Abstract
HK-2 human renal proximal tubule cells (RPTC) are commonly used in the in vitro study of “normal” RPTCs. We recently discovered that HK-2 cells are uncoupled from dopamine-1 receptor (D1R) adenylyl cyclase (AC) stimulation. We hypothesized that G protein coupled receptor kinase type 4 (GRK4) single nucleotide polymorphisms (SNPs) may be responsible for the D1R/AC uncoupling in HK-2. This hypothesis was tested by genotyping GRK4 SNPs, measuring D1-like receptor agonist (fenoldopam)stimulated cAMP accumulation, quantifying D1R inhibition of sodium transport, and testing the ability of GRK4 siRNA to reverse the D1R/AC uncoupling. We compared HK-2 to 2 normally coupled human RPTC cell lines (nRPTC) and 2 uncoupled RPTC cell lines (uRPTC). The HK-2 cell line was found to have 4 out of 6 potential GRK4 SNPs known to uncouple the D1R from AC (namely R65L, A142V, and A486V). AC response to fenoldopam stimulation was increased in the two nRPTC cell lines (FEN 2.02±0.05-fold and 2.33±0.19-fold over control, P<0.001, N=4), but not in the two uncoupled or HK-2 cell lines. GRK4 siRNA rescued the fenoldopam-mediated AC stimulation in the uncoupled cells, including HK-2. The expected fenoldopam -mediated inhibition of sodium hydrogen exchanger type 3 was absent in HK-2 (N=6) and uRPTCs (N=6), but was observed in the two nRPTCs (−25.41±4.7% and −27.36±2.70% (P<0.001, N=6)), which express wild-type GRK4. Despite the fact that HK-2 cells retain many functional characteristics of RPTCs, they are not normal from the perspective of dopaminergic function.
Keywords: HK-2, renal proximal tubule, dopamine receptors, GRK4, NHE3, FRET, NaKATPase
Introduction
The HK-2 human renal proximal tubule cell (RPTC) has been the subject of research interest since it was first isolated and cultured in 1984,1 with over 170 publications using this cell line listed in PubMed. The relevance of using RPTCs of human origin to study human physiology and pathophysiology over using those derived from non-human sources, such as the MDCK (dog), LLC-PK (pig), LLC-RK1 (rabbit) and OK (opossum) can not be overemphasized. Initially, primary RPTC lines were used to study renal cellular physiology since they retained many of their in vivo characteristics in vitro.2 However, primary cell lines cannot be sustained in long-term culture, which makes inter-assay comparisons difficult.1, 3 Therefore, transformed cell lines were generated using immortalizing virus (e.g. SV40, human papilloma virus (HPV)) which extended their growth potential beyond the 8–15 passage limit of primary cells.4–5 Transformed RPTCs contain many of the functional and morphological characteristics of RPTCs in primary culture.5–6 There are instances where the HK-2 do not always accurately represent normal human RPTC physiology. For example, when cultured in a 3-dimensional matrix, HK-2 form aggregates or cysts similar to primary cultures of RPTCs from autosomal dominant polycystic kidney disease, as compared to primary cultures of RPTCs from normal kidney which form tubular structures.7
While using HK-2 cells as normal controls for human primary renal proximal tubule cells, we recently discovered that HK-2 cells are uncoupled from dopaminergic stimulation of adenylyl cyclase (AC). Because we have previously reported that the uncoupling of the dopamine-1 receptor (D1R) from AC is due to variants of single nucleotide polymorphisms (SNPs) of G protein-coupled receptor kinase type 4 (GRK4), we hypothesized that the uncoupling of D1R to AC in HK-2 cells may also be caused by GRK4 SNPs.8 This hypothesis was tested in HK-2 cells by genotyping GRK4 SNPs, measuring D1-like receptor agonist (fenoldopam) stimulated AC, quantifying D1R inhibition of sodium transport, and testing the ability of GRK4 siRNA to rescue the D1R/AC coupling. We compared the HK-2 RPTCs with human RPTCs that are normally coupled (nRPTC) or uncoupled (uRPTC) from AC in order to determine if HK-2 is suitable for the study of normal dopaminergic activity and cellular function.
Materials and Methods
(Please see http://hyper.ahajournals.org for Expanded Methods in Online Supplement)
Cell lines
Using previously published methods, we cultured HK-2 cells (ATCC®CRL-2190™), and compared them to 4 immortalized human RPTC lines routinely used in our laboratory (2 normally D1R/AC coupled nRPTC lines i14 and i16, and 2 uncoupled uRPTC lines i2 and i25).9–10 Our cell lines were obtained from IRB-approved normal tissue from nephrectomies in human subjects. Cell culture, characterization and immortalization procedures have been described.4, 8–9, 11–13 Each cell line has been genotyped for 3 single nucleotide polymorphisms (SNPs) to GRK4 (R65L (exon 3), A142V (exon 5), A486V (exon 14)) (Table 1) using established methods.14 In order to obviate the possibility that cell culture conditions were responsible for the aberrant behavior of the HK-2, we cultured HK-2 in keratinocyte-free media as suggested by the supplier, as well as in media used routinely in our laboratory for growing immortalized human renal proximal tubular cells.9, 12 Furthermore, we obtained fresh HK-2 cells from the ATCC (Manassas, VA) and compared them to our stock cells originally obtained from the ATCC several years ago.
Table 1.
Sequencing of Cell Lines for GRK4 SNPs
| Cell Line | R65L (exon 3) | A142 (exon 5) | A486 (exon 14) |
|---|---|---|---|
| HK-2 | HETERO* | HOMO | HETERO |
| i2 (uRPTC) | HOMO† | HOMO | WILD |
| i25 (uRPTC) | HETERO | HETERO | HOMO |
| i14 (nRPTC) | WILD‡ | WILD | WILD |
| i16 (NRPTC) | WILD | WILD | WILD |
HOMO = homozygous variant,
HETERO = heterozygous variant,
WILD = wild-type pmoles cAMP/mg Protein
Immunofluorescent Staining of HK-2 and Human RPTC for Proximal Tubule Characteristics
Markers of proximal tubules used were: Lotus tetragonolobus agglutinin (LTA), γglutamyl transpeptidase (GGT), megalin (MEG), aminopeptidase A (APA), aminopeptidase N (APN / CD13) and villin. Levels of genes expressed in proximal tubule as well as other segments include NHE3 (proximal tubule and thick ascending limb15 and caveolin-1 (proximal tubule, distal tubule, glomerulus and cortical collecting duct).16–17 Markers of cells from other nephron segments which served as negative controls were Tamm-Horsfall protein (THP) 18 and sodium chloride co-transporter (NCC).19 Controls for non-specific secondary antibody binding were performed for each cell type. See the Online Supplement and Table S1 for details about the antibodies and staining.
