Abstract
Obesity, sedentary lifestyles, and antiretroviral therapies may predispose HIV-infected children to poor physical fitness. Estimated peak oxygen consumption (VO2 peak), maximal strength and endurance, and flexibility were measured in HIV-infected and uninfected children. Among HIV-infected children, anthropometric and HIV disease-specific factors were evaluated to determine their association with VO2 peak. Forty-five HIV-infected children (mean age 16.1 years) and 36 uninfected children (mean age 13.5 years) participated in the study. In HIV-infected subjects, median viral load was 980 copies/ml (IQR 200–11,000 copies/ml), CD4% was 28% (IQR 15–35%), and 82% were on highly active antiretroviral therapy (HAART). Compared to uninfected children, after adjusting for age, sex, race, body fat, and siblingship, HIV-infected children had lower VO2 peak (25.92 vs. 30.90 ml/kg/min, p<0.0001), flexibility (23.71% vs. 46.09%, p=0.0003), and lower-extremity strength-to-weight ratio (0.79 vs. 1.10 kg lifted/kg of body weight, p=0.002). Among the HIV-infected children, a multivariable analysis adjusting for age, sex, race, percent body fat, and viral load showed VO2 peak was 0.30 ml/kg/min lower per unit increase in percent body fat (p<0.0001) and VO2 peak (SE) decreased 29.45 (±1.62), 28.70 (±1.87), and 24.09 (±0.75) ml/kg/min across HAART exposure categories of no exposure, <60, and ≥60 months, respectively (p<0.0001). HIV-infected children had, in general, lower measures of fitness compared to uninfected children. Factors negatively associated with VO2 peak in HIV-infected children include higher body fat and duration of HAART ≥60 months. Future studies that elucidate the understanding of these differences and mechanisms of decreased physical fitness should be pursued.
Introduction
Regular physical activity is linked to many positive health outcomes with evidence of its multidimensional benefits among children and adolescents.1 Active children have greater levels of cardiovascular fitness when compared to their nonactive counterparts.2 Most striking is the disparity of fitness between healthy children and children with chronic illnesses.3 Cardiovascular fitness in children with chronic disease can positively impact disease-specific outcomes, yet their medical conditions often marginalize them.4,5 Families, caregivers, and medical providers often conservatively restrict the amount of physically demanding activities for these children based on limited data.6,7 This approach to chronic disease is compounded by the growing trend in the United States in which most children are less active than the preceding generations,2,8–10 in part because of media-driven sedentary behaviors.
Although researchers have examined the disparate levels of fitness in some populations of children with chronic illness including childhood cancer survivors11–16 and children with cardiac and pulmonary dysfunction,17,18 little is known about the physical fitness of children and adolescents living with the human immunodeficiency virus (HIV). With the advent of highly active antiretroviral therapy (HAART), HIV-infected children have developed unfavorable metabolic profiles that put them at risk for future cardiovascular disease.19–21 Although the evidence is conflicting, some have speculated that antiretrovirals (ARV) may be associated with mitochondrial toxicity that impacts organs such as muscle and liver.22 Thus, HIV-infected children may be predisposed to deconditioning due to a combination of both psychosociological and physiological factors including a predisposition to a sedentary lifestyle due to the nature of their disease and/or long-term exposure to HAART.
Cardiometabolic risk factors and adiposity are prevalent among HIV-infected children19–21 and exercise can help control some of these risks.1,2 We therefore sought to compare peak oxygen consumption (VO2 peak), maximal strength and endurance, and flexibility of HIV-infected and uninfected children to determine if clinical and HIV-specific factors are associated with physical fitness.
Materials and Methods
Subjects
HIV-infected subjects were recruited from the Pediatric Special Immunology and Adolescent Health Clinics at the University of Miami, Miami, FL between July 2005 and December 2009. HIV infection was confirmed by repeatedly positive serum enzyme-linked immunosorbent assays confirmed by Western blot assays, repeatedly positive HIV RNA or DNA polymerase chain reaction (PCR) assays, or by HIV culture. HIV-uninfected children were recruited from the general pediatric population or were siblings of the patients. At the time of recruitment, the HIV-infected children were encouraged to ask uninfected friends or siblings to participate in the protocol as voluntary subjects. All subjects (both infected and uninfected) were eligible for this study if they were without acute infection, were between 7 and 20 years of age, were medically cleared to participate in the exercise testing protocol, and were capable of following instructions. Prior to enrollment, informed consent and assent were obtained from the participants' parents or legal guardians and the participant, respectively. This study was approved by the University of Miami's Human Subjects Research Office.
Anthropometric and clinical measurements
Demographic information was collected from patients' clinic charts and/or questionnaires at testing. Anthropometric measurements included height, weight, and hip and waist circumference. Body weight was measured to the nearest 0.1 kg and height was measured to the nearest 0.1 cm. These values were used to calculate body mass index (BMI) [weight (kg)/ height2 (m2)]. Age- and sex-specific BMI percentiles and z-scores were calculated based on the Centers for Disease Control and Prevention (CDC) growth charts.23 Dual energy X-ray absorptiometry (DXA) scans assessed body composition and regionalization of body fat (GE/Lunar Prodigy, Madison, WI; enCORE 2006 software version 10.50.086) using standard methods.24,25 Waist and hip circumferences were measured using a nonstretchable plastic tape measure according to standard methods.26 Waist and hip measurements were taken three times and the average was determined. All measures were taken by the same research dietician.
Fasting serum levels of total cholesterol, high-density lipoprotein (HDL)-cholesterol, low-density lipoprotein (LDL)-cholesterol, triglycerides, glucose, insulin, and hemoglobin A1c were measured on the HIV-infected participants after an overnight fast and analyzed at the Diabetes Research Institute Clinical Chemistry Laboratory at the University of Miami on a Cobas 6000 analyzer (Roche Diagnostics, Indianapolis, IN) using the manufacturer's reagents and procedures. The homeostatic model assessment of insulin resistance (HOMA-IR) score was calculated: [fasting insulin (μU/ml)×fasting glucose (mmol/liter)]/22.5.27
HIV-specific factors
For HIV-infected subjects, we collected HIV-specific factors at the time of the study including CDC pediatric HIV disease stage,28 CD4 T-lymphocyte percent, and plasma HIV-1 RNA concentration by quantitative HIV-1 RNA PCR (Amplicor HIV-1 Monitor test, Roche Diagnostic Systems, Branchburg, NJ). Type of ARV [nucleoside reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs) and duration of each and of HAART (over the prior 60 months] were documented.
