Abstract
The effects of soluble Nef protein on CD4+ T cells were examined. CD4+-T-cell cultures exposed to soluble Nef were analyzed for apoptosis by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling and hallmarks of apoptosis including cytoplasmic shrinkage, nuclear fragmentation, DNA laddering, and caspase activation. We observed dose- and time-dependent inductions of apoptosis. DNA laddering and activated caspase 3 were also evident. Cells treated with Nef/protein kinase inhibitor complexes were protected from Nef-induced apoptosis, suggesting possible roles for protein kinases in the apoptosis pathway. Similarly, cells treated with Nef/anti-Nef antibody complexes were protected from Nef-induced apoptosis. The cellular receptor responsible for Nef-induced apoptosis was identified through antibody- and ligand-blocking experiments as a receptor commonly involved in viral entry. CXCR4 antibodies, as well as the endogenous ligand SDF-1α, were effective in blocking Nef-induced apoptosis, while CCR5 and CD4 antibodies were ineffective. Moreover, a CXCR4-deficient cell line, MDA-MB-468, which was resistant to Nef-induced apoptosis, became sensitive upon transfection with a CXCR4-expressing vector. This study suggests that extracellular Nef protein could contribute to the decline of CD4 counts prior to and during the onset of AIDS in patients with human immunodeficiency virus type 1 infections.
The most consistent pathogenic feature of human immunodeficiency virus type 1 (HIV-1) infection is the gradual depletion of CD4+ T lymphocytes, a phenomenon that renders the host susceptible to opportunistic infections arising from the dysregulation and dysfunction of the immune system (13, 27, 35, 36). Elimination of CD4+ T lymphocytes leads to the occurrence of a wide range of opportunistic infections, as well as proliferation of virus-transformed and malignant cells. A direct consequence of decline of cell-mediated immunity is an elevation in the viral load in plasma and circulating, peripheral blood T lymphocytes, as the HIV-infected individual progresses from the asymptomatic stage of infection to advanced AIDS.
It has been proposed that the depletion of T lymphocytes is directly linked to the viral load and the process of T-cell depletion is induced by viral infectivity (9, 64). However, Anderson et al. (3) suggested that this model for pathogenesis made too many assumptions. They suggested that a careful examination of the relevant data with a minimum number of assumptions leads to the conclusion that direct killing of infected cells did not adequately explain the consequences of HIV-1 infection.
An alternate hypothesis to explain T lymphocyte depletion is that it is a result of programmed cell killing or apoptosis. Finkel et al. reported that apoptosis was observed in CD4+ T lymphocytes in infected individuals and is observed predominantly in uninfected or bystander cells, with a distinct lack of cell killing in the productively infected cells themselves (18). The “bystander effect” directly implicates viral proteins or indirectly implicates virally stimulated cellular factors as mediators of apoptosis. Several studies support the argument for the bystander effect and implicate Nef. In one study, infected cells were protected from virus-induced cell death through major histocompatibility complex down-regulation induced by intracellularly expressed Nef protein (11). The infected cells continued to produce viral particles, while the surrounding uninfected CD4+ T lymphocytes died (11, 47). A number of other studies support the hypothesis that endogenous Nef is involved in bystander effects. It has been suggested that Nef protects the HIV-1-infected host cell from proapoptotic signals through interference with apoptosis signal-regulating kinase 1 (ASK1) while simultaneously promoting the killing of bystander cells through the induction of FasL (20). It was shown that ASK1, which is a serine/threonine kinase, is essential for the complete signaling of Fas and tumor necrosis factor (TNF) alpha death-signaling pathways. However, in the presence of Nef, the formation of Nef/ASK1 complexes prevents the normal interaction of ASK1 with the death-signaling receptors. Other researchers report a direct association of HIV-1 Nef with the zeta chain of the T-cell receptor complex and the requirement of both of these proteins for HIV-mediated up-regulation of FasL (65). Their data suggest that Nef can form a signaling complex with the T-cell receptor of T lymphocytes, thereby bypassing the requirement for antigen to initiate T-cell activation and subsequent up-regulation of FasL expression. Long-term stable expression of Nef, which mimics persistent or latent infection in vivo, was observed to confer resistance against anti-Fas antibody-induced apoptosis through inhibition of caspase 3 and caspase 8 activation (66). HIV-1 Nef has been suggested to augment viral replication by prolonging the viability of infected cells through the blocking of p53-mediated apoptosis (23).
Transgenic animal models have been developed to study effects of HIV expression on in vivo host systems (mouse [14-17, 21, 24, 25, 33, 59] or rat [52]). In these studies, lymphocyte depletion, as well as most of the organ system dysfunction found in AIDS patients, was observed. In mice transgenic for nef, lymphocyte depletion, as well as most of the tissue-specific dysfunction found in AIDS patients, was observed (14-17, 21, 24, 25, 33, 59), and the nef-transgenic mice progressed to death as in typical HIV infections (54). These effects required only the expression of Nef in the target cells.
A second premise of the bystander effect model implicates viral proteins or indirectly implicates virally stimulated cellular factors as mediators of bystander cell death. The N-terminal portion of Nef has been found to be involved in apoptosis of uninfected lymphocytes (5, 31). Okada and colleagues have shown that soluble, exogenous Nef protein, in complex with anti-Nef antibodies, causes destruction of a broad spectrum of uninfected lymphoid cells (i.e., CD4+ and CD8+ T lymphocytes, B lymphocytes, macrophages, and neutrophils), leading to suppression of the immune system (46-49). This limited body of literature hints that Nef may be one of the viral factors directly involved in host pathogenesis. Reports made by Fujii et al. also suggest that soluble Nef protein may require the presence of anti-Nef antibody to facilitate the induction of apoptosis in T helper cells (19).
In the following studies, the effects of soluble Nef protein on immortalized T-cell lines, as well as on human peripheral blood mononuclear cells (PBMCs), were examined. Nef protein, in the absence of antibody, was found to be apoptotic to these and other cell types through the CXCR4 receptor.
MATERIALS AND METHODS
Proteins and antibodies.
RANTES and SDF-1a were obtained from Chemicon (Temecula, Calif.). Fasudil (HA1077) and H7 were obtained from Sigma (St. Louis, Mo.). E-TOXATE was obtained from Sigma. The following antibodies were used: monoclonal mouse anti-human fusin clone 12G5, murine immunoglobulin G2a (IgG2a) (CXCR4) (Research Diagnostics Inc., Flanders, N.J.); monoclonal mouse anti-human CCR5 clone 2D7/CCR5, murine immunoglobulin G2a (PharMingen, San Diego, Calif.); monoclonal mouse anti-human CD4 antibody (American Bio-Technologies, Inc., Cambridge, Mass.); mouse IgG (Sigma); monoclonal mouse anti-caspase 3 antibody (Active Motif, Carlsbad, Calif.); rabbit anti-HIV-1 Nef antiserum (Ab-1; NIH AIDS Research and Reference Reagent Program, Rockville, Md.); rabbit anti-HIV-1 Nef antiserum GF7 (Ab-2; gift from Warner Greene).
Nef-expressing plasmids.
The HIV-1 nef gene used for protein expression in bacteria was subcloned from pNL43 (nucleotide [nt] 8743 to 9632), using a forward primer [5′-GGG GGG AAG CCT C(A/T) ATG GGT GGC AAG TGG TCA AAA AGT AGT GT-3′] engineered to contain the NdeI restriction site (underlined) while retaining the start codon, and a reverse primer [5′-GGA GGG CTG C(A/G) TCA GTG ATG GTG ATG GTG ATG TCC GCC GGA TCC ACC GCA GTT CTT GAA GTA CTC CGG-3′] that was designed to contain a linker region, followed by a hexahistidine tag, a stop codon and the PstI restriction site (underlined). The PCR product was cloned into the pRSETB expression vector using the NdeI and PstI restriction sites. This digestion removed the N-terminal histidine tag, T7 gene 10 leader and Xpress Epitope from the pRSETB vector. The recombinant plasmid, which contained a C-terminal hexahistidine tag, was referred to as pRSETB-Nef. Vectors for the eukaryotic expression of HIV-1, HIV-2, and simian immunodeficiency virus (SIV) Nef were produced by amplifying the Nef coding regions from pNL4-3 (nt 8743 to 9632), HIV7312A (nt 8896 to 10085), and SIVMM239 (nt 9137 to 10414), respectively. The following primer pairs were used: HIV-1 forward primer, 5′-CCT AGA AGA ATA AGA CAG GGC, and reverse primer, 5′-CAC TAC TTG AAG CAC TCA AGG C; HIV-2 forward primer, 5′-GAA GAA GGA GGT GGA AAC GAC G, and reverse primer, 5′-AAG TGC TGG TGA GAG TCT AGC; SIV forward primer, 5′-GCT CCT GGC CTT GGC AGA TAG, and reverse primer, 5′-GCT TAC TTC TAA AAT GGC AGC. The PCR products were cloned into the pcDNA3.1/V5-His topo vector (Invitrogen, Palo Alto, Calif.) according to the manufacturer's instructions.
Expression and purification of Nef. (i) Bacterially expressed Nef.
HIV-1 Nef protein was expressed in BL21 DE3 Escherichia coli cells from pRSETB-Nef. Bacteria were grown for 16 h in Luria-Bertani medium containing ampicillin (100 μg/ml) and chloramphenicol (34 μg/ml). The protein was not IPTG (isopropyl-β-d-thiogalactopyranoside) induced due to its toxicity. Bacterial cells were chemically disrupted using BugBuster protein extract reagent plus benzonase nuclease (Novagen, Madison, Wis.), while the synthesized Nef protein was protected from degradation using the protease inhibitors phenylmethylsulfonyl fluoride (Calbiochem, San Diego, Calif.) and Protease Arrest (Genotech, San Francisco, Calif.). Recombinant HIV-1 Nef was purified using the His-Bind Kit (Novagen), and the purified protein was eluted using a 4 M imidazole wash. The eluted Nef protein was dialyzed and concentrated by centrifugation at 2,000 × g using the ultrafree-15 centrifugal filter provided by Millipore (Fisher, Tustin, Calif.), with a molecular mass cutoff of 5 kDa. The purified recombinant HIV-1 Nef protein resolved at approximately 32 kDa, with a smaller amount of the dimeric form occurring at approximately 50 kDa (data not shown). Surface-enhanced laser desorption-ionization analysis under nondenaturing conditions revealed the existence of Nef protein monomers, dimers, and trimers (data not shown) consistent with previous reports (4). This suggests that the recombinant protein possessed secondary and tertiary structures that normally exist in the native protein.
