluences resembling that of the control. There was no evidence of additional cytotoxicity as compared to control when the Trypan Blue exclusion test was performed upon completion of the experiment (data not shown).
Innate inhibitory effect of AF against HIV-1
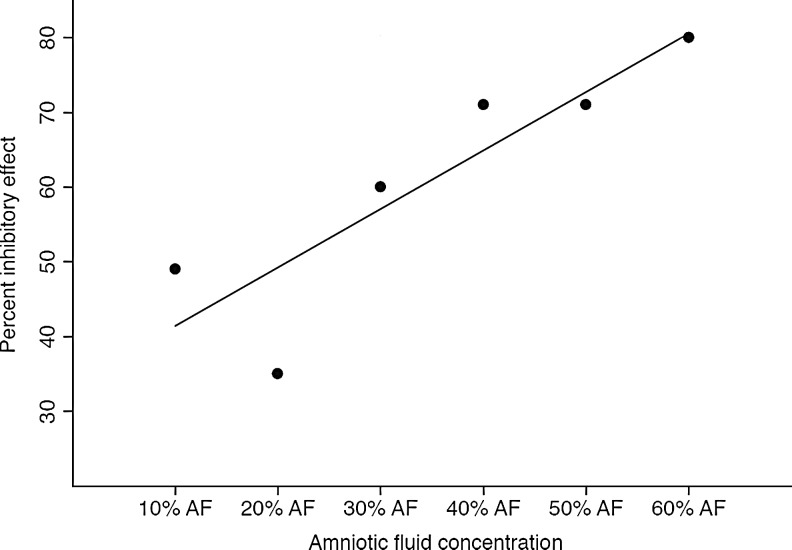
We examined the activity of AF in inhibiting replication of HIV-1 in an in vitro culture of PHA-stimulated PBMCs. AF was added to the cell culture after infection of cells and removal of excess virus to evaluate its role in preventing HIV replication. To assess a possible interferon-like effect, we pretreated the cells for 24 h with AF followed by removal of AF with a wash and then infection of the cells. In these experiments we observed only minimal activity and it was not pursued further (data not shown). Single donor amniotic fluid exhibited dose-dependent anti-HIV activity ranging from 12%, 33%, and 71% in the presence of 10%, 20%, and 50% AF, respectively (data not shown). Figure 1 shows dose-dependent activity of amniotic fluid using a pool from three different donors at concentrations of 10–60% resulting in an inhibitory activity ranging from 35% to 80%>
FIG. 1.
Amniotic fluid exhibited a dose-dependent anti-HIV-1 activity in vitro as seen on day 4 of infection. The percent inhibitory effect against HIV-1BA-L increased in the presence of a higher concentration of amniotic fluid (AF) with 80% inhibitory activity in the presence of 60% AF (n=3, R2=0.78).
As shown in Table 1, we measured the anti-HIV activity using 20% and 50% AF concentrations of all 12 samples. The addition of 50% AF showed a consistent and significant inhibitory activity ≥90% as compared to controls in all samples. At 20% AF, 6 of 13 samples exerted a significant inhibitory with a median of 68%. Of note, both AF samples obtained from patient 10 during amniocentesis and at the time of cesarean delivery had a consistent anti-HIV activity of 63–66% at 20% and 93–96% at 50% AF.
Table 1.
Lack of Correlation of HNP1-3 Concentration with Percent Inhibitory Activity in the Presence of 20% and 50% Amniotic Fluid
| Sample ID | GAa(weeks) | HNP1-3 (pg/ml) | 20% AFb | 50% AFc |
|---|---|---|---|---|
| 1 | 40.7 | 4,187 | 72% | 93% |
| 2 | 38.6 | 7,226 | 0% | 91% |
| 3 | 38.0 | 3,040 | 75% | 90% |
| 4 | 38.7 | 26,283 | 85% | 99% |
| 5 | 39.1 | >250,000 | 0% | 92% |
| 6 | 38.9 | 18,681 | 0% | 98% |
| 7 | 40.0 | 3,700 | 0% | 93% |
| 8 | 38.9 | 5,146 | 68% | 97% |
| 9 | 39.0 | <41 | 0% | 97% |
| 10A | 36.8 | <41 | 66% | 93% |
| 10B | 36.9 | <41 | 62% | 96% |
| 11 | 38.3 | 9,833 | 0% | 98% |
| 12 | 39.1 | 5,187 | 40% | 97% |
Gestational age (GA) at the time of delivery.
