Abstract
New HIV infections among younger men who have sex with men (MSM) in the United States are escalating. Data on HIV infections in college students are limited. In 2010, three MSM college students presented to our clinic with primary HIV infection (PHI) in a single month. To determine the number of college students among new HIV diagnoses, we reviewed clinical characteristics and molecular epidemiology of HIV-diagnosed individuals from January to December 2010 at the largest HIV clinic in Southern New England. PHI was defined as acute HIV infection or seroconversion within the last 6 months. Of 66 individuals diagnosed with HIV in 2010, 62% were MSM and 17% were academic students (12% college or university, 5% other). Seventy-three percent of students were MSM. Compared to nonstudents, students were more likely to be younger (24 versus 39 years), born in the United States (91% versus 56%), have another sexually transmitted disease (45% versus 11%), and present with PHI (73% versus 16%, all p-values<0.05). Thirty percent of individuals formed eight transmission clusters including four students. MSM were more likely to be part of clusters. Department of Health contact tracing of cluster participants allowed further identification of epidemiological linkages. Given these high rates of PHI in recently diagnosed students, institutions of higher education should be aware of acute HIV presentation and the need for rapid diagnosis. Prevention strategies should focus on younger MSM, specifically college-age students who may be at increased risk of HIV infection.
HIV infection continues to increase in the United States, disproportionally affecting younger men who have sex with men (MSM) and minority populations.1 Individuals less than 30 years old account for up to 35% of new infections, more than any other age group.2 College students represent a young, understudied population with multiple HIV risk factors. Approximately 18.2 million students are enrolled in higher education in the United States, of whom 75% are less than 30 years old.3 Students are at increased HIV risk because of multiple sexual partners, low condom use, and drug and alcohol use.3–5 Despite these behaviors, earlier and more recent reports suggested low HIV prevalence in college students compared to the general population,5–7 while higher rates have been observed in subpopulations such as MSM who frequently engage in risky behaviors.8–10 Sexual networks may extend outside campuses and involve groups of MSM with higher infection prevalence.11 Recent data on epidemiology and transmission of HIV among college students in the United States are lacking.
Previous reports,12,13 including here in Rhode Island,14 demonstrated transmission networks and their role in HIV spread. In a single month during 2010, three Southern New England college students (“index” patients) presented to our clinic with primary HIV infection (PHI).15,16 Concerned for an acute HIV epidemic, we reviewed all newly diagnosed individuals at our clinic during 2010 with a focus on college and other academic students, PHI, HIV transmission clusters, and drug resistance. We hypothesized that the three index cases were representative of a larger HIV-infected student population, and that their identification and characterization would provide information for prevention opportunities.
The Samuel and Esther Chester Immunology Center in Providence, Rhode Island was the main study site and is the largest HIV outpatient clinic in the state providing comprehensive care to over 1,500 patients. The clinic does not specifically serve college students. Patients were included if they were (1) HIV positive, (2) ≥18 years old, and (3) diagnosed during 2010. Demographic, clinical, and laboratory data were collected from patient records. Acute infection was defined as a negative enzyme-linked immunosorbent assay (ELISA) and detectable RNA at presentation, or positive ELISA and indeterminate Western blot.16 Recent, nonacute HIV infection was defined as seroconversion within 6 months as evidenced by a current positive ELISA and negative, 6 month prior ELISA, or patient-reported clinical syndrome consistent with acute retroviral syndrome in the prior 6 months15,16 (including fever, lymphadenopathy, pharyngitis, rash, myalgias, and/or arthralgias17–20). PHI was defined as acute or recent infection. The study was approved by the Lifespan institutional review board.
HIV pol genotyping was performed with ViroSeq v2.0 (Celera Corporation, Alameda, CA) at Quest Diagnostics, Department of Molecular Diagnostics, Chantilly, VA. Sequences were handled using ClustalW,21 Bioedit,22 and SQUAT.23 Neighbor-joining phylogenetic analysis was with Phylip24 and MEGA,25 using the F84 evolution model, transversion/transition ratio 2:1, and 1000 replicate bootstraps. Genetic distances were measured using SynScan.26 Clustering was defined as high (>99%) bootstraps and small (<0.05) genetic distances. Subtyping and resistance interpretation were performed using REGA,27 Stanford Database tools,28 and the International AIDS Society mutation list.29 Statistical significance (p<0.05) was determined by Fisher's exact test for categorical variables and the Mann–Whitney nonparametric test for continuous variables.
