Abstract
Purpose of review
Asthma prevalence and severity are greater in women than in men, and mounting evidence suggests this is in part related to female steroid sex hormones. Of these, estrogen has been the subject of much study. This review highlights recent research exploring the effects of estrogen in allergic disease.
Recent findings
Estrogen receptors are found on numerous immunoregulatory cells and estrogen’s actions skew immune responses toward allergy. It may act directly to create deleterious effects in asthma, or indirectly via modulation of various pathways including secretory leukoprotease inhibitor, transient receptor potential vanilloid type 1 ion channel and nitric oxide production to exert effects on lung mechanics and inflammation. Not only do endogenous estrogens appear to play a role, but environmental estrogens have also been implicated. Environmental estrogens (xenoestrogens) including bisphenol A and phthalates enhance allergic sensitization in animal models and may enhance development of atopic disorders like asthma in humans.
Summary
Estrogen’s role in allergic disease remains complex. As allergic diseases continue to increase in prevalence and affect women disproportionately, gaining a fuller understanding of its effects in these disorders will be essential. Of particular importance may be effects of xenoestrogens on allergic disease.
Keywords: asthma, environmental estrogen, estrogen, xenoestrogen
INTRODUCTION
It has long been postulated that the female hormones, estrogen and progesterone, play an active role in allergic disease in women. This seems plausible because of the clear differences in incidence, severity, and fluctuations in allergic disorders in women as compared with men. As the roles these hormones play have not been fully elucidated, defining their effects in allergic disease is an active area of exploration.
Sex/age effects on asthma suggest an effect of female hormones
The prevalence of asthma is greater in boys than in girls during prepubescent ages [1,2]; however, after puberty, this trend reverses [3,4,5▪]. The prevalence of asthma and incidence of asthma exacerbations are consistently higher in women during early to mid-adulthood than in men [6,7▪,8]. Not only is prevalence greater in women, but the disease is also more severe. Physician visits, hospitalizations, and deaths due to asthma are more likely to occur in women than in men [9▪▪]. Women taking hormone replacement therapy had significantly greater rates of physician-diagnosed asthma compared with matched controls [10]. Finally, 30–40% of the women with asthma have perimenstrual worsening during phases of rapid changes in estrogen and progesterone concentrations [11]. There is evidence that not only do endogenous estrogens exert effects in allergic disease, but also that exogenous compounds with estrogenic activity (xenoestrogens) may also play a role in asthma and other allergic disorders. Environmental estrogens act as ‘imperfect’ estrogens on numerous organ systems [12]. This may, at least partly, occur because they largely signal via understudied membrane forms of classical estrogen receptors [13] and act via nongenomic signaling pathways. Alternatively, developmental stage may dictate differences in susceptibility due to alterations in the production and metabolism of endogenous estrogens and androgens, which use the same metabolic machineries.
ESTROGEN RECEPTORS ON IMMUNOREGULATORY CELLS
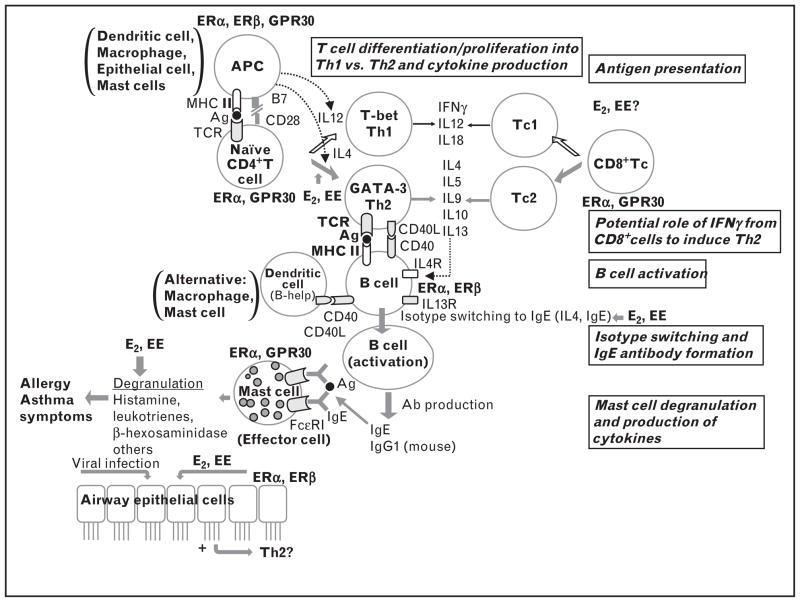
Estrogens have significant effects on several components of immune function. Estrogens act primarily via receptors. Estrogen receptors α, β (ERα, ERβ) and G protein-coupled receptor (GPR) 30 expression is reported on immunomodulatory cells (Fig. 1) [14,15▪,16–18,19▪▪,20–22,23▪,24,25]. Giannoni et al. [26] reported higher expression of ERα and ERβ on cord blood mononuclear cells, as well as progesterone receptors. These classical receptors were initially described as nuclear receptors. In the last decade, these receptors were detected on the cell membrane and effects occur within seconds to minutes [27]. Another class of orphan (without clear ligand assignments) receptors, the estrogen receptor-related receptor expression has not been detected on the immune cells. Estrogens can have potential effects on each step of allergic sensitization: antigen presentation, type 2 T helper cells (Th2) polarization, isotype switching to immunoglobulin E (IgE), and mast cell degranulation via classical estrogen receptors.