(ii) Eukaryotic cell-expressed Nef.
HEK 293 cells (107) were transfected with 6 μg of plasmid DNA from the HIV-1, HIV-2, or SIV vectors described above using Effectene transfection reagent (QIAGEN) according to the manufacturer's recommendations. Cultures were then allowed to grow and express the appropriate Nef protein into the medium for 48 to 96 h. The conditioned medium was then collected, analyzed for Nef protein, and used in our assays.
Determination of cytotoxic contaminants in purified bacterial Nef protein preparations.
The final Nef preparations were tested for traces of lipopolysaccharide or other cytotoxic bacterial molecules by two methods.
(i) Limulus lysate assay.
E-TOXATE working solution (100 μl) was added to an equal volume of Nef protein using aseptic conditions. The solutions were gently mixed and incubated at 37°C for 1 h. Once the incubation began, the tube was kept stationary. At 1 h the tube was inverted 180°, while being observed for evidence of gel formation. A positive test was the formation of a hard gel permitting complete inversion of the tube. All other results, such as soft gels, turbidity, increases in viscosity, or clear liquid were considered negative. The test was sensitive to 0.05 to 0.1 endotoxin unit per ml. This test was negative (data not shown), indicating the absence of lipopolysaccharide.
(ii) Parallel synthesis of human-derived CRABP.
Cellular retinoic acid-binding protein (CRABP) was synthesized in parallel with Nef protein synthesis and then tested by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) for its ability to induce apoptosis in Jurkat cell cultures. The CRABP protein preparation was found to be negative for apoptotic activity (data not shown), thereby confirming the absence of bacterium-derived cytotoxic agents in the Nef protein preparation.
Tissue culture. (i) Cell types.
Jurkat and H9 cells are CD4+-T-cell lines derived from human T-cell leukemia and human cutaneous T-cell lymphoma cells, respectively, and were obtained from the NIH AIDS Research and Reference Reagent Program. Fresh human PBMCs were obtained from Cambrex Bio Science (Walkersville, Md.). MDA-MB-468 and MDA-MB-231 cells are derived from human breast adenocarcinoma and human breast carcinoma, respectively, and were obtained from the American Type Culture Collection (Manassas, Va.). HEK 293 cells are derived from a human primary embryonic kidney transformed by adenovirus type 5 and were obtained from the AIDS Research and Reference Reagent Program.
(ii) Cell culturing.
Jurkat, H9, MDA-MB-468, and MDA-MB-231 cells were maintained in RPMI 1640 medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum, streptomycin (100 U/ml), penicillin (100 U/ml), l-glutamine (2 mM), and HEPES-buffered saline solution (30 μM). PBMCs were maintained in LGM-3 medium (Cambrex Bio Science), supplemented with interleukin-2 (30 U/μl), allowing subpopulations to adhere to the flask while T-cell populations remained in suspension. After 24 h, the medium containing suspended cells was centrifuged to harvest suspension cells, which comprised primarily T cells. HEK 293 cells were maintained in Dulbecco's modified Eagle medium (Gibco, Carlsbad, Calif.) supplemented with 10% heat-inactivated fetal bovine serum and gentamicin (100 U/ml). This cell population was 84% CD4+ and 79% CXCR4+ as measured by immunocytochemical staining, with the vast majority of the CXCR4+ cells colabeling for CD4 in cells simultaneously labeled for both markers. Alternatively, cells were treated with various concentrations and for various times with purified recombinant HIV-1 Nef or eukaryotic cell-conditioned medium containing Nef protein expressed from transfected Nef constructs at 37°C.
(iii) Transfection of MDA-MB-468 cells with CXCR4 expression plasmid.
MDA-MB-468 cells in log phase were pelleted and resuspended in RPMI 1640 (without serum) at a concentration of 4.5 × 106 cells per 400-μl aliquot. CXCR4 plasmid DNA (Pc-Fusin [2.5 μg]; NIH AIDS Research and Reference Reagent Program, McKessonHBOC BioServices, Rockville, Md.) was added to each aliquot of cells, transferred to 0.4-cm-diameter electroporation cuvettes (Bio-Rad Labs, Hercules, Calif.), and electroporated at 250 V, 960 μF of capacitance pulse, and 200 Ω of resistance (Gene Pulser; Bio-Rad, Richmond, Calif.). Cells were allowed to rest at room temperature (RT) for 10 min, transferred to microcentrifuge tubes with 400 μl of fresh RPMI 1640, and pelleted at 200 × g for 5 min to remove debris. The cells were resuspended in 1 ml of RPMI 1640 (with serum) on plates and incubated at 37°C for 48 h to allow expression of the transfected gene.
TUNEL assay.
Cultures were assayed for apoptosis using a TUNEL assay as described previously (29). The cells were visualized by epifluorescence on a computer-controlled microscope system (Zeiss microscope; Carl Zeiss, Thornwood, N.Y.). Microscopic images were processed using a charge-coupled device camera, MC 100 SPOT (Photonic Science, East Sussex, United Kingdom). Further image processing was conducted by utilizing the Image-Pro Plus 2.0 software (Media Cybernetics, Silver Spring, Md.).
Immunocytochemistry.
Cultures were rinsed twice with phosphate-buffered saline (PBS) containing 0.1% glycine to reduce intrinsic fluorescence and blocked, with or without fixing, in 1% goat serum in PBS containing 0.3% Triton X-100 at RT for 1 h. Primary antibody (1:250) was added to the blocking solution, and the slides were incubated overnight at 4°C. The slides were rinsed three times with 1× PBS containing 1% Triton X-100 at RT, and the secondary antibody, 1:200 Texas Red anti-mouse IgG (heavy plus light chain) (Vector Laboratories, Burlingame, Calif.), was added to the blocking solution and incubated at RT for 1 h. The slides were then rinsed three times with 1× PBS containing 1% Triton X-100 at RT, and in those experiments where we stained unfixed cells, the cells were subsequently fixed in 4% paraformaldehyde at RT for 60 min. Finally, the slides were rinsed three times with 1× PBS, briefly dried, and mounted, and excess oil was removed and slides were visualized or stored in the refrigerator. Cells were visualized by epifluorescence on a Zeiss microscope (Carl Zeiss) using a charge-coupled device camera, MC 100 SPOT (Photonic Science). Further image processing was performed utilizing the Image-Pro Plus 4.1 for Windows software (Media Cybernetics) and Adobe Photoshop software (version 5.0.2; Adobe Systems, San Jose, Calif.).
Morphological analysis.
A characteristic of apoptosis is the occurrence of visible nuclear fragmentation, which is distinguished from necrosis in which nuclear fragmentation is not typical. Following treatments, cells were analyzed for nuclear fragmentation by light microscopy as described previously (29). Additionally, cells were analyzed for nuclear fragmentation following TUNEL treatment, since nuclear fragmentation was easier to detect when cells were fluorescently labeled.
DNA laddering-agarose gel electrophoresis.
Another hallmark of apoptosis is internucleosomal cleavage, leading to the generation of discrete DNA fragments that are multiples of approximately 100 bp. Jurkat cell cultures were either untreated or treated with extracellular HIV-1 Nef for 24 h at 37°C. Nuclear extracts were obtained as described previously (29). Briefly, cellular and nuclear membranes were ruptured using lysis buffer (1.0% Nonidet P-40 [NP-40; Sigma]; 50 mM Tris-HCl, pH 7.5; 20 mM EDTA buffer) at RT for 2 min. The nuclear extracts were centrifuged at 15,340 × g for 20 min at 4°C, and then RNA was removed by performing RNase A (Sigma) digestion at 37°C for 2 h. All protein present was eliminated from the mixture by proteinase K (Promega, Madison, Wis.) treatment at 56°C for 2 h. DNA was precipitated overnight in 4 M ammonium acetate and a 0.7 volume of isopropanol at −20°C. The DNA pellet was then washed three times with 70% ethanol. Purified DNA was air dried, resuspended in sterile water, and quantitated by spectrophotometry (HP 8453 spectrophotometer; HP ChemStation, Palo Alto, Calif.). A 20-μg aliquot of DNA from each treatment was prepared in gel loading buffer that gave a final concentration of 0.02% bromophenol blue, 5% glycerol, 0.1% sodium dodecyl sulfate (SDS), and 50 μg of ethidium bromide. The buffered DNA samples were resolved on a neutral 1.5% agarose gel at 6 V/cm for 4 h. The molecular weights of the DNA species were determined by parallel resolution of HindIII-digested bacteriophage lambda DNA and a 100-bp DNA ladder (Promega).
Protein assay.