Physical fitness assessment
Physical fitness tests were performed at the University of Miami Pediatric Cardiac Rehabilitation Program and over two visits with at least 48 h rest between sessions. On the first day, subjects completed upper and lower body strength, flexibility, and muscular endurance. On the subsequent visit, subjects completed the cardiorespiratory fitness test.
Cardiorespiratory fitness was determined indirectly by VO2 peak. Each participant performed a metabolic stress test to volitional exhaustion on a motor-driven treadmill. Participants were familiarized with the treadmill and protocol on a visit prior to the actual exercise testing. The study utilized the modified Balke protocol maintaining a constant speed of 3.3 mph with increasing grade elevation of 1 degree every minute until the conclusion of the test.29 The VO2 peak value is estimated from normative tables using the final stage that is completed by the child on the treadmill. This protocol has been validated in both adult and pediatric studies.30 The Rate of Perceived Exertion (RPE) scale was used during the test to assess perception of effort by the participant. VO2 peak was achieved if the participant could not maintain workload, RPE reached at least 17, or if the exercise physiologist administering the test determined maximal effort had been reached.31
To evaluate upper and lower body strength, all participants performed a 1-Repetition Maximum (1-RM) chest press and leg press. Utilizing a modified protocol, each participant completed three sets: (1) five repetitions at light weight, (2) three repetitions at moderate weight, and (3) as many repetitions at a predicted near maximal weight.32 1-RM was calculated using the Mayhew regression equation.32 Weight values were selected and monitored by trained pediatric exercise physiologists and trainers.
Flexibility was assessed with the modified sit and reach test that is validated for hamstring flexibility in both children and adolescents.33 Standardized testing protocol is described by the President's Council on Exercise and used during this assessment.34 The test was repeated three times and the best value was recorded. Absolute values were converted to normative percentiles derived from the National Presidential Fitness Program.34
Muscular endurance was evaluated by the sit-up test. Standardized testing protocol is described by the President's Council on Exercise and used during this assessment.34 When instructed, the subjects initiated the test and performed as many complete sit-ups as they could during a 60-s timed period. Absolute values were converted to normative percentiles derived from the National Presidential Fitness Program.34
Statistical analysis
Multiple regressions examined the relationship between HIV status (i.e., infected versus uninfected) and VO2 peak as well as flexibility, sit-ups, and body strength. The same methodology was used on a subset of the subjects (i.e., infected) to examine the relationship between three HAART therapy categories (i.e., no exposure, <60 months, and ≥60 months) and VO2 peak. Both models controlled for age, sex, race (black versus nonblack), and percentage of body fat. Since some of the HIV-infected subjects recruited uninfected siblings into the study, the statistical model that compared HIV status groups also accounted for the nonindependence of sibling pairs via the model estimation technique (i.e., General Estimating Equations). The natural log of viral load was also included in the model for HIV-infected subjects. Model stability and model assumption tenability were examined and the individual predictive contribution of each independent variable was described using several statistical criteria including standard statistical tests, partial R2 squares, Cook's D statistic (i.e., influence analysis), and bootstrapped stepwise regressions. Statistical computations were performed using a combination of statistical software including SAS version 9.2, JMP version 9.0 (SAS Institute, Cary, NC), and STATA version 8.2 (StataCorp, College Station, TX).
Due to the observational nature of this investigation, results of the statistical test are given as exact probabilities (i.e., p values) and reflect the probability of encountering the observed effect or one greater under the assumption that the observed estimated error structure is valid and unbiased. To evaluate the stability of the results, two statistical data perturbation techniques were employed. The first technique examined the results of generating 1000 bootstrap samples (i.e., subjects sampled with replacement) from the original observations and subsequently performing a stepwise regression on each bootstrapped sample.35 The number of times each independent variable entered the multiple regression equation over the 1000 samples was recorded. A forward selection criterion was used with entry and retained p values set at 0.10 and 0.05, respectively. For the second technique, Cook's influence statistic (i.e., D) for each observation was determined from the residuals of both multiple regression models and observations with the highest influence (10% of the data) were dropped and the models reestimated. Dropping these observation ensures that the relationship observed in the full data set was not an artifact resulting from a few overly influential observations.
Results
We enrolled 45 HIV-infected and 36 uninfected controls. HIV infection was acquired perinatally in 42 children and horizontally in the remaining three. Unadjusted demographic, anthropometric, and fitness characteristics of the two groups are shown in Table 1. The HIV-infected group was older (16.1 years) than the uninfected group (13.5 years), p<0.0001, and had a greater proportion of blacks (78% vs. 39%, p=0.0004). Based on normalized values, HIV-infected subjects were significantly shorter in stature than uninfected controls (height z-score –0.84 and –0.02, respectively, p=0.003). Raw weight between the two groups was approximately equal (63.2 vs. 62.1 kg). However, uninfected subjects were significantly heavier than HIV-infected subjects, with normalized weight z-scores of 1.03 and 0.16, respectively, p=0.03. VO2 peak was higher in the uninfected group (30.85 ml/kg/min) compared to HIV-infected subjects (25.96 ml/kg/min, p=0.0006). Flexibility was also higher in the uninfected group (45.37 vs. 24.29 %-ile, p<0.0001).
Table 1.
