Abstract
Herpes simplex virus-2 (HSV-2) suppression with acyclovir or valacyclovir reduces HIV-1 viral RNA levels; one hypothesis is that HSV-2 suppression reduces immune activation. We measured T cell immune activation markers among women participating in a randomized placebo-controlled trial of valacyclovir to reduce HIV-1 RNA levels among pregnant women. Although valacyclovir was associated with lower HIV-1 RNA levels, the distribution of both CD4+ and CD8+ CD38+HLA-DR+ T cells was not different among women taking valacyclovir when compared to women taking placebo. Further study is needed to understand the mechanism of HIV-1 RNA reduction following herpes suppression among those coinfected with HIV-1 and HSV-2.
Introduction
Coinfection with herpes simplex virus type 2 (HSV-2) and HIV-1 is associated with higher HIV-1 plasma RNA levels, faster disease progression, and increased T cell immune activation.1 Use of suppressive antivirals such as valacyclovir in HIV-1/HSV-2 coinfection reduces episodes of genital ulcers and genital shedding of HIV-1,2 reduces plasma HIV-1 viral load,3 and modestly slows HIV-1 disease progression.4 One theory to explain the effects of anti-herpes agents on HIV-1 is that suppression of herpes virus reduces immune activation, which is an important component of HIV-1 pathogenesis and is related to increased HIV-1 disease progression and poor T cell recovery.5 Reduction of systemic immune activation may confer clinical benefits to persons with HIV-1. HIV-1-infected persons in Africa have been noted to have high levels of systemic immune activation6 and those with the lowest immune activation have improved CD4 responses to antiretroviral therapy.7 Coinfecting pathogens can increase immune activation; HSV-2 coinfection is highly prevalent in Africa and is implicated in HIV-1 transmission, making it a desirable target for suppression. We measured T cell activation markers during a randomized controlled trial of valacyclovir for herpes suppression in pregnant and breastfeeding Kenyan women coinfected with HIV-1 and HSV-2. We hypothesized that women taking valacyclovir over long periods of time would have measurable reductions in T cell immune activation markers.
Materials and Methods
This study was nested within a randomized, double-blind clinical trial, evaluating the efficacy of 500 mg valacyclovir twice daily or matching placebo for reducing maternal HIV-1 RNA levels in plasma, genital secretions, and breast milk, as described by Drake et al.8 From 2007 to 2009, pregnant, HIV-1-seropositive, HSV-2-seropositive women seeking antenatal care were recruited and followed at a public health clinic in Nairobi, Kenya. Eligible women were ≥18 years of age, WHO stage 1 or 2, with a CD4 count >250 cells/μl. HIV-1-seronegative nonpregnant female subjects ≥18 years of age were recruited at voluntary counseling and testing centers in Nairobi from 2009 to 2010 as controls for a different cohort. Both study protocols were approved by the Kenyatta National Hospital Ethical Review Committee and the University of Washington Institutional Review Board. The clinical trial was registered (ClinicalTrials.gov, identifier NCT 00530777).
At 34 weeks gestation, participants provided written informed consent and were randomized; study drugs were taken from 34 weeks until 1 year postpartum. In addition to study drug, mothers received short-course prevention of mother-to-child (PMTCT) antiretroviral (ARV) regimens according to Kenyan national guidelines. Blood specimens were collected at all study visits; CD4 counts and HIV-1 RNA levels were determined at enrollment, 6 months and 12 months postpartum. T cell immune activation markers were measured at 6 and 12 months postpartum. HIV-1-seronegative female participants in the control population gave written consent, were enrolled, and gave a blood specimen at one time point only.