FIGURE 1.
Flow diagram of immune development leading to allergic sensitization with known expression of estrogen receptors by each immune cell type. Environmental estrogens (EEs) have potential effects on each step of allergic sensitization: antigen presentation, Th2 polarization, isotype switching to IgE and mast cell degranulation via ERα, ERβ and G-protein coupled receptor 30 (GPR30) [14,15▪,16–18,19▪▪,20–22,23▪,24,25]. ER, estrogen receptor; IgE, immunoglobulin E.
Estrogens may enhance antigen-presenting cell function to develop allergic diseases
Specific immunity is a delicately balanced process that requires efficient sampling for potential pathogenicity of invading and colonizing microbes and other sources of environmental macromolecules, as well as the ability to mobilize those host defenses best adapted to protect against the type of pathogen encountered. For example, harmless environmental proteins may cause allergic asthma when naive T helper cells erroneously respond to them with the development of Th2-specific responses, a type of response that normally protects against helminthic infection. T cells only recognize antigen when they are presented as protein-derived peptides in association with class II major histocompatibility complex (MHC) encoded molecules expressed by antigen-presenting cells (APCs). Recent research indicates that APCs also sway the immune response toward the most effective type of T helper cells. APCs that support Th2 development include ‘conventional’ APCs (e.g. dendritic cells, B lymphocytes, and monocytes/macrophages) and nonconventional APCs (e.g. eosinophils, basophils, and mast cells and some endothelial cells). Both estradiol and bisphenol A enhance the production of dendritic cell populations, which preferentially promote Th2 responses [28,29].
Although all nucleated cells express class I MHC molecules, only selected cell types express class II MHC molecules and can act as APCs. Mast cells normally express MHC class I and also express MHC class II molecules on activation, as well as adhesion molecules (ICAM-1 and ICAM-3) and co-stimulatory molecules (CD43, CD80, CD86, and CD40L), which allow them to interact with T and B lymphocytes [30]. After antigen uptake and processing, a major prerequisite for mast cell antigen presentation to T cells is an interaction of these cells via the MHC complex with the TCR on CD4+ or CD8+ lymphocytes.
Estrogens may polarize T cells to type 2 T helper cell response
The differentiation of naive CD4+ T cells to type 1 T helper cells or Th2 is determined by the coordinated action of multiple signals induced by stimulation of the TCR, co-stimulatory molecules, and cytokine receptors. It is accompanied by extensive re-organization of the chromatin structure around the IFNγ or the IL-4/IL-5/IL-13 loci, respectively. In patients with asthma, CD4+ T cells producing IL-4, IL-5, and IL-13 have been identified in bronchoalveolar lavage (BAL) and airway biopsies. After antigen challenge in patients with allergic asthma, Th2 lymphocytes are increased in the airways. The presence of IL-4-producing and IL-5-producing cells was shown to correlate with airway hyperreactivity. Thus, the association of Th2 with their effects in the respiratory tract has suggested that Th2 lymphocytes orchestrate the characteristic inflammatory response that results in asthma. Using an animal model of asthma (BALB/c mice), Cai et al. [31] showed that estrogens induced production of IL-5 and IL-13 from mediastinal lymph nodes. The production of these cytokines was suppressed using estrogen receptor antagonists tamoxifen or ICI182,780. This group also showed that estrogens induced eosinophilic inflammation in peripheral blood and BAL fluid.
Estrogens promote the class switching of B cells to immunoglobulin E synthesis
Naive B cells that have not yet encountered antigen express immunoglobulin M and immunoglobulin D on their surface. During an immune response, B cells can express different immunoglobulin heavy chain isotypes sharing the same variable–diversity–joining (VDJ) region. This isotype-switching recombination allows a B-cell clone to produce antibodies with the same specificity for antigens but with different effector functions. To switch to a particular isotype, a B cell needs two signals: cytokine-dependent and CD40-dependent. The interaction between IL-4 and its receptor delivers the first signal for switching to IgE. The engagement of CD40 on B cells by CD154 expressed on T cells provides the second signal required for switching to IgE. In addition, IL-4 (but not IL-13) and IgE increase the expression of the α-chain of FcεRI in nasal mast cells, resulting in a potential amplification loop. Thus, activation of mast cells by allergen–antibody receptor complexes can not only induce degranulation but also enhance and perpetuate the production of IgE and its high-affinity receptor FcεRI in that complex. This positive feedback mechanism may explain why treating of allergic diseases with injections of small amounts of the sensitizing allergen may actually increase the production of allergen-specific IgE, particularly early in the immunization process. Enhancing effects of soy isoflavone on the production of allergen-specific IgE from splenocytes was detected in an animal model (BALB/c mice) [32].