Unadjusted Demographic, Anthropometric, and Fitness Clinical Characteristics by HIV Status
| Variable | HIV-infected N=45 | Uninfected N=36 | p-value* |
|---|---|---|---|
| Demographics | |||
| Age (years), mean (SD) | 16.1 (2.66) | 13.5 (3.01) | <0.0001 |
| Sex (male), n (%) | 24 (53) | 22 (61) | 0.48 |
| Race (black), n (%) | 35 (78) | 14 (39) | 0.0004 |
| Anthropometrics—mean (SD) | |||
| Height (cm) | 159.48 (9.70) | 154.93 (13.46) | 0.08 |
| Height z-score | −0.84 (1.31) | −0.02 (1.14) | 0.003 |
| Weight (kg) | 63.22 (17.98) | 62.11 (23.65) | 0.83 |
| Weight z-score | 0.16 (1.94) | 1.03 (1.39) | 0.03 |
| BMI (kg/m2) | 24.71 (6.38) | 25.37 (7.50) | 0.70 |
| BMI z-score | 0.60 (1.44) | 1.05 (1.39) | 0.20 |
| Waist circumference (cm) | 82.84 (13.62) | 85.97 (15.91) | 0.49 |
| Hip circumference (cm) | 93.81 (13.87) | 95.75 (15.19) | 0.65 |
| Waist:hip ratio | 0.88 (0.08) | 0.90 (0.08) | 0.59 |
| Total body fat (%) | 28.36 (13.01) | 31.41 (13.29) | 0.39 |
| Fitness—mean (SD) | |||
| VO2 peak (ml/kg/min) | 25.96 (6.33) | 30.85 (6.53) | 0.0006 |
| Flexibility (%) | 24.29 (22.12) | 45.37 (27.18) | <0.0001 |
| Muscular endurance, sit-ups (%) | 13.55 (12.79) | 17.03 (17.15) | 0.30 |
| Upper body strength ratio (1-RM/kg) | 0.54 (0.23) | 0.50 (0.20) | 0.30 |
| Lower body strength ratio (1-RM/kg) | 0.89 (0.47) | 0.98 (0.44) | 0.33 |
p-values were adjusted by siblingship.
BMI, body mass index.
RM, repetition maximum.
HIV disease-specific and metabolic characteristics for HIV-infected subjects are shown in Table 2. Forty-two percent (n=19) of the HIV-infected subjects had viral loads less than 400 copies/ml [median viral load=980; interquartile range (IQR)=200–11,000] and the median CD% was 28% (IQR=15–35%). Over one-quarter of the HIV-infected children had triglyceride, LDL-cholesterol, and HDL-cholesterol values in the abnormal range. Approximately 15% had abnormal hemoglobin A1C levels.
Table 2.
Clinical and Metabolic Characteristics of the HIV-Infected Children
| HIV-specific disease characteristics | |
|---|---|
| CDC stage, n (%) | |
| Stage N | 4 (9) |
| Stage A | 10 (22) |
| Stage B | 18 (40) |
| Stage C | 13 (29) |
| CD4 %, median (25th, 75th) | 28 (15–35) |
| Viral load (copies/ml), median (25th, 75th) | 980 (200–11,000) |
| Viral load (copies/ml) <400, n (%) | 19 (42) |
| Antiretroviral therapy exposures | Never exposed | Exposed <60 months | Exposed ≥60 months |
|---|---|---|---|
| NRTI, n (%) | 4 (9) | 9 (20) | 32 (71) |
| NNRTI, n(%) | 21 (47) | 20 (44) | 4 (9) |
| PI, n (%) | 9 (20) | 16 (36) | 20 (44) |
| HAART, n (%) | 8 (18) | 9 (20) | 28 (62) |
| Metabolic characteristics | N | Median | IQR | Percent abnormal |
|---|---|---|---|---|
| Glucose, fasting (mg/dl) | 37 | 79 | 72–88 | 5.40a |
| Insulin, fasting (MIU/ml) | 26 | 9 | 6–13 | 19.23a |
| Hemoglobin A1C (%) | 27 | 5.2 | 5.1–5.7 | 14.81a |
| Cholesterol (mg/dl) | 38 | 146.5 | 126–167 | 13.15b |
| Triglycerides (mg/dl) | 38 | 87 | 62–113 | 28.95b |
| HDL-cholesterol (mg/dl) | 33 | 43 | 35–46 | 39.39b |
| LDL-cholesterol (mg/dl) | 33 | 77 | 62–108 | 25.80b |
| HOMA-IR | 25 | 1.60 | 1.24–2.83 | 12.00c |
As determined by reference laboratory; glucose ≥100 mg/dl, insulin >25 MIU/ml, hemoglobin A1C ≥6.1%, HOMA-IR >4.
Reference 78.
Reference 79.
IQR, interquartile range; HAART, highly active antiretroviral therapy; HOMA-IR, homeostatic model assessment of insulin resistance; LDL, low-density lipoprotein; HDL, high-density lipoprotein; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
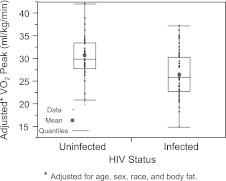
Age, sex, race, percent body fat, and sibling-adjusted fitness outcomes are compared between the HIV-infected and uninfected groups and are shown in Table 3 and graphically depicted in Fig. 1. Mean VO2 peak was lower in the HIV-infected group (25.92 ml/kg/min vs. 30.90 ml/kg/min; p<0.0001). Flexibility and lower body strength ratio were also lower in the HIV-infected group (p=0.0003 and p=0.002, respectively).
Table 3.
Adjusted Physical Fitness Measures by HIV Status
| Physical fitness measure (estimated mean, SE) | HIV-infected (N=45) | Uninfected (N=36) | p-value |
|---|---|---|---|
| VO2 peak (ml/kg/min) | 25.92 (0.79) | 30.90 (0.80) | <0.0001 |
| Flexibility (%) | 23.71 (3.07) | 46.09 (4.71) | 0.0003 |
| Muscular endurance, sit-ups (%) | 13.75 (2.29) | 16.78 (2.11) | 0.39 |
| Upper body strength ratio (1-RM/kg) | 0.51 (0.04) | 0.54 (0.03) | 0.66 |
| Lower body strength ratio (1-RM/kg) | 0.79 (0.07) | 1.10 (0.07) | 0.002 |
Means adjusted by age, sex, race, percent body fat, and siblingship.
RM, repetition maximum.
FIG. 1.
VO2 peak estimated mean by HIV status.
