Abstract
HeLa cells were stably transfected with a cDNA clone encoding the B1 isoform of the mouse FcγRII receptor (hereafter referred to as HeLa-FcRII cells). The receptor was expressed at high level at the plasma membrane in about 90% of the cells. These cells bound and internalized mouse monoclonal virus-neutralizing antibodies 8F5 and 3B10 of the subtype immunoglobulin G2a (IgG2a) and IgG1, respectively. Binding of the minor-group human rhinovirus type 2 (HRV2) to its natural receptors, members of the low-density lipoprotein receptor family, is dependent on the presence of Ca2+ ions. Thus, chelating Ca2+ ions with EDTA prevented HRV2 binding, entry, and infection. However, upon complex formation of 35S-labeled HRV2 with 8F5 or 3B10, virus was bound, internalized, and degraded in HeLa-FcRII cells. Furthermore, challenge of these cells with HRV2-8F5 or HRV2-3B10 complexes resulted in de novo synthesis of viral proteins, as shown by indirect immunofluorescence microscopy. These data demonstrate that minor-group receptors can be replaced by surrogate receptors to mediate HRV2 cell entry, delivery into endosomal compartments, and productive uncoating. Consequently, the conformational change and uncoating of HRV2 appears to be solely triggered by the low-pH (pH ≤ 5.6) environment in these compartments.
Human rhinoviruses (HRVs), members of the picornavirus family, are the main cause of recurrent mild upper respiratory infections known as the common cold. Their capsid measures about 30 nm in diameter and is composed of 60 copies each of the viral proteins VP1, -2, -3, and -4. It encloses a single-stranded positive-sense RNA genome of some 7,200 bases (28). HRVs are divided into two receptor groups. The major-group HRVs (91 serotypes) bind intercellular adhesion molecule 1 (ICAM-1) (37), whereas the minor group includes 10 serotypes that bind to the low-density lipoprotein receptor (LDLR), the LDLR-related protein (LRP), and the very-low-density lipoprotein receptor (VLDLR). HRV87 has been recently found to phylogenetically belong to the enterovirus genus despite its inactivation at low pH, which is typical for HRVs (30); it also uses a yet-uncharacterized receptor and therefore does not belong to either group. ICAM-1 belongs to the immunoglobulin superfamily and is structurally unrelated to LDLR (34).
A hallmark of members of the LDLR family is the presence of various numbers of ligand-binding repeats at the N terminus. These consist of roughly 40 amino acids, each containing three disulfide bridges. The LDLR, VLDLR, and LRP, have 7, 8, and 31 such repeats, respectively. These repeats compose the ligand-binding domains that recognize a number of structurally and functionally diverse ligands (24). The receptors further contain domains with similarity to the epidermal growth factor precursor, YWDT motifs within a β-propeller structure that is responsible for acid-dependent release of the ligands in endosomes (29), a transmembrane region, and a cytoplasmic domain with NPXY internalization motifs. A number of other receptors belong to this family, but these do not appear to play a role in rhinovirus uptake. HeLa cells that are frequently used to study HRVs express all three known minor-group receptors as well as ICAM-1.
HRV type 2 (HRV2), a prototype minor-group virus, enters cells by receptor-mediated endocytosis, presumably dissociates from its receptors in the mildly acidic milieu (pH 6.5 to 6.0) in early endosomes (4), and is subsequently delivered to late endosomes. The more acidic pH (≤5.6) in the lumen of late endosomes induces a conformational change of the viral capsid. This results in release of the viral RNA and RNA penetration into the cytoplasm, where replication occurs (25, 31). For HRV2, the modification of the capsid at low pH exhibits very similar kinetics between 4 and 34°C and is independent of whether the virus is in solution or bound to plasma membrane receptors (4). Thus, in contrast to ICAM-1, which catalyzes uncoating of major-group HRVs, minor-group receptors appear not to be required for the conformational modification and uncoating.
To assess whether minor-group receptors are indeed dispensable for HRV2 infection, we studied virus binding and internalization via mouse Fc-γ receptors (FcR) in conjunction with mouse anti-HRV2 monoclonal antibodies (MAbs). Three distinct classes of FcRs are known: FcRI, FcRII, and FcRIII. In the mouse, each class is encoded by a single gene, with alternative splicing giving rise to different isoforms. The classes differ in affinity and specificity toward the immunoglobulin G (IgG) subclasses. FcRI binds monomeric IgGs (with high affinities), while FcRII and FcRIII bind IgG immune complexes with low and medium affinities, respectively (9, 20). We have chosen FcRII, because well-characterized rat MAbs specific for this receptor are available (38). Two isoforms of this receptor are known, FcRIIB1 and FcRIIB2, which are predominantly expressed in lymphocytes and macrophages, respectively (20). Whereas the function of FcRIIB2 is the internalization and thus clearance of immune complexes from the circulation, FcRIIB1 is a so-called inhibitory receptor as it blocks B-cell activation (9, 26).