Jurkat cell cultures were examined for the activation of caspase 3. Jurkat cell cultures were either untreated or treated with extracellular HIV-1 Nef for 1, 3, 6, 12, 18, 24, 30, or 36 h. Cells were harvested and extracts were obtained by disrupting cells using lysis buffer (1.0% Nonidet P-40 [NP-40; Sigma]; 50 mM Tris-HCl, pH 7.5; 20 mM EDTA buffer) at RT for 2 min. Proteins were resolved on SDS-polyacrylamide gels (4 to 20% acrylamide) and transferred to polyvinylidene difluoride membranes by electroblotting. Membranes were then washed in Tris-buffered saline (25 mM Tris-HCl, 0.15 M NaCl, pH 7.5) with 0.05% (wt/vol) Tween 20, blocked for nonspecific binding with 5% (wt/vol) dry milk, and then probed using antibody buffers in 1% (wt/vol) dry milk. Caspase was assayed using mouse anti-caspase 3 antibody (Active Motif) as primary antibody and anti-mouse antibody as secondary antibody. The mouse anti-caspase 3 antibody recognized both inactive or pro-caspase 3 and the larger catalytic subunit of active caspase 3. Nef protein was visualized using rabbit anti-HIV-1 Nef antiserum (NIH AIDS Research and Reference Reagent Program) as the primary antibody and horseradish peroxidase-labeled goat anti-rabbit IgG (heavy plus light chain) (Pierce, Rockford, Ill.) as the secondary antibody. Visualization of protein-antibody complexes was achieved by incubation in the ECL Plus Western Blotting Detection System solutions (Amersham Biosciences, Piscataway, N.J.), followed by exposure to photographic film (BioMax film; Fisher Scientific, Pittsburgh, Pa.). Images were scanned into Adobe Photoshop 5.0.2, and densitometry was performed using Scion Imaging software, Release Beta 3b (Scion Corporation, Frederick, Md.).
Data analysis.
Microsoft Excel was used for the numerical, graphical, and statistical analyses of all data obtained. Utilities included the Student t test (two-tailed distribution; unpaired), standard deviation, and standard error. Significance of results was determined by comparing treated and untreated samples, where P values were set at less that 0.05.
RESULTS
Soluble Nef protein induces apoptosis.
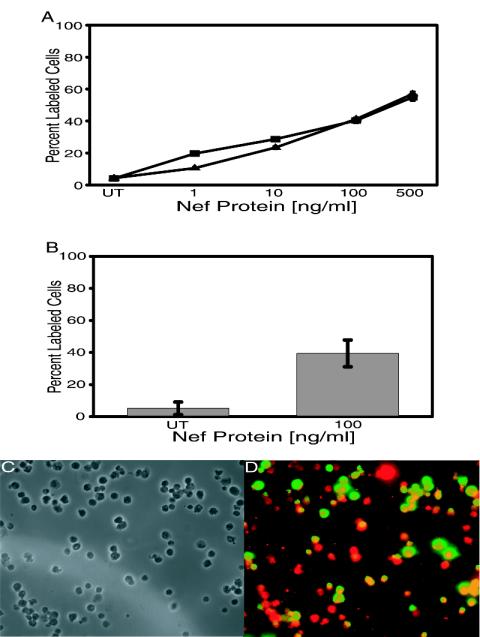
Jurkat cell cultures were exposed to extracellular Nef protein at concentrations ranging from 1 to 500 ng of Nef/ml of medium for a period of 24 h. The cultures were subsequently screened for apoptosis by TUNEL assay. Figure 1A shows that soluble Nef protein induced apoptosis in Jurkat cell cultures in a dose-dependent fashion, where the minimal treatment of 1 ng of Nef/ml of medium induced 19.69% and the maximal treatment of 500 ng of Nef/ml of medium induced 54.94% apoptosis, compared to 4.09% apoptosis in untreated cells. Treatment of Jurkat cells with 10 ng of Nef/ml of medium, which is representative of the level of soluble Nef that circulates in HIV-1-infected individuals (19), resulted in significant apoptosis (28.68%).
FIG. 1.
Nef-induced apoptosis as measured by dose response in lymphocytic cells. (A) Jurkat or H9 cell cultures were untreated or were treated with 1, 10, 100, or 500 ng of Nef/ml of medium. After 24 h, cells were assayed for apoptosis by TUNEL labeling, and then percent apoptosis was determined by fluorescent microscopic analysis of 10 fields per slide. Percent apoptosis was calculated as the number of FITC-labeled cells per total cell count. Data from two experiments performed in triplicate with 12 individually treated cell sets were pooled to generate average values and were used to determine standard errors (error bars). UT represents untreated cells. (B) PBMCs were either untreated or exposed to soluble Nef protein for 24 h. Apoptotic cells were detected by TUNEL, and then percent apoptosis in treated cells was compared to levels in untreated cells. Data from two experiments performed in triplicate with 12 individually treated cell sets were pooled to generate average values and were used to determine standard errors (error bars). (C and D) Representative images of Nef-treated PBMCs described for panel B. Images were taken via phase and fluorescence microscopy and arranged via Adobe Photoshop software (version 5.0.2; Adobe Systems). (C) Matched-phase image for panel D, which is the combined fluorescent images for TUNEL (FITC, green) and CD4 staining (Texas Red). Cells fluorescing yellow were simultaneously stained for TUNEL and CD4. Magnification, ×240.
H9 cell cultures were also treated in a similar dose-dependent manner, and apoptosis was determined by TUNEL (Fig. 1A). In contrast to 4.33% apoptosis in untreated H9 cells, 1, 10, 100, and 500 ng of Nef/ml of medium induced 10.54%, 23.40%, 41.25%, and 56.93% apoptosis, respectively. The occurrence of apoptosis in H9 cell cultures in a Nef-induced dose-dependent fashion was similar to that observed in Jurkat cell cultures, suggesting that the effects are common to CD4+-T-cell cultures and not reserved to any one specific CD4+-T-cell line.
Since Jurkat and H9 cell cultures are immortalized lymphocytic cells, it was important to demonstrate apoptosis in primary PBMCs. As was observed in the CD4+-T-cell lines, soluble Nef protein induced apoptosis in unstimulated human PBMCs at levels similar to those observed in the cell lines (Fig. 1B). Unstimulated human PBMCs treated with 100 ng of Nef/ml of medium exhibited 41.13% apoptosis compared to 3.93% apoptosis in untreated cells. To show that Nef was causing apoptosis in the T-cell subpopulation, Nef-treated PBMCs were processed for TUNEL (fluorescein isothiocyanate FITC] [green]) and then stained using a CD4 primary antibody and a secondary antibody tagged with Texas Red (red). Figure 1C and D represent one typical field of these Nef-treated PBMCs, showing a combined FITC-Texas Red image. As can be seen, there are many CD4+ cells that display TUNEL staining indicating Nef-induced apoptosis (yellow stained cells). We observed 56% of the CD4+ cells with TUNEL labeling after 24 h of exposure. As discussed above in Materials and Methods, the vast majority of CXCR4+ cells colabeled with CD4, and we observed a pattern similar to that shown in Fig. 1D for simultaneously TUNEL/CXCR4 stained cells (data not shown). Thus, the ability of soluble Nef to kill unstimulated human CD4+ T cells suggests that soluble Nef may be capable of killing bystander CD4+ T cells in HIV-1-infected humans.
The kinetics of HIV-1 Nef-induced apoptosis.
The apoptotic activity of extracellular Nef protein on Jurkat cell cultures as a function of time was examined (Fig. 2A). Soluble Nef protein killed CD4+ T cells in a time-dependent fashion, with significant apoptosis observed in the 6 h following initial protein exposure. At 36 h postexposure, more than three-quarters of the cells had undergone apoptosis, and the overall rate of apoptosis was essentially linear over the entire time measured.
FIG. 2.
Nef-induced apoptosis as a function of time. (A) Jurkat cell cultures were either untreated (▪) or exposed to soluble HIV-1 Nef (□) for various times. Cells were then harvested, assayed for apoptosis by TUNEL, and analyzed by fluorescence microscopy. Data from two experiments performed in triplicate with six individually treated cell sets were pooled to generate average values and were used to determine standard errors. (B) Jurkat cell cultures were exposed to 100 ng of Nef/ml of medium for 12, 24, and 36 h and subsequently harvested and assayed by TUNEL. Images were taken via fluorescence microscopy and arranged via Adobe Photoshop software (version 5.0.2; Adobe Systems). Subpanels A, C, and E represent untreated Jurkat cell cultures at 12, 24, and 36 h, respectively, after initial medium replenishment. Subpanels B, D, and F depict Jurkat cell cultures after 12, 24, and 36 h exposure to soluble Nef protein (100 ng/ml). Magnification, ×280. The image was formatted using Adobe Photoshop 5.0.2 software.
Nef protein induces apoptosis as opposed to necrosis.
Having detected apoptosis by TUNEL, we were interested in providing more conclusive evidence for the occurrence of apoptosis. To this end, we screened Nef-treated CD4+ T cells for classic markers used in the identification of apoptosis. We initially observed cytoplasmic shrinkage in Nef-treated cells (data not shown). Since apoptosis is characterized by cytoplasmic shrinkage of cells, while necrosis features cytoplasmic bloating (58), our observations suggested the occurrence of apoptosis.
Another morphological characteristic of apoptosis is nuclear fragmentation due to the actions of activated cellular proteases. In our experiments, nuclear fragmentation was readily observed after 18 h of exposure to soluble Nef protein (data not shown). Furthermore, apoptosis also features DNA cleavage that can be detected by the labeling of the 3′ end of nicked DNA via TUNEL. TUNEL-labeled Jurkat cells were observed as early as 6 h post-Nef treatment. Pictures depict TUNEL-labeled cells at 12, 24, and 36 h post-Nef treatment (Fig. 2B, panels b, d, and f), compared to little or no TUNEL labeling in untreated cells (Fig. 2B, panels a, c, and e). Since we observed some degree of cytoplasmic shrinkage, and identified the presence of nicked DNA, these results suggest the occurrence of apoptosis as opposed to necrosis, which is exemplified by the preservation of nuclear morphology in swollen cells (58).