During 2010, 66 individuals presented with a new diagnosis of HIV (Table 1). Major HIV risk factors were MSM (41/66, 62%) followed by heterosexual behaviors (23/66, 35%). Sixty-two percent (41/66) were born in the United States. Mean CD4 counts and viral load on presentation were 457 cells/μl (24%) and 161,827 copies/ml, respectively. Twenty-six percent (17/66) presented with PHI and 17% (11/66) had another sexually transmitted disease (STD) at diagnosis.
Table 1.
Characteristics of HIV-1-Infected Individuals Diagnosed in 2010
| All N=66a | Students N=11 | Nonstudents N=55 | p-value | |
|---|---|---|---|---|
| Age (average) | 37 years | 24 years | 39 years | <0.01 |
| Male | 51 (77%) | 8 (73%) | 43 (78%) | 0.70 |
| MSM | 41 (62%) | 8 (73%) | 33 (60%) | 0.51 |
| PHI | 17 (26%) | 8 (73%) | 9 (16%) | <0.01 |
| TDRa | 10 (16%) | 2 (20.0%) | 8 (16%) | 0.66 |
| Non-Subtype Ba | 7 (12%) | 0 (0%) | 7 (14%) | 0.59 |
| Foreign born | 25 (38%) | 1 (9%) | 24 (44%) | 0.041 |
| CD4 (average) | 457 | 759 | 397 | <0.01 |
| PVL (average) | 161,827 | 93,998 | 175,393 | <0.01 |
| STD | 11 (17%) | 5 (46) | 6 (11%) | 0.014 |
| Clustera | 16 (26%) | 4 (40%) | 12 (24%) | 0.43 |
Sequence data were available for 61 of the 66 patients. For categories that are based on sequence data (TDR, Subtype, Cluster), the total number is based on 61 available sequences.
MSM, men who have sex with men; PHI, primary HIV-1 infection; TDR, transmitted drug resistance; STD, sexually transmitted diseases, other than HIV; CD4 units, cells/μl; PVL, HIV plasma viral load (copies/ml).
Seventeen percent (11/66) of the cohort were students of higher education (eight college/university students, three high-school equivalency students). Ten students were born in the United States and one was from the Caribbean. Of United States born individuals, 24% (10/41) were students. Seventy-three percent of students (8/11) were males, all MSM. Eighty percent of students (8/10) were nonwhite (four African-Americans, three Hispanics, one Asian; missing information on race/ethnicity for one student). Four students were from the same institution and all were MSM. Student status was not associated with sexual orientation or other HIV risk factors. Students were significantly more likely to be born in the United States (91% versus 56%, p=0.04), have another STD on presentation (45% versus 11%, p<0.05), and present with PHI (73% versus 16%, p<0.05) than nonstudents. Of the three index students, two presented with acute infection, one had a positive ELISA but indeterminate Western blot, and one had a high viral load and acute retroviral syndrome in the past month. Seven of the eight students with PHI (88%) reported recent symptoms consistent with acute retroviral syndrome including the three index students.
Of the eight MSM students, six reported meeting partners online and one reported visiting a bathhouse. In contrast, 52% (17/33) of MSM nonstudents reported visiting a bathhouse (p=0.06). There was no difference between student and nonstudent MSM in drug use, mental illness, or internet use to meet partners.
Sixty-one of the 66 patients (92%) had available genotypes (three were unsuccessful including one student, one had HIV-2, and one was not requested). Seven of 61 (11%) patients had a non-B subtype (subtype G: 1, G/B: 2, CRF02_AG: 1, AG/AE: 1, A/AE: 1). None of the patients infected with a non-B subtype reported being a student. Of patients with a non-B subtype, two were from the United States and the other five were from Africa. Of 61 available sequences, transmitted drug resistance was 16% (10/61; 95% CI 0.090–0.28; Fig. 1). Student status was not associated with drug resistance (p=0.66). There was no transmitted drug resistance observed in non-B subtypes.