Similar approaches toward usage of surrogate receptors have been pursued previously to either internalize virus into cells devoid of the natural receptor or to propagate virus carrying mutations abrogating binding. Foot-and-mouth disease virus (FMDV), another picornavirus causing devastating disease in cattle, was shown to infect Chinese hamster ovary (CHO) cells via FcRIIB2 when complexed to a neutralizing antibody (18). Akio Nomoto's group used the high-affinity FcRI for infection of mouse cells whose poliovirus receptor homologue does not bind the virus (1).
For minor-group HRVs the situation is more complicated. Members of the LDLR are highly conserved throughout evolution and are ubiquitously expressed. Therefore, apart from differences in affinity, minor-group HRVs bind to cells of many species, but most of them, in particular HRV2, do not replicate (27). Therefore, a human cell line had to be used in surrogate receptor studies; however, this either required the prevention of endocytosis via the natural receptors or neutralization of the virus by suitable antibodies. To prevent binding of HRV2 to LDLRs, the Ca2+ ions present in each one of the ligand binding repeats (8) were chelated. In addition, neutralizing MAbs were used. Under these conditions, complexes between HRV2 and MAbs were taken up and initiated infection in HeLa cells only upon expression of the murine FcRIIB1 receptor.
MATERIALS AND METHODS
Materials.
All chemicals were purchased from Sigma (St. Louis, Mo.) unless stated otherwise. Tissue culture media and supplements were from Gibco (Invitrogen Corp., Paisley, United Kingdom). Tissue culture media for growth of HeLa cells and of 2.4G2 hybridoma in suspension were from Sigma. Tissue culture plates and flasks were from Iwaki (Bibby Sterilin, Stone, Staffordshire, United Kingdom). Alexa Fluor secondary antibodies were obtained from Molecular Probes (Eugene, Oreg.).
Cells and viruses.
HeLa-H1 cells (American Type Culture Collection [ATCC], Manassas, Va.) were used for establishment of HeLa-FcRII, stably expressing the mouse IgG FcγRIIB1 receptor. Cells were grown in monolayers in minimal essential medium (MEM) supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM l-glutamine, penicillin G sodium salt (100 U/ml), and streptomycin sulfate (100 μg/ml). For suspension culture, minimal essential medium modified for suspension (S-MEM) containing 7% horse serum (HS) with the same additives was used. 2.4G2 hybridoma cells, secreting a rat IgG2b MAb against the mouse FcγRII ectodomain, were obtained from the ATCC (HB-197) and cultivated in Dulbecco's modified essential medium (4,500 mg/liter of glucose) supplemented with 5% FCS, 5% HS, and gentamicin (100 μg/ml). These cells were maintained at a density between 105 and 106 viable cells/ml. HRV2, originally obtained from the ATCC, was propagated and labeled with 35S-labeled cysteine/methionine (American Radiolabeled Chemicals, Inc., St. Louis, Mo.) as described previously (23).
Stable transfection of HeLa-H1 cells with FcRII.
The cDNA encoding FcγRII-B1, one of the two murine FcRII isoforms (15), was excised from pCB6 (kindly provided by W. Hunziker, Institute of Molecular and Cell Biology, Singapore, Republic of Singapore) and inserted into the eukaryotic expression vector pEF-Puro using standard procedures. The plasmid was amplified and then transfected stably in HeLa-H1 cells (2 μg of DNA; 3 × 105 cells) using GeneJammer Transfection Reagent (Stratagene, La Jolla, Calif.). After selection in puromycin (2 μg/ml) for 2 to 3 weeks, single resistant clones were expanded and screened for FcRII expression by immunofluorescence. Clone E1/1, whose expression rate was high, was selected for further experiments and designated HeLa-FcRII.
Production of MAb 2.4G2.
2.4G2 hybridoma cells (106/ml) were washed, transferred into protein-free medium (PFHM II; Gibco) containing (gentamicin 100 μg/ml), and cultivated for about 14 days without removing the medium. Fresh medium was only added when the cell density exceeded 106/ml. 2.4G2 was purified from the supernatant by (NH4)2SO4 precipitation (final saturation, 55%) followed by dialysis against 10 mM NaPO4 buffer. The material was lyophilized and dissolved in water to give the desired concentration (stock, 2.5 mg/ml).
Antibody subtype determination.