Estrogens promote the degranulation of mast cell/basophils
In the immediate hypersensitivity reaction, poly-valent allergen cross-links IgE bound to mast cells through FcεRI inducing release of preformed mediators and cytokines by a process of degranulation and induction of the synthesis of prostaglandins, leukotrienes, and additional cytokines. The release of the major mediators of acute hypersensitivity (e.g. histamine, cysteinyl leukotrienes) is an obligatory event in allergic reactions.
Mast cells also degranulate in response to stimuli other than the FcεRI-mediated pathways, including a Ca2+ ionophore and compound 48/80. We found that exposure to physiological doses of estradiol [27] and a panel of environmental estrogens [13] induced the release of the preformed granular protein β-hexosaminidase, induced leukotriene C4 synthesis and release, and enhanced IgE-dependent release of these mediators. Consistently with our finding, more mast cells and higher histamine concentrations were observed in the estrous stage than in the progestrous stage and diestrous stage in the mammary glands of nonsensitized female Wister rats [33]. Ovariectomy decreased the mast cell number and histamine concentration, which were reconstituted by exogenous estradiol. We and our colleagues have shown that some endogenous and environmental estrogens (e.g., 10−8 to 10−6 mol/l estradiol, nonylphenol, and octylphenol) promote Th2 responses by increasing IL4, transcription factor GATA3, and MHC class II expression and decreasing IFNγ production by CD4+CD8+ thymocytes, naive CD4+ T cells, or spleen dendritic cells from mice. In addition to the pro-allergic FcεRI, mast cells also express the inhibitory receptor FcγRIIB on their surface. It is possible that inhibitors of allergic reactions act by inducing production of this receptor.
ESTROGEN EFFECTS ON ALLERGIC DISEASE
The role of female hormones in allergic disease has perhaps been most intensely studied in asthma. Early menarche has been demonstrated to be a risk factor for asthma in adult women [34,35▪]. Menarche at age 10 years or earlier was associated with reported presence of wheezing and multiple symptoms of asthma. Further, early menarche was found to be associated with decreased forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC).
Among women with asthma, up to 30–40% in some series have reported worsening of asthma symptoms at specific times of the menstrual cycle. The perimenstrual phase, shortly before and during the first few days of the menstrual period, has been identified as a time during which symptoms may commonly worsen. This may be attributable to a variety of factors including fluctuations in lung mechanics over the menstrual cycle and increasing inflammation during the perimenstrual period. Further, women with perimenstrual asthma have been noted to have more severe asthma and comorbid conditions such as aspirin-induced exacerbations of asthma [36].
Estrogen affects lung function and mechanics
It has been reported that FEV1 and FVC are lowest in the periovulatory time of the cycle when estradiol levels are high [9▪▪]. In fact, a linear decrease in FEV1 as ovulation approaches during the follicular phase has been demonstrated [37▪]. Additionally increased bronchial hyperreactivity has been identified in the periovulatory period and especially in the perimenstrual period [38]. Further characterization of lung function during the luteal phase is not as clearly defined. Some have found FEV1 and FVC to be greatest during the perimenstrual period [39], whereas others have noted a trend toward further slight decreases in FEV1 during the luteal phase among women with asthma [37▪].
There is evidence that estrogen is able to induce smooth muscle relaxation in the airway [40]. Expression of both α and β estrogen receptors has been demonstrated on human bronchial epithelial cells (BECs) [19▪▪]. Townsend et al. demonstrated that activation of these receptors by estradiol and by specific agonists of ERα and ERβ resulted in increased production of nitric oxide in BECs. Further, estrogen receptor stimulation by agonists resulted in relaxation in bronchial rings that were previously treated with acetylcholine to induce constriction. This evidence stands in contradistinction to findings that high estrogen levels tend to coincide with worse asthma symptoms, but supports the findings of others that exacerbations are more frequent when estrogen levels are low.
Additionally, sex hormones have been shown to exert effects on bronchial epithelium cilia. Progesterone receptors are present on the cilia of human airway epithelial cells in both men and women [41▪]. Progesterone binding to these epithelial receptors leads to decreased cilia beat frequency. However, when estradiol is present, this effect is abrogated, signifying interplay between the female hormones in regulation of cilia beat frequency [41▪]. This may have important effects on mucociliary clearance in asthma, particularly during hormonal fluctuations over the menstrual phases in women of reproductive age.
Estrogen acts on intermediary factors in allergic disease
Apart from its direct effects on immune and airway cells, estrogen may modulate other processes that influence disease. Secretory leukoprotease inhibitor (SLPI) is a serine protease inhibitor that may guard against tissue injury by a host of proteases including neutrophil elastase. It has additionally been found to exert some immunomodulatory effects apart from its inhibition of protease activity. SLPI levels are increased in allergic asthma and levels may be upregulated by estradiol [42]. SLPI expression has also been shown to increase with high levels of circulating progesterone. SLPI levels have been found to be higher in asthmatic patients than in nonasthmatic individuals. It is postulated that these elevated levels of SLPI may function to protect the airways from inflammation in asthma. This protective effect of SLPI has been demonstrated in a mouse model of allergic asthma [42].