Maternal HIV-1 serostatus was determined with rapid-testing protocols and confirmed using enzyme-linked immunosorbent assay (ELISA) (Vironostika HIV Uni-Form II, bioMérieux). HSV-2 serostatus was determined using HerpeSelect ELISA (Focus Technologies) with positive result defined as an index value ≥3.5. HIV-1 RNA assays were performed using a transcription-mediated amplification method validated for Kenyan HIV-1 subtypes (Gen-Probe). Immune activation markers were measured on fresh whole blood specimens; specimens were surface-stained with fluorochrome-conjugated antibodies, and each specimen was run with isotype controls on a 4-color flow cytometer (FACSCalibur, BectonDickinson). The following two premixed staining combinations were used: anti-CD4-FITC/anti-CD38-PE/anti-CD3-PerCP/anti-HLA-DR-APC, and anti-CD8-FITC/anti-CD38-PE/anti-CD3-PerCP/anti-HLA-DR-APC (BectonDickinson). Single-stain controls and unstained controls were run daily. Flow cytometry results were interpreted using FlowJo and a logical gating strategy was used to define activated cells.
Baseline characteristics (including CD4 counts and HIV-1 RNA levels) of women in each study arm with immune activation results were compared by chi-square and Wilcoxon rank-sum tests. Distributions of activated cells, defined as CD38+, HLA-DR+ and combined CD38+HLA-DR+, in subsets of CD4+ and CD8+ T cells were compared between randomization arms using nonparametric tests. The same analyses were done to compare T cell subsets between HIV-1-seropositive participants and HIV-1-seronegative controls. Analyses were done using Stata 10.
Results
Of the 148 women enrolled in this study, 12 (8%) were lost to follow-up and 19 (13%) came to the clinic when the laboratory was closed; 117 women (79%) had at least one immune activation result and are described in this analysis. The women had a median age of 25, and most had one prior pregnancy, primary school education or less, and lived in poverty (Table 1). Consistent with enrollment criteria, all women were WHO stage 1 or 2, and 89% never had an HIV-1-related illness. At 6 months postpartum, 51% of study women were breastfeeding; by 12 months postpartum, 13% of study women were breastfeeding. Two women initiated ART by 12 months postpartum. Control women (n=31) had similar median numbers of sexual partners and children, but had more education and were more likely to be employed. Only eight (27%) of the control women were HSV-2 seropositive, compared to 100% of the study women, and control women were less likely to ever have an STI. Four (13%) control women were breastfeeding.
Table 1.
Clinical and Demographic Characteristics
| |
Placebo (n=58) |
Valacyclovir (n=59) |
Control (n=31) |
|---|---|---|---|
| Characteristic at baseline | Median (IQR) or N (%) | ||
| Age (years) | 25 (22–29) | 25 (23–31) | 24 (21–30) |
| No. of pregnancies | 2 (1–2) | 1 (0–3) | 1 (0–3) |
| Monthly rent (US$) | 21 (13–33) | 27 (20–33)a | 32 (27–40) |
| Employed | 15 (26%) | 16 (27%) | 14 (45%) |
| Education (years) | 8 (7–10) | 8 (8–12) | 12 (8–12) |
| Lifetime sex partners | 3 (2–5) | 3 (2–4) | 3 (2–4) |
| History of STI | 15 (26%) | 15 (25%) | 2 (7%) |
| HSV-2 antibody positive | 58 (100%) | 59 (100%) | 8 (27%) |
| WHO Stage | |||
| Stage 1 | 51 (88%) | 55 (93%) | n/a |
| Stage 2 | 7 (12%) | 4 (7%) | n/a |
| CD4 (cells/μl) | 488 (341–598) | 466 (351–560) | n/a |
| Log10 HIV-1 plasma RNA (copies/ml) | 3.77 (3.31–4.46) | 3.99 (3.15–4.35) | n/a |
n=58.
GUD, genital ulcer disease; STI, sexually transmitted infection.