Enzyme-linked immunosorbent assay plates (Nalge Nunc International, Rochester, N.Y.) were coated with 100 μl of purified HRV2 (∼0.1 μg/ml) in Tris-buffered saline, pH 7.4, containing 2 mM CaCl2 (TBSC) at 4°C overnight. After unspecific binding sites were blocked with 2% bovine serum albumin in TBSC the plates were incubated with 100 μl of the anti-HRV2-MAbs (∼1 μg/ml diluted in TBSC) for 2 h at room temperature (RT). After washing, wells were incubated with goat anti-mouse isotype-specific antibodies (Sigma) for 30 min at RT followed by alkaline phosphatase-conjugated rabbit anti-goat antiserum (1:5,000). After 15 min at RT, the plates were washed, bound antibody was detected with p-nitrophenylphosphate (1 mg/ml) in 100 mM glycine buffer (pH 10.5), and the extinction was measured at 405 nm (reference wavelength, 550 nm) in a Microplate Reader (Dynatech Laboratories Inc., Chantilly, Va.).
Antibody binding and internalization.
Cells were grown on coverslips overnight until half-confluent and were then washed and preincubated in serum-free MEM for 30 min at 37°C to deplete endogenous IgG. They were then incubated with 8F5 (40 μg/ml) or 3B10 (15 μg/ml)—at the respective minimal concentrations required to completely neutralize 106 PFU/ml of HRV2—for 1 h at 4°C (binding assay) or for 20 or 60 min at 34°C (internalization assay), and this was followed by ice-cold phosphate-buffered saline (PBS) washes prior to fixation. As a control, the IgG3 anti-HRV2 MAb 5H5 (40 μg/ml) was used when indicated. Experiments in the absence of Ca2+ were carried out with cells grown in suspension: 105 cells were washed and depleted of endogenous IgG by incubation in 1 ml of serum-free MEM for 30 min at 37°C. Cells were then incubated either with 3B10 or 8F5 in 500 μl of MEM containing 10 mM EDTA for 1 h at 4°C (binding) or for 20 min at 34°C (internalization). To determine 8F5 internalization, cells were subsequently transferred into MEM without EDTA and further incubated for 40 min at 34°C. Cells were spun onto adhesive slides (Starfrost Adhäsiv; Laboroptik GmbH, Friedrichsdorf, Germany), washed and further processed for immunofluorescence microscopy.
Indirect immunofluorescence microscopy.
Cells were extensively washed with ice-cold PBS++ (PBS containing 1 mM CaCl2 and 1 mM MgCl2), fixed with 4% paraformaldehyde in PBS at RT for 30 min, quenched with 50 mM NH4Cl in PBS for 10 min at RT, and washed three times with PBS. Only if the cells had to be permeabilized were they incubated with 0.1% Triton X-100 in PBS for 5 min at RT and washed three times with PBS. Unspecific binding sites were blocked with 0.5% bovine serum albumin in PBS for 15 min at RT. FcRII was detected with rat anti-FcRII MAb 2.4G2 (50 μg/ml) followed by Alexa 568-conjugated goat anti-rat IgG (1:600). HRV2 was detected with 8F5 (32) at 5 μg/ml followed by Alexa 488-conjugated goat anti-mouse IgG (1:1,000). Cells were mounted in Immuno Fluore Mounting Medium (ICN Pharmaceuticals, Inc., Costa Mesa, Calif.) and viewed using a Zeiss Axioplan 2 (Carl Zeiss, Jena, Germany). Images were processed by using Zeiss AxioVision software and Adobe Photoshop software.
Fluorescence-activated cell sorter analysis of FcRII expression and antibody binding.
Confluent parental HeLa cells or HeLa-FcRII cells grown in six-well plates (∼106 cells) were washed and preincubated in serum-free MEM for 30 min at 37°C to deplete endogenous IgG. Cells were cooled, scraped, transferred into fluorescence-activated cell sorter tubes, and incubated with 8F5 (40 μg/ml) or 3B10 (15 μg/ml) for 1 h at 4°C, and this was followed by washing with ice-cold PBS prior to fixation with 4% paraformaldehyde in PBS. Cells were washed and incubated with Alexa 488-conjugated goat anti-mouse IgG (1:400), and this was followed by incubation with rat anti-FcRII MAb 2.4G2 (100 μg/ml) and Alexa 568-conjugated goat anti-rat IgG (1:300). The final cell pellet was resuspended in 300 μl of PBS, and cells were immediately analyzed by flow cytometry. Each sample was measured six times. A dual-laser FACScalibur (Becton Dickinson Immunocytometry Systems) equipped with argon-ion and red-diode lasers was used. Alexa 488 fluorescence (excitation wavelength, 499 nm) was measured using a 530-nm band-pass filter (30-nm bandwidth), and Alexa 568 fluorescence (excitation wavelength, 579 nm) was measured using a 585-nm band-pass filter (42-nm bandwidth). Forward light scatter and 90° (side) scatter, along with both fluorescence values, were collected in list mode using 256-channel resolution. Data for 10,000 cells were collected. In dual-fluorescence histograms, threshold parameters were set after analyzing unlabeled HeLa-FcRII cells. Thus, a region was defined for Alexa 488 and/or Alexa 568 fluorescence-positive events.