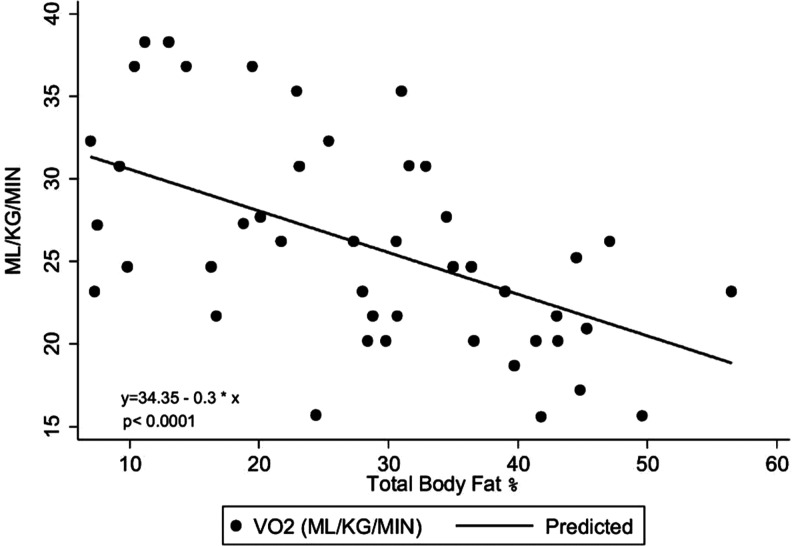
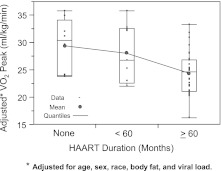
For the HIV-infected group alone, we performed a multivariable analysis to determine factors that were independently associated with VO2 peak (Table 4). Factors that were considered for this analysis included anthropometric, body composition measures, and HIV-specific variables, as previously defined. VO2 peak was negatively associated with percent body fat (a 1% increase in body fat was associated with a decrease of 0.30 ml/kg/min VO2 peak) (p<0.0001) (Fig. 2). Percent body fat was the primary predictor of VO2 peak (partial R2=0.23) accounting for the majority of the total explained variance (full model R2=0.51). Adjusted mean (SE) VO2 peaks were 29.45 (±1.62), 28.70 (±1.87), and 24.09 (±0.75) ml/kg/min for no exposure, <0 months, and ≥60 months of HAART exposure, respectively (p<0.0001). The HAART effect accounted for approximately 12% of the total variation in VO2 peak. Figure 3 gives a distributional comparison for adjusted VO2 peak by duration of HAART therapy. Since the duration of HAART therapy was ordered, the overall effect was tested for trend (assuming equal spacing) using orthogonal polynomials. The linear component accounted for 74% of the total variation attributable to the HAART duration effect (p=0.03). The remaining variation left over for the quadratic term (i.e., lack of fit) was nonsignificant (p=0.42).
Table 4.
Multivariable Determinants of VO2 Peak (ml/kg/min) Among 45 HIV-Infected Children
| |
Multivariable model |
|||
|---|---|---|---|---|
| Variable (units) | Estimate | SE | z-value | p-value |
| Age (years) | −0.43 | 0.27 | −1.60 | 0.11 |
| Sex (male vs. female) | 2.72 | 1.64 | 1.66 | 0.10 |
| Race (black vs. nonblack) | 2.93 | 1.96 | 1.50 | 0.13 |
| Body fat (percent) | −0.30 | 0.06 | −4.69 | <0.0001 |
| HAART durationa (months) | ||||
| Never exposed | ref | ref | ref | ref |
| Exposed <60 months | −0.75 | 2.62 | −0.29 | 0.77 |
| Exposed ≥60 months | −5.37 | 1.71 | −3.13 | 0.002 |
| Log viral load | −0.17 | 0.30 | −0.58 | 0.56 |
NRTI and PI ≥60 months were also associated with lower VO2 peak [an estimate of 6.8 ml/kg/min decrease in VO2 peak for NRTI; p=0.0009 (compared to never exposed) and 5.2 ml/kg/min decrease in VO2 peak for PI; p=0.002 (compared to never exposed)]. However, including the individual classes of ARV in the model was found to be collinear with HAART. Furthermore, the overall effect in VO2 peak was higher when HAART was considered.
Other covariates considered for the model included CD4 percent, metabolic laboratories, and other ARV classes.
HAART, highly active antiretroviral therapy.
FIG. 2.
Relationship between VO2 peak and percent fat among 45 HIV-infected children.
FIG. 3.
VO2 peak estimated mean for HIV-infected children by category of highly active antiretroviral therapy (HAART) exposure.
Although additional information was available for NRTI, NNRTI, and PI therapy duration, including the individual classes of ARVs in the statistical model created a high degree of multicollinearity with HAART (i.e., whole part relationships). Furthermore, the overall effect on VO2 peak was higher when HAART was considered since HAART effectively constitutes the principal component of the other ARV therapies.
For the dependent variable VO2 peak, over the 1000 bootstrapped stepwise regressions, HIV status and HAART entered and remained in their respective multiple regression models over 97% of the time. When 10% of the subjects were dropped based on their degree of influence on the estimates of the multiple regression models, the results (i.e., p-values, partial correlations, adjusted means) remained virtually unchanged. Further examination of the residuals indicated a normal distribution with constant variance across all levels of the predicted values.
Discussion
Cardiorespiratory fitness is one of the most widely used measures of fitness and is a measure of overall physical health. Our study shows that medically stable HIV-infected children have lower cardiorespiratory fitness, flexibility, and lower extremity strength than a contemporary cohort of healthy children. Higher body fat percentage and prolonged exposure to HAART were independent clinical factors associated with lower VO2 peak in HIV-infected children. While decreased VO2 peak in HIV-infected children is consistent with other studies of cardiovascular fitness in HIV-positive adolescents and adults,36–38 decrements in cardiorespiratory fitness as related to HAART exposure and body composition have not been reported previously.
Cardiovascular disease is now the leading cause of mortality for HIV-infected adults in developed nations39 with ARVs associated with twice the risk of cardiovascular disease when compared to treatment-naive patients.40 HIV-infected children are at similar risk with studies showing higher rates of cardiovascular disease risk factors among HIV-infected children compared to controls.19,41,42 Low physical functioning may either contribute to cardiovascular risk43,44 or be a result of it.45 In our study, HIV-infected children who had metabolic abnormalities including higher hemoglobin A1C, triglycerides, and insulin were associated with lower VO2 peak, although these associations did not remain significant when other factors, such as body fat and ARV exposures, were accounted for (data not shown). Physical activity interventions that increase VO2 peak can improve some of these cardiovascular disease risk factors in HIV-infected patients46,47 and can be an important adjunct therapy with medications.48
We found that higher body fat is independently associated with lower physical functioning. This is not an unexpected finding.49 However, HIV-infected children are at particular risk for changes in body fat distribution and trunk adiposity when compared to their non-HIV-infected peers.19,50,51 This body fat distribution, in turn, is associated with cardiovascular disease risk 52,53 as well as some antiretroviral therapies.50 HIV-infected children and young adults also have an increased prevalence of overall obesity54,55 that follows contemporary patterns.56 The extent to which low physical capacity and fitness contribute to higher levels of adiposity or are a result of it cannot be determined by our analyses. Since sedentary activities such as television viewing and video games are correlated with obesity,57 decreased physical activity,58 and lower exercise capacity,59 interventions that modify these habits could alter the trajectory that HIV-infected children are following, which is similar to their noninfected peers.