When compared to the main study results, which reported a relative reduction of HIV-1 RNA of 0.53 log10 copies/ml at 1 year postpartum among women randomized to valacyclovir,8 the subset of women in this nested study randomized to valacyclovir also had significant relative reductions in plasma HIV-1 RNA levels of 0.6 log10 copies/ml at 6 months postpartum and 0.3 log10 copies/ml at 12 months postpartum. Only seven (12%) women taking valacyclovir and 11 (19%) women taking placebo had a prior history of genital ulcer disease (GUD). During follow-up, illness was common in both study arms: five (4%) women were treated for tuberculosis (two valacyclovir, three placebo), three (3%) women were treated for herpes zoster (one valacyclovir, two placebo), 18 (15%) received treatment for malaria (five valacyclovir, 13 placebo), and 23 (20%) received treatment for diarrhea or helminths (14 valacyclovir, nine placebo). Three women died prior to 6 months postpartum and are not included in this analysis. Monitoring showed high adherence (>85%) to study drugs as previously reported.9
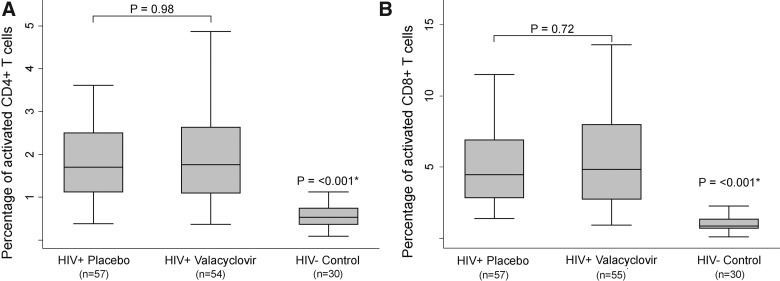
Immune activation did not differ between women randomized to valacyclovir and women taking placebo. At 6 months postpartum, the median percentage of CD38+HLA-DR+ CD4+ T cells was 2.0% in the valacyclovir arm vs. 2.0% in the placebo arm (p=0.75); at 12 months postpartum, the median percent CD38+HLA-DR+ CD4+ T cells was 1.7% (p=0.98) in both study arms. Similarly, at 6 months postpartum, the median percentage of CD38+HLA-DR+ CD8+ T cells was 4.9% among women taking valacyclovir, compared to 5.0% among women taking placebo (p=0.95), and at 12 months postpartum, there was again no significant difference between study arms: 4.8% of cells were activated among women taking valacyclovir, compared to 4.5% among women taking placebo (p=0.72). At both 6 and 12 months postpartum, percentages of CD38 and HLA-DR activated T cells were uniformly higher among HIV-1-seropositive women in the study when compared to HIV-1-seronegative female controls (Fig. 1).
FIG. 1.
Univariate comparison of T cell activation markers. T cell activation in human immunodeficiency virus-1 (HIV-1)-seropositive study participants randomized to valacyclovir or placebo and HIV-1-seronegative Kenyan women. This figure describes activated CD38+HLA-DR+ CD4+ T cells (A) and CD38+HLA-DR+ CD8+ T cells (B) among study participants randomized to take valacyclovir or placebo, measured at the 12 month time point, and a control group of HIV-1-seronegative women, measured at a single time point. Each central bar represents the median value, each box represents the 25th through 75th percentile (interquartile) range, and the whiskers include 1.5 times the interquartile range below the 25th percentile and above the 75th percentile values. p-values were calculated using the Wilcoxon rank-sum test comparing medians. *Women in the control group were compared to all study participants at 12 months.
Discussion
We did not see any differences in immune activation between the two study arms, although women in the valacyclovir arm had significantly lower HIV-1 plasma RNA levels. This result differs from studies of other coinfections, including tuberculosis, helminths, and malaria, which have shown reductions in both HIV-1 RNA levels and systemic immune activation after treatment.10,11 HIV-1-seropositive persons with HSV-2 who take acyclovir or valacyclovir in clinical trials have sustained decreases in HIV-1 RNA levels and reduced disease progression; our results suggest that these findings may not be related to reduced immune activation.