Determination of cAMP Accumulation
cAMP was measured both by a commercial ELISA (Cayman Chemical) and an intracellular real-time kinetic fluorescence resonance energy transfer (FRET) cAMP sensor (ICUE3) as previously described.10, 20 Agonists used were fenoldopam (FEN, 1 µmol/L, D1-like receptor agonist), and isoproterenol (ISO, 1 µmol/L, beta-adrenergic receptor agonist) in order to compare alternative adenylyl cyclase G protein coupled receptor pathways.
Rescue of cAMP Coupling following Transfection with GRK4 siRNA
100 nmol/L GRK4 siRNA (target sequence 5’ AATACAAAGAGAAAGTCAA 3’) or scrambled control (5’AGAAGATAAGAACAATAAC 3’) was transfected for 24 hours into cells by electroporation along with the ICUE3 cAMP biosensor plasmid. Details of transfection have been previously published. 10
Immunofluorescent Staining for GRK4 following Transfection with GRK4 siRNA
The cell lines were stained for GRK4 after being transfected with GRK4 siRNA or SCR control as stated above (without the ICUE biosensor). Immunofluorescent staining was performed as described above, using the Santa Cruz GRK4 antibody (SC-13079) at a 1:100 dilution. Identical exposures were taken at 100x magnification and quantitated using Slidebook v.4.2 software.
NHE3-mediated Sodium Accumulation Assay
NHE3 activity as a function of sodium accumulation was measured with modifications of our previous method.21 Details of our current method are in the Online Supplement. Briefly, cells were loaded with a sodium ion indicator, sodium benzofuran isophthalate (SBFI), and treated for 30 minutes with combinations of the following drugs: ouabain (OUB, 100 µmol/L, NaKATPase inhibitor), fenoldopam (FEN, 1 µmol/L, D1-like receptor agonist), LE300 (10 µmol/L, D1-like receptor antagonist), EIPA (10 µmol/L, NHE inhibitor), S3226 (10 µmol/L, NHE3 inhibitor) 22–23 and cariporide (HOE-642, 10 µmol/L, NHE1 inhibitor).24 The specificity of the assay was verified by the positive inhibition seen with S3226 (NHE3 selective) and negative inhibition seen with cariporide (NHE1 selective). Time-lapse ratiometric images were acquired every three minutes. Each intracellular sodium measurement (mmol/L) is derived from the emission at 510 nm when excited at 340 or 380 nm. Internal calibration of sodium concentration was performed according to the manufacturer’s instructions in Slidebook™ version 4.2, using calibration buffers described previously.21
NaKATPase-mediated Sodium Efflux Assay
The detailed method used to measure the rate of sodium efflux was perfomed simultaneously on the five cell lines as previously published.10 In brief, cells were cultured in glass bottom collagen-coated Matrical plates (Spokane, WA) and were serum-starved overnight prior to loading with a sodium ion indicator, sodium benzofuran isophthalate (SBFI, 5µM, Molecular Probes, OR). After a 30 minute recovery, cells were washed and incubated in potassium-free HEPES media (20 mM HEPES pH 7.4, 130 mM NaCl, 1 mM CaCl, 1 mM MgCl) to raise the internal sodium concentration. VEH, FEN, or OUB were added, then directly before imaging, EIPA (10 uM final concentration, a selective inhibitor of the Na+/H+ exchanger 3 (NHE3),) and KCl (2.7 mM final concentration) were added to all wells as 10X stock. Changes in intracellular sodium concentration were measured by live multiwell ratiometric fluorescence imaging of SBFI and internally calibrated using the ratio imaging module of Slidebook™ version 4.2.
Statistical Analysis
The data are expressed as mean ± SE. Comparisons within and among groups were made by repeated measures or factorial ANOVA, respectively, followed by Holm-Sidak or Duncan’s test. T-test was used for two-group comparisons. P values of <0.05 were considered significant.
Results
Our RPTC lines, as well as the HK-2 line, were characterized for their renal proximal tubule origin using a series of selective stains for proximal tubular proteins and carbohydrates (online supplement Figure S1). All of the primary and immortalized cell lines showed positive staining for the proximal tubule markers and were definitively negative for the markers for other nephron sites.
The HK-2 cells had virtually non-measurable expression of a proximal tubule specific marker, APN25, a markedly reduced expression of another proximal tubule specific marker, Lotus tetragonolobus agglutinin (LTA)26 and a slightly reduced expression of another proximal tubule specific marker, APA 27, when compared to our cell lines i2, i25, i14, and i16 (Figure S1, top panel).
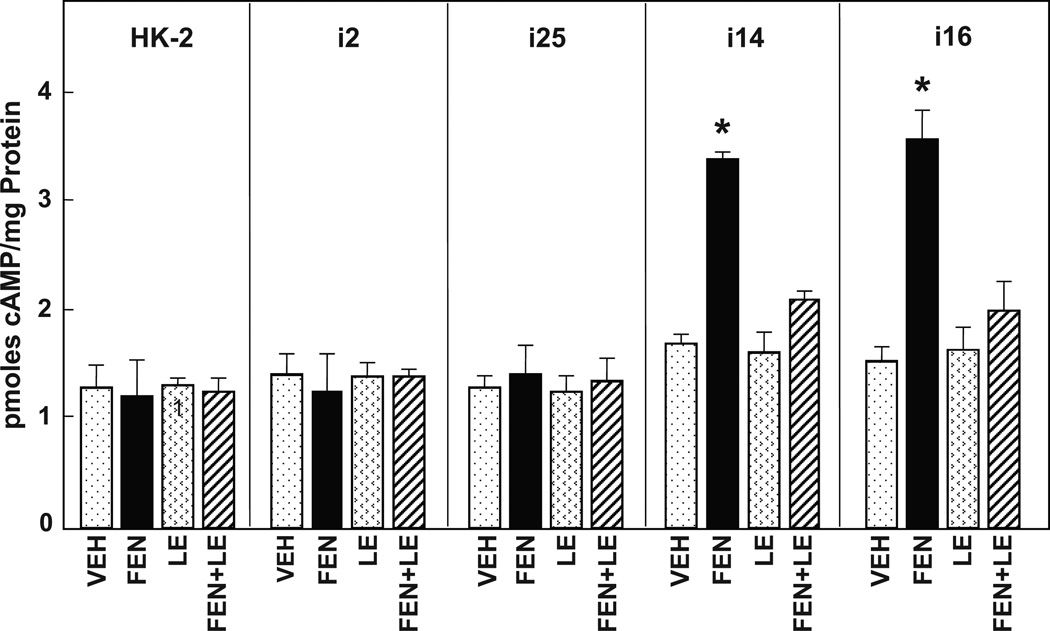
In order to determine if the D1R were normally coupled to AC in HK-2 cells, we employed two methods for measuring intracellular cAMP.10 An established ELISA method to measure cAMP extracted from the cell cytoplasm (Figure 1) was compared to the intracytoplasmic fluorescent resonant energy transfer (FRET)-based sensor (Figure 2). We compared HK-2 with two normally coupled (nRPTC) and two uncoupled (uRPTC) lines, in their response to the dopamine D1-like receptor agonist fenoldopam (FEN, 1 µmol/L, 30 min), or DMSO vehicle (VEH) control. Figure 1 shows that there was no response to FEN stimulation in HK-2, which is similar to that seen in the uncoupled uRPTC lines (i2 and i25). However, there was a greater than 2-fold increase in cAMP accumulation (ELISA) following fenoldopam stimulation in nRPTCs (i14 and i16) (2.02 +/− 0.05 and 2.33 +/− 0.19-fold, respectively; P<0.001, N=4 vs VEH). This effect was blocked by the D1-like receptor antagonist, LE300 (10 µmol/L), indicating that the stimulatory effect was via D1-like receptors. LE-300 had no effect when added alone. The HK-2 cells showed a similar lack of AC stimulation by FEN when measured using a cAMP FRET Biosensor, ICUE3. D1-like stimulation with fenoldopam (FEN, 1 µmol/L, 30 min) caused a significant rise in intracellular cAMP levels in nRPTCs (i14, i16), but not in HK-2 cells or uRPTCs (i2, i25) (Figure 2).