Percent inhibitory effect of 20% amniotic fluid (AF) on day 4 of infection.
Percent inhibitory effect of 50% amniotic fluid on day 4 of infection.
A total of 13 AF samples were collected from 12 women at term (36.9–40.7 weeks). Patient 10 had two samples collected: sample A during amniocentesis and sample B collected at the time of cesarean section. At 20% AF, samples from half of the subjects and at 50% samples from all subjects exhibited significant anti-HIV activity as determined on day 4 of infection. HNP1-3 concentration was highly variable and ranged from undetectable to >250,000 pg/ml.
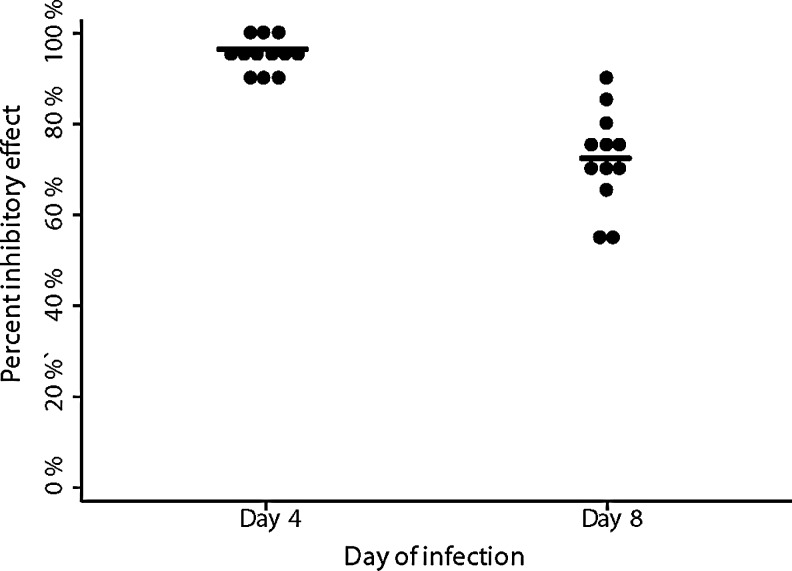
We then evaluated the durability of anti-HIV activity of AF in vitro over 8 days. As seen in Fig. 2, the addition of AF at 50% showed a consistent 90–99% (median 95%) inhibitory effect on HIV-1 replication on day 4 of infection, which decreased to 54–91% (median 72%) by day 8.
FIG. 2.
Percent inhibitory effect of 50% amniotic fluid decreased significantly from day 4 (median=95%) to day 8 (median=72%) of infection (n=12). Each sample is represented by a single dot with the median represented as the straight line through each group.
Preservation of proteins and peptides in AF via addition of PIC
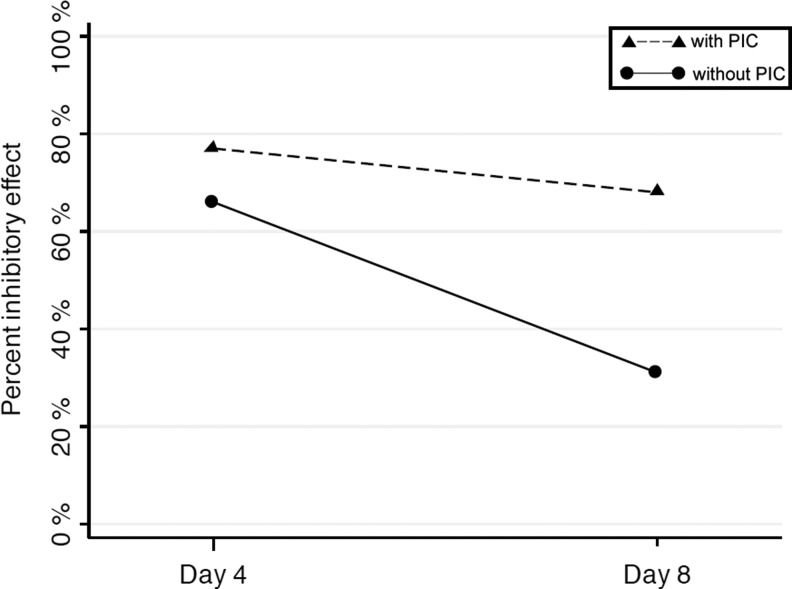
The in vitro inhibitory effect of AF decreased from day 4 to 8. This observation suggested that the in vitro loss of activity might be associated with potential degradation of peptides and proteins over time. We therefore repeated the experiment using PIC to preserve the proteins and peptides in some AF samples. The concentration of PIC in wells was maintained at a dilution of less than 1:200 or more dilute without significant cytotoxic effects, based on preliminary studies (see above). HIV-1 replication in the presence of PIC and without the addition of AF was comparable to the control, confirming the lack of its inhibitory effect on HIV-1 aspartyl protease activity. The activity of AF alone was compared to the addition PIC on days 4 and 8 of infection. As demonstrated in Fig. 3, with the addition of PIC, inhibitory effect was maintained at a mean of ≥77% on day 4 and ≥68 on day 8 (n=3). These data indicated that anti-HIV activity of the cell-free portion of AF was preserved over a prolonged period via addition of PIC.