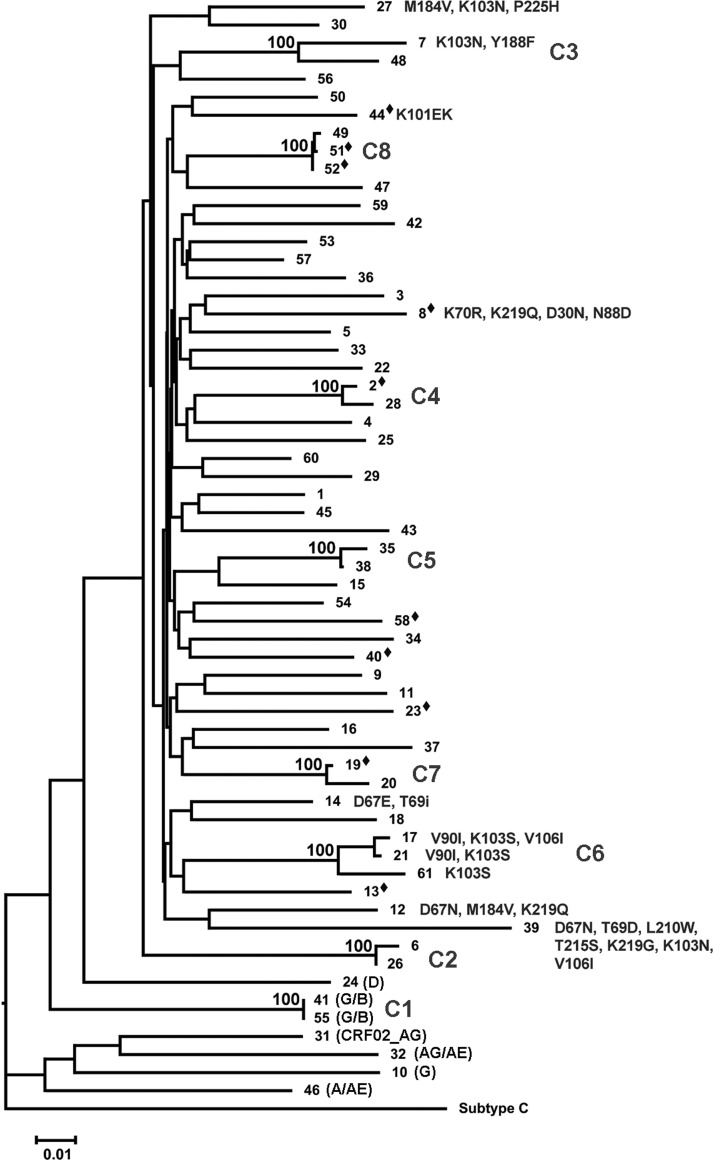
FIG. 1.
Phylogenetic analysis of newly HIV diagnosed individuals. A pol neighbor-joining phylogenetic tree of patients included in the study rooted by HIV-1 subtype C (accession number U46016.1). A 1% distance scale is shown on the bottom left. Numbers to the right of tree branches represent patient samples. Numbers on top of tree branches represent percent bootstrap support (1000 replicates). C1 through C8 identify the eight transmission clusters. Letters in parentheses represent non-B subtypes. Resistance mutations are shown to the right of the sample number. Diamonds indicate students. Identical sequences in cluster A were investigated and verified.
Thirty percent (18/61) of individuals formed eight distinct transmission clusters, two with three individuals and six with two individuals (Fig. 1). Clusters remained similar after removal of drug resistance positions to avoid convergent evolution. Mean genetic distance among clusters was 1.7%. Four students were included in clusters. This was not significantly different from nonstudents (p=0.43). One cluster was composed of two index students. This association was confirmed by epidemiologic contact tracing conducted by the Rhode Island Department of Health, and led to identification of other contacts. Being part of a cluster was not associated with being male, place of birth, PHI, STDs, drug resistance, or student status. MSM were significantly more likely to be part of a cluster compared to other risk groups (p=0.02). Four individuals within clusters had drug resistance mutations, all nonnucleoside reverse transcriptase inhibitor (NNRTI) associated. Three were in the same cluster (F) with some overlapping resistance mutations, and one was in a two-patient cluster (C) with no resistance overlap.