Another potential effect of female hormones is on the transient receptor potential vanilloid type 1 (TRPV1). This ion channel has been implicated in the pathophysiology of chronic cough and cough hypersensitivity syndrome (including cough variant asthma) [43,44▪]. It is stimulated by such triggers as bradykinin, prostaglandin, heat, and acid, leading to increased afferent nerve responses causing cough. Morice et al. identified a greater likelihood of women developing cough hypersensitivity syndrome. It has been theorized that one explanation for this increased incidence is linked to the finding that estrogen may increase activation of TRPV1 [45].
Contamination of the human fetus and infant by environmental pollutants
Certain chemicals that accumulate in the mother’s tissues are transferred to their babies through the placenta and breastfeeding. This is particularly true for lipid-soluble pollutants, because breast milk has higher lipid content than serum. The production of BPA, monomer of bicarbonate plastic, has increased each year since its first production in 1950s to 7300 tons in 1991 and 856 000 tons in 2003, according to the National Toxicology Program [46]. This rapid increase in industrial production of BPA in the USA began about one human generation before asthma prevalence began to increase. Free BPA has been found in human fluids and tissues in concentrations of up to approximately 100 ng/ml [47]. Epidemiological studies have revealed that the concentrations of environmental estrogens in the breast milk and a body burden in the second-born infant are lower than that in the first [48,49]. Interestingly, other epidemiological studies indicate that the first-born child is at a higher risk of developing asthma than subsequent children [50,51].
We reported that maternal BPA exposure enhanced the development of experimental asthma with allergen-specific IgE, eosinophilic inflammation, and airway hyperresponsiveness in the pups, which had ‘suboptimal’ sensitization and fetal exposure was necessary to induce these asthma phenotypes using BALB/C mice model [47,52▪▪]. Bauer et al. [53▪] found the enhanced airway lymphatic and lung inflammation in only females not in males of perinatal BPA-exposed adult C57BL/6 mouse model. Numerous additional studies suggest a relationship between xenoestrogen exposure and development of allergic disease (Table 1) [2,13,17,27,47,52▪▪,54,55▪, 56–58,59▪,60▪].
Table 1.
Evidence for an association between environmental estrogen exposures and the development of asthma
| Types of studies and topic and type of patients | Findings of study | Finding in asthma |
|---|---|---|
| Human epidemiology | Significant positive association between urinary BPA and lifetime prevalence of allergic asthma [54] | 10-fold increase in BPA was independently associated with higher likelyhood of allergic asthma in females |
| All patients with urinary BPA measurements in NHANES 2005–2006 survey | ||
| Human epidemiology | Significant positive association between mid-gestational urinary excretion of BPA and incidence of wheeze by age 6 months [55▪] | Wheezing in the first 6 months of life is positively associated with subsequent episodes of wheezing (asthma) |
| Pregnant women and their children followed up for 3 years | ||
| Human epidemiology | Male children have higher level of BPA in amniotic fluid than female children [56] | |
| Several relatively small group studies of urinary BPA | ||
| Human epidemiology | Urinary BPA is higher in children than in adults [57] | Age at diagnosis of asthma (80% by age 5 years) |
| NHANES | ||
| Human epidemiology | Urinary BPA is lowest in Mexican Hispanics and highest in African–Americans [2,58] | Ethnicity order for asthma prevalence same as order for urinary BPA |
| NHANES | ||
| Small-animal study | Perinatal exposure to BPA via dams’ drinking water [47] | Pups from BPA exposed were more likely to develop each manifestation of allergic asthma |
| Small-animal study | Prenatal vs. postnatal exposure to maternal BPA [52▪▪] | Prenatal exposure required to develop after postnatal allergen sensitization |
| Cell culture experiments on mouse and human mast cells to which endogenous and EEs were added | Both forms of estrogens induce partial release of mediators and augments release induced by IgE –allergen exposure [13,27] | About 40% of women have perimenstrual worsening of their asthma symptoms |
| Small-animal model | Urinary phthalate secretion relationship to allergy and asthma; the phthalate DEHP increases the IgE response to OVA [17,59▪,60▪] | Urinary phthalate level is associated with asthma prevalence |
The table gives a summary of growing body of evidence suggesting a relationship between environmental estrogens and the development of asthma. EEs, environmental estrogens; IgE, immunoglobulin E.