Figure 1. Comparison of fenoldopam-stimulated coupling efficiency to adenylyl cyclase in HK-2 and immortalized human cell lines, uRPTC (i2, i25) and nRPTC (i14, i16).
cAMP accumulation was compared in HK-2, i2, i25, i14, and i16. Innate cAMP accumulation was similar in all cell lines (Vehicle (VEH)-treated cells). The D1-like receptor agonist fenoldopam (FEN, 1 µmol/L, 30 min) stimulated cAMP accumulation in the coupled cell lines i14 and i16 (*P<0.001 vs others, N=6/group) but not in the uncoupled HK-2, i2, or i25 cells. The D1-like receptor antagonist LE300 (10 µmol/L) had no effect alone, but reversed the FEN stimulation observed in i14 and i16.
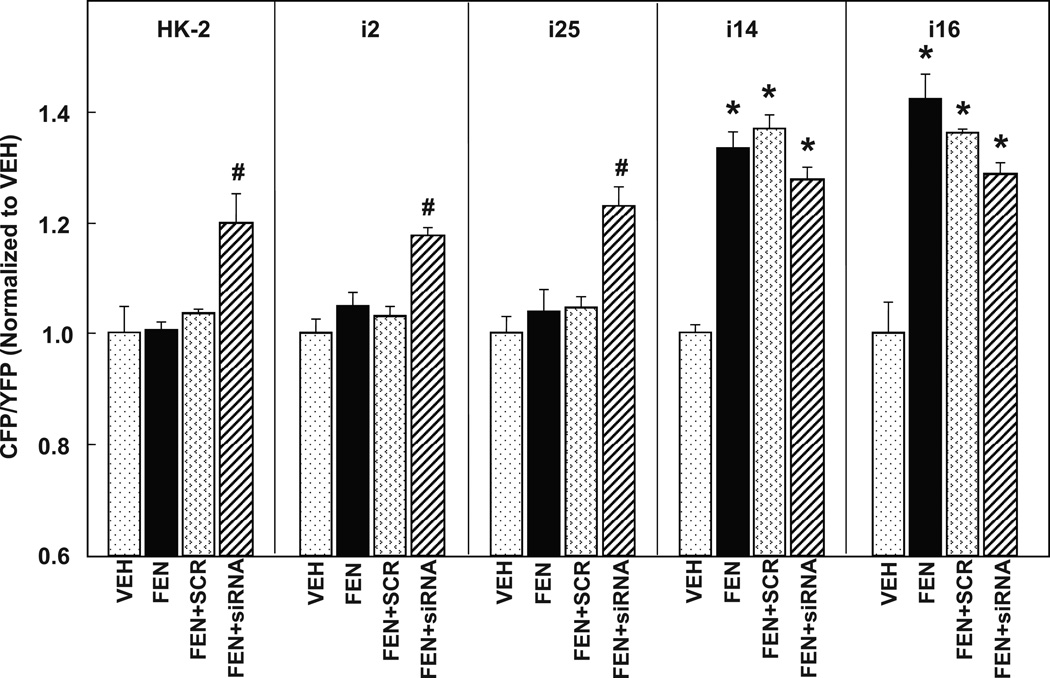
Figure 2. Failure of FEN-stimulated cAMP accumulation in HK-2 cells can be reversed by the silencing of G protein-coupled receptor kinase type 4 (GRK4).
Intracellular cAMP accumulation was measured in real-time using a cAMP FRET Biosensor, ICUE3. Similar to cAMP accumulation demonstrated in Figure 1, FEN (1 µmol/L, 30 min) increased cAMP in i14 and i16 (*P<0.001 vs VEH, N=6/group), but not in HK-2, i2 or i25. Scrambled RNA control (SCR) had no effect on the FEN-stimulated cAMP accumulation in the coupled cell lines, i14 and i16. GRK4 siRNA rescued FEN-stimulated cAMP accumulation in HK-2, i2, and i25 cell lines, although not to control levels (#P<0.05 vs FEN + SCR, N=4/group).
This lack of response to fenoldopam by the uncoupled cells was not due to an intrinsic defect in their Gs coupling, as is shown in the online supplement Figure S2. The beta-adrenergic receptor agonist isoproterenol (ISO, 1 µmol/L, 30 min) significantly increased cAMP accumulation in each of the cell lines (*P<0.001 vs VEH, N=10). In addition, the response of the uRPTCs i2 and i25 was significantly higher than that seen in their normally coupled counterparts, i14 and i16, or the HK-2 (**P<0.01, N=10 vs the other 3 cell lines, P< 0.001 from VEH).