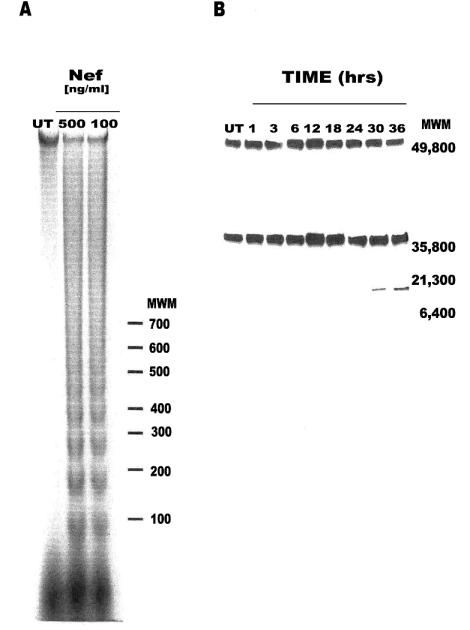
Further confirmation of apoptosis was provided by the detection of DNA laddering in Jurkat cell cultures exposed to soluble Nef protein (Fig. 3A). The clear distinction between DNA bands of approximately 100-bp differences in molecular size on agarose gel provides evidence of extensive internucleosomal cleavage of the genomic DNA, which is a common phenomenon in apoptotic cells.
FIG. 3.
Hallmarks of apoptosis. (A) DNA from Jurkat cell extracts was resolved by agarose gel electrophoresis, stained with ethidium bromide, and visualized by exposure to UV light. Pictures were taken using the DP12 Microscope Digital Camera System (Olympus Optical Co., Ltd., Melville, N.Y.), and the negative image was generated via Adobe Photoshop software (version 5.0.2). (B) Jurkat cell cultures were either untreated or exposed to extracellular Nef for various times. Caspase 3 activation was tested by Western blot analysis. The high-molecular-mass band is pro-caspase 3 (32 kDa), and the large catalytic subunit of active caspase 3 is 17 kDa. Tubulin (50 kDa) is the gel-loading control. Prestained SDS-polyacrylamide gel standard (broad range) (Bio-Rad Labs) was used as the molecular weight marker. The image was formatted using Adobe Photoshop software (version 5.0.2).
Finally, the occurrence of apoptosis, not necrosis, was supported by the detection of the larger catalytic subunit of active caspase 3 (Fig. 3B) after 24 h of exposure of Jurkat cell cultures to soluble Nef protein. The high-molecular-mass pro-caspase 3 (32 kDa) was observed at all time points examined. The large catalytic subunit of active caspase 3 (17 kDa) was detected at 30 and 36 h posttreatment. The 50-kDa tubulin band was assayed as a gel-loading control. Overexposure of the filter did not result in detection of the active form of caspase 3 at or before 24 h posttreatment (data not shown). Caspase 3 is a late or effector caspase, being generated from its pro-nonenzymatic form by initiator caspase-mediated cleavage (10, 51, 55, 56, 60), such as caspase 8 and 9. Caspase 3 is often referred to as the “executioner,” as its presence authenticates the occurrence of apoptosis and confirms that the cell is destined or programmed for cell death (10, 51, 55, 56, 60).
Evidence of the specificity of Nef-induced apoptosis.
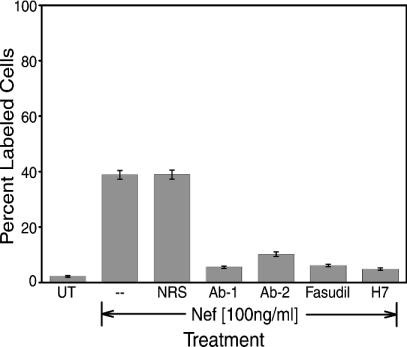
Nef protein was preincubated with HIV-1 Nef polyclonal antiserum for 2 h at 4°C, Jurkat cell cultures were exposed to the Nef/antiserum mixture for 24 h, and apoptosis was determined by TUNEL assay. Two different Nef polyclonal antibodies were used in this study. In both cases, the anti-Nef antiserum blocked Nef-induced apoptosis, as demonstrated by the reduced the number of TUNEL-labeled cells (Fig. 4). Antibody pretreatment dropped Nef-induced apoptosis from 38.85% in the absence of antibody to 5.53% in the presence of Ab-1 and 10.21% in the presence of Ab-2. Alternatively, pretreatment of Nef protein with normal rabbit serum had no effect on the amount Nef-induced apoptosis (Fig. 4). This confirms that Nef protein, and not a contaminant, was responsible for inducing the apoptosis that we documented. It also shows that these recombinant anti-Nef antibodies are protective against Nef-induced apoptosis.
FIG. 4.
Inhibitory molecules suppress HIV-1 Nef-induced apoptosis in Jurkat cell cultures. Nef was preincubated with anti-Nef antibody (1:4,000 dilutions), fasudil (20 mM), or H7 (20 mM) for 2 h at 4°C. Then, Jurkat cells were left untreated or were treated with Nef protein, Nef anti-Nef antibody, Nef-fasudil, or Nef-H7 cocktail. After 24 h at 37°C, cells were harvested and then assayed by TUNEL. Ab-1 is the NIH polyclonal antibody, and Ab-2 is the polyclonal antibody from Warner Greene. Fasudil and H7 are protein kinase inhibitors. Data from two experiments performed in triplicate were pooled to generate average values and were used to determine standard errors (error bars). Abbreviations: UT, untransfected; NRS, normal rabbit serum.
Nef protein induced signaling.
Okada and colleagues (48, 49) have reported that protein kinase inhibitors can block Nef-induced apoptosis. Fasudil hydrochloride (HA 1077) is a protein kinase C inhibitor (1, 48, 49), while H7 is a protein kinase A, C, and G inhibitor, as well as a serine kinase inhibitor (28, 48, 50). We preincubated soluble Nef protein with either fasudil hydrochloride or H7 for 2 h at 4°C, followed by the exposure of Jurkat cell cultures to that solution for 24 h. Fasudil and H7 blocked Nef-induced apoptosis, reducing the amount of apoptosis to 6.14% and 4.85%, respectively (Fig. 4, bars 6 and 7, respectively). These findings suggest that Nef may be kinase dependent.
The effects of exogenous eukaryotic cell-expressed Nef protein.
The evidence presented above clearly suggests that bacterially expressed HIV-1 Nef protein induces apoptosis in T helper cells. However, bacterially expressed Nef protein is not identical to Nef synthesized by virally infected eukaryotic lymphocytes (e.g., the bacterially expressed Nef protein is not myristylated). To resolve this concern, HIV-1 nef cDNA was transfected and expressed in HEK 293 cells. The cell-conditioned supernatant was collected, examined by Western blot analysis, and found to contain Nef protein (Fig. 5A). In all cases, Nef protein was expressed and shed into the extracellular supernatant. Additionally, the Nef proteins synthesized in eukaryotic cells (Fig. 5A, lanes 3 to 5) were identical in molecular weight (as per Western analysis) to the analogous bacterially expressed proteins (Fig. 5A, lane 1). Finally, Nef protein was expressed into the extracellular supernatant in significant quantities. Densitometry analysis of the bands in Fig. 5A, and numerical analysis of those numbers relative to the readings for bacterially expressed Nef standard in lane 1, allowed quantitation of the concentrations of each 293-expressed Nef protein. Supernatant concentrations for HIV-1 Nef, HIV-2 Nef, and SIV Nef protein were determined to be 21.4, 19.8, and 19.5 μg/ml of supernatant, respectively.
FIG. 5.
Apoptotic effects of eukaryotic cell-expressed Nef protein. HEK 293 cells were either untransfected or transfected with plasmids containing HIV-1, HIV-2, or SIV nef reading frames. Cultures were incubated for 36 h, and the cell-conditioned supernatant was removed and stored. (A) The changes in Nef-induced apoptosis are not the result of variations in Nef protein expression. Western analysis of 40 μl of HEK 293-conditioned medium was performed using rabbit anti-Nef antibody. Lane 1, HIV Nef protein (1 μg); lane 2, untransfected HEK 293 cell-conditioned medium; lane 3, HIV-1 nef-transfected HEK 293 cell-conditioned medium; lane 4, HIV-2 nef-transfected HEK 293 cell-conditioned medium; lane 5, SIV nef-transfected HEK 293 cell-conditioned medium. (B) Jurkat cell cultures were exposed for 24 h to cell-conditioned supernatant from HIV-1, HIV-2, or SIV nef-transfected cells alone, or pretreated for 2 h with a 1:250 dilution of rabbit anti-Nef antibody. Cultures were then assayed for apoptosis by TUNEL. Bars: Media, unconditioned medium; bNef, bacterially expressed and purified Nef protein; UT, cell-conditioned medium from untransfected 293 cells; HIV-1, cell-conditioned medium from HIV-1 nef-transfected 293 cells; HIV-2, cell-conditioned medium from HIV-2 nef-transfected 293 cells; SIV, cell-conditioned medium from SIV nef-transfected 293 cells; αNef Ab, pretreated with Nef antibody. Error bars show the standard error of measurement. Data from at least two experiments performed in triplicate were pooled to generate average values and were used to determine standard errors (error bars).
Jurkat cell cultures were exposed to a 10-fold dilution of (i) the Nef-containing supernatant or (ii) the Nef-containing supernatant preincubated for 2 h with a 1:250 dilution of rabbit anti-Nef antibody (Ab-1) for 24 h, and apoptosis was determined by TUNEL assay (Fig. 5B). The supernatant from HIV-1 nef-transfected cells induced 41.6% apoptosis, compared to supernatants from the untransfected cell control (Fig. 5B), which induced 0.57%. The Nef antibody blocked the effect of the supernatant (Fig. 5B, compare bars 4 and 9). Preincubation of the supernatant with normal rabbit serum had no effect on HIV-1 nef-transfected cell supernatant-induced apoptosis (data not shown). From these data, it is evident that HIV-1 Nef expressed by eukaryotic cells is cytotoxic and apoptotic to CD4+ T cells.