CONCLUSION
Female hormones appear to play a significant role in allergic disease, with estrogen effects being most well studied. Estrogen’s influences on immune cells favor the allergic response promoting Th2 polarization, encouraging class switching of B cells to IgE production and prompting mast cell and basophil degranulation. The potential role for estrogen in asthma is supported by epidemiologic evidence of increased asthma prevalence and severity in adult women and by associating estrogen with changes in airway mechanics and inflammation. However, the mechanism by which it acts may be quite complex, with effects dependent on the concentrations of hormone present and the concomitant presence or absence of other factors such as progesterone. Further, exogenous compounds with estrogenic activity may influence allergic disease as well. As allergic diseases continue to become more prevalent and affect women disproportionately, further delineation of the functions of female hormones in these disorders will be essential.
KEY POINTS.
Asthma prevalence and severity are greater in women than in men, and a large body of evidence suggests that this is related to estrogen.
Estrogen receptors are found on numerous immunoregulatory cells and estrogen’s actions skew the immune response toward allergy.
Estrogen may act directly to create deleterious effects in asthma, or may affect other intermediaries to exert effects on lung mechanics and airway inflammation.
Environmental estrogens (xenoestrogens) may enhance development of atopic disorders like asthma.
Acknowledgments
The authors thank Dr Randall Goldblum for his assistance in the preparation of this manuscript. The work by T.M.-H. cited herein was supported by ES016428 from National Institute of Environmental Health Science (NIEHS), and pilot projects from the NIEHS Center Grant P30 ES006676 and 1UL1RR029876-01 from the National Center for Research Resources, NIH.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 123).
- 1.CDC (Centers for Disease Control and Prevention) National Health Interview Survey. NHIS; Atlanta, GA: 2010. [Accessed 3 September 2012]. http://www.cdc.gov/asthma/nhis/default.htm. [Google Scholar]
- 2.Akinbami L, Moorman J, Liu X. Asthma prevalence, healthcare use, and mortality: United States, 2005–2009. [Accessed 3 September 2012];National Health Statistics Reports. 2011 32:1–15. http://www.cdc.gov/nchs/data/nhsr/nhsr032.pdf. [PubMed] [Google Scholar]
- 3.Almqvist C, Worm M, Leynaert B. Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy. 2008;63:47–57. doi: 10.1111/j.1398-9995.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- 4.Osman M, Hansell A, Simpson C, et al. Gender-specific presentations for asthma, allergic rhinitis and eczema in primary care. Prim Care Respir J. 2007;16:28–35. doi: 10.3132/pcrj.2007.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5▪.Choi I. Gender-specific asthma treatment. Allergy Asthma Immunol Res. 2011;3:74–80. doi: 10.4168/aair.2011.3.2.74. This review highlights sex differences in asthma prevalence, pathophysiology, and treatment effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mancuso CA, Peterson MG, Gaeta TJ, et al. Time to seeking emergency department care for asthma: self-management, clinical features at presentation, and hospitalization. J Asthma. 2012;49:275–281. doi: 10.3109/02770903.2012.661011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7▪.Leynaert B, Sunyer J, Garcia-Esteban R, et al. Gender differences in prevalence, diagnosis and incidence of allergic and nonallergic asthma: a population-based cohort. Thorax. 2012;67:625–631. doi: 10.1136/thoraxjnl-2011-201249. This study found an increased prevalence of asthma in women vs. men over 35 years of age. Additionally, incidence of adult onset asthma was greater in women and was largely due to nonatopic asthma. [DOI] [PubMed] [Google Scholar]
- 8.Vink N, Postma D, Schouten J, et al. Gender differences in asthma development and remission during transition through puberty: the TRacking Adolescents’ Individual Lives Survey (TRAILS) study. J Allergy Clin Immunol. 2010;126:498–504. doi: 10.1016/j.jaci.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 9▪▪.Tam A, Morrish D, Wadsworth S, et al. The role of female hormones on lung function in chronic lung diseases. BMC Womens Health. 2011;11:24. doi: 10.1186/1472-6874-11-24. This article reviews the effects of sex hormones in asthma, chronic obstructive pulmonary disease, and cyctic fibrosis. It focuses its exploration of asthma on hormonal influences on adaptive immunity, nitric oxide, and lung function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dratva J. Use of oestrogen only hormone replacement therapy associated with increased risk of asthma onset in postmenopausal women. Evid Based Med. 2010;15:190–191. doi: 10.1136/ebm1135. [DOI] [PubMed] [Google Scholar]