Duration of HAART was independently associated with lower exercise capacity in HIV-infected children. We also found that greater exposures to NRTIs and PIs affected VO2 peak. These exposures were highly correlated with each other and with HAART. We found the best overall predictor of lower VO2 peak was HAART exposure. Antiretroviral agents impact mitochondrial function through a number of different mechanisms.60–65 NRTIs cause mtDNA polymerase-γ inhibition and/or mtRNA depletion, which lead to impaired OXPHOS, altered ATP levels, cell apoptosis, and peripheral subcutaneous lipoatrophy with or without visceral fat accumulation. Protease inhibitors lead to inhibition of Glut4,66 insulin resistance,67 inhibition of transcription factors,68 cellular apoptosis,65 lipoprotein lipase inhibition,68,69 and decreased adipocyte differentiation.70 NNRTIs such as efavirenz can suppress lipogenic pathways of adipocytes in vitro.71 Since mitochondria are the major source of energy for skeletal muscle, mitochondrial dysfunction can affect exercise performance in other disorders.72–75 Some reports show this association in HIV-infected adults,76 although other studies have not found this to be true.77 We did not measure lactate levels that might indicate mitochondrial dysfunction. Since HAART exposure includes simultaneous exposures to multiple drugs from different classes, it is difficult to ascertain the specific effects from each class or specific agent in our study. Larger studies may be able to explore this.
This study was primarily a convenience sample and the HIV cohort was not strictly matched with controls. Although the results were adjusted for age, sex, and race, a priori matching of controls to HIV-infected participants on these characteristics may yield different results. The results are specific to the children in one center, however, the demographic profile of HIV-infected children is often consistent across centers, with greater than 50% being black, non-Hispanic. This study cannot define causality because of its cross-sectional design. We have tried to account for clinical factors associated with the outcomes, but it is possible that there are other factors, such as poor general health or sedentary lifestyle, that influence the outcomes and may influence our reported associations.78,79 The results presented above show findings should be investigated in larger studies.
In summary, we evaluated the relationship between HIV infection and physical fitness in children and found that HIV-infected children have lower VO2 peak, body strength, and flexibility. These findings provide evidence that children with HIV infection are less physically fit than healthy peers. Factors associated with decreased VO2 peak in HIV-infected children include increased total body fat percentage and greater duration of HAART. HAART may induce muscle and metabolic abnormalities that contribute to decreased exercise performance. Physical activity interventions may improve fitness and potentially decrease cardiovascular risk in HIV-infected children and should be considered as part of medical management.
Acknowledgments
This work was supported by grants from the National Institute of Health, National Heart, Lung and Blood Institute (1RO1HL095127). Partial funding for laboratory and imaging work as well as assistance with general study coordination was provided by the University of Washington's CVD and Metabolic Complications of HIV/AIDS Data Coordinating Center (5R01HL095126).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Lakka TA. Laaksonen DE. Physical activity in prevention and treatment of the metabolic syndrome. Appl Physiol Nutr Metab. 2007;32:76–88. doi: 10.1139/h06-113. [DOI] [PubMed] [Google Scholar]
- 2.Steele RM. Brage S. Corder K. Wareham NJ. Ekelund U. Physical activity, cardiorespiratory fitness, and the metabolic syndrome in youth. J Appl Physiol. 2008;105:342–351. doi: 10.1152/japplphysiol.00072.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Brussel M. van der Net J. Hulzebos E. Helders PJ. Takken T. The Utrecht approach to exercise in chronic childhood conditions: The decade in review. Pediatr Phys Ther. 2011;23:2–14. doi: 10.1097/PEP.0b013e318208cb22. [DOI] [PubMed] [Google Scholar]
- 4.Pianosi P. Leblanc J. Almudevar A. Peak oxygen uptake and mortality in children with cystic fibrosis. Thorax. 2005;60:50–54. doi: 10.1136/thx.2003.008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis JA. McBride MG. Chrisant MR. Patil SM. Hanna BD. Paridon SM. Longitudinal assessment of cardiovascular exercise performance after pediatric heart transplantation. J Heart Lung Transplant. 2006;25:626–633. doi: 10.1016/j.healun.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Majnemer A. Shevell M. Law M, et al. Participation and enjoyment of leisure activities in school-aged children with cerebral palsy. Dev Med Child Neurol. 2008;50:751–758. doi: 10.1111/j.1469-8749.2008.03068.x. [DOI] [PubMed] [Google Scholar]
- 7.Clark NM. Feldman CH. Evans D, et al. Managing better: Children, parents, and asthma. Patient Educ Couns. 1986;8:27–38. doi: 10.1016/0738-3991(86)90024-8. [DOI] [PubMed] [Google Scholar]
- 8.Herman KM. Craig CL. Gauvin L. Katzmarzyk PT. Tracking of obesity and physical activity from childhood to adulthood: The Physical Activity Longitudinal Study. Int J Pediatr Obes. 2009;4:281–288. doi: 10.3109/17477160802596171. [DOI] [PubMed] [Google Scholar]
- 9.Position AHAS Exercise (Physical Activity) and Children. American Heart Association. http://www.americanheart.org/print_presenter.jhtml?identifier=4596. [Jan 15;2012 ]. http://www.americanheart.org/print_presenter.jhtml?identifier=4596
- 10.Froberg K. Andersen LB. Mini review: physical activity and fitness and its relations to cardiovascular disease risk factors in children. Int J Obes (Lond) 2005;29(Suppl 2):S34–39. doi: 10.1038/sj.ijo.0803096. [DOI] [PubMed] [Google Scholar]