If acyclovir therapy does not reduce systemic immune activation, then how does it reduce HIV-1 RNA levels? There is evidence that valacyclovir interacts directly with HIV-1 reverse transcriptase if phosphorylated by human herpesviruses in tissues,12 although it remains unclear why no acyclovir resistance develops in vivo;13 valacyclovir may also suppress HIV-1 replication indirectly by reducing HSV-2, causing a cascade of effects including a reduction in target cells for HIV-1 replication and less HSV-2 upregulation of HIV-1 transcription factors.14
The lack of effect of valacyclovir on immune activation that we observed may be unique to HSV-2 when compared to other coinfections. The long duration of inflammation associated with HSV-2 lesions15 and the steady shedding of HSV-2 even among people who are asymptomatic16 or being prophylactically treated17 suggest that anti-herpes medications are not optimally effective in the mucosal sites where the immune activation is potentially generated. This could partly explain the failure of acyclovir to protect against HIV-1 transmission and acquisition in large randomized trials.18,19
Our results contrast with a recent trial of valganciclovir in persons with HIV-1 and cytomegalovirus (CMV) coinfection, which showed reductions in immune activation markers among those with poor CD4 response to ART who completed 8 weeks of CMV suppression.20 CMV-specific cells dominate the circulating memory T cell repertoire21 and results from these two studies may signal that CMV may be a more important coinfection than HSV-2 in amplifying systemic immune activation. In addition, the HIV-1/CMV coinfected subjects were on ART, which could make the immune activation more easily downregulated.
The strengths of our study are randomization to reduce confounding, prolonged (>12 months) follow-up of participants, excellent adherence to valacyclovir, a large sample with adequate power to detect clinically significant differences in immune activation, and inclusion of an appropriate local control population. The weaknesses include lack of pre-valacyclovir measures of immune activation and unpredictable effects of recent pregnancy and breastfeeding on immune activation, although these are mitigated by the randomized trial design. Maternal illness is a confounder that could influence immune activation, but appears to be evenly distributed across study arms. We measured only two commonly cited markers of T cell activation, but other markers might reveal different results. This cohort of postpartum women with CD4 >250 cells/μl was relatively healthy, and our results may not be generalizable to other populations, or populations with lower CD4 counts.
In conclusion, 500 mg twice daily valacyclovir taken over 12 months in a group of asymptomatic HIV-1/HSV-2 coinfected African women failed to reduce systemic immune activation markers. Valacyclovir's role in controlling HIV-1 is promising, and new drugs are in development to exploit this potential.22 Further research should be pursued to clarify how anti-herpes medications improve outcomes in HIV-1/HSV-2 coinfected individuals.
Acknowledgments
We gratefully acknowledge the women who participated in this study, our study staff, and the staff of Mathare North Health Centre in Nairobi. We especially thank our laboratory staff, John Gatimu and Daniel N. Matemo.
This work was supported by US National Institutes of Health (NIH) research grants (R03 HD 057773, R03 HD 057773-02S1, R01 AI076105, K24 AI087399, R01 AI06843-05 to C.F., K24 HD054314 to G.J.S., K24 AI071113 and PO1 AI30731 to A.W.); the University of Washington Center for AIDS Research (CFAR) (P30 AI027757), a Puget Sound Partners for Global Health Research and Technology Grant to B.A.R., and University of Washington Royalty Research Fund Grant #4027. GlaxoSmithKline donated study drug and matched placebo, but had no other role in the study. A.L.D. was supported by an NIH-funded University of Washington CFAR Training Grant (T32AI07140-32). A.C.R. and A.Y.L. were scholars in the International AIDS Research and Training Program, funded by the Fogarty International Center, NIH (D43-TW000007). A.C.R. was a Fogarty International Clinical Research Fellow funded by the Fogarty International Center, NIH (R24 TW007988).
Author Disclosure Statement
Dr. Wald has received grant support from GlaxoSmithKline and Antigenics and has consulted for AiCuris and Agenus. All other authors report no disclosures.