- 11.Thornton J, Lewis J, Lebrun CM, Licskai CJ. Clinical characteristics of women with menstrual-linked asthma. Respir Med. 2012;106:1236–1243. doi: 10.1016/j.rmed.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Braun JM, Hauser R. Bisphenol A and children’s health. Curr Opin Pediatr. 2011;23:233–239. doi: 10.1097/MOP.0b013e3283445675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narita S, Goldblum RM, Watson CS, et al. Environmental estrogens induce mast cell degranulation and enhance IgE-mediated release of allergic mediators. Env Health Perspectives. 2007;115:48–52. doi: 10.1289/ehp.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pierdominici M, Maselli A, Colasanti T, et al. Estrogen receptor profiles in human peripheral blood lymphocytes. Immunol Lett. 2010;132:79–85. doi: 10.1016/j.imlet.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 15▪.Seillet C, Laffont S, Tremollieres F, et al. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor α signaling. Blood. 2012;119:454–464. doi: 10.1182/blood-2011-08-371831. The authors demonstrate in a human study that estrogens have regulatory effects on the innate functions of plasmacytoid dentritic cells. This may be an additional explanation for some of the sex differences in allergic and autoimmune diseases. [DOI] [PubMed] [Google Scholar]
- 16.Yu X, Ma H, Barman SA, et al. Activation of G protein-coupled estrogen receptor induces endothelium-independent relaxation of coronary artery smooth muscle. Am J Physiol Endocrinol Metab. 2011;301:E882–E888. doi: 10.1152/ajpendo.00037.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doucet DR, Bonitz RP, Feinman R, et al. Estrogenic hormone modulation abrogates changes in red blood cell deformability and neutrophil activation in trauma hemorrhagic shock. J Trauma. 2010;68:35–41. doi: 10.1097/TA.0b013e3181bbbddb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Douin-Echinard V, Calippe B, Billon-Gales A, et al. Estradiol administration controls eosinophilia through estrogen receptor-alpha activation during acute peritoneal inflammation. J Leukoc Biol. 2011;90:145–154. doi: 10.1189/jlb.0210073. [DOI] [PubMed] [Google Scholar]
- 19.Townsend EA, Meuchel LW, Thompson MA, et al. Estrogen increases nitric oxide production in human bronchial epithelium. J Pharmacol Exp Ther. 2011;339:815–824. doi: 10.1124/jpet.111.184416. Study demonstrated increased nitric oxide production by acutely dissociated bronchial epithelial cells upon exposure to estrogen and estrogen receptor agonists. Townsend et al. also demonstrated relaxation of acetylcholine-treated bronchial rings after exposure to estrogen receptor agonists. This supports earlier data that estrogenic compounds can actually induce airway smooth muscle relaxation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪▪.Lambert KC, Curran EM, Judy BM, et al. Estrogen receptor alpha (ERalpha) deficiency in macrophages results in increased stimulation of CD4+ T cells while 17beta-estradiol acts through ERalpha to increase IL-4 and GATA-3 expression in CD4+ T cells independent of antigen presentation. J Immunol. 2005;175:5716–5723. doi: 10.4049/jimmunol.175.9.5716. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki T, Shimizu T, Yu HP, et al. Tissue compartment-specific role of estrogen receptor subtypes in immune cell cytokine production following trauma-hemorrhage. J Appl Physiol. 2007;102:163–168. doi: 10.1152/japplphysiol.00964.2006. [DOI] [PubMed] [Google Scholar]
- 22.Blasko E, Haskell CA, Leung S, et al. Beneficial role of the GPR30 agonist G-1 in an animal model of multiple sclerosis. J Neuroimmunol. 2009;214:67–77. doi: 10.1016/j.jneuroim.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23▪.Du S, Sandoval F, Trinh P, et al. Estrogen receptor-beta ligand treatment modulates dendritic cells in the target organ during autoimmune demyelinating disease. Eur J Immunol. 2011;41:140–150. doi: 10.1002/eji.201040796. The authors demonstrated for the first time an effect of ERβ ligand on dendritic cells in the target organ in experimental autoimmune encephalitis mouse model, a prototype cell-mediated autoimmune disorder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catley MC, Birrell MA, Hardaker EL, et al. Estrogen receptor beta: expression profile and possible anti-inflammatory role in disease. J Pharmacol Exp Ther. 2008;326:83–88. doi: 10.1124/jpet.108.136275. [DOI] [PubMed] [Google Scholar]
- 25.Kovats S. Estrogen receptors regulate an inflammatory pathway of dendritic cell differentiation: mechanisms and implications for immunity. Horm Behav. 2012 doi: 10.1016/j.yhbeh.2012.04.011. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giannoni E, Guignard L, Knaup RM, et al. Estradiol and progesterone strongly inhibit the innate immune response of mononuclear cells in newborns. Infect Immun. 2011;79:2690–2698. doi: 10.1128/IAI.00076-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaitsu M, Narita S, Lambert KC, et al. Estradiol activates mast cells via a nongenomic estrogen receptor-alpha and calcium influx. Mol Immunol. 2007;44:1987–1995. doi: 10.1016/j.molimm.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uemura Y, Liu TY, Narita Y, et al. 17 Beta-estradiol (E2) plus tumor necrosis factor-alpha induces a distorted maturation of human monocyte derived dendritic cells and promotes their capacity to initiate T-helper 2 responses. Hum Immunol. 2008;69:149–157. doi: 10.1016/j.humimm.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Guo H, Liu T, Jiao S, et al. Bisphenol A in combination with TNF-alpha selectively induces Th2 cell-promoting dendritic cells in vitro with an estrogen-like activity. Cell Mol Immunol. 2010;7:227–234. doi: 10.1038/cmi.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshimura S, Bondeson J, Foxwell BM, et al. Effective antigen presentation by dendritic cells is NF-kappaB dependent: coordinate regulation of MHC, co-stimulatory molecules and cytokines. Int Immunol. 2001;13:675–683. doi: 10.1093/intimm/13.5.675. [DOI] [PubMed] [Google Scholar]