In summary, we demonstrate a high prevalence of college and other academic students among patients diagnosed with HIV in 2010 at the largest HIV clinic in Southern New England: 17% of all, and 24% of United States born, patients. Twelve percent attended a college or university. Students were mostly younger, born in the United States, and likely to present with PHI and another STD. The prevalence of United States academic students among new HIV diagnoses has not been widely studied. HIV among college students was previously described in North Carolina11,30 in 2000–2003 during which time 11% of new HIV diagnoses in men 18–30 years old were college students. HIV infection rates were especially high among African-Americans (87%) and MSM (92%), with 14% presenting with PHI.11 The number of students here is higher and supports high rates of nonwhite (82%) and MSM status (73%) as HIV risk factors in college and other academic students. A considerably larger proportion of college students in our series (73%) also presented with PHI. These combined data suggest that MSM college students are at higher risk for HIV infection then the general college population, especially MSM who are Hispanic or African-American.31
The prevalence of students among new HIV diagnoses may be increasing in Southern New England. In our previous 2007 study, college and other academic students consisted of only 6% (2/35) of the cohort.14 These data likely underrepresent true HIV prevalence among high-risk students. Of the almost 25% of undiagnosed HIV infections in the United States,32 it is possible that many individuals are infected in college or other academic settings and diagnosed later in life. Low rates of reported HIV testing by institutions and individuals may account for missed HIV diagnosis opportunities. College health services should be educated regarding presentation and diagnosis of acute HIV, which may mimic other nonspecific presentations. Given that such services are already present at many higher learning institutions and the active involvement of MSM and lesbian/gay/bisexual/transgender (LGBT) student groups on campuses, implementation of focused HIV screening may offer feasible and important interventions that can prevent HIV infections both during college and later in life.
The molecular epidemiology of HIV can suggest the existence of sexual transmission networks among students. HIV pol sequences contain enough genetic diversity to reconstruct phylogenetic clusters,14,33 however, they cannot and do not intend to reflect epidemiologic linkages and do not necessarily indicate direct HIV transmission among its members. Evaluation of transmission clusters within our patient sample demonstrated some clustering, similar to our prior study in 2007.14 MSM students did form clusters, but not with students from other schools. This is in contrast to previous epidemiologic investigations of HIV transmission clusters on college campuses in the Southeastern United States, which showed diffuse networks across numerous colleges.34 In larger studies, sexual networks among MSM included many more individuals and found significant clustering in this population.13,35 Given the limited time frame, the observed clusters here likely underrepresent larger MSM sexual networks present on academic campuses. Sexual networks are responsible for transmission of HIV and propagation of the epidemic.13 A larger molecular and contact tracing analysis is needed to further evaluate these networks in our community.
The main limitation of this study is the small sample size. However, our observation is concerning and warrants further attention. A second limitation relates to the retrospective nature of data collection, which limits the ability to define specific behaviors or perform detailed risk assessments. The study is also limited by the self-reporting of sexual contacts and risk behaviors associated with HIV transmission. There is the potential underreporting of sexual contacts or other behaviors due to social desirability bias. This may limit our understanding of transmission clusters, but these biases are noted to be a barrier across all studies of this type.
Our study demonstrates that college and other adult education students comprise a significant percentage of new HIV diagnoses at our clinic, higher than in previous years. This trend parallels a national concern of rising HIV rates among younger MSM and suggests targeting this population in prevention strategies. Propagation of HIV infection is occurring in specific MSM sexual networks and may differ between students and nonstudents. Characterization of these transmission networks can lead to targeted prevention interventions to disrupt HIV transmission among high-risk populations. College and other academic students, particularly MSM, are an understudied population that represents a significant proportion of recent and acute infections in Southern New England. Students are a potentially captive audience in which HIV and other STD prevention interventions could be useful, especially given the focus of academic institutions on education. Health services at colleges and other academic institutions should consider implementing HIV prevention programs, as well as education on recognition of acute HIV symptoms for the general student population and targeted high-risk groups such as MSM.