- 11.Jarfelt M. Kujacic V. Holmgren D. Bjarnason R. Lannering B. Exercise echocardiography reveals subclinical cardiac dysfunction in young adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49:835–840. doi: 10.1002/pbc.21289. [DOI] [PubMed] [Google Scholar]
- 12.Adamsen L. Quist M. Midtgaard J, et al. The effect of a multidimensional exercise intervention on physical capacity, well-being and quality of life in cancer patients undergoing chemotherapy. Support Care Cancer. 2006;14:116–127. doi: 10.1007/s00520-005-0864-x. [DOI] [PubMed] [Google Scholar]
- 13.De Caro E. Fioredda F. Calevo MG, et al. Exercise capacity in apparently healthy survivors of cancer. Arch Dis Child. 2006;91:47–51. doi: 10.1136/adc.2004.071241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams MJ. Lipsitz SR. Colan SD, et al. Cardiovascular status in long-term survivors of Hodgkin's disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–3148. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 15.Black P. Gutjahr P. Stopfkuchen H. Physical performance in long-term survivors of acute leukaemia in childhood. Eur J Pediatr. 1998;157:464–467. doi: 10.1007/s004310050854. [DOI] [PubMed] [Google Scholar]
- 16.Miller TL. Milton-Miller A. Lopez-Mitnik G, et al. Exercise capacity in long-term survivors of pediatric cancer. American Society of Clinical Oncology Annual Meeting; Orlando, FL. Jun;2009 ; 2009. (submitted 1/2009, Abstract Control #: 35354, Permanent Abstract ID #: 10026, Accepted as a Discussion Poster in the Pediatric Cancer session on 6/1/09 from 8 to 12 with slide presentation order #13 from 11 to 12 on Level 3, W304E, Poster Board #R14, Level 2 West Hall C). [Google Scholar]
- 17.Somarriba G. Extein J. Miller TL. Exercise rehabilitation in pediatric cardiomyopathy. Prog Pediatr Cardiol. 2008;25:91–102. doi: 10.1016/j.ppedcard.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hager A. Hess J. Comparison of health related quality of life with cardiopulmonary exercise testing in adolescents and adults with congenital heart disease. Heart. 2005;91:517–520. doi: 10.1136/hrt.2003.032722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller TL. Orav EJ. Lipshultz SE, et al. Risk factors for cardiovascular disease in children infected with human immunodeficiency virus-1. J Pediatr. 2008;153:491–497. doi: 10.1016/j.jpeds.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma TS. Messiah S. Fisher S. Miller TL. Lipshultz SE. Accelerated cardiovascular disease and myocardial infarction risk in patients with the human immunodeficiency virus. J Cardiometab Syndr. 2008;3:93–97. doi: 10.1111/j.1559-4572.2008.07635.x. [DOI] [PubMed] [Google Scholar]
- 21.McDonald CL. Kaltman JR. Cardiovascular disease in adult and pediatric HIV/AIDS. J Am Coll Cardiol. 2009;54:1185–1188. doi: 10.1016/j.jacc.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crain MJ. Chernoff MC. Oleske JM, et al. Possible mitochondrial dysfunction and its association with antiretroviral therapy use in children perinatally infected with HIV. J Infect Dis. 2010;202:291–301. doi: 10.1086/653497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prevention. CfDCa. CDC table for calculated body mass index values for selected heights and weights for ages 2 to 20 years. http://www.cdc.gov/nccdphp/dnpa/healthyweight/assessing/bmi/00binaries/bmi-tables.pdf. [Feb 5;2012 ]. http://www.cdc.gov/nccdphp/dnpa/healthyweight/assessing/bmi/00binaries/bmi-tables.pdf
- 24.Koo WW. Hammami M. Hockman EM. Validation of bone mass and body composition measurements in small subjects with pencil beam dual energy X-ray absorptiometry. J Am Coll Nutr. 2004;23:79–84. doi: 10.1080/07315724.2004.10719346. [DOI] [PubMed] [Google Scholar]
- 25.Margulies L. Horlick M. Thornton JC. Wang J. Ioannidou E. Heymsfield SB. Reproducibility of pediatric whole body bone and body composition measures by dual-energy X-ray absorptiometry using the GE Lunar Prodigy. J Clin Densitom. 2005;8:298–304. doi: 10.1385/jcd:8:3:298. [DOI] [PubMed] [Google Scholar]
- 26.National Health Nutrition Examination Survey III Body Measurements (Anthropometry) http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/anthro.pdf. [Feb 1;2012 ]. http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/anthro.pdf
- 27.Matthews DR. Hosker JP. Rudenski AS, et al. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 28.Caldwell MB. Oxtoby MJ. Simonds RJ. Revised classification system for human immunodeficiency virus infection in children less than 13 years of age. MMWR CDC Surveill Summ. 19941994:1–12. [Google Scholar]
- 29.Balke B. Ware RW. An experimental study of physical fitness of Air Force personnel. US Armed Forces Med J. 1959;10:675–688. [PubMed] [Google Scholar]
- 30.Froelicher VF., Jr Lancaster MC. The prediction of maximal oxygen consumption from a continuous exercise treadmill protocol. Am Heart J. 1974;87:445–450. doi: 10.1016/0002-8703(74)90169-0. [DOI] [PubMed] [Google Scholar]
- 31.Medicine ACoS. ACSM's Guidelines for Exercise Testing and Prescription. 7th. 2006. [DOI] [PubMed]
- 32.Mayhew JL. Ball TE. Bowen JC. Relative muscular endurance performance as a predictor of bench press strength in college men and women. Sports Med Training Rehabil. 1992;3:195–201. [Google Scholar]
- 33.Castro-Pinero J. Chillon P. Ortega FB. Montesinos JL. Sjostrom M. Ruiz JR. Criterion-related validity of sit-and-reach and modified sit-and-reach test for estimating hamstring flexibility in children and adolescents aged 6–17 years. Int J Sports Med. 2009;30:658–662. doi: 10.1055/s-0029-1224175. [DOI] [PubMed] [Google Scholar]
- 34.Sports PsCoPFa: 1985 National School Population Fitness Survey. Public Health Service. Office of the Assistant Secretary for Health; Washington, DC: 1986. US Department of Health and Human Services. [Google Scholar]