The Nef protein of HIV-2 and SIV contain additional amino acids when compared to HIV-1 Nef. A CLUSTAL alignment of HIV-1, HIV-2, and SIV Nef sequences group the extra amino acids into two regions. One region is close to the core of Nef, and the other is at the C terminus (38). We were interested in determining whether the Nef proteins from these different viruses possessed similar apoptotic activities in CD4+ T lymphocytes. HIV-2 and SIV nef cDNAs were transfected and expressed in HEK 293 cells, and the supernatants from these two groups of cells were collected, examined by Western blot analysis, and determined to contain Nef protein (Fig. 5A, lanes 4 and 5). Approximately equivalent amounts of HIV-1, HIV-2, and SIV Nef protein were found to be expressed into the supernatant by transfected HEK 293 cells (Fig. 5A, compare lanes 3, 4, and 5). Jurkat cell cultures were then exposed to supernatants from either HIV-2 or SIV nef transfections for 24 h, and apoptosis was determined by TUNEL assay (Fig. 5B, bars 5 and 6). HIV-1 Nef induced 41.6% apoptosis while HIV-2 and SIV Nef induced 37.5% and 29.1% apoptosis, respectively. When the supernatants were preincubated for 2 h with a 1:250 dilution of rabbit anti-Nef antibody (Ab-1) and then put on Jurkat cultures for 24 h, the rabbit anti-Nef antibody blocked the apoptotic effect of the supernatants (Fig. 5B, compare bars 5 and 6 with bars 10 and 11). Preincubation of the supernatants with normal rabbit serum had no effect on HIV-2 or SIV nef-transfected cell supernatant-induced apoptosis (data not shown). These data clearly demonstrate that both HIV-2 and SIV Nef proteins similarly induce apoptosis in CD4+ T cells.
Nef-induced apoptosis is driven through the CXCR4 receptor.
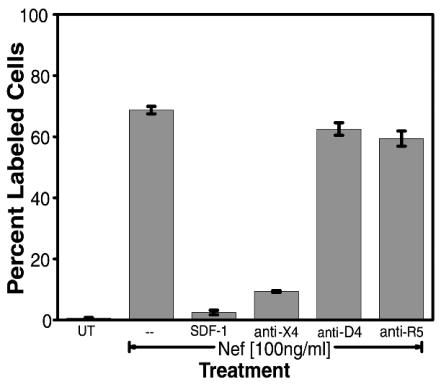
Nef protein can induce apoptosis in T lymphocytes and a number of other cell types (unpublished results). However, the exact mechanism is unclear. It had previously been shown in our laboratory and others that HIV-1 gp120-induced apoptosis is mediated through the chemokine receptors CXCR4 and CCR5 (28, 30, 34, 42, 68). Additionally, HIV-1 Tat protein has been shown to mimic chemokine features and to bind with a number of chemokine receptors, including CXCR4 (2, 63). Thus, we postulated that HIV-1 Nef protein might also interact with chemokine receptors. We screened for potential competition between HIV-1 Nef-induced apoptosis and CCR5 or CXCR4 ligands (RANTES or SDF-1α) or antibodies to these chemokine receptors. Jurkat cell cultures were either untreated or pretreated with the natural ligand for CXCR4, SDF-1α, or antibodies to CXCR4, CCR5, or CD4. Subsequently, the cultures were treated with HIV-1 Nef for 24 h, and apoptosis was determined by TUNEL assay (Fig. 6). Pretreatment of cultures with SDF-1α or anti-CXCR4 antibody significantly reduced the amount of Nef-induced apoptosis in these cultures (Fig. 6). Alternatively, pretreatment with anti-CD4 antibody or with anti-CCR5 antibody had no effect on Nef-induced apoptosis (Fig. 6). The CCR5 monoclonal antibody, which was the same isotype as the CXCR4 monoclonal antibody, stands as an isotype control for this experiment.
FIG. 6.
Nef-induced apoptosis is mediated by CXCR4 molecules. Jurkat cultures were either untreated (UT) or pretreated with the natural ligand for CXCR4, SDF-1α, or antibodies to CXCR4 (anti-X4), CD4 (anti-D4), or CCR5 (anti-R5). Subsequently, cultures were treated with HIV-1 Nef for 24 h, and apoptosis was then determined for all cultures by TUNEL. Error bars show the standard error of measurement. Data from two experiments performed in triplicate were pooled to generate average values and were used to determine standard errors.
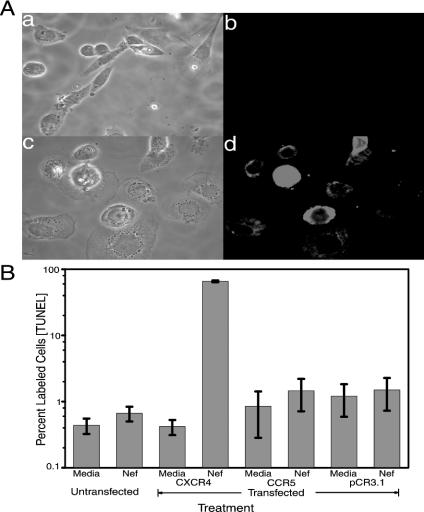
The breast tumor cell line MDA-MB-468 does not express CXCR4 mRNA or protein (43; unpublished results). If Nef-induced apoptosis acts through this receptor, the MDA-MB-468 line should be refractory to Nef-induced apoptosis. Alternatively, if the receptor were transfected into and expressed in MDA-MB-468, the transfected line would then be susceptible to Nef-induced apoptosis. Thus, MDA-MB-468 cultures were either untransfected or transiently transfected with a CXCR4 cDNA clone, a CCR5 clone, or empty vector pCR3.1. At 48 h posttransfection, half the CXCR4-transfected cultures were assayed for cell surface expression of CXCR4 receptor by immunocytochemistry analysis (Fig. 7A). The CXCR4-transfected cultures clearly showed cell surface staining with the CXCR4 antibody indicative of cell surface expression of the CXCR4 protein in these cells (Fig. 7A, panel d). No cell surface staining was observed with untransfected cells (Fig. 7A, panel b). The other half of the cultures were treated with HIV-1 Nef for 24 h and subsequently assayed for apoptosis by TUNEL assay (Fig. 7B). Untransfected MDA-MB-468 cells were refractory for Nef-induced apoptosis (Fig. 7B, bar 2). In contrast, MDA-MB-468 cells expressing the CXCR4 were susceptible to Nef-induced apoptosis (Fig. 7B). Apoptosis was not observed in cultures transfected with either a CCR5 clone or the empty vector pCR3.1 (Fig. 7B). CCR5 receptor was expressed on the cell surface of the CCR5-transfected MDA-MB-468 cells (data not shown).
FIG. 7.
Surface expression of CXCR4 molecules in a CXCR4-deficient cell line restores sensitivity to Nef-induced apoptosis. MDA-MB-468 cultures were either untransfected or transiently transfected with 1 μg of a CXCR4 cDNA clone. (A) At 48 h posttransfection, half the CXCR4-transfected cultures were assayed for cell surface expression of CXCR4 receptor by immunocytochemical assay using an anti-CXCR4 antibody. Subpanels: a, phase image of untransfected MDA-MB-468 cells; b, anti-CXCR4 immunostained image of untransfected MDA-MB-468 cells; c, phase image of CXCR4-transfected MDA-MB-468 cells; d, anti-CXCR4 immunostained image of CXCR4-transfected MDA-MB-468 cells. Magnification, ×240. (B) MDA-MB-468 cultures were either untransfected (Media), transiently transfected with 1 μg of a CXCR4 cDNA clone, transiently transfected with 1 μg of a CCR5 cDNA clone, or transfected with 1 μg of the empty vector pCR3.1. At 48 h posttransfection, cultures were treated with HIV-1 Nef for 24 h and subsequently assayed for apoptosis by TUNEL. At 48 h posttransfection, cultures were treated with medium (Media) or with Nef protein. Error bars show the standard error of measurement. Data from two experiments performed in triplicate were pooled to generate average values and were used to determine standard errors.
DISCUSSION
In this study, we report that extracellular HIV-1 Nef protein induced apoptosis in CD4+ T lymphocytes in vitro. These data are consistent with the bystander effect scenario (18) and fit the second premise of that scenario, implicating a viral protein as the mediator of bystander cell death. Nef is synthesized early during initial viral infection and could contribute to the depletion of T helper cells through a bystander effect.
Dose-response experiments with bacterially expressed Nef and experiments with HEK 293-expressed Nef revealed that exposure of the T-cell lines Jurkat and H9 to soluble HIV-1 Nef protein resulted in significant apoptosis. In the dose-response experiments, apoptosis was observed to be directly proportional to Nef protein levels. More than 25% apoptosis was observed in all T cells examined at 24 h and at the average level of soluble Nef protein (10 ng/ml) detected in the sera of several HIV-1-infected patients (19). In confirmatory experiments, the occurrence of similar and significant levels of apoptosis in unstimulated human PBMCs following exposure to soluble HIV-1 Nef protein over 24 h was observed. These findings suggest that (i) exogenous Nef is capable of killing lymphocytes through apoptosis and (ii) the levels of Nef protein present in the sera of HIV-1-infected patients (19) are sufficient to induce apoptosis in lymphocytes in vitro.
Although it is not clear how a membrane-associated protein such as Nef might be secreted from an HIV-1-infected cell, secretion into culture medium of Nef produced by recombinant plasmids has been observed. As mentioned above, Nef was detected in culture medium of Saccharomyces cerevisiae infected with HIV-1 plasmid constructs (41). Nef was also detected in the medium from the MOLT-4 lymphocyte cell line transfected with a Nef-producing baculovirus vector (19). Nef protein was quantitated in 32 patient serum samples using a sandwich enzyme-linked immunosorbent assay procedure. The concentration of Nef protein in 21 patient sera varied between 5 and 10 ng/ml, with the concentration being between 0.1 and 5 ng of Nef/ml in 6 patient sera and no detectable Nef protein in 5 patient sera. This evidence clearly suggests that Nef can be secreted extracellularly and that variable levels of serum-associated Nef protein can be observed in different infected individuals.