We then investigated the role of GRK4 SNPs in the uncoupling of AC in the HK2, i2 and i25 cell lines by treating the cells with either scrambled (SCR) or siRNA to GRK4 (Figure 2) in addition to fenoldopam. siRNA to GRK4 rescued the uncoupling of AC in the HK-2, i2, and i25 cell lines (P<0.05, N=4 vs FEN + SCR), suggesting that GRK4 SNPs mediated the uncoupling of the D1-like receptor to AC. siRNA slightly decreased the FEN stimulation of AC in nRPTCs (i14 and i16), which is in agreement with our reports that wild-type GRK4 is needed for normal D1R function. 28–29
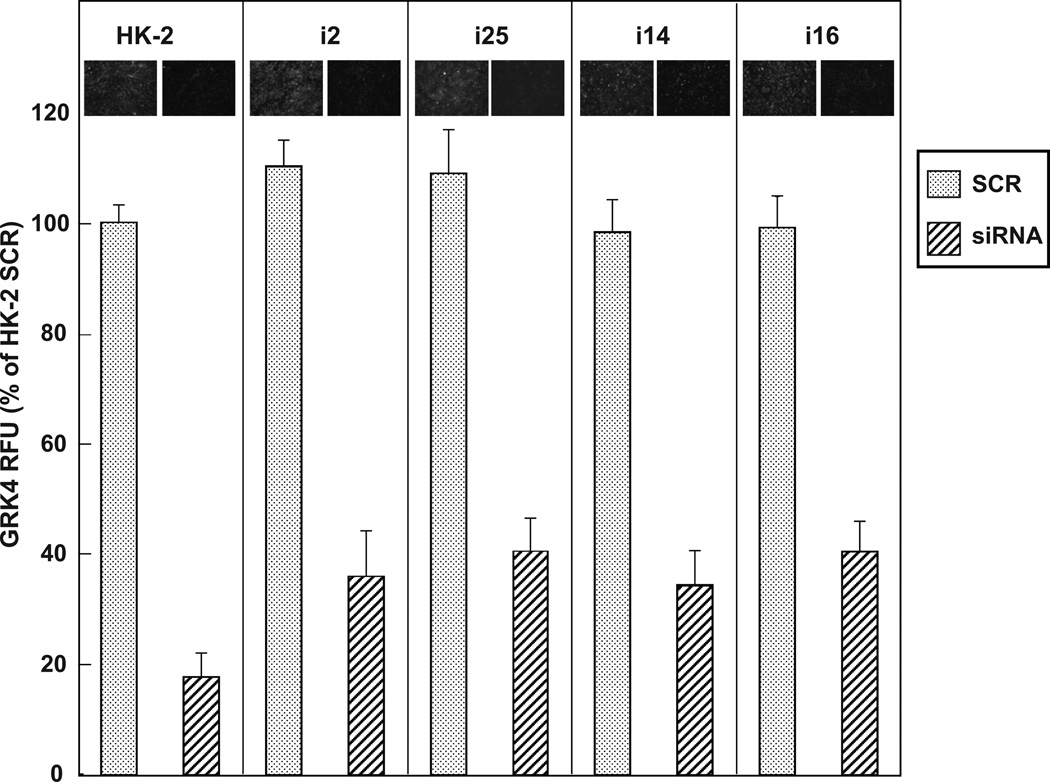
Figure 3 demonstrates that basal levels of GRK4 were similar among the 5 cell lines. GRK4 expression was quantified and equivalent immunoreactive staining was observed in all cell lines. This figure also demonstrates the efficacy of the GRK4 siRNA used in Figure 2. The cell lines showed a 63–83% reduction in GRK4 expression level after they were transfected with 100 nmol/L GRK4 siRNA for 24 hours, compared to the SCR transfected cells. Equivalent expression levels of GRK4 were seen between the SCR control cells and mock transfected cells without siRNA (data not shown).
Figure 3. GRK4 immunofluorescence in HK-2, uRPTC i2 and i25, and nRPTC i14 and i16, that were transfected with GRK4 siRNA or the scrambled control (SCR).
Cells were transfected with 100 nmol/L GRK4 siRNA or SCR and 24 hr later were stained for GRK4. GRK4 siRNA significantly knocked down GRK4 expression in each of the cell lines (P<0.001, N=6). Representative images of GRK4 expression with SCR or GRK4 siRNA are shown above each bar in the graph.
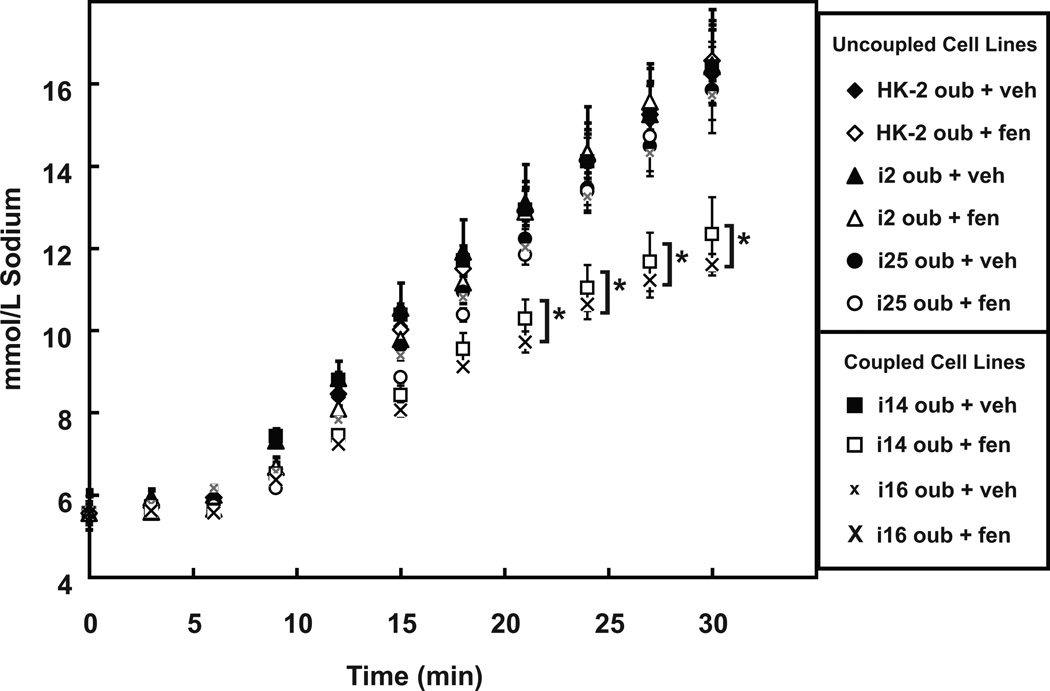
Since a normal action of dopamine is to inhibit sodium transport via NHE3,30 we investigated whether D1R/AC uncoupling decreased intracellular sodium accumulation in these cells. Figure 4 shows the effect of ouabain (OUB, 100 µmol/L) in increasing intracellular sodium concentration by preventing sodium efflux via inhibition of NaKATPase activity. This effect was reduced by FEN (10 µmol/L) in nRPTC lines i14 and i16 (P<.05 vs OUB + VEH, N=6 at the 21-, 24-, 27- and 30-minute time points). However, in the HK-2 cells or uRPTCs i2 and i25, FEN had no effect on the ouabainmediated increase in intracellular sodium. These results are summarized in the online supplement Figure S3, with the OUB + FEN data expressed as a percentage of the OUB + VEH control for each cell line at the 30-minute time point. At this time point, the inhibition in coupled RPTC lines was a 25.41±4.67% decrease for i14 and a 27.36±2.70% decrease for i16 (P<0.001 vs OUB + VEH, N=6).
Figure 4. FEN reduces intracellular sodium accumulation in i14 and i16 but not in HK-2, i2, or i25 cell lines.