- 35.Diaconis P. Efron B. Computer-intensive methods in statistics. Sci Am May. 1983:116–130. [Google Scholar]
- 36.Miller TL. Somarriba G. Kinnamon DD. Weinberg GA. Friedman LB. Scott GB. The effect of a structured exercise program on nutrition and fitness outcomes in human immunodeficiency virus-infected children. AIDS Res Hum Retroviruses. 2010;26:313–319. doi: 10.1089/aid.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dolan SE. Frontera W. Librizzi J, et al. Effects of a supervised home-based aerobic and progressive resistance training regimen in women infected with human immunodeficiency virus: A randomized trial. Arch Intern Med. 2006;166:1225–1231. doi: 10.1001/archinte.166.11.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cade WT. Peralta L. Keyser RE. Aerobic capacity in late adolescents infected with HIV and controls. Pediatr Rehabil. 2002;5:161–169. doi: 10.1080/1363849021000039362. [DOI] [PubMed] [Google Scholar]
- 39.Grinspoon SK. Grunfeld C. Kotler DP, et al. State of the science conference: Initiative to decrease cardiovascular risk and increase quality of care for patients living with HIV/AIDS: Executive summary. Circulation. 2008;118:198–210. doi: 10.1161/CIRCULATIONAHA.107.189622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein D. Hurley LB. Quesenberry CP., Jr Sidney S. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? J Acquir Immune Defic Syndr. 2002;30:471–477. doi: 10.1097/00126334-200208150-00002. [DOI] [PubMed] [Google Scholar]
- 41.Miller TL. Grant YT. Almeida DN. Sharma T. Lipshultz SE. Cardiometabolic disease in human immunodeficiency virus-infected children. J Cardiometab Syndr. 2008;3:98–105. doi: 10.1111/j.1559-4572.2008.07651.x. [DOI] [PubMed] [Google Scholar]
- 42.Rhoads MP. Lanigan J. Smith CJ. Lyall EG. Effect of specific ART drugs on lipid changes and the need for lipid management in children with HIV. J Acquir Immune Defic Syndr. 2011;57:404–412. doi: 10.1097/QAI.0b013e31821d33be. [DOI] [PubMed] [Google Scholar]
- 43.Lobelo F. Pate RR. Dowda M. Liese AD. Daniels SR. Cardiorespiratory fitness and clustered cardiovascular disease risk in U.S. adolescents. J Adolesc Health. 2010;47:352–359. doi: 10.1016/j.jadohealth.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 44.Tanha T. Wollmer P. Thorsson O, et al. Lack of physical activity in young children is related to higher composite risk factor score for cardiovascular disease. Acta Paediatr. 2011;100:717–721. doi: 10.1111/j.1651-2227.2011.02226.x. [DOI] [PubMed] [Google Scholar]
- 45.Berry JD. Willis B. Gupta S, et al. Lifetime risks for cardiovascular disease mortality by cardiorespiratory fitness levels measured at ages 45, 55, and 65 years in men. The Cooper Center Longitudinal Study. J Am Coll Cardiol. 2011;57:1604–1610. doi: 10.1016/j.jacc.2010.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogalha C. Luz E. Sampaio E, et al. A randomized, clinical trial to evaluate the impact of regular physical activity on the quality of life, body morphology and metabolic parameters of patients with AIDS in Salvador, Brazil. J Acquir Immune Defic Syndr. 2011;57(Suppl 3):S179–185. doi: 10.1097/QAI.0b013e31821e9bca. [DOI] [PubMed] [Google Scholar]
- 47.Fillipas S. Cherry CL. Cicuttini F. Smirneos L. Holland AE. The effects of exercise training on metabolic and morphological outcomes for people living with HIV: A systematic review of randomised controlled trials. HIV Clin Trials. 2010;11:270–282. doi: 10.1310/hct1105-270. [DOI] [PubMed] [Google Scholar]
- 48.Yarasheski KE. Cade WT. Overton ET, et al. Exercise training augments the peripheral insulin-sensitizing effects of pioglitazone in HIV-infected adults with insulin resistance and central adiposity. Am J Physiol Endocrinol Metab. 2011;300:E243–251. doi: 10.1152/ajpendo.00468.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raudsepp L. Pall P. Physical growth and fatness as related to physical activity in preadolescent girls. Coll Anthropol. 1999;23:53–58. [PubMed] [Google Scholar]
- 50.Arpadi SM. Bethel J. Horlick M, et al. Longitudinal changes in regional fat content in HIV-infected children and adolescents. AIDS. 2009;23:1501–1509. doi: 10.1097/QAD.0b013e32832b7e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacobson DL. Patel K. Siberry GK, et al. Body fat distribution in perinatally HIV-infected and HIV-exposed but uninfected children in the era of highly active antiretroviral therapy: Outcomes from the Pediatric HIV/AIDS Cohort Study (PHACS) Am J Clin Nutr. 2011;94:1485–1495. doi: 10.3945/ajcn.111.020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mokha JS. Srinivasan SR. Dasmahapatra P, et al. Utility of waist-to-height ratio in assessing the status of central obesity, related cardiometabolic risk profile among normal weight, overweight/obese children: The Bogalusa Heart Study. BMC Pediatr. 2010:11. doi: 10.1186/1471-2431-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lake JE. Wohl D. Scherzer R, et al. Regional fat deposition and cardiovascular risk in HIV infection: The FRAM study. AIDS Care. 2011;23:929–938. doi: 10.1080/09540121.2010.543885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mulligan K. Harris DR. Monte D, et al. Obesity and dyslipidemia in behaviorally HIV-infected young women: Adolescent Trials Network study 021. Clin Infect Dis. 2010;50:106–114. doi: 10.1086/648728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hendricks KM. Willis K. Houser R. Jones CY. Obesity in HIV-infection: Dietary correlates. J Am Coll Nutr. 2006;25:321–331. doi: 10.1080/07315724.2006.10719542. [DOI] [PubMed] [Google Scholar]
- 56.Skelton JA. Cook SR. Auinger P. Klein JD. Barlow SE. Prevalence and trends of severe obesity among US children and adolescents. Acad Pediatr. 2009;9:322–329. doi: 10.1016/j.acap.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Must A. Bandini LG. Tybor DJ. Phillips SM. Naumova EN. Dietz WH. Activity, inactivity, and screen time in relation to weight and fatness over adolescence in girls. Obesity. 2007;15:1774–1781. doi: 10.1038/oby.2007.211. [DOI] [PubMed] [Google Scholar]