- 31.Cai Y, Zhou J, Webb DC. Estrogen stimulates Th2 cytokine production and regulates the compartmentalisation of eosinophils during allergen challenge in a mouse model of asthma. Int Arch Allergy Immunol. 2012;158:252–260. doi: 10.1159/000331437. [DOI] [PubMed] [Google Scholar]
- 32.Sakai T, Furoku S, Nakamoto M, et al. The soy isoflavone equol enhances antigen-specific IgE production in ovalbumin-immunized BALB/c mice. J Nutr Sci Vitaminol (Tokyo) 2010;56:72–76. doi: 10.3177/jnsv.56.72. [DOI] [PubMed] [Google Scholar]
- 33.Jing H, Wang Z, Chen Y. Effect of oestradiol on mast cell number and histamine level in the mammary glands of rat. Anat Histol Embryol. 2012;41:170–176. doi: 10.1111/j.1439-0264.2011.01120.x. [DOI] [PubMed] [Google Scholar]
- 34.Salam M, Wenten M, Gilliland F. Endogenous and exogenous sex steroid hormones and asthma and wheeze in young women. J Allergy Clin Immunol. 2006;117:1001–1007. doi: 10.1016/j.jaci.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 35▪.Macsali F, Gomez Real F, Plana E, et al. Early age at menarche, lung function, and adult asthma. Am J Respir Crit Care Med. 2011;183:8–14. doi: 10.1164/rccm.200912-1886OC. This study examined the relationship between age of onset of menses and asthma in adulthood. It found that women with menarche prior to age 10 had lower lung function and were more likely to have wheeze, multiple symptoms of asthma, and higher asthma score. This supports prior evidence that female sex hormones impart increased risk of asthma. [DOI] [PubMed] [Google Scholar]
- 36.Sabry E. Relation of perimenstrual asthma with disease severity and other allergic comorbidities: the first report of perimenstrual asthma prevalence in Saudi Arabia. Allergol Immunopathol. 2011;39:23–26. doi: 10.1016/j.aller.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 37▪.Wegienka G, Hasiec E, Boushey H, et al. Studying forced expiratory volume at 1 s over menstrual segments in asthmatic and nonasthmatic women: assessing protocol feasibility. BMC Res Notes. 2012;5:261. doi: 10.1186/1756-0500-5-261. This pilot study demonstrated decrease in FEV1 over the course of the follicular phase of the menstrual cycle, implicating high estrogen levels in decreased lung function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dratva J, Schindler C, Curjuric I, et al. Perimenstrual increase in bronchial hyperreactivity in premenopausal women: results from the population-based SAPALDIA 2 cohort. J Allergy Clin Immunol. 2010;125:823–829. doi: 10.1016/j.jaci.2009.12.938. [DOI] [PubMed] [Google Scholar]
- 39.Farha S, Asosingh K, Laskowski D, et al. Effects of the menstrual cycle on lung function variables in women with asthma. Am J Respir Crit Care Med. 2009;180:304–310. doi: 10.1164/rccm.200904-0497OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Townsend EA, Thompson MA, Pabelick CM, Prakash YS. Rapid effects of estrogen on intracellular Ca2+ regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2010;298:L521–L530. doi: 10.1152/ajplung.00287.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41▪.Jain R, Ray J, Pan J, Brody S. Sex hormone-dependent regulation of cilia beat frequency in airway epithelium. Am J Respir Crit Care Med. 2012;46:446–453. doi: 10.1165/rcmb.2011-0107OC. This study demonstrated a decrease in cilia beat frequency in human tracheal epithelial cells induced by progesterone. It also identified an inhibition of this progesterone effect when estradiol was also present. An important point is the potential complex interplay of hormones. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKiernan P, McElvaney N, Greene C. SLPI and inflammatory lung disease in females. Biochem Soc Trans. 2011;39:1421–1426. doi: 10.1042/BST0391421. [DOI] [PubMed] [Google Scholar]
- 43.Morice AH. The cough hypersensitivity syndrome: a novel paradigm for understanding cough. Lung. 2010;188 (Suppl 1):S87–S90. doi: 10.1007/s00408-009-9185-z. [DOI] [PubMed] [Google Scholar]
- 44▪.Morice AH, McGarvey LP, Dicpinigaitis PV. Cough hypersensitivity syndrome is an important clinical concept: a pro/con debate. Lung. 2012;190:3–9. doi: 10.1007/s00408-011-9351-y. This article is an interesting review of the concept of cough hypersensitivity syndrome, its utility as a new paradigm and its shortfalls for clinical application. [DOI] [PubMed] [Google Scholar]