FIG. 3.
Percent inhibitory activity of amniotic fluid against HIV-1BA-L decreased over time in vitro (solid line). The activity was maintained with the addition of protease inhibitor cocktail (PIC) (dashed line), suggesting a role for the protein fraction of amniotic fluid.
Concentration of HNP1-3 in amniotic fluid
We measured the concentration of HNP1-3 since it has both broad antiviral and specific anti-HIV activity. As shown in Table 1, HNP1-3 concentration ranged from undetectable (<41 pg/ml) to >250,000 pg/ml with a median 5,146 pg/ml. Anti-HIV activity in AF samples was detected in the absence of a detectable concentration of HNP1-3. Viral inhibitory activity did not correlate directly with HNP1-3 level. Of interest, patient 10 with two AF samples obtained during amniocentesis and again at the time of cesarean section had similar values in both specimens.
Discussion
We observed a dose-dependent innate anti-HIV activity exhibited by the cell-free portion of amniotic fluid collected from HIV-negative women. This activity was prolonged in the presence of protease inhibitory cocktail suggesting that anti-HIV-1 factors might reside in the protein portion of AF. Significant anti-HIV-1 activity was present in all AF tested in this experiment, although there was slight variation among samples from different women. This activity did not correlate with HNP1-3 levels and was present even in the absence of detectable levels of HNP1-3, suggesting additional factors may contribute to anti-HIV-1 activity of AF.
Direct evidence for nonspecific antimicrobial activity in AF has been available as early as 1949.11 However, the antiviral properties of innate immune factors in AF are less well understood. In 1977, Pacsa and Pejtsik showed inhibition of HSV and poliovirus growth using AF samples with undetectable viral-specific IgG levels. Seven of 44 samples showed definite antiviral activity against HSV-1, poliovirus-1, or both in a microneutralization test. Amniotic fluid inhibited growth of HSV-1 more effectively than poliovirus-1. Ultrafiltration of AF samples showed that the active substance belonged to the protein fraction of samples.12 Further investigation of innate antiviral activity of AF has been scant over the past several decades. Our results are consistent and for the first time extend these findings to activity against HIV-1, an important agent among perinatally transmitted infections. Samples in this study were collected from a cohort of HIV-negative women, which allowed us to isolate the activity to innate immune factors, as compared to AF from HIV-positive women where the presence of immunoglobulins and antiretroviral drugs in AF may also contribute to inhibition of HIV replication.
Addition of PIC inhibits protein and peptide breakdown and was able to prolong in vitro anti-HIV activity of AF, supporting previous reports that the activity belongs to the protein fraction of AF.
Recent evidence describes an abundance of antimicrobial peptides (AMPs) in AF.13,14 Antimicrobial peptides are effector molecules of the innate immune system with the ability to directly kill bacteria, fungi, and enveloped viruses. Several AMPs isolated from AF are known to inhibit HIV-1 activity in vitro, acting prior,15 at the level of viral fusion and entry,16 after binding,17 or during replication.15,18,19 It is postulated that AMPs contribute to maintaining sterility of the fetal environment by their ubiquitous presence in AF, vernix,14 and the cervical mucus plug.20 Current understanding of the origins of these peptides is limited but speculations include release from the fetal lungs and skin. Some of the important peptides isolated from AF include α-defensins (HNP1-3), lactoferrin, secretory leukocyte protease inhibitors (SLPI), lysozyme, and LL-37.13,14 Since HNP1-3 are AMPs with broad antiviral and anti-HIV activity and have a readily available ELISA, we measured their concentration as a potential candidate substance to explain the antiviral activity of AF.