Detection of de novo-synthesized viral proteins upon internalization of virus-antibody complexes in the absence of Ca2+.
106 PFU of HRV2, corresponding to a multiplicity of infection (MOI) of 10 when added to the cells (105), was preincubated with or without anti-HRV2 MAbs (8F5 at 40 μg/ml and 3B10 at 15 μg/ml) in 500 μl of MEM with or without 10 mM EDTA for 1 h at RT. These solutions containing virus alone or virus-MAb complexes were then incubated with 105 HeLa-FcRII cells grown in suspension (following depletion of endogenous IgG) for 20 min at 34°C. Taking into account the Ca2+ and Mg2+ present in the medium, this results in a concentration of free EDTA of about 7 mM. After extensive washing with EDTA-containing MEM and PBS, cells were transferred into eight-well Lab-Tek glass chamber slides (Nalge Nunc International) and further incubated for 18 h at 34°C in 250 μl of MEM with supplements (but without EDTA). De novo synthesis of viral proteins and nucleus staining were monitored by immunofluorescence, and the ratio between the total cell number and the number of cells expressing viral proteins was determined by examining about 1,500 to 2,000 cells.
Binding of HRV2-Ab complexes.
35S-labeled HRV2 (6 × 104 cpm) was preincubated with or without MAbs (8F5, 40 μg/ml; 3B10, 15 μg/ml) for 1 h at RT in 500 μl of MEM containing 10 mM EDTA under rotation and then cooled to 4°C. Subsequently, 5 × 106 HeLa-FcRII suspension cells (following depletion of endogenous IgG) in 1 ml of MEM-10 mM EDTA were added and mixed, and the suspensions were divided into three aliquots (500 μl each) and incubated for 1 h at 4°C. Cells were washed three times with PBS, and the cell pellets were lysed in 50 μl of RIPA buffer (50 mM Tris-HCl [pH 7.5] containing 150 mM NaCl, 1 mM EDTA, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and 1% Triton X-100) for 10 min on ice. Lysates were mixed with 10 volumes of scintillation cocktail Emulsifier-safe (Packard-PerkinElmer Life Sciences Inc., Boston, Mass.) for counting.
Lysosomal degradation of MAb-complexed 35S-labeled HRV2.
35S-labeled HRV2 (3 × 105 cpm) was preincubated with MAbs (8F5, 40 μg/ml; 3B10, 15 μg/ml) for 1 h at RT in 500 μl of MEM containing 10 mM EDTA. These samples were then added to 107 suspension-grown HeLa-FcRII cells (depleted of endogenous IgG and suspended in 1 ml MEM, containing EDTA), mixed, and divided into three aliquots (500 μl each) to be used for parallel determinations. As a control, 35S-labeled HRV2 preincubated in the absence of antibodies was used. After incubation for 20 min at 34°C either in the presence or in the absence of calcium, cells were pelleted and washed with EDTA-containing MEM followed by PBS, and each aliquot was resuspended in 1 ml of MEM (without EDTA) containing 2% HS. A 500-μl aliquot of this suspension was analyzed immediately for trichloroacetic acid (TCA)-precipitable counts. The remaining 500-μl suspensions were further incubated for 220 min at 34°C (chase). To determine total cell-associated and TCA-soluble counts after the 20-min internalization period, cells were pelleted and resuspended in 100 μl of MEM supplemented with 2% HS. Proteins were precipitated by addition of an equal volume of ice-cold 10% TCA, and precipitable and nonprecipitable radioactivities were quantified by liquid scintillation counting after neutralization with NaOH. In the samples from the 220-min chase, total TCA-precipitable and -nonprecipitable radioactivity was determined in the entire cell suspensions (including the supernatant).
RESULTS
Functional expression of murine FcRIIB1 in HeLa H1 cells.
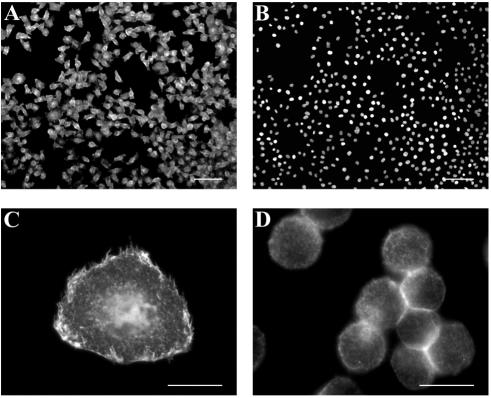
HeLa H1 cells were stably transfected with a cDNA clone encoding the B1 isoform of the mouse FcRII receptor under the control of the EF1α promoter. Since FcRII receptors are normally expressed in macrophages and lymphocytes (20), we first determined whether in HeLa cells the protein was also correctly transported to the plasma membrane in a functional conformation. Surface expression was confirmed by examination of selected clones by indirect immunofluorescence microscopy using rat MAb 2.4G2 followed by secondary Alexa 568-conjugated goat anti-rat IgG. As exemplified for clone E1/1 in Fig. 1A (FcRII staining at the plasma membrane) and 1B (nuclear staining), about 80 to 90% of the cells transfected with the FcRII cDNA plasmid expressed FcRII at a high level. Figure 1C shows that substantial amounts of the receptor were present at the plasma membrane. Nontransfected HeLa-H1 cells were not stained with the antibody (Fig. 2B). This demonstrates that FcRII molecules are correctly transported to the cell surface and are available for antibody binding. Clone E1/1 was used for all further experiments and termed HeLa-FcRII.