There are several possible mechanistic scenarios that might explain Nef secretion. Previous studies have shown that SIV envelope is shed from cells by two mechanisms, one involving shedding from the cell surface and the second involving secretion without transitory appearance on the cell surface as part of a gp41-gp120 complex (57). Tat protein is also secreted into the extracellular environment (for a review, see reference 2). These leaderless proteins, which lack secretory sequences, have been shown to be secreted extracellularly through a nonclassical pathway (for a review, see reference 45). Evidence suggests that modifications like myristoylation may play a role in leaderless secretion by making the protein more hydrophobic, improving its affinity for membranes. It is possible that Nef is secreted through a mechanism such as this. The “Trojan horse” hypothesis proposed by Gould et al., which suggests that HIV exploits exosomal secretion for viral biogenesis and cell-cell transmission, could be another mechanism (22). Exosomal secretion of viral proteins not packaged in viral particles, including Nef, would lead to Nef secretion. Both of these hypotheses are testable, and these experiments are currently under way.
In time course assays Nef induced apoptosis within a few hours of exposure, and more than three-quarters of all cells were apoptotic by 36 h. The essentially linear induction of apoptosis over time was suggestive of a sustained and stable interaction (direct or indirect) between Nef and CXCR4, which caused signaling through that receptor and induced an apoptotic program. The data suggest that the susceptibility to apoptosis of any particular cell in the population has a time component, hinting that apoptosis susceptibility may be related to a particular state of the cells, such as a specific point in the cell cycle. An alternative scenario could be that the Nef/T-cell CXCR4 interactions may involve the endocytic pathway, where soluble Nef is sufficiently small to be taken up in a coated pit and targeted to specific compartments of the cell, such as the mitochondrion, by endocytic vesicles. However, this endocytic scenario is unsupported by previously published data on intracellular Nef suggesting that it exhibits antiapoptotic activities by its ability to inhibit ASK1 involved in mediating apoptosis stimulated by Fas-FasL and TNF-TNF receptor interactions (20, 23).
Examination of the morphology of T cells exposed to Nef protein provided further support for the induction of apoptosis. Cytoplasmic shrinkage and nuclear fragmentation were evident, with greater exposure times producing elevated levels of nuclear fragmentation and apoptotic bodies. We believe that aspartic acid cleaving caspases were activated upon cellular exposure to Nef protein, as caspase 3, a terminal enzyme in caspase pathways (see reference 10 for a review), was detected by Western analysis after approximately 1 day of exposure to Nef protein.
Polyclonal anti-Nef antibodies were protective against Nef-induced apoptosis, when Nef was preincubated with the antibody prior to cellular treatment. These data suggest that Nefantibodies can be protective against Nef-induced apoptosis; however, one would have to postulate that if extracellular Nef were involved in T-cell depletion, Nef antibodies synthesized by HIV-1-infected individuals are not always able to prevent a loss of T helper cells. These results are in direct contrast to the work of Fujii et al. (19), who reported that the presence of cross-linking anti-Nef antibody was actually required for Nef-induced apoptosis. One possible explanation for the disparity between their results and ours may be related to the structure of the bacterially expressed Nef protein. If the purified protein was not fully folded, it may be able to induce apoptosis without interactions with an antibody that could act to stabilize the structure. Analysis of our bacterially expressed Nef by surface-enhanced laser desorption-ionization revealed the presence of Nef protein dimers, trimers, and even tetramers, which was highly indicative of a stable protein in the proper conformational integrity. Apoptosis was also observed using soluble extracellular Nef expressed from HEK 293 cells transfected with HIV-1, HIV-2, or SIV nef genes. The protective effect of pretreatment of the supernatants with polyclonal anti-Nef antibody was indicative that soluble Nef in the supernatants was driving the supernatant-induced apoptosis observed. In this case, the expression of these nef constructs was also able to fully restore infectivity to an HIV-1 virus lacking a nef gene.
Observations made in this study that are consistent with those made in earlier studies suggest that protein kinase inhibitors prevent the progression of the apoptotic pathway (28, 48, 49). We have previously shown that gp120-induced apoptosis in human umbilical vein endothelial cell cultures is mediated through CXCR4 (29). Protein kinase C was found to be involved at multiple points in the gp120-induced apoptotic pathway, and the CXCR4 receptor phorbol ester-induced endocytosis pathway played a role in this effect. These data indicated that gp120-receptor interactions with CXCR4, and natural ligand-receptor interactions with CXCR4, induce different downstream effects (28). The results presented here and the results from Okada et al. suggest that Nef could similarly be acting through a protein kinase-dependent pathway (48, 49), possibly the same gp120-induced apoptosis pathway.
We have presented morphological and biochemical evidence that Nef induces hallmarks of apoptotic cell killing and not necrotic cell killing. Apoptosis is the mechanism responsible for the physiological deletion of cells. It is characterized by distinctive morphological changes in the nucleus and cytoplasm, endonucleolytic cleavage of genomic DNA at internucleosomal sites, and caspase activation. All of these features were observed in Nef-treated lymphocytes. Caspase 3 activation is especially indicative of apoptosis in lymphocytic cell lines (10). Apoptosis normally serves as a balance to mitosis in regulating the size of animal tissues and in mediating pathological processes associated with tumor growth (37, 53). In this case it appears that HIV-1 produces several proteins that can disrupt this balance.
Two other primate retroviruses are known to carry the nef gene, namely, HIV-2 and SIV. There are differences between the Nef amino acid sequences in all three viruses (38), yet interestingly, all three versions of this viral protein were found to be capable of inducing apoptosis in CD4+ cell cultures (Fig. 6). Thus, the apoptotic activity of soluble Nef protein is well conserved among the primate lentiviruses, as well as among the different strains of the viruses that currently exist, again suggesting a key role for Nef-induced apoptosis.
Our molecular competition analysis revealed that SDF-1α, the endogenous ligand for CXCR4, was protective against Nef protein-induced apoptosis. Similarly, in the presence of anti-CXCR4 receptor antibodies, Nef protein was unable to induce apoptosis. One explanation could be that both SDF-1a and the anti-CXCR4 antibody induce a signal through CXCR4 that competes with Nef-induced apoptosis, and Nef interacts with some as yet unknown surface factor. However, Nanki and Lipsky (44) showed that the same CXCR4 antibody we used blocked the up-regulation of activation markers, proliferation, and cytokine production in anti-CD3-activated CD4+ T cells with SDF-1a. It is difficult to explain how an antibody could be inducing a signal that blocks both proliferation and apoptosis. Alternatively, it is easier to envision that the antibody physically blocks both proliferation and apoptosis through binding to CXCR4. This suggests, but does not confirm, that Nef as well as SDF-1a physically interacts with CXCR4. Certainly, CD4 and CCR5 receptors were not involved in facilitating Nef-induced apoptosis, as incubation of these receptors with their respective antibodies offered little protection to cells against Nef-induced apoptosis (Fig. 6).
The breast tumor cell line MDA-MB-468 has been shown to be negative for CXCR4 mRNA and protein (43; unpublished results). Competition results suggested that Nef-induced apoptosis acts through this chemokine receptor. If CXCR4 is the targeted receptor for Nef-induced apoptosis, MDA-MB-468 should be, and was found to be, refractory to Nef-induced apoptosis. Thus, having CXCR4 available is a requirement for Nef-induced apoptosis. One would predict that MDA-MB-468, transfected with a vector expressing CXCR4, would then be made susceptible to Nef-induced apoptosis. In fact, that is what was observed (Fig. 6). Alternatively, MDA-MB-468 cells were still refractory to Nef-induced apoptosis on transfection with empty vector or CCR5 expressing vector. Finally, MDA-MB-468 cells transfected with the CXCR4-expressing vector displayed no differences in cell growth patterns and were not apoptotic on exposure to SDF-1a or vMIP-II (data not shown).
This evidence clearly indicates that soluble Nef protein acts through CXCR4 to induce apoptosis. One possibility is that Nef interacts with the CXCR4 receptor and directly induces apoptosis. However, it is also possible that Nef binds to another cell surface factor(s), and through this other factor induces apoptosis through CXCR4. Additional studies will be required to determine which possibility is correct. Nef-induced apoptosis occurs in lymphocytes, as observed for gp120-induced apoptosis (6-8, 12, 26, 28-30, 32, 40, 61, 62), although Nef-induced lymphocytic apoptosis does not require cofactors as does gp120-induced lymphocytic apoptosis. Thus, three HIV proteins, Nef, gp120, and Tat (2, 63), have been shown to induce an apoptotic signal (Nef and gp120) or a proliferative signal (Tat) through CXCR4.
We have generated considerable data showing that Nef protein induces apoptosis in CD4+ T cells. This evidence, and evidence from other studies discussed above, begins to build the case that exogenous HIV-1 Nef protein, independent of viral replication and the viral life cycle, could be involved in the depletion of CD4+ T cells. As Nef is synthesized very early during HIV-1 infections, it is possible that this protein could target T helper cells for programmed cell death early on during viral infection. Interestingly, HIV-1 Nef protein induces T-helper-cell depletion in an in vivo transgenic mouse model (14-17, 21, 24, 25, 33, 59), although it is not clear whether (i) this effect is due to intracellular or extracellular Nef protein or (ii) this effect is a developmental consequence of constitutive expression of the transgene or a model for Nef effect in HIV infection. Further, in patients receiving highly active combination antiretroviral therapy and with undetectable plasma viral RNA levels, several studies have shown (i) a stable pool of T cells harboring latent replication-competent virus (39) and (ii) detectable levels of early viral spliced mRNA in the PBMCs (67). One can speculate that, in light of the accumulated evidence concerning extracellular Nef, even in this scenario, expression of low levels of Nef could cause continued, albeit slower, depletion of T cells. Thus, additional studies (i) into the mechanics of Nef-induced apoptosis and (ii) to definitively determine whether soluble Nef-induced apoptosis leads to depletion of CD4+ T cells in vivo would be useful. These studies should lead to confirmation of the hypothesis that Nef-induced apoptosis drives some portion of viral pathogenesis, and subsequently to the development of therapeutic agents that target Nef protein. These agents would impede the rate and levels of Nef-driven apoptosis in CD4+ T lymphocytes and therefore reduce virus-associated pathogenesis. For example, identification of the apoptotic motif on Nef protein would allow development of an antibody vaccine. Consequently, the studies discussed above are ongoing.