HNP1-3 are short cationic peptides primarily produced in neutrophil granules. In the perinatal environment, they are present in AF,21,22 breast milk,23 cervical mucus plug,20 and vernix.14 The concentration of HNP1-3 increases significantly in the presence of infections including sepsis24 and intrauterine infections.21 Increasing HNP1-3 concentration in breast milk has been associated with decreased risk of HIV transmission among breastfed infants.23 In vitro anti-HIV activity of HNP1-3 has been well established. HNP1-3 exhibit an anti-HIV effect at physiologic and nontoxic concentrations (1) in the presence of serum via target cell modification and inhibition of HIV-1 replication, and (2) in the absence of serum via direct inactivation of virions.15,25 We used a quantitative ELISA assay to measure HNP1-3 levels in AF. In a previous study of AF from nonlaboring women collected at term gestation, the HNP1-3 concentration was determined using the same ELISA kit manufacturer. Results were comparable to our findings and ranged from 1.31 to 42.46 ng/ml with median of 5.56 ng/ml.22 The physiologic concentration of HNP1-3 in AF is lower in comparison to other reported specimen including plasma from healthy donors (median 25, range 25–213 ng/ml),24 human vaginal fluid (VF) from HIV-uninfected female sex workers (median 255, range 0.05–4658 ng/ml),26 and cervical mucus plug collected in healthy women at the time of labor (median 3.4 μM∼11,220 ng/ml).20
Concentrations of HNP1-3, which has been reported to result in significant inhibition of HIV-1, range from 1,000 ng/ml15 to 60 μM (∼200 μg/ml).27 This inhibitory effect is highly variable and depends on the strain of HIV, presence of serum, and quantity of virus. We used HIV-1BA-L, a macrophage trophic (R5) laboratory strain. In this experiment we found significant inhibitory activity against HIV when AF was added after infection and in the presence of serum. This suggests that the potentially limiting role of HNP1-3 may have been limited to target cell modification and postentry restriction of replication. It is also possible that the detectable concentration of HNP1-3 in AF is too low to account for its anti-HIV-1 activity. Furthermore, this activity was also present in the absence of detectable levels of HNP1-3. Our findings suggest that HNP1-3 is likely not responsible for innate anti-HIV-1 activity of AF in isolation.
Cole et al. reported intrinsic anti-HIV-1 activity of human VF from healthy donors against both BAL (R5-tropic) and IIIB (X4-tropic) HIV-1 laboratory strains. This inhibitory effect was ablated with the removal of cationic polypeptides from VF. Eighteen cationic polypeptides were identified in vaginal secretion including HNP1-3, lactoferrin, and SLPI. Physiologic concentrations of 13 of the cationic polypeptides were not active alone against HIV. However, the addition of the entire polypeptide extract was highly restorative to the depleted vaginal fluid, suggesting a synergistic role for polypeptides of VF in inhibiting HIV-1 infection.28,29 Findings by Levinson et al. support the additive effect of innate AMP in cervicovaginal secretions. The ability of individual samples to neutralize HIV was strongly associated with higher levels of total innate immune factors including HNP1-3, SLPI, and LL-37.26
AMPs can be degraded by proteases in vitro, resulting in decrease or loss of their biological activity. In vitro protease activity may be limited via preserving samples in cold temperature (<4°C), as was done in this study. Protease inhibitors can further prevent degradation of peptides and prolong duration of peptide and protease activity in vitro. We selected a PIC with broad specificity. None of its components was known to exhibit an inhibitory effect on HIV-1 protease activity, as was confirmed in this experiment. Addition of PIC prolonged the inhibitory effect of AF, suggesting innate immune factors with anti-HIV activity are contained in the protein portion of AF.