References
- 1.Sheth PM. Sunderji S. Shin LY, et al. Coinfection with herpes simplex virus type 2 is associated with reduced HIV-specific T cell responses and systemic immune activation. J Infect Dis. 2008;197(10):1394–1401. doi: 10.1086/587697. [DOI] [PubMed] [Google Scholar]
- 2.Ouedraogo A. Nagot N. Vergne L, et al. Impact of suppressive herpes therapy on genital HIV-1 RNA among women taking antiretroviral therapy: A randomized controlled trial. AIDS. 2006;20(18):2305–2313. doi: 10.1097/QAD.0b013e328010238d. [DOI] [PubMed] [Google Scholar]
- 3.Nagot N. Ouedraogo A. Foulongne V, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356(8):790–799. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 4.Lingappa JR. Baeten JM. Wald A, et al. Daily aciclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: A randomised placebo-controlled trial. Lancet. 2010;375(9717):824–833. doi: 10.1016/S0140-6736(09)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawn SD. Butera ST. Folks TM. Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1 infection. Clin Microbiol Rev. 2001;14(4):753–777. doi: 10.1128/CMR.14.4.753-777.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clerici M. Butto S. Lukwiya M, et al. Immune activation in Africa is environmentally-driven and is associated with upregulation of CCR5. Italian-Ugandan AIDS Project. AIDS. 2000;14(14):2083–2092. doi: 10.1097/00002030-200009290-00003. [DOI] [PubMed] [Google Scholar]
- 7.Hunt PW. Cao HL. Muzoora C, et al. Impact of CD8+ T-cell activation on CD4+ T-cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS. 2011;25(17):2123–2131. doi: 10.1097/QAD.0b013e32834c4ac1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drake AL. Roxby AC. Ongecha-Owuor F, et al. Valacyclovir suppressive therapy reduces plasma and breast milk HIV-1 RNA levels during pregnancy and postpartum: A randomized trial. J Infect Dis. 2012;205(3):366–375. doi: 10.1093/infdis/jir766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roxby AC. Drake AL. Ongecha-Owuor F, et al. Effects of valacyclovir on markers of disease progression in postpartum women co-infected with HIV-1 and herpes simplex virus-2. PLoS ONE. 2012;7(6):e38622. doi: 10.1371/journal.pone.0038622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Modjarrad K. Vermund SH. Effect of treating co-infections on HIV-1 viral load: A systematic review. Lancet Infect Dis. 2010;10(7):455–463. doi: 10.1016/S1473-3099(10)70093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnabas RV. Webb EL. Weiss HA. Wasserheit JN. The role of coinfections in HIV epidemic trajectory and positive prevention: A systematic review and meta-analysis. AIDS. 2011;25(13):1559–1573. doi: 10.1097/QAD.0b013e3283491e3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lisco A. Vanpouille C. Tchesnokov EP, et al. Acyclovir is activated into a HIV-1 reverse transcriptase inhibitor in herpesvirus-infected human tissues. Cell Host Microbe. 2008;4(3):260–270. doi: 10.1016/j.chom.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baeten JM. Lingappa J. Beck I, et al. Herpes simplex virus type 2 suppressive therapy with acyclovir or valacyclovir does not select for specific HIV-1 resistance in HIV-1/HSV-2 dually infected persons. J Infect Dis. 2010;203(1):117–121. doi: 10.1093/infdis/jiq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mole L. Ripich S. Margolis D. Holodniy M. The impact of active herpes simplex virus infection on human immunodeficiency virus load. J Infect Dis. 1997;176(3):766–770. doi: 10.1086/517297. [DOI] [PubMed] [Google Scholar]
- 15.Zhu J. Hladik F. Woodward A, et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med. 2009;15(8):886–892. doi: 10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiffer JT. Wald A. Selke S. Corey L. Magaret A. The kinetics of mucosal herpes simplex virus-2 infection in humans: Evidence for rapid viral-host interactions. J Infect Dis. 2011;204(4):554–561. doi: 10.1093/infdis/jir314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiffer JT. Magaret A. Selke S. Corey L. Wald A. Detailed analysis of mucosal herpes simplex virus-2 replication kinetics with and without antiviral therapy. J Antimicrob Chemother. 2011;66(11):2593–2600. doi: 10.1093/jac/dkr346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Celum C. Wald A. Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362(5):427–439. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watson-Jones D. Weiss HA. Rusizoka M, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med. 2008;358(15):1560–1571. doi: 10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunt PW. Martin JN. Sinclair E, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis. 2011;203(10):1474–1483. doi: 10.1093/infdis/jir060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sylwester AW. Mitchell BL. Edgar JB, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202(5):673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanpouille C. Lisco A. Derudas M, et al. A new class of dual-targeted antivirals: Monophosphorylated acyclovir prodrug derivatives suppress both human immunodeficiency virus type 1 and herpes simplex virus type 2. J Infect Dis. 2010;201(4):635–643. doi: 10.1086/650343. [DOI] [PMC free article] [PubMed] [Google Scholar]