FIG. 1.
FcRII expression in stably transfected HeLa-H1 cells. After transfection with pEF-Puro carrying the cDNA encoding murine FcRII and selection, clones were isolated and expanded. Cells grown on coverslips were incubated at 4°C with MAb 2.4G2 followed by Alexa 568-conjugated goat anti-rat IgG and viewed under a Zeiss Axiovert fluorescence microscope. Clone E1/1 is shown as an example. Comparison of the FcRII expression (A) and the nuclear staining of the same cells (B) shows that about 90% of the transfected cells express FcRII at the cell surface. Nuclei were stained with Hoechst dye. (C) At a higher magnification, plasma membrane staining can be seen. (D) FcRII expression in HeLa-FcRII cells grown in suspension. Bar, 100 μm in panels A and B; 20 μm in panels C and D.
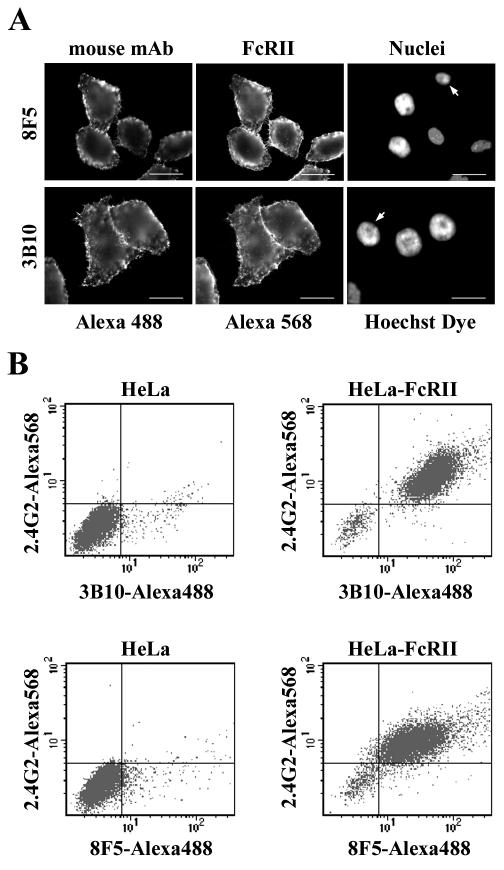
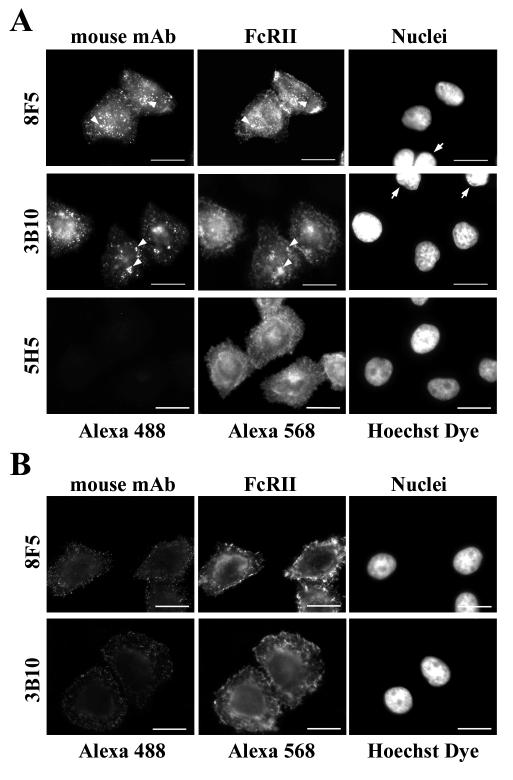
FIG. 2.
MAbs 8F5 and 3B10 bind to FcRII. (A) HeLa-FcRII cells were grown on coverslips, incubated with 8F5 (40 μg/ml) or with 3B10 (15 μg/ml) at 4°C for 1 h, washed, fixed, and stained with Alexa 488-conjugated goat anti-mouse antibodies. Subsequently, cells were incubated with 2.4G2 followed by Alexa 568-conjugated goat anti-rat antibody. Nuclei were stained with Hoechst dye. Note the absence of Alexa 488 staining in cells that do not express FcRII (arrows). Bar, 20 μm. (B) Parental HeLa cells or HeLa-FcRII cells were incubated with 8F5 (40 μg/ml) or 3B10 (15 μg/ml) at 4°C for 1 h, washed, fixed, and stained with Alexa 488-conjugated goat anti-mouse antibodies followed by incubation with 2.4G2 and Alexa 568-conjugated goat anti-rat antibodies. Cells were analyzed by flow cytometry (for details, see Materials and Methods). One typical experiment out of three is shown. Note that only those cells that express FcRII bound the MAbs.