Acknowledgments
Philip A. Chan and Rami Kantor are supported by grants (1K23AI096923-01 and R01AI66922, respectively) from the National Institute of Allergy and Infectious Diseases at the National Institutes of Health. Additional support was provided by a research training program funded by the National Institute of Mental Health (T32 MH078788, PI: Larry Brown) and the Lifespan/Tufts/Brown Center for AIDS Research funded by the National Institute of Allergy and Infectious Diseases (P30AI042853, PI: Charles Carpenter). We would like to thank Paul Loberti, Sutopa Chowdhury, and the Rhode Island Department of Health for their support of this study.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hall HI. Song R. Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300(5):520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control (CDC) Subpopulation estimates from the HIV incidence surveillance system—United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57(36):985–989. [PubMed] [Google Scholar]
- 3.U.S. Department of Education. National Center for Education Statistics. http://nces.ed.gov. 2011. http://nces.ed.gov
- 4.Lewis JE. Malow RM. Ireland SJ. HIV/AIDS risk in heterosexual college students. A review of a decade of literature. J Am Coll Health. 1997;45(4):147–158. doi: 10.1080/07448481.1997.9936875. [DOI] [PubMed] [Google Scholar]
- 5.American College Health Association-National College Health Assessment Spring 2008 Reference Group Data Report (abridged): The American College Health Association. J Am Coll Health. 2009;57(5):477–488. doi: 10.3200/JACH.57.5.477-488. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control (CDC). Youth Risk Behavior Surveillance: National College Health Risk Behavior Survey—United States, 1995. MMWR CDC Surveill Summ. 1997;46(6):1–56. [PubMed] [Google Scholar]
- 7.Gayle HD. Keeling RP. Garcia-Tunon M, et al. Prevalence of the human immunodeficiency virus among university students. N Engl J Med. 1990;323(22):1538–1541. doi: 10.1056/NEJM199011293232206. [DOI] [PubMed] [Google Scholar]
- 8.Mansergh G. Marks G. Age and risk of HIV infection in men who have sex with men. AIDS. 1998;12(10):1119–1128. doi: 10.1097/00002030-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Crepaz N. Marks G. Mansergh G, et al. Age-related risk for HIV infection in men who have sex with men: Examination of behavioral, relationship, and serostatus variables. AIDS Educ Prev. 2000;12(5):405–415. [PubMed] [Google Scholar]
- 10.Fisher M. Pao D. Brown AE, et al. Determinants of HIV-1 transmission in men who have sex with men: A combined clinical, epidemiological and phylogenetic approach. AIDS. 2010;24(11):1739–1747. doi: 10.1097/QAD.0b013e32833ac9e6. [DOI] [PubMed] [Google Scholar]
- 11.Hightow LB. MacDonald PDM. Pilcher CD, et al. The unexpected movement of the HIV epidemic in the Southeastern United States: Transmission among college students. J Acquir Immune Defic Syndr. 2005;38(5):531–537. doi: 10.1097/01.qai.0000155037.10628.cb. [DOI] [PubMed] [Google Scholar]
- 12.Brenner BG. Roger M. Moisi DD, et al. Transmission networks of drug resistance acquired in primary/early stage HIV infection. AIDS. 2008;22(18):2509–2515. doi: 10.1097/QAD.0b013e3283121c90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenner BG. Roger M. Routy J-P, et al. High rates of forward transmission events after acute/early HIV-1 infection. J Infect Dis. 2007;195(7):951–959. doi: 10.1086/512088. [DOI] [PubMed] [Google Scholar]
- 14.Chan PA. Tashima K. Cartwright CP, et al. Transmitted drug resistance and molecular epidemiology in antiretroviral naive HIV type 1-infected patients in Rhode Island. AIDS Res Hum Retroviruses. 2011;27:275–281. doi: 10.1089/aid.2010.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cates W., Jr Chesney MA. Cohen MS. Primary HIV infection—a public health opportunity. Am J Public Health. 1997;87(12):1928–1930. doi: 10.2105/ajph.87.12.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescent. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2011. pp. 1–167.