Previous investigation of the role of AF in in utero transmission of HIV has focused on the adaptive immune system. Analysis of AF from HIV-1-positive women has shown the presence of HIV-1-specific immunoglobulin G with relative proportions of 3- to 10-fold higher than plasma when levels were adjusted as proportions of total immunoglobulin content. Levels were variable depending on the individual subject and the specific antigen measured (HIV-1 p24 or gp120). Similar studies in macaques showed SIV-neutralizing antibody in plasma and AF.10 In a previous study, administration of HIV hyperimmune immunoglobulin was associated with a lack of in utero transmission in a small number of cases as compared to infusions of intravenous immunoglobulin (IVIG) without HIV antibody.30 Our group has examined the role of maternal autologous neutralizing antibody (aNAB) in selective perinatal transmission of HIV-1. Mothers with detectable aNAB were less likely to transmit HIV-1 in utero and the infecting strain was more closely related to maternal aNAB escape variants, suggesting neutralizing antibody is an important immune correlate of protection from HIV-1 infection.31 In a related study, phylogenetic analysis of HIV-1 strains isolated from infants' first gastric aspirate collected from two mother–baby pairs with intrauterine transmission revealed a distant genetic relatedness to maternal strains at time of birth. This finding suggests the viral variant was potentially able to access the fetal stomach earlier in pregnancy, supporting the hypothesis of its acquisition via swallow of infected AF.32
In the absence of antiviral treatment, approximately 90–93% of mothers do not transmit HIV in utero. Thus, in most cases of maternal HIV infection, the maternal fetal environment including physical and immunological barriers—the placenta, amniotic fluid, vernix, and cervical mucus plug—is sufficient to prevent in utero acquisition.20,33 The fetal–placental unit provides a highly specific barrier to entry of maternal infections through the placenta.34
To our knowledge, this is the first study showing the presence of an innate anti-HIV activity in AF from healthy HIV-negative women. There is a paucity of information about immunoprotective properties of AF and whether these can be enhanced to prevent in utero transmission of infections. Natural35 or induced36 perturbations in innate immune defenses may play a role in transmission of intrauterine HIV-1 and other perinatal infections. In HIV-infected women the antiviral effects of amniotic fluid likely consist of a combination of HIV-1-specific antibody and innate immune factors such as we describe. Currently, we are actively looking for other substances that may contribute to the innate antiviral activity of AF and its potentially synergistic activity with adaptive immune factors in AF collected from HIV-infected women.
In summary, our results are consistent with the hypothesis that normal AF exhibits innate anti-HIV activity in vitro and that this effect is in part contained in the protein fraction. Although the factor/factors responsible for this activity are not known, it is likely that an array of AMPs or other factors may synergize to exhibit this effect and may provide the first line of defense. A better understanding of natural and acquired protective factors can aid in the development of safe and effective methods to prevent in utero transmission of HIV and other perinatal infections.
Acknowledgments
We thank Dr. Robert Lehrer for his guidance and support and the families who kindly participated in this study. We also thank Laura Wennstrom Sheehan for her invaluable assistance. P24 assays were provided by the Center for AIDS Research Virology Core Lab that is supported by the National Institutes of Health Award AI-28697 and by the UCLA AIDS Institute and the UCLA Council of Bioscience Resources. This work was supported by the National Institutes of Health (U01-AI069401-01), American Academy of Pediatrics (20093565), UCLA AIDS Institute and the UCLA Center for AIDS Research (AI28697), and National Institute of Child Health and Human Development (T32 HD007549). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, AAP, UCLA AIDS Institute, or NICHD.
Data were presented at the 2011 American Academy of Pediatrics National Conference & Exhibition, Boston, MA, 17th Conference on Retroviruses and Opportunistic Infections, San Francisco, CA, and 2010 Pediatric Academic Societies, Vancouver, Canada, 2010
Author Disclosure Statement
No competing financial interests exist.