- 58.Hager RL. Television viewing and physical activity in children. J Adolesc Health. 2006;39:656–661. doi: 10.1016/j.jadohealth.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 59.Tremblay MS. Leblanc AG. Kho ME, et al. Systematic review of sedentary behaviour and health indicators in school-aged children and youth. Int J Behav Nutr Phys Activity. 2011;8:98. doi: 10.1186/1479-5868-8-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mynarcik D. Wei LX. Komaroff E. Ferris R. McNurlan M. Gelato M. Chronic loss of subcutaneous adipose tissue in HIV-associated lipodystrophy may not be associated with accelerated apoptosis. J Acquir Immune Defic Syndr. 2005;38:367–371. [PubMed] [Google Scholar]
- 61.McComsey GA. Paulsen DM. Lonergan JT, et al. Improvements in lipoatrophy, mitochondrial DNA levels and fat apoptosis after replacing stavudine with abacavir or zidovudine. AIDS. 2005;19:15–23. doi: 10.1097/00002030-200501030-00002. [DOI] [PubMed] [Google Scholar]
- 62.Vernochet C. Azoulay S. Duval D. Guedj R. Ailhaud G. Dani C. Differential effect of HIV protease inhibitors on adipogenesis: Intracellular ritonavir is not sufficient to inhibit differentiation. AIDS. 2003;17:2177–2180. doi: 10.1097/01.aids.0000088160.01779.2b. [DOI] [PubMed] [Google Scholar]
- 63.Walker UA. Setzer B. Venhoff N. Increased long-term mitochondrial toxicity in combinations of nucleoside analogue reverse-transcriptase inhibitors. AIDS. 2002;16:2165–2173. doi: 10.1097/00002030-200211080-00009. [DOI] [PubMed] [Google Scholar]
- 64.Carr A. Miller J. Law M. Cooper DA. A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy: Contribution to protease inhibitor-related lipodystrophy syndrome. AIDS. 2000;14:F25–32. doi: 10.1097/00002030-200002180-00001. [DOI] [PubMed] [Google Scholar]
- 65.Domingo P. Matias-Guiu X. Pujol RM, et al. Subcutaneous adipocyte apoptosis in HIV-1 protease inhibitor-associated lipodystrophy. AIDS. 1999;13:2261–2267. doi: 10.1097/00002030-199911120-00008. [DOI] [PubMed] [Google Scholar]
- 66.Hruz PW. Yan Q. Struthers H. Jay PY. HIV protease inhibitors that block GLUT4 precipitate acute, decompensated heart failure in a mouse model of dilated cardiomyopathy. FASEB J. 2008;22:2161–2167. doi: 10.1096/fj.07-102269. [DOI] [PubMed] [Google Scholar]
- 67.Dube MP. Parker RA. Tebas P, et al. Glucose metabolism, lipid, and body fat changes in antiretroviral-naive subjects randomized to nelfinavir or efavirenz plus dual nucleosides. AIDS. 2005;19:1807–1818. doi: 10.1097/01.aids.0000183629.20041.bb. [DOI] [PubMed] [Google Scholar]
- 68.Mallon PW. Unemori P. Sedwell R, et al. In vivo, nucleoside reverse-transcriptase inhibitors alter expression of both mitochondrial and lipid metabolism genes in the absence of depletion of mitochondrial DNA. J Infect Dis. 2005;191:1686–1696. doi: 10.1086/429697. [DOI] [PubMed] [Google Scholar]
- 69.Bastard JP. Caron M. Vidal H, et al. Association between altered expression of adipogenic factor SREBP1 in lipoatrophic adipose tissue from HIV-1-infected patients and abnormal adipocyte differentiation and insulin resistance. Lancet. 2002;359:1026–1031. doi: 10.1016/S0140-6736(02)08094-7. [DOI] [PubMed] [Google Scholar]
- 70.Caron M. Auclair M. Sterlingot H. Kornprobst M. Capeau J. Some HIV protease inhibitors alter lamin A/C maturation and stability, SREBP-1 nuclear localization and adipocyte differentiation. AIDS. 2003;17:2437–2444. doi: 10.1097/00002030-200311210-00005. [DOI] [PubMed] [Google Scholar]
- 71.Rodriguez de la Concepcion ML. Yubero P. Domingo JC, et al. Reverse transcriptase inhibitors alter uncoupling protein-1 and mitochondrial biogenesis in brown adipocytes. Antivir Ther. 2005;10:515–526. [PubMed] [Google Scholar]
- 72.Schmiedel J. Jackson S. Schafer J. Reichmann H. Mitochondrial cytopathies. J Neurol. 2003;250:267–277. doi: 10.1007/s00415-003-0978-3. [DOI] [PubMed] [Google Scholar]
- 73.Elliot DL. Buist NR. Goldberg L. Kennaway NG. Powell BR. Kuehl KS. Metabolic myopathies: Evaluation by graded exercise testing. Medicine (Baltimore) 1989;68:163–172. [PubMed] [Google Scholar]
- 74.Taivassalo T. Jensen TD. Kennaway N. DiMauro S. Vissing J. Haller RG. The spectrum of exercise tolerance in mitochondrial myopathies: A study of 40 patients. Brain. 2003;126:413–423. doi: 10.1093/brain/awg028. [DOI] [PubMed] [Google Scholar]
- 75.Flaherty KR. Wald J. Weisman IM, et al. Unexplained exertional limitation: Characterization of patients with a mitochondrial myopathy. Am J Respir Crit Care Med. 2001;164:425–432. doi: 10.1164/ajrccm.164.3.2005110. [DOI] [PubMed] [Google Scholar]
- 76.Duong M. Dumas JP. Buisson M, et al. Limitation of exercise capacity in nucleoside-treated HIV-infected patients with hyperlactataemia. HIV Med. 2007;8:105–111. doi: 10.1111/j.1468-1293.2007.00439.x. [DOI] [PubMed] [Google Scholar]
- 77.Roge BT. Calbet JA. Moller K, et al. Skeletal muscle mitochondrial function and exercise capacity in HIV-infected patients with lipodystrophy and elevated p-lactate levels. AIDS. 2002;16:973–982. doi: 10.1097/00002030-200205030-00003. [DOI] [PubMed] [Google Scholar]
- 78.Hickman TB. Briefel RR. Carroll MD, et al. Distributions and trends of serum lipid levels among United States children and adolescents ages 4–19 years: Data from the Third National Health and Nutrition Examination Survey. Prev Med. 1998;27:879–890. doi: 10.1006/pmed.1998.0376. [DOI] [PubMed] [Google Scholar]
- 79.Valerio G. Licenziati MR. Iannuzzi A, et al. Insulin resistance and impaired glucose tolerance in obese children and adolescents from Southern Italy. Nutr Metab Cardiovasc Dis. 2006;16:279–284. doi: 10.1016/j.numecd.2005.12.007. [DOI] [PubMed] [Google Scholar]