Having established that FcRII was expressed at the surface of HeLa-FcRII cells when grown in monolayers, we next determined the distribution of FcRII in cells grown in suspension culture. HeLa-FcRII cells propagated in a spinner flask in suspension medium were spun onto slides, and plasma membrane expression of FcRII was monitored with MAb 2.4G2. Except for a different morphology of the cells cultured in suspension, the FcRII molecules were also expressed at the cell surface (Fig. 1D). To also reveal intracellular receptor, cells were permeabilized prior to immunostaining but virtually no intracellular receptor was observed (data not shown). This agrees well with the exclusive surface expression of the FcRIIB1 isoform (22) and suggests that endocytosis of HRV2 complexed with anti-HRV2 MAbs might occur via FcRII. Therefore, we next examined whether the FcRII molecules expressed by HeLa-FcRII cells were able to bind and internalize anti-HRV2 MAbs.
Anti-HRV2 MAbs bind to and are internalized by HeLa-FcRII cells regardless of the presence of Ca2+.
Subtyping of the available HRV2-neutralizing MAbs revealed various subtypes, including IgG1, IgG2a, and IgG3 (data not shown). However, since 8F5 (IgG2a) and 3B10 (IgG1) are by far the best characterized (10, 11), we decided to carry out all further experiments with these antibodies. First, we verified the subtype specificity of mouse IgGs for the receptor expressed in HeLa-FcRII. Cells were grown on coverslips, washed, depleted of endogenous IgG, and incubated at 4°C for 1 h with commercially available mouse IgG preparations. Cells were fixed and processed for detection of mouse MAbs and FcRII. In accordance with the literature (9), fluorescence microscopy revealed that only antibodies of IgG1, IgG2a, and IgG2b subtype bound to the FcRII-expressing cells (data not shown). Next, we determined whether MAbs 8F5 and 3B10 were bound. The antibodies were used at 40 and 15 μg/ml, respectively, concentrations that completely neutralized 106 PFU of HRV2/ml (see below). Binding was again monitored by indirect immunofluorescence microscopy. As depicted in Fig. 2A, 8F5 as well as 3B10 bound to HeLa-FcRII cells. It is important to point out that only cells expressing the FcRII bound the MAbs, whereas a small number of cells not expressing the receptor (Fig. 2A) as well as parental HeLa cells did not (Fig. 2B).
FcRII expression and anti-HRV2 MAb binding were also quantified by flow cytometry. As shown in Fig. 2B, FcRII was not present in the parental HeLa cells; consequently, they did not bind anti-HRV2 MAbs. On the other hand, 90 to 95% of HeLa-FcRII cells expressed FcRII at the cell surface. The double-positive events indicate that these cells bound 8F5 as well as 3B10. Therefore, antibody binding is clearly dependent on the presence of FcRII.
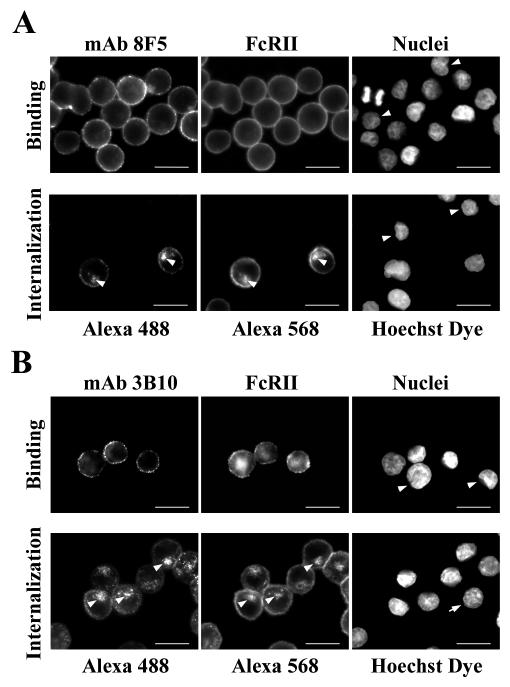
Having established the binding of 8F5 and 3B10 to HeLa-FcRII cells, we next explored whether the anti-HRV2 antibodies were internalized by incubation with 8F5 or 3B10 in serum-free culture medium at 34°C. Immunofluorescence microscopy showed perinuclear localization of the anti-HRV2 MAbs 8F5 and 3B10 only in permeabilized cells (Fig. 3A). The MAbs were only internalized by those cells which expressed FcRII. Furthermore, antibody-induced FcRII-internalization is revealed by colocalization (Fig. 3A). In contrast, only barely visible plasma membrane staining of the antibodies was observed in nonpermeabilized cells (Fig. 3B). An IgG3 antibody (5H5) that does not bind to FcRII was not internalized (Fig. 3A), further substantiating the specific binding and internalization of 8F5 and 3B10 via FcRII. Thus, in agreement with our previous data on IgG uptake in trophoblast cells (7), fluid-phase endocytosis is under the detection limit at the antibody concentrations used in this experiment. Therefore, these data demonstrate that the MAbs were concentrated by internalization via receptor-mediated endocytosis.