Intracellular sodium concentration was determined using the sodium sensitive dye SBFI. The ouabain (OUB, 100 µmol/L plus vehicle, VEH)increased intracellular accumulation, by inhibition of sodium efflux via NaKATPase, was linear over a 30 minute time interval. OUB + FEN (1 µmol/L) reduced Na+ accumulation (*P<0.05, N=6) in only the i14 (□) and i16 (x) coupled cell lines.
Additional controls for the sodium influx assay are provided in the online supplement Figure S4. This figure depicts the change in intracellular sodium over the 30-minute time interval tested, in response to various pharmacological agents by themselves or in combination with OUB. FEN (1 µmol/L), cariporide (HOE, 10 µmol/L) and LE300 (10 µmol/L) by themselves had no effect on sodium influx over VEH alone. OUB (100 µmol/L) increased intracellular sodium while the NHE3 inhibitors EIPA (10 µmol/L) and S2336 (10 µmol/L) by themselves decreased intracellular sodium concentrations, relative to VEH. The combination of OUB + FEN inhibited sodium influx by 32+/−0.48% (P<0.05, N=6) compared to OUB alone. This effect of fenoldopam was reversed by LE300 (OUB + FEN + LE300), confirming the specificity of the FEN effect via D1-like receptors. The addition of either NHE3 inhibitor EIPA or S3226 almost completely blocked the OUB-mediated increase in intracellular sodium concentration. The addition of the NHE1 inhibitor, cariporide (HOE) did not reduce the OUB response, indicating that the increase in intracellular sodium concentration with OUB is not through NHE1 but rather through NHE3.
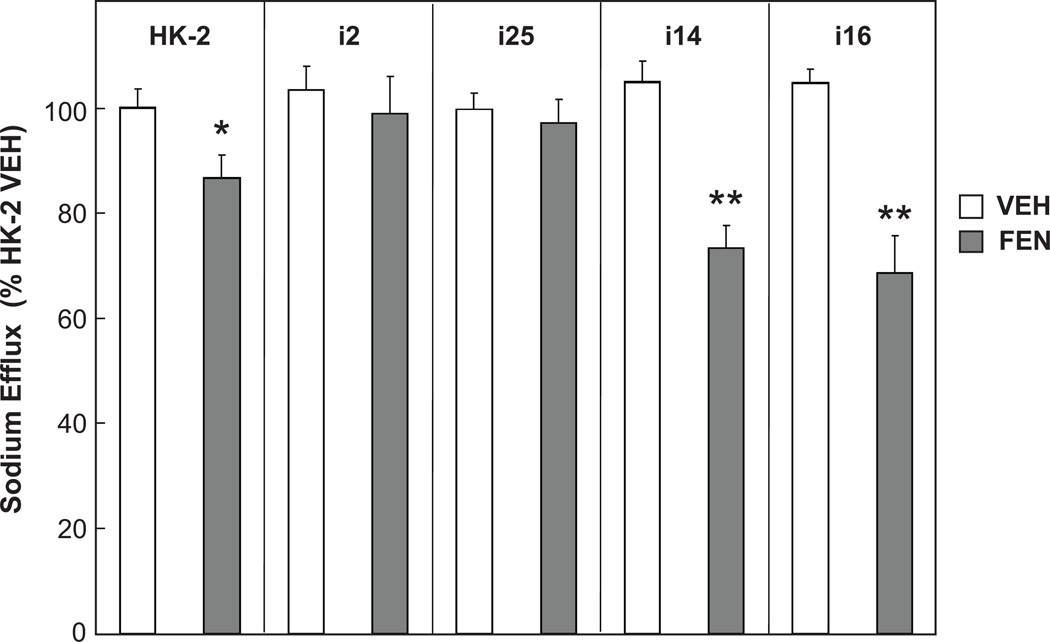
Since dopaminergic stimulation of RPTCs also causes an inhibition of NaKATPase activity,31 we compared these 5 cell lines in our NaKATPase-dependent sodium efflux assay. Fenoldopam inhibited sodium efflux to a greater extent in normally coupled renal proximal tubule cells compared to the uncoupled cell lines (Figure 5). The two adenylyl cyclase coupled cell lines i14 and i16 had a large decrease in sodium efflux upon agonist stimulation (**P<0.01 vs VEH, ANOVA, Holm-Sidak test, n=6/group) but this was not seen in the two uncoupled renal proximal tubule cells (i2 and i25). HK-2 cells displayed a fenoldopam-mediated reduction in sodium efflux, although it was significantly less than that seen in i14 and i16 (* P<0.05 vs VEH, i14 and i16, ANOVA, Holm-Sidak test, n=6/group).
Figure 5. Fenoldopam-mediated reduction in NaKATPase-dependent sodium efflux is greater in nRPTCs than uRPTCs.
Cells labeled with the sodium sensitive dye SBFI were sodium loaded by incubating the cells in potassium-free media, and ratiometric fluorescence images were collected simultaneously on an automated confocal fluorescence microscope. The two adenylyl cyclase normally coupled cell lines i14 and i16 had a large decrease in sodium influx upon agonist stimulation (**P<0.01 vs VEH, ANOVA, Holm-Sidak test, n=6/group) but the two uncoupled renal proximal tubule cells (i2 and i25) had no decrease. HK2 cells displayed a small but significant fenoldopam-mediated reduction in sodium efflux although it was less than i14 and i16 (* P<0.05 vs VEH, i14 and i16, ANOVA, Holm-Sidak test, n=6/group).
The three GRK4 SNPs R65L, A142V, and A486V are associated with human essential hypertension or salt-sensitive hypertension 8, 14, 32–35, and cause the uncoupling of the D1R to AC. Table 1 shows that the HK-2 cell line is homozygous variant at exon 5, and heterozygous variant at exons 3 and 14, as compared to our control cell lines i14 and i16, which are wild-type at all 3 GRK4 exons and have normal adenylyl cyclase coupling.
Discussion
The HK-2 cell line has been relied on as a surrogate for “normal” renal proximal tubular physiology for over two decades. HK-2 cells have been reported to express parathyroid hormone receptor which participates in the proliferative response after energy depletion36, but other cellular parameters do not always show concordance with primary cultures of RPTCs (e.g., low basal angiotensinogen, basal NF-kB and STAT3 activities, and total protein expression).37 In the current study, we found that HK-2 cells had virtually non-measurable expression of APN25, and a reduced expression of the proximal tubule specific markers LTA27 and APA 25, when compared to our human RPTC lines i2, i25, i14, and i16. The differential expression of cell surface markers led us to examine dopamine-stimulated AC activity and sodium transport in HK-2 cells.
In the current studies, we demonstrate the novel finding that the D1-like stimulation in the HK-2 is uncoupled from D1-like stimulation of AC as well as D1-like stimulation of sodium influx (NHE3 activity), and displayed a blunted D1-like stimulated reduction in sodium efflux (NaKATPase activity). The fact that beta-adrenergic receptor stimulation of AC remains intact suggests that the uncoupling phenomenon is unique to the D1-like receptors. Similar to our previous findings, the siRNA silencing of GRK4 (which normally prevents the phosphorylation and inhibition of the D1-like receptors) restores D1-like coupling to AC in the uncoupled cells.