References
- 1.World Health Organization and UNAIDS. Global summary of AIDS epidemic. 2009. [Sep 17;2012 ]. www.who.int/hiv/data/2009_global_summary.png www.who.int/hiv/data/2009_global_summary.png
- 2.Bryson YJ. Luzuriaga K. Sullivan JL. Wara DW. Proposed definitions for in utero versus intrapartum transmission of HIV-1. N Engl J Med. 1992;327:1246–1247. doi: 10.1056/NEJM199210223271718. [DOI] [PubMed] [Google Scholar]
- 3.Deville J. Bryson Y. Perinatal transmission of HIV: Recognition and treatment interventions. Curr Infect Dis Rep. 2001;3:388–396. doi: 10.1007/s11908-001-0080-x. [DOI] [PubMed] [Google Scholar]
- 4.Revello MG. Gerna G. Pathogenesis and prenatal diagnosis of human cytomegalovirus infection. J Clin Virol. 2004;29:71–83. doi: 10.1016/j.jcv.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Mundy DC. Schinazi RF. Gerber AR. Nahmias AJ. Randall HW., Jr Human immunodeficiency virus isolated from amniotic fluid. Lancet. 1987;2:459–460. doi: 10.1016/s0140-6736(87)91001-4. [DOI] [PubMed] [Google Scholar]
- 6.Tess BH. Rodrigues LC. Newell ML. Dunn DT. Lago TD. Breastfeeding, genetic, obstetric and other risk factors associated with mother-to-child transmission of HIV-1 in Sao Paulo State, Brazil. Sao Paulo Collaborative Study for Vertical Transmission of HIV-1. AIDS. 1998;12:513–520. doi: 10.1097/00002030-199805000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Fazely F. Sharma PL. Fratazzi C, et al. Simian immunodeficiency virus infection via amniotic fluid: A model to study fetal immunopathogenesis and prophylaxis. J Acquir Immune Defic Syndr. 1993;6:107–114. [PubMed] [Google Scholar]
- 8.Nielsen K. Boyer P. Dillon M, et al. Presence of human immunodeficiency virus (HIV) type 1 and HIV-1-specific antibodies in cervicovaginal secretions of infected mothers and in the gastric aspirates of their infants. J Infect Dis. 1996;173:1001–1004. doi: 10.1093/infdis/173.4.1001. [DOI] [PubMed] [Google Scholar]
- 9.Lewis DB. Gern JE. Hill HR, et al. Newborn immunology: Relevance to the clinician. Curr Probl Pediatr Adolesc Health Care. 2006;36:189–204. doi: 10.1016/j.cppeds.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Jaspan HB. Robinson JE. Amedee AM. Van Dyke RB. Garry RF. Amniotic fluid has higher relative levels of lentivirus-specific antibodies than plasma and can contain neutralizing antibodies. J Clin Virol. 2004;31:190–197. doi: 10.1016/j.jcv.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Bowdler NC. Galask RP. Amniotic fluid, its relation to infections. In: Pastorek JG, editor. Obstetric and Gynecologic Infectious Disease. Raven Press; New York: 1994. pp. 417–425. [Google Scholar]
- 12.Pacsa AS. Impairment of immunity during pregnancy and antiviral effect of amniotic fluid. Lancet. 1977;1:330–331. doi: 10.1016/s0140-6736(77)91134-5. [DOI] [PubMed] [Google Scholar]
- 13.Akinbi HT. Narendran V. Pass AK. Markart P. Hoath SB. Host defense proteins in vernix caseosa and amniotic fluid. Am J Obstet Gynecol. 2004;191:2090–2096. doi: 10.1016/j.ajog.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Yoshio H. Tollin M. Gudmundsson GH, et al. Antimicrobial polypeptides of human vernix caseosa and amniotic fluid: Implications for newborn innate defense. Pediatr Res. 2003;53:211–216. doi: 10.1203/01.PDR.0000047471.47777.B0. [DOI] [PubMed] [Google Scholar]
- 15.Chang TL. Vargas J., Jr DelPortillo A. Klotman ME. Dual role of alpha-defensin-1 in anti-HIV-1 innate immunity. J Clin Invest. 2005;115:765–773. doi: 10.1172/JCI200521948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Strate BW. Beljaars L. Molema G. Harmsen MC. Meijer DK. Antiviral activities of lactoferrin. Antiviral Res. 2001;52:225–239. doi: 10.1016/s0166-3542(01)00195-4. [DOI] [PubMed] [Google Scholar]
- 17.McNeely TB. Dealy M. Dripps DJ. Orenstein JM. Eisenberg SP. Wahl SM. Secretory leukocyte protease inhibitor: A human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J Clin Invest. 1995;96:456–464. doi: 10.1172/JCI118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee-Huang S. Maiorov V. Huang PL, et al. Structural and functional modeling of human lysozyme reveals a unique nonapeptide, HL9, with anti-HIV activity. Biochemistry. 2005;44:4648–4655. doi: 10.1021/bi0477081. [DOI] [PubMed] [Google Scholar]