- 45.Patberg K. The female preponderance to cough hypersensitivity syndrome: another clue pointing to the role of TRPV1 in cough. Lung. 2011;189:257–258. doi: 10.1007/s00408-011-9295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Center for the Evaluation of Risks to Human Reproduction. National Toxicology Program. U.S. Department of Health and Human Service; Nov 26, 2007. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A; pp. 1–384. [Google Scholar]
- 47.Midoro-Horiuti T, Tiwari R, Watson CS, Goldblum RM. Maternal exposure to bisphenol A enhances susceptibility to experimental allergic asthma in their pups. Environ Health Perspect. 2010;118:273–277. doi: 10.1289/ehp.0901259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takebayashi T, Abraham J, Murthy GG, et al. Role of tachykinins in airway responses to ozone in rats. J Appl Physiol. 1998;85:442–450. doi: 10.1152/jappl.1998.85.2.442. [DOI] [PubMed] [Google Scholar]
- 49.Lorber M, Phillips L. Infant exposure to dioxin-like compounds in breast milk. Environ Health Perspect. 2002;110:A325–A332. doi: 10.1289/ehp.021100325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmitz R, Atzpodien K, Schlaud M. Prevalence and risk factors of atopic diseases in German children and adolescents: findings from the German Health Interview and Examination Survey for Children and Adolescents (KiGGS) Pediatr Allergy Immunol. 2012 doi: 10.1111/j.1399-3038.2012.01342.x. (in press) [DOI] [PubMed] [Google Scholar]
- 51.Lee SY, Kwon JW, Seo JH, et al. Prevalence of atopy and allergic diseases in Korean children: associations with a farming environment and rural lifestyle. Int Arch Allergy Immunol. 2012;158:168–174. doi: 10.1159/000330820. [DOI] [PubMed] [Google Scholar]
- 52▪▪.Nakajima Y, Goldblum RM, Midoro-Horiuti T. Fetal exposure to bisphenol A as a risk factor for the development of childhood asthma: an animal model study. Environ Health. 2012;11:8. doi: 10.1186/1476-069X-11-8. The authors demonstrated that prenatal exposures to bisphenol A encourage development of experimental allergic asthma. They also demonstrated a key finding of delayed expression of BPA-metabolizing enzymes, which may explain fetal susceptibility to the effects of BPA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53▪.Bauer SM, Roy A, Emo J, et al. The effects of maternal exposure to bisphenol A on allergic lung inflammation into adulthood. Toxicol Sci. 2012;7:e38448. doi: 10.1093/toxsci/kfs227. A mouse model of allergic asthma demonstrated evidence of persistent alterations in inflammatory parameters, but calls into question the long-term effect of prenatal and early life bisphenol A exposure in airway responsiveness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vaidya S, Kulkarni H. Association of urinary bisphenol A concentration with allergic asthma: results from the National Health and Nutrition Examination Survey 2005–2006. J Asthma. 2012;49:800–806. doi: 10.3109/02770903.2012.721041. [DOI] [PubMed] [Google Scholar]
- 55▪.Spanier AJ, Kahn RS, Kunselman AR, et al. Prenatal exposure to bisphenol A and child wheeze from birth to 3 years of age. Environ Health Perspect. 2012;120:916–920. doi: 10.1289/ehp.1104175. This was the first study of the association of prenatal bisphenol A exposure and wheeze in childhood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schonfelder G, Wittfoht W, Hopp H, et al. Parent bisphenol A accumulation in the human maternal–fetal–placental unit. Environ Health Perspect. 2002;110:A703–A707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.CDC (Centers for Disease Control and Prevention) Fourth National Report on Human Exposure to Environmental Chemicals Updated Tables. U.S. Department of Health and Human Services CDC; Sep, 2012. pp. 1–241. [Google Scholar]
- 58.Holguin F, Mannino DM, Anto J, et al. Country of birth as a risk factor for asthma among Mexican Americans. Am J Respir Crit Care Med. 2005;171:103–108. doi: 10.1164/rccm.200402-143OC. [DOI] [PubMed] [Google Scholar]
- 59▪.Hsu NY, Lee CC, Wang JY, et al. Predicted risk of childhood allergy, asthma, and reported symptoms using measured phthalate exposure in dust and urine. Indoor Air. 2012;22:186–199. doi: 10.1111/j.1600-0668.2011.00753.x. This study reported that exposure to elevated levels of phthalate in settled dust was associated with allergy or asthma. An important implication is that controlling environmental exposure to phthalates may be a practical avoidance measure in the management of allergic disease. [DOI] [PubMed] [Google Scholar]
- 60▪.Guo J, Han B, Qin L, et al. Pulmonary toxicity and adjuvant effect of di-(2-exylhexyl) phthalate in ovalbumin-immunized BALB/c mice. PLoS One. 2012;7:e39008. doi: 10.1371/journal.pone.0039008. This study examined the role of DEHP exposure in combination with allergen exposure in airway reactivity. An important demonstration is that DEHP may promote allergic asthma by adjuvant effect in a dose-dependent manner rather than acting alone to promote substantial inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]