Zhang et al demonstrated normal coupling between D1R-like stimulation with fenoldopam and inhibition of NaKATPase activity in HK-2 cells .38 Their results suggest the presence of functional D1-like receptors in HK-2 cells, yet in our study HK-2 cells show a reduced inhibition of NaKATPase activity compared to normally coupled RPTC cells. Because GRK4 may not regulate D5R, the other D1-like receptor, 39 it is possible that the cell culture conditions of Zhang et al increased the expression of the D5R, overcoming the impaired D1R function, thus allowing the fenoldopam to work through the D5R. PMA, a known stimulator of the protein kinase C (PKC) pathway, has been shown to inhibit NaKATPase independently of cAMP.40 PTH has also been shown to inhibit NaKATPase independently of cAMP.41 However, the role of the D5R in the fenoldopam-mediated inhibition of NHE3 or NaKATPase in HK-2 cells remains to be further evaluated. One other possibility for our results differing from Dr. Zhang’s is that they may be using an entirely different subclone of HK-2 cells. 42, 43 The present findings demonstrate that GRK4 may be a potential therapeutic target, and that uncoupled cells may serve as a tool for screening for compounds with therapeutic potential. Moreover, there are significant cost and efficiency benefits to using cells expressing the intracellular cAMP sensor ICUE3 for high throughput screens. The D1R expressed in the HK-2 cell was uncoupled from AC similar to our two D1R uncoupled cell models.9, 11, 44 The presence of gene variants at three exons in GRK4 in the HK-2 cell line is in keeping with the known effects of GRK4 variants on uncoupling of the D1R to AC. We have previously demonstrated that variants R65L and A142V independently reduce the coupling of the D1R to AC by approximately 50%, while A486V is more potent at reducing D1R/AC coupling.8 When the expression of GRK4 was decreased with antisense oligonucleotides, normal coupling was restored between the D1R and AC.11 The fact that the uncoupling could also be reversed by the addition of siRNA to GRK4 in HK-2 cells suggests that both the HK-2 and our uncoupled cell lines possess similar mechanisms responsible for the uncoupling phenomenon.
We have reported that GRK4 gene variants are associated with an increased incidence of hypertension in several populations.14, 34, 45–46 These reports have been corroborated by others, in hypertensive Han Chinese32 and a white Australian cohort35 but not in an European cohort.47 However, in the same European population, polymorphisms in the GRK4 promoter were found to affect transcriptional activity.31, 48 The possibility that increased GRK4 expression may also be a cause of hypertension is supported by in vivo studies in rats: chronic renal silencing of GRK4 in spontaneously hypertensive rats (that over-express GRK4 but do not have an altered GRK4 genotype) resulted in about a 30% reduction in blood pressure.49 Thus, there is a need for human cell lines with various GRK4 polymorphisms to test the impact of these gene variants on RPTC function. Future studies will focus on the association between GRK4 genotype and the hypertension/salt sensitivity phenotype.
Supplementary Material
Perspectives.
Renal cell culture models must be used with caution since they may contain genes that are not associated with normal cell function. Moreover, the disease phenotypes that are encoded in the genome and possibly expressed, are usually not available for the various renal cell lines that are available to study. In these studies, we demonstrated that the widely used HK-2 cell line carries homozygous SNPs at one GRK4 allele, and heterozygous SNPs at 2 other alleles that are associated with either hypertension and/or salt sensitivity. The presence of these variants was associated with uncoupling of the D1R from AC, similar to that found in two human RPTC lines carrying more than three GRK4 variants. Two control cell lines that do not carry the GRK4 variants had normal D1R/AC coupling. These results suggest that the HK-2 cell line may be a good model for cellular physiology associated with GRK4 variants only when used in conjunction with additional cell models.
Acknowledgments
Sources of Funding: HL074940, HL23081, DK39308, HL68686, and HL092196.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest/Disclosure: R.A.F. and Pedro A. Jose were awarded a US Patent (No. 6,660,474) on "GRK variants in essential hypertension" which has been assigned to Hypogen, Inc.
REFERENCES
- 1.Detrisac CJ, Sens MA, Garvin AJ, Spicer SS, Sens DA. Tissue culture of human kidney epithelial cells of proximal tubule origin. Kidney International. 1984;25:383–390. doi: 10.1038/ki.1984.28. [DOI] [PubMed] [Google Scholar]
- 2.Baer PC, Bereiter-Hahn J, Schubert R, Geiger H. Differentiation status of human renal proximal and distal tubular epithelial cells in vitro: Differential expression of characteristic markers. Cells Tissues Organs. 2006;184:16–22. doi: 10.1159/000096947. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PD, Dillingham MA, Breckon R, Anderson RJ. Defined human renal tubular epithelia in culture: growth, characterization, and hormonal response. Am J Physiol. 1985;248:F436–F443. doi: 10.1152/ajprenal.1985.248.3.F436. [DOI] [PubMed] [Google Scholar]
- 4.Racusen LC, Monteil C, Sgrignoli A, Lucskay M, Marouillat S, Rhim JG, Morin JP. Cell lines with extended in vitro growth potential from human renal proximal tubule: characterization, response to inducers, and comparison with established cell lines. J Lab Clin Med. 1997;129:318–329. doi: 10.1016/s0022-2143(97)90180-3. [DOI] [PubMed] [Google Scholar]
- 5.Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994;45:48–57. doi: 10.1038/ki.1994.6. [DOI] [PubMed] [Google Scholar]
- 6.Racusen LC, Wilson PD, Hartz PA, Fivush BA, Burrow CR. Renal proximal tubular epithelium from patients with nephropathic cystinosis: immortalized cell lines as in vitro model systems. Kidney Int. 1995;48:536–543. doi: 10.1038/ki.1995.324. [DOI] [PubMed] [Google Scholar]