- 19.Bergman P. Walter-Jallow L. Broliden K. Agerberth B. Soderlund J. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr HIV Res. 2007;5:410–415. doi: 10.2174/157016207781023947. [DOI] [PubMed] [Google Scholar]
- 20.Hein M. Valore EV. Helmig RB. Uldbjerg N. Ganz T. Antimicrobial factors in the cervical mucus plug. Am J Obstet Gynecol. 2002;187:137–144. doi: 10.1067/mob.2002.123034. [DOI] [PubMed] [Google Scholar]
- 21.Heine RP. Wiesenfeld H. Mortimer L. Greig PC. Amniotic fluid defensins: Potential markers of subclinical intrauterine infection. Clin Infect Dis. 1998;27:513–518. doi: 10.1086/514691. [DOI] [PubMed] [Google Scholar]
- 22.Espinoza J. Chaiworapongsa T. Romero R, et al. Antimicrobial peptides in amniotic fluid: Defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003;13:2–21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- 23.Kuhn L. Trabattoni D. Kankasa C, et al. Alpha-defensins in the prevention of HIV transmission among breastfed infants. J Acquir Immune Defic Syndr. 2005;39:138–142. [PMC free article] [PubMed] [Google Scholar]
- 24.Panyutich AV. Panyutich EA. Krapivin VA. Baturevich EA. Ganz T. Plasma defensin concentrations are elevated in patients with septicemia or bacterial meningitis. J Lab Clin Med. 1993;122:202–207. [PubMed] [Google Scholar]
- 25.Klotman ME. Chang TL. Defensins in innate antiviral immunity. Nat Rev Immunol. 2006;6:447–456. doi: 10.1038/nri1860. [DOI] [PubMed] [Google Scholar]
- 26.Levinson P. Kaul R. Kimani J, et al. Levels of innate immune factors in genital fluids: Association of alpha defensins and LL-37 with genital infections and increased HIV acquisition. AIDS. 2009;23:309–317. doi: 10.1097/QAD.0b013e328321809c. [DOI] [PubMed] [Google Scholar]
- 27.Chang TL. Klotman ME. Defensins: Natural anti-HIV peptides. AIDS Rev. 2004;6:161–168. [PubMed] [Google Scholar]
- 28.Cole AM. Cole AL. Antimicrobial polypeptides are key anti-HIV-1 effector molecules of cervicovaginal host defense. Am J Reprod Immunol. 2008;59:27–34. doi: 10.1111/j.1600-0897.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 29.Venkataraman N. Cole AL. Svoboda P. Pohl J. Cole AM. Cationic polypeptides are required for anti-HIV-1 activity of human vaginal fluid. J Immunol. 2005;175:7560–7567. doi: 10.4049/jimmunol.175.11.7560. [DOI] [PubMed] [Google Scholar]
- 30.Stiehm ER. Lambert JS. Mofenson LM, et al. Efficacy of zidovudine and human immunodeficiency virus (HIV) hyperimmune immunoglobulin for reducing perinatal HIV transmission from HIV-infected women with advanced disease: Results of Pediatric AIDS Clinical Trials Group protocol 185. J Infect Dis. 1999;179:567–575. doi: 10.1086/314637. [DOI] [PubMed] [Google Scholar]
- 31.Dickover R. Garratty E. Yusim K. Miller C. Korber B. Bryson Y. Role of maternal autologous neutralizing antibody in selective perinatal transmission of human immunodeficiency virus type 1 escape variants. J Virol. 2006;80:6525–6533. doi: 10.1128/JVI.02658-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickover R. Nielsen K. Yusim K. Korber B. Jin J. Bryson Y. Perinatal transmission of HIV-1 via amniotic fluid, vaginal secretions, blood: A molecular analysis. 12th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2005. [Google Scholar]
- 33.Hein M. Helmig RB. Schonheyder HC. Ganz T. Uldbjerg N. An in vitro study of antibacterial properties of the cervical mucus plug in pregnancy. Am J Obstet Gynecol. 2001;185:586–592. doi: 10.1067/mob.2001.116685. [DOI] [PubMed] [Google Scholar]
- 34.Miller RK. Polliotti BM. Laughlin T, et al. Role of the placenta in fetal HIV infection. Teratology. 2000;61:391–394. doi: 10.1002/(SICI)1096-9926(200005)61:5<391::AID-TERA14>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 35.Hollox EJ. Barber JC. Brookes AJ. Armour JA. Defensins and the dynamic genome: What we can learn from structural variation at human chromosome band 8p23.1. Genome Res. 2008;18:1686–1697. doi: 10.1101/gr.080945.108. [DOI] [PubMed] [Google Scholar]
- 36.Wang TT. Nestel FP. Bourdeau V, et al. Cutting edge: 1,25-Dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]