FIG. 3.
MAbs 8F5 and 3B10 are internalized into HeLa-FcRII cells. Cells were incubated at 34°C with 8F5 (40 μg/ml), 3B10 (15 μg/ml), or the control antibody 5H5 subtype IgG3 (40 μg/ml) for 60 min (8F5, 5H5) or for 20 min (3B10). Cells were washed, permeabilized (A) or nonpermeabilized (B), and processed for immunofluorescence microscopy as in Fig. 2. Note the perinuclear colocalization of MAbs and FcRII in permeabilized cells, indicative of localization in late endosomes (arrowheads), and the absence of any antibody uptake in FcRII-negative cells (right-hand panels, arrows). The control antibody 5H5 was not internalized by HeLa-FcRII cells. Bar, 20 μm.
The latter experiments were carried out with adherent cells and consequently in the presence of calcium ions. Each ligand-binding repeat in the extracellular domain of the members of the LDLR family builds an octagonal cage chelating a Ca2+ ion. This is required to maintain their native conformation, and in the absence of Ca2+, the affinity for the ligands is lost (5, 6). Therefore, HRV2 fails to bind to cells in the presence of EDTA at concentrations sufficient to complex the Ca2+ ions (17). Hence, binding and internalization of HRV2 via its natural receptors LDLR, VLDLR, and LRP are strictly Ca2+ dependent. EDTA, when added to the medium at a concentration of 10 mM during incubation of the virus with the cells, effectively inhibited binding and viral uptake (see below). As cells readily detach from the substrate in the absence of Ca2+, we rather used cells grown in suspension for the following experiments aimed at determining whether antibody binding to FcRII was Ca2+ dependent.
MAbs were incubated with HeLa-FcRII cells in the presence of EDTA at 4°C for binding, or at 34°C for internalization. After the cells were spun onto slides, they were fixed and MAbs and FcRII were visualized by indirect immunofluorescence microscopy as described above. All cells expressing FcRII unambiguously showed plasma membrane staining of 8F5 (Fig. 4A) and of 3B10 (Fig. 4B), indicating that binding of IgG to FcRII is not dependent on the presence of calcium ions. By the same token, complexation of Ca2+ did not inhibit internalization (lower panels in Fig. 4) as revealed by perinuclear colocalization of the antibodies and FcRII (Fig. 4). Taken together, these results show that the mouse anti-HRV2 MAbs bind to HeLa-FcRII and are internalized even in the absence of calcium ions.
FIG. 4.
Binding and internalization of antibodies is Ca2+ independent. HeLa-FcRII were grown in suspension and incubated with 8F5 (A) and 3B10 (B) in medium containing 10 mM EDTA either at 4°C for 1 h (binding, upper panels) or at 34°C for 60 min (8F5) or 20 min (3B10) (internalization, lower panels). Cells were washed, fixed, spun onto microscope slides and processed for indirect immunofluorescence microscopy as in Fig. 2. Upon internalization, colocalization of the antibodies with FcRII was observed (arrowheads). Again, cells devoid of FcRII do not bind and internalize antibodies (arrows). Bar, 20 μm.
HRV2 coated with MAbs binds to HeLa-FcRII cells in the absence of calcium.
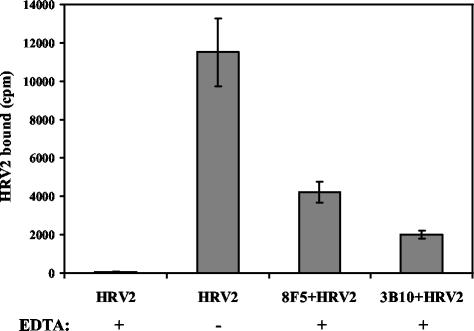
As shown below, the presence of 10 mM EDTA in the medium blocked HRV2 binding to HeLa cells. Therefore, to avoid HRV2 uptake via the natural receptors, the following experiments were performed in medium containing 10 mM EDTA. HeLa-FcRII cells grown in suspension were washed, depleted of endogenous IgG, and incubated for 1 h at 34°C with 35S-labeled HRV2 alone or with 35S-HRV2 complexed to 8F5 or 3B10. Cells were washed, and cell-associated radioactivity was determined by liquid scintillation counting. As seen in Fig. 5, in the absence of MAb, almost no virus was cell associated, confirming that 10 mM EDTA effectively prevented HRV2 binding to its natural receptors. In contrast, if MAb-complexed 35S-HRV2 was presented to the HeLa-FcRII cells in EDTA-containing medium, about 27% (8F5) and 14% (3B10) of the total 35S-labeled HRV2 became cell associated. Control incubations showed that MAb-complexed 35S-HRV2 as well as 35S-HRV2 alone failed to bind to parental HeLa cells in the absence of calcium (data not shown). These results clearly demonstrate that both antibodies mediate binding of HRV2 to FcRII-expressing HeLa cells.