- 7.Elberg G, Guruswamy S, Logan CJ, Chen L, Turman MA. Plasticity of epithelial cells derived from human normal and ADPKD kidneys in primary cultures. Cell Tissue Res. 2008;331:495–508. doi: 10.1007/s00441-007-0521-4. [DOI] [PubMed] [Google Scholar]
- 8.Felder RA, Sanada H, Xu J, Yu PY, Wang Z, Watanabe H, Asico LD, Wang W, Zheng S, Yamaguchi I, Williams SM, Gainer J, Brown NJ, Hazen-Martin D, Wong LJ, Robillard JE, Carey RM, Eisner GM, Jose PA. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci USA. 2002;99:3872–3877. doi: 10.1073/pnas.062694599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanada H, Jose PA, Hazen-Martin D, Yu PY, Xu J, Bruns DE, Phipps J, Carey RM, Felder RA. Dopamine-1 receptor coupling defect in renal proximal tubule cells in hypertension. Hypertension. 1999;33:1036–1042. doi: 10.1161/01.hyp.33.4.1036. [DOI] [PubMed] [Google Scholar]
- 10.Gildea JJ, Israel JA, Johnson AK, Zhang J, Jose PA, Felder RA. Caveolin-1 and dopamine-mediated internalization of NaKATPase in human renal proximal tubule cells. Hypertension. 2009;54:1070–1076. doi: 10.1161/HYPERTENSIONAHA.109.134338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe H, Xu J, Bengra C, Jose PA, Felder RA. Desensitization of human renal D1 dopamine receptors by G protein-coupled receptor kinase 4. Kidney Int. 2002;62:790–798. doi: 10.1046/j.1523-1755.2002.00525.x. [DOI] [PubMed] [Google Scholar]
- 12.Gildea JJ, Wang X, Jose PA, Felder RA. Differential D1 and D5 receptor regulation and degradation of the angiotensin type 1 receptor. Hypertension. 2008;51:360–366. doi: 10.1161/HYPERTENSIONAHA.107.100099. [DOI] [PubMed] [Google Scholar]
- 13.Han W, Li H, Villar VA, Pascua AM, Dajani MI, Wang X, Natarajan A, Quinn MT, Felder RA, Jose PA, Yu P. Lipid rafts keep NADPH oxidase in the inactive state in human renal proximal tubule cells. Hypertension. 2008;51:481–487. doi: 10.1161/HYPERTENSIONAHA.107.103275. [DOI] [PubMed] [Google Scholar]
- 14.Sanada H, Yatabe J, Midorikawa S, Hashimoto S, Watanabe T, Moore JH, Ritchie MD, Williams SM, Pezzullo JC, Sasaki M, Eisner GM, Jose PA, Felder RA. Single-nucleotide polymorphisms for diagnosis of salt-sensitive hypertension. Clin Chem. 2006;52:352–360. doi: 10.1373/clinchem.2005.059139. [DOI] [PubMed] [Google Scholar]
- 15.Amemiya M, Loffing J, Lotscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney Int. 1995;48:1206–1215. doi: 10.1038/ki.1995.404. [DOI] [PubMed] [Google Scholar]
- 16.Campbell L, Gumbleton M, Griffiths DF. Caveolin-1 overexpression predicts poor disease-free survival of patients with clinically confined renal cell carcinoma. Br J Cancer. 2003;89:1909–1913. doi: 10.1038/sj.bjc.6601359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breton S, Lisanti MP, Tyszkowski R, McLaughlin M, Brown D. Basolateral distribution of caveolin-1 in the kidney. Absence from H+-atpase-coated endocytic vesicles in intercalated cells. J Histochem Cytochem. 1998;46 doi: 10.1177/002215549804600209. 205214. [DOI] [PubMed] [Google Scholar]
- 18.Baer PC, Geiger H. Human renal cells from the thick ascending limb and early distal tubule: characterization of primary isolated and cultured cells by reverse transcription polymerase chain reaction. Nephrology (Carlton) 2008;13:316–321. doi: 10.1111/j.1440-1797.2008.00927.x. [DOI] [PubMed] [Google Scholar]
- 19.Knepper MA, Brooks HL. Regulation of the sodium transporters NHE3, NKCC2 and NCC in the kidney. Curr Opin Nephrol Hypertens. 2001;10:655–659. doi: 10.1097/00041552-200109000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Violin JD, DiPilato LM, Yildirim N, Elston TC, Zhang J, Lefkowitz RJ. beta2adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J Biol Chem. 2008;283:2949–2961. doi: 10.1074/jbc.M707009200. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki S, Siragy HM, Gildea JJ, Felder RA, Carey RM. Production and role of extracellular guanosine cyclic 3', 5' monophosphate in sodium uptake in human proximal tubule cells. Hypertension. 2004;43:286–291. doi: 10.1161/01.HYP.0000112421.18551.1e. [DOI] [PubMed] [Google Scholar]
- 22.Hropot M, Juretschke HP, Langer KH, Schwark JR. S3226, a novel NHE3 inhibitor, attenuates ischemia-induced acute renal failure in rats. Kidney Int. 2001;60:2283–2289. doi: 10.1046/j.1523-1755.2001.00058.x. [DOI] [PubMed] [Google Scholar]
- 23.Vallon V, Schwark JR, Richter K, Hropot M. Role of Na(+)/H(+) exchanger NHE3 in nephron function: micropuncture studies with S3226, an inhibitor of NHE3. Am J Physiol Renal Physiol. 2000;278:F375–F379. doi: 10.1152/ajprenal.2000.278.3.F375. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Jung YS, Han W, Kim MY, Namkung W, Lee BH, Yi KY, Yoo SE, Lee MG, Kim KH. Pharmacodynamic characteristics and cardioprotective effects of new NHE1 inhibitors. Eur J Pharmacol. 2007;567:131–138. doi: 10.1016/j.ejphar.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 25.Ronco P, Antoine M, Baudouin B, Geniteau-Legendre M, Lelongt B, Chatelet F, Verroust P, Vandewalle A. Polarized membrane expression of brush-border hydrolases in primary cultures of kidney proximal tubular cells depends on cell differentiation and is induced by dexamethasone. J Cell Physiol. 1990;145 doi: 10.1002/jcp.1041450206. 222237. [DOI] [PubMed] [Google Scholar]
- 26.Terada N, Ohno N, Yamakawa H, Seki G, Fujii Y, Baba T, Ohara O, Ohno S. Immunoelectron microscopic localization of protein 4.1B in proximal S1 and S2 tubules of rodent kidneys. Med Electron Microsc. 2004;37:45–51. doi: 10.1007/s00795-003-0236-x. [DOI] [PubMed] [Google Scholar]
- 27.Helbert MJ, Dauwe SE, Van der Biest I, Nouwen EJ, De Broe ME. Immunodissection of the human proximal nephron: flow sorting of S1S2S3, S1S2 and S3 proximal tubular cells. Kidney Int. 1997;52:414–428. doi: 10.1038/ki.1997.348. [DOI] [PubMed] [Google Scholar]
- 28.Gildea JJ, Yatabe J, Sasaki M, Jose PA, Felder RA. G-protein coupled receptor kinase 4 (GRK4) polymorphisms block receptor recruitment to cell membranes. Hypertension. 2006;48:e85. [Google Scholar]