Acknowledgments
We acknowledge the helpful discussions and editing of William Roth. We acknowledge the NIH AIDS Research and Reference Reagent Program for providing the anti-Nef antibody.
This work was supported by NIH/NIGMS/MBRS (grant 58268) and NIH/NCRR/RCMI (grant G12-RR03034).
REFERENCES
- 1.Akari, H., Y. Yamamoto, H. Hiwada, Y. Saito, N. Takayanagi, A. H. Koyama, and A. Adachi. 1998. Suppression of HIV-1 replication in peripheral blood mononuclear cells by fasudil. J. Med. Investig. 44:211-214. [PubMed] [Google Scholar]
- 2.Albini, A., S. Ferrini, R. Benelli, S. Sforzini, D. Giunciuglio, M. G. Aluigi, A. E. Proudfoot, S. Alouani, T. N. Wells, G. Mariani, R. L. Rabin, J. M. Farber, and D. M. Noonan. 1998. HIV-1 Tat protein mimicry of chemokines. Proc. Natl. Acad. Sci. USA 95:13153-13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, R. W., M. S. Ascher, and H. W. Sheppard. 1998. Direct HIV cytopathicity cannot account for CD4 decline in AIDS in the presence of homeostasis: a worst-case dynamic analysis. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 17:245-252. [DOI] [PubMed] [Google Scholar]
- 4.Arold, S., F. Hoh, S. Domergue, C. Birck, M. A. Delsuc, M. Jullien, and C. Dumas. 2000. Characterization and molecular basis of the oligomeric structure of HIV-1 nef protein. Protein Sci. 9:1137-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azad, A. A. 2000. Could Nef and Vpr proteins contribute to disease progression by promoting depletion of bystander cells and prolonged survival of HIV-infected cells? Biochem. Biophys. Res. Commun. 267:677-685. [DOI] [PubMed] [Google Scholar]
- 6.Bagetta, G., M. T. Corasaniti, L. Berliocchi, M. Navarra, A. Finazzi-Agro, and G. Nistico. 1995. HIV-1 gp120 produces DNA fragmentation in the cerebral cortex of rat. Biochem. Biophys. Res. Commun. 211:130-136. [DOI] [PubMed] [Google Scholar]
- 7.Bagetta, G., M. T. Corasaniti, L. Berliocchi, R. Nistico, A. M. Giammarioli, W. Malorni, L. Aloe, and A. Finazzi-Agro. 1999. Involvement of interleukin-1beta in the mechanism of human immunodeficiency virus type 1 (HIV-1) recombinant protein gp120-induced apoptosis in the neocortex of rat. Neuroscience 89:1051-1066. [DOI] [PubMed] [Google Scholar]
- 8.Biard-Piechaczyk, M., V. Robert-Hebmann, V. Richard, J. Roland, R. A. Hipskind, and C. Devaux. 2000. Caspase-dependent apoptosis of cells expressing the chemokine receptor CXCR4 is induced by cell membrane-associated human immunodeficiency virus type 1 envelope glycoprotein (gp120). Virology 268:329-344. [DOI] [PubMed] [Google Scholar]
- 9.Coffin, J. M. 1995. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science 267:483-489. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, G. M. 1997. Caspases: the executioners of apoptosis. Biochem. J. 326:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins, K. L., B. K. Chen, S. A. Kalams, B. D. Walker, and D. Baltimore. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397-401. [DOI] [PubMed] [Google Scholar]
- 12.Corasaniti, M. T., M. C. Strongoli, S. Piccirilli, R. Nistico, A. Costa, A. Bilotta, P. Turano, A. Finazzi-Agro, and G. Bagetta. 2000. Apoptosis induced by gp120 in the neocortex of rat involves enhanced expression of cyclooxygenase type 2 and is prevented by NMDA receptor antagonists and by the 21-aminosteroid U-74389G. Biochem. Biophys. Res. Commun. 274:664-669. [DOI] [PubMed] [Google Scholar]
- 13.Dalgleish, A. G., P. C. Beverley, P. R. Clapham, D. H. Crawford, M. F. Greaves, and R. A. Weiss. 1984. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312:763-767. [DOI] [PubMed] [Google Scholar]
- 14.Dickie, P. 1996. HIV type 1 Nef perturbs eye lens development in transgenic mice. AIDS Res. Hum. Retrovir. 12:177-189. [DOI] [PubMed] [Google Scholar]
- 15.Dickie, P. 2000. Nef modulation of HIV type 1 gene expression and cytopathicity in tissues of HIV transgenic mice. AIDS Res. Hum. Retrovir. 16:777-790. [DOI] [PubMed] [Google Scholar]
- 16.Dickie, P., J. Felser, M. Eckhaus, J. Bryant, J. Silver, N. Marinos, and A. L. Notkins. 1991. HIV-associated nephropathy in transgenic mice expressing HIV-1 genes. Virology 185:109-119. [DOI] [PubMed] [Google Scholar]
- 17.Dickie, P., F. Ramsdell, A. L. Notkins, and S. Venkatesan. 1993. Spontaneous and inducible epidermal hyperplasia in transgenic mice expressing HIV-1 Nef. Virology 197:431-438. [DOI] [PubMed] [Google Scholar]
- 18.Finkel, T. H., G. Tudor-Williams, N. K. Banda, M. F. Cotton, T. Curiel, C. Monks, T. W. Baba, R. M. Ruprecht, and A. Kupfer. 1995. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat. Med. 1:129-134. [DOI] [PubMed] [Google Scholar]
- 19.Fujii, Y., K. Otake, M. Tashiro, and A. Adachi. 1996. Soluble Nef antigen of HIV-1 is cytotoxic for human CD4+ T cells. FEBS Lett. 393:93-96. [DOI] [PubMed] [Google Scholar]
- 20.Geleziunas, R., W. Xu, K. Takeda, H. Ichijo, and W. C. Greene. 2001. HIV-1 Nef inhibits ASK1-dependent death signalling providing a potential mechanism for protecting the infected host cell. Nature 410:834-838. [DOI] [PubMed] [Google Scholar]
- 21.Goudreau, G., S. Carpenter, N. Beaulieu, and P. Jolicoeur. 1996. Vacuolar myelopathy in transgenic mice expressing human immunodeficiency virus type 1 proteins under the regulation of the myelin basic protein gene promoter. Nat. Med. 2:655-661. [DOI] [PubMed] [Google Scholar]
- 22.Gould, S. J., A. M. Booth, and J. E. Hildreth. 2003. The Trojan exosome hypothesis. Proc. Natl. Acad. Sci. USA 100:10592-10597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenway, A. L., D. A. McPhee, K. Allen, R. Johnstone, G. Holloway, J. Mills, A. Azad, S. Sankovich, and P. Lambert. 2002. Human immunodeficiency virus type 1 Nef binds to tumor suppressor p53 and protects cells against p53-mediated apoptosis. J. Virol. 76:2692-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanna, Z., D. G. Kay, M. Cool, S. Jothy, N. Rebai, and P. Jolicoeur. 1998. Transgenic mice expressing human immunodeficiency virus type 1 in immune cells develop a severe AIDS-like disease. J. Virol. 72:121-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanna, Z., D. G. Kay, N. Rebai, A. Guimond, S. Jothy, and P. Jolicoeur. 1998. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell 95:163-175. [DOI] [PubMed] [Google Scholar]
- 26.Hesselgesser, J., D. Taub, P. Baskar, M. Greenberg, J. Hoxie, D. L. Kolson, and R. Horuk. 1998. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr. Biol. 8:595-598. [DOI] [PubMed] [Google Scholar]
- 27.Hoxie, J. A., J. D. Alpers, J. L. Rackowski, K. Huebner, B. S. Haggarty, A. J. Cedarbaum, and J. C. Reed. 1986. Alterations in T4 (CD4) protein and mRNA synthesis in cells infected with HIV. Science 234:1123-1127. [DOI] [PubMed] [Google Scholar]
- 28.Huang, M. B., and V. C. Bond. 2000. Involvement of protein kinase C in HIV-1 gp120-induced apoptosis in primary endothelium. J. Acquir. Immune Defic. Syndr. 25:375-389. [DOI] [PubMed] [Google Scholar]
- 29.Huang, M. B., M. Hunter, and V. C. Bond. 1999. Effect of extracellular human immunodeficiency virus type 1 glycoprotein 120 on primary human vascular endothelial cell cultures. AIDS Res. Hum. Retrovir. 15:1265-1277. [DOI] [PubMed] [Google Scholar]
- 30.Huang, M. B., M. Khan, M. Garcia-Barrio, M. Powell, and V. C. Bond. 2001. Apoptotic effects in primary human umbilical vein endothelial cell cultures caused by exposure to virion-associated and cell membrane-associated HIV-1 gp120. J. Acquir. Immune Defic. Syndr. 27:213-221. [DOI] [PubMed] [Google Scholar]
- 31.Ikuta, K., M. Kameoka, and R. B. Luftig. 1997. AIDS pathogenesis: the role of accessory gene mutations, leading to formation of long-lived persistently infected cells and/or apoptosis-inducing HIV-1 particles. Virus Res. 52:145-156. [DOI] [PubMed] [Google Scholar]
- 32.Jekle, A., O. T. Keppler, E. De Clercq, D. Schols, M. Weinstein, and M. A. Goldsmith. 2003. In vivo evolution of human immunodeficiency virus type 1 toward increased pathogenicity through CXCR4-mediated killing of uninfected CD4 T cells. J. Virol. 77:5846-5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jolicoeur, P., D. G. Kay, M. Cool, S. Jothy, N. Rebai, and Z. Hanna. 1999. A novel mouse model of HIV-1 disease. Leukemia 13(Suppl. 1):S78-S80. [DOI] [PubMed] [Google Scholar]
- 34.Kaul, M., and S. A. Lipton. 1999. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc. Natl. Acad. Sci. USA 96:8212-8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klatzmann, D., F. Barre-Sinoussi, M. T. Nugeyre, C. Dauguet, E. Vilmer, C. Griscelli, F. Brun-vezinet, C. Rouzioux, J. C. Gluckman, and J. C. Chermann. 1984. Selective tropism of lymphadenopathy associated virus (LAV) for helper-inducer T lymphocytes. Science 225:59-63. [DOI] [PubMed] [Google Scholar]
- 36.Klatzmann, D., E. Champagne, S. Chamaret, J. Gruest, D. Guetard, T. Hercend, J. C. Gluckman, and L. Montagnier. 1984. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 312:767-768. [DOI] [PubMed] [Google Scholar]
- 37.Klimiuk, P. A., K. Bernacka, and A. Kuryliszyn-Moskal. 1995. Apoptosis in autoimmune diseases. Rocz. Akad. Med. Bialymst. 40:227-232. [PubMed] [Google Scholar]
- 38.Kuiken, C. L., B. Foley, B. Hahn, P. A. Marx, F. McCutchan, J. W. Mellors, S. Wolinksy, and B. Korber (ed.). 2001. HIV sequence compendium 2001. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, N.Mex.