- 17.Hecht FM. Busch MP. Rawal B, et al. Use of laboratory tests and clinical symptoms for identification of primary HIV infection. AIDS. 2002;16(8):1119–1129. doi: 10.1097/00002030-200205240-00005. [DOI] [PubMed] [Google Scholar]
- 18.Daar ES. Little S. Pitt J, et al. Diagnosis of primary HIV-1 infection. Los Angeles County Primary HIV Infection Recruitment Network. Ann Intern Med. 2001;134(1):25–29. doi: 10.7326/0003-4819-134-1-200101020-00010. [DOI] [PubMed] [Google Scholar]
- 19.Daar ES. Pilcher CD. Hecht FM. Clinical presentation and diagnosis of primary HIV-1 infection. Curr Opin HIV AIDS. 2008;3(1):10–15. doi: 10.1097/COH.0b013e3282f2e295. [DOI] [PubMed] [Google Scholar]
- 20.Schacker T. Collier AC. Hughes J. Shea T. Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125(4):257–264. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 21.Thompson JD. Gibson TJ. Plewniak F. Jeanmougin F. Higgins DG. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25(24):4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall TA. Bioedit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 23.Delong AK. Wu M. Bennett D, et al. Sequence quality analysis tool for HIV type 1 protease, reverse transcriptase. AIDS Res Hum Retroviruses. 2011. http://www.ncbi.nlm.nih.gov/pubmed/21916749. [Dec 30;2011 ]. http://www.ncbi.nlm.nih.gov/pubmed/21916749 [DOI] [PMC free article] [PubMed]
- 24.Feldstein J. PHYLIP (Phylogeny Inference Package) University of Washington; Seattle, WA: 2009. version 3.69. Department of Genetics. [Google Scholar]
- 25.Tamura K. Peterson D. Peterson N, et al. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzales MJ. Dugan JM. Shafer RW. Synonymous-non-synonymous mutation rates between sequences containing ambiguous nucleotides (Syn-SCAN) Bioinformatics. 2002;18(6):886–887. doi: 10.1093/bioinformatics/18.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Oliveira T. Deforche K. Cassol S, et al. An automated genotyping system for analysis of HIV-1 and other microbial sequences. Bioinformatics. 2005;21(19):3797–3800. doi: 10.1093/bioinformatics/bti607. [DOI] [PubMed] [Google Scholar]
- 28.Stanford HIV Drug Resistance Database. hivdb.stanford.edu. [Aug 31;2010 ]. hivdb.stanford.edu
- 29.Johnson VA. Brun-Vézinet F. Clotet B, et al. Update of the drug resistance mutations in HIV-1: December 2010. Top HIV Med. 2010;18(5):156–163. [PubMed] [Google Scholar]
- 30.Centers for Disease Control (CDC) HIV transmission among black college student and non-student men who have sex with men—North Carolina, 2003. MMWR Morb Mortal Wkly Rep. 2004;53(32):731–734. [PubMed] [Google Scholar]
- 31.Hall HI. Hughes D. Dean HD. Mermin JH. Fenton KA. HIV Infection—United States, 2005 and 2008. MMWR Surveill Summ. 2011;60(Suppl 0):87–89. [PubMed] [Google Scholar]
- 32.Centers for Disease Control (CDC) HIV prevalence estimates—United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57(39):1073–1076. [PubMed] [Google Scholar]
- 33.Hué S. Clewley JP. Cane PA. Pillay D. HIV-1 pol gene variation is sufficient for reconstruction of transmissions in the era of antiretroviral therapy. AIDS. 2004;18(5):719–728. doi: 10.1097/00002030-200403260-00002. [DOI] [PubMed] [Google Scholar]
- 34.Hightow LB. Leone PA. Macdonald PDM, et al. Men who have sex with men and women: A unique risk group for HIV transmission on North Carolina College campuses. Sex Transm Dis. 2006;33(10):585–593. doi: 10.1097/01.olq.0000216031.93089.68. [DOI] [PubMed] [Google Scholar]
- 35.Yerly S. Junier T. Gayet-Ageron A, et al. The impact of transmission clusters on primary drug resistance in newly diagnosed HIV-1 infection. AIDS. 2009;23(11):1415–1423. doi: 10.1097/QAD.0b013e32832d40ad. [DOI] [PubMed] [Google Scholar]