FIG. 5.
HRV2 binds to HeLa-FcRII cells via MAbs in the absence of Ca2+. HeLa-FcRII suspension cells were incubated with 35S-labeled HRV2 alone or with HRV2 complexed to MAbs 8F5 and 3B10, respectively, in the presence or absence of 10 mM EDTA for 1 h at 4°C. Cells were pelleted and washed, and bound radioactivity was determined by liquid scintillation counting. Data given are means ± standard deviations (error bars) from one typical experiment out of three independent experiments, each carried out in triplicate.
Internalization and degradation of MAb-complexed HRV2 in HeLa-FcRII cells.
In order to differentiate between bound and internalized virus it is necessary to remove virus-antibody complexes from the plasma membrane. However, various low-pH buffers (pH 4.0 to 2.0) failed to dissociate FcR-IgG complexes. It was thus impossible to determine whether MAb-complexed HRV2 was internalized or remained bound to the plasma membrane. Therefore, we assessed whether MAb-coated HRV2 was degraded upon prolonged incubation.
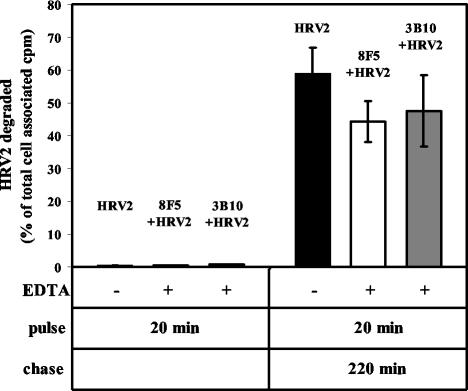
As demonstrated by immunofluorescence microscopy and cell fractionation in the parental HeLa cells, HRV2 arrives in lysosomes 25 to 30 min after uptake as indicated by rapid degradation and the appearance of TCA-soluble material (3, 25). To study whether MAb-complexed HRV2 is indeed transferred to lysosomes we determined the TCA-soluble radioactivity upon incubation of 35S-HRV2-MAb complexes with HeLa-FcRII cells. After depletion of endogenous IgG, cells were incubated for 20 min at 34°C with 8F5- or 3B10-complexed 35S-HRV2 in medium containing 10 mM EDTA. For control purposes HeLa-FcRII cells were incubated with 35S-HRV2 in medium without EDTA under the same conditions. The cells were then washed with EDTA-containing medium and either immediately analyzed or further incubated in fresh medium without EDTA for 220 min. TCA-soluble as well as precipitable radioactivity in the cell pellets and supernatants was determined by liquid scintillation counting. Figure 6 shows that less than 1% of cell-associated virus was degraded within 20 min, irrespective of whether it was taken up via its natural receptors or via FcRII when complexed with MAb. About 25, 15, and 20% of total virus became cell associated during this time period for virus without antibody, virus complexed to 8F5, and virus complexed to 3B10, respectively. After a chase of 220 min the control incubation showed about 50% degradation of total cell-associated virus. A similar percentage of degraded virus was found in the samples from HeLa-FcRII-associated 8F5-35S-HRV2 and 3B10-35S-HRV2. This implies that HeLa-FcRII cells readily internalize MAb-HRV2 complexes and that these complexes follow the endocytic route to lysosomes.
FIG. 6.
Antibody-complexed virus is degraded in lysosomes. HeLa-FcRII cells grown in suspension were incubated for 20 min at 34°C with either 35S-HRV2 (in the absence of EDTA; control) or 35S-HRV2 complexed to 8F5 and 3B10, respectively, in the presence of EDTA to inhibit binding of the virus to its natural receptors. Cells were pelleted and washed, and incubation was continued for 220 min in normal medium. Total TCA-soluble and -precipitable counts were determined by liquid scintillation counting (see Materials and Methods). Degraded virus (TCA-soluble counts) is given as a percentage of cell-associated radioactivity after the 20-min incubation. The means ± standard deviations (error bars) of three independent experiments each carried out in triplicate are shown.
Antibody-complexed HRV2 infects HeLa-FcRII cells.