- 39.Lambotte, O., A. Demoustier, M. G. de Goer, C. Wallon, J. Gasnault, C. Goujard, J. F. Delfraissy, and Y. Taoufik. 2002. Persistence of replication-competent HIV in both memory and naive CD4 T cell subsets in patients on prolonged and effective HAART. AIDS 16:2151-2157. [DOI] [PubMed] [Google Scholar]
- 40.Lannuzel, A., J. V. Barnier, C. Hery, V. T. Huynh, B. Guibert, F. Gray, J. D. Vincent, and M. Tardieu. 1997. Human immunodeficiency virus type 1 and its coat protein gp120 induce apoptosis and activate JNK and ERK mitogen-activated protein kinases in human neurons. Ann. Neurol. 42:847-856. [DOI] [PubMed] [Google Scholar]
- 41.Macreadie, I. G., L. A. Castelli, A. Lucantoni, and A. A. Azad. 1995. Stress- and sequence-dependent release into the culture medium of HIV-1 Nef produced in Saccharomyces cerevisiae. Gene 162:239-243. [DOI] [PubMed] [Google Scholar]
- 42.Meucci, O., A. Fatatis, A. A. Simen, T. J. Bushell, P. W. Gray, and R. J. Miller. 1998. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc. Natl. Acad. Sci. USA 95:14500-14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muller, A., B. Homey, H. Soto, N. Ge, D. Catron, M. E. Buchanan, T. McClanahan, E. Murphy, W. Yuan, S. N. Wagner, J. L. Barrera, A. Mohar, E. Verastegui, and A. Zlotnik. 2001. Involvement of chemokine receptors in breast cancer metastasis. Nature 410:50-56. [DOI] [PubMed] [Google Scholar]
- 44.Nanki, T., and P. E. Lipsky. 2000. Cutting edge: stromal cell-derived factor-1 is a costimulator for CD4+ T cell activation. J. Immunol. 164:5010-5014. [DOI] [PubMed] [Google Scholar]
- 45.Nickel, W. 2003. The mystery of nonclassical protein secretion: a current view on cargo proteins and potential export routes. Eur. J. Biochem. 270:2109-2119. [DOI] [PubMed] [Google Scholar]
- 46.Okada, H., S. Morikawa, and M. Tashiro. 1998. HIV-1 Nef binding protein expressed on the surface of murine blood cells. Med. Microbiol. Immunol. (Berlin) 186:201-207. [DOI] [PubMed] [Google Scholar]
- 47.Okada, H., R. Takei, and M. Tashiro. 1997. HIV-1 Nef protein-induced apoptotic cytolysis of a broad spectrum of uninfected human blood cells independently of CD95(Fas). FEBS Lett. 414:603-606. [DOI] [PubMed] [Google Scholar]
- 48.Okada, H., R. Takei, and M. Tashiro. 1997. Nef protein of HIV-1 induces apoptotic cytolysis of murine lymphoid cells independently of CD95 (Fas) and its suppression by serine/threonine protein kinase inhibitors. FEBS Lett. 417:61-64. [DOI] [PubMed] [Google Scholar]
- 49.Okada, H., R. Takei, and M. Tashiro. 1998. Inhibition of HIV-1 Nef-induced apoptosis of uninfected human blood cells by serine/threonine protein kinase inhibitors, fasudil hydrochloride and M3. FEBS Lett. 422:363-367. [DOI] [PubMed] [Google Scholar]
- 50.Quick, J., J. A. Ware, and P. E. Driedger. 1992. The structure and biological activities of the widely used protein kinase inhibitor, H7, differ depending on the commercial source. Biochem. Biophys. Res. Commun. 187:657-663. [DOI] [PubMed] [Google Scholar]
- 51.Raff, M. 1998. Cell suicide for beginners. Nature 396:119-122. [DOI] [PubMed] [Google Scholar]
- 52.Reid, W., M. Sadowska, F. Denaro, S. Rao, J. Foulke, Jr., N. Hayes, O. Jones, D. Doodnauth, H. Davis, A. Sill, P. O'Driscoll, D. Huso, T. Fouts, G. Lewis, M. Hill, R. Kamin-Lewis, C. Wei, P. Ray, R. C. Gallo, M. Reitz, and J. Bryant. 2001. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc. Natl. Acad. Sci. USA 98:9271-9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silvestris, F., D. Ribatti, B. Nico, N. Silvestris, A. Romito, and F. Dammacco. 1995. Apoptosis or programmed cell death: regulatory and pathophysiological mechanisms. Ann. Ital. Med. Int. 10:7-13. (In Italian.) [PubMed] [Google Scholar]
- 54.Simard, M. C., P. Chrobak, D. G. Kay, Z. Hanna, S. Jothy, and P. Jolicoeur. 2002. Expression of simian immunodeficiency virus nef in immune cells of transgenic mice leads to a severe AIDS-like disease. J. Virol. 76:3981-3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slee, E. A., C. Adrain, and S. J. Martin. 1999. Serial killers: ordering caspase activation events in apoptosis. Cell Death Differ. 6:1067-1074. [DOI] [PubMed] [Google Scholar]
- 56.Slee, E. A., M. T. Harte, R. M. Kluck, B. B. Wolf, C. A. Casiano, D. D. Newmeyer, H. G. Wang, J. C. Reed, D. W. Nicholson, E. S. Alnemri, D. R. Green, and S. J. Martin. 1999. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J. Cell Biol. 144:281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spies, C. P., and R. W. Compans. 1993. Alternate pathways of secretion of simian immunodeficiency virus envelope glycoproteins. J. Virol. 67:6535-6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Studzinski, G. P. (ed.). 1999. Apoptosis: a practical approach, p. 1-16. Oxford University Press, Oxford, United Kingdom.
- 59.Thomas, F. P., C. Chalk, R. Lalonde, Y. Robitaille, and P. Jolicoeur. 1994. Expression of human immunodeficiency virus type 1 in the nervous system of transgenic mice leads to neurological disease. J. Virol. 68:7099-7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thornberry, N. A., and Y. Lazebnik. 1998. Caspases: enemies within. Science 281:1312-1316. [DOI] [PubMed] [Google Scholar]
- 61.Tuosto, L., M. S. Montani, S. Lorenzetti, E. Cundari, S. Moretti, G. Lombardi, and E. Piccolella. 1995. Differential susceptibility to monomeric HIV gp120-mediated apoptosis in antigen-activated CD4+ T cell populations. Eur. J. Immunol. 25:2907-2916. [DOI] [PubMed] [Google Scholar]
- 62.Ullrich, C. K., J. E. Groopman, and R. K. Ganju. 2000. HIV-1 gp120- and gp160-induced apoptosis in cultured endothelial cells is mediated by caspases. Blood 96:1438-1442. [PubMed] [Google Scholar]
- 63.Vene, R., R. Benelli, D. M. Noonan, and A. Albini. 2000. HIV-Tat dependent chemotaxis and invasion, key aspects of tat mediated pathogenesis. Clin. Exp. Metastasis 18:533-538. [DOI] [PubMed] [Google Scholar]
- 64.Wain-Hobson, S. 1997. Down or out in blood and lymph? Nature 387:123-124. [DOI] [PubMed] [Google Scholar]
- 65.Xu, X. N., B. Laffert, G. R. Screaton, M. Kraft, D. Wolf, W. Kolanus, J. Mongkolsapay, A. J. McMichael, and A. S. Baur. 1999. Induction of Fas ligand expression by HIV involves the interaction of Nef with the T cell receptor zeta chain. J. Exp. Med. 189:1489-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoon, K., J. G. Jeong, and S. Kim. 2001. Stable expression of human immunodeficiency virus type 1 Nef confers resistance against Fas-mediated apoptosis. AIDS Res. Hum. Retrovir. 17:99-104. [DOI] [PubMed] [Google Scholar]
- 67.Zhang, J., and C. S. Crumpacker. 2001. Human immunodeficiency virus type 1 RNA in peripheral blood mononuclear cells of patients receiving prolonged highly active antiretroviral therapy. J. Infect. Dis. 184:1341-1344. [DOI] [PubMed] [Google Scholar]
- 68.Zheng, J., A. Ghorpade, D. Niemann, R. L. Cotter, M. R. Thylin, L. Epstein, J. M. Swartz, R. B. Shepard, X. Liu, A. Nukuna, and H. E. Gendelman. 1999. Lymphotropic virions affect chemokine receptor-mediated neural signaling and apoptosis: implications for human immunodeficiency virus type 1-associated dementia. J. Virol. 73:8256-8267. [DOI] [PMC free article] [PubMed] [Google Scholar]