- 29.Villar VA, Jones JE, Armando I, Palmes-Saloma C, Yu P, Pascua AM, Keever L, Arnaldo FB, Wang Z, Luo Y, Felder RA, Jose PA. G protein-coupled receptor kinase 4 (GRK4) regulates the phosphorylation and function of the dopamine D3 receptor. J Biol Chem. 2009;284:21425–21434. doi: 10.1074/jbc.M109.003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moe OW, Amemiya M, Yamaji Y. Activation of protein kinase A acutely inhibits and phosphorylates Na/H exchanger NHE-3. J Clin Invest. 1995;96:2187–2194. doi: 10.1172/JCI118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chibalin AV, Pedemonte CH, Katz AI, Feraille E, Berggren PO, Bertorello AM. Phosphorylation of the catalyic alpha-subunit constitutes a triggering signal for Na+,K+-ATPase endocytosis. J Biol Chem. 1998;273:8814–8819. doi: 10.1074/jbc.273.15.8814. [DOI] [PubMed] [Google Scholar]
- 32.Gu D, Su S, Ge D, Chen S, Huang J, Li B, Chen R, Qiang B. Association study with 33 single-nucleotide polymorphisms in 11 candidate genes for hypertension in Chinese. Hypertension. 2006;47:1147–1154. doi: 10.1161/01.HYP.0000219041.66702.45. [DOI] [PubMed] [Google Scholar]
- 33.Williams SM, Addy JH, Phillips JA, 3rd, Dai M, Kpodonu J, Afful J, Jackson H, Joseph K, Eason F, Murray MM, Epperson P, Aduonum A, Wong LJ, Jose PA, Felder RA. Combinations of variations in multiple genes are associated with hypertension. Hypertension. 2000;36:2–6. doi: 10.1161/01.hyp.36.1.2. [DOI] [PubMed] [Google Scholar]
- 34.Lohmueller KE, Wong LJ, Mauney MM, Jiang L, Felder RA, Jose PA, Williams SM. Patterns of genetic variation in the hypertension candidate gene GRK4: ethnic variation and haplotype structure. Ann Hum Genet. 2006;70:27–41. doi: 10.1111/j.1529-8817.2005.00197.x. [DOI] [PubMed] [Google Scholar]
- 35.Speirs HJ, Katyk K, Kumar NN, Benjafield AV, Wang WY, Morris BJ. Association of G-protein-coupled receptor kinase 4 haplotypes, but not HSD3B1 or PTP1B polymorphisms, with essential hypertension. J Hypertens. 2004;22:931–936. doi: 10.1097/00004872-200405000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Ocana A, Galbraith SC, Van Why SK, Yang K, Golovyan L, Dann P, Zager RA, Stewart AF, Siegel NJ, Orloff JJ. Expression and role of parathyroid hormone-related protein in human renal proximal tubule cells during recovery from ATP depletion. J Am Soc Nephrol. 1999;10:238–244. doi: 10.1681/ASN.V102238. [DOI] [PubMed] [Google Scholar]
- 37.Satou R, Gonzalez-Villalobos RA, Miyata K, Ohashi N, Urushihara M, Acres OW, Navar LG, Kobori H. IL-6 augments angiotensinogen in primary cultured renal proximal tubular cells. Mol Cell Endocrinol. 2009;311:24–31. doi: 10.1016/j.mce.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Yuan Z, Ge H, Ren Y. Effects of long-term ouabain treatment on blood pressure, sodium excretion, and renal dopamine D(1) receptor levels in rats. J Comp Physiol B. 2010;180:117–124. doi: 10.1007/s00360-009-0391-z. [DOI] [PubMed] [Google Scholar]
- 39.Zeng C, Luo Y, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. Perturbation of D1 dopamine and AT1 receptor interaction in spontaneously hypertensive rats. Hypertension. 2003;42:787–792. doi: 10.1161/01.HYP.0000085334.34963.4E. [DOI] [PubMed] [Google Scholar]
- 40.Efendiev R, Budu CE, Cinelli AR, Bertorello AM, Pedemonte CH. Intracellular Na+ regulates dopamine and angiotensin II receptors availability at the plasma membrane and their cellular responses in renal epithelia. J Biol Chem. 2003;278:28719–28726. doi: 10.1074/jbc.M303741200. [DOI] [PubMed] [Google Scholar]
- 41.Ribeiro CP, Mandel LJ. Parathyroid hormone inhibits proximal tubule Na(+)-K(+)-ATPase activity. Am J Physiol. 1992;262:F209–F216. doi: 10.1152/ajprenal.1992.262.2.F209. [DOI] [PubMed] [Google Scholar]
- 42.Gekle M, Wunsch S, Oberleithner H, Silbernagl S. Characterization of two MDCK-cell subtypes as a model system to study principal cell and intercalated cell properties. Pflugers Arch. 1994;428:157–162. doi: 10.1007/BF00374853. [DOI] [PubMed] [Google Scholar]
- 43.Sodium in processed foods. JAMA. 1983;249:784–789. [PubMed] [Google Scholar]
- 44.Felder RA, Jose PA. Mechanisms of disease: the role of GRK4 in the etiology of essential hypertension and salt sensitivity. Nat Clin Pract Nephrol. 2006;2 doi: 10.1038/ncpneph0301. 637650. [DOI] [PubMed] [Google Scholar]
- 45.Bengra C, Mifflin TE, Khripin Y, Manunta P, Williams SM, Jose PA, Felder RA. Genotyping of essential hypertension single-nucleotide polymorphisms by a homogeneous PCR method with universal energy transfer primers. Clin Chem. 2002;48:2131–2140. [PubMed] [Google Scholar]
- 46.Williams SM, Ritchie MD, Phillips JA, 3rd, Dawson E, Prince M, Dzhura E, Willis A, Semenya A, Summar M, White BC, Addy JH, Kpodonu J, Wong LJ, Felder RA, Jose PA, Moore JH. Multilocus analysis of hypertension: a hierarchical approach. Hum Hered. 2004;57:28–38. doi: 10.1159/000077387. [DOI] [PubMed] [Google Scholar]
- 47.Staessen JA, Kuznetsova T, Zhang H, Maillard M, Bochud M, Hasenkamp S, Westerkamp J, Richart T, Thijs L, Li X, Brand-Herrmann SM, Burnier M, Brand E. Blood pressure and renal sodium handling in relation to genetic variation in the DRD1 promoter and GRK4. Hypertension. 2008;51:1643–1650. doi: 10.1161/HYPERTENSIONAHA.107.109611. [DOI] [PubMed] [Google Scholar]
- 48.Hasenkamp S, Telgmann R, Staessen JA, Hagedorn C, Dordelmann C, Bek M, Brand-Herrmann SM, Brand E. Characterization and functional analyses of the human G protein-coupled receptor kinase 4 gene promoter. Hypertension. 2008;52:737–746. doi: 10.1161/HYPERTENSIONAHA.108.114512. [DOI] [PubMed] [Google Scholar]
- 49.Sanada H, Yatabe J, Midorikawa S, Katoh T, Hashimoto S, Watanabe T, Xu J, Luo Y, Wang X, Zeng C, Armando I, Felder RA, Jose PA. Amelioration of genetic hypertension by suppression of renal G protein-coupled receptor kinase type 4 expression. Hypertension. 2006;47:1131–1139. doi: 10.1161/01.HYP.0000222004.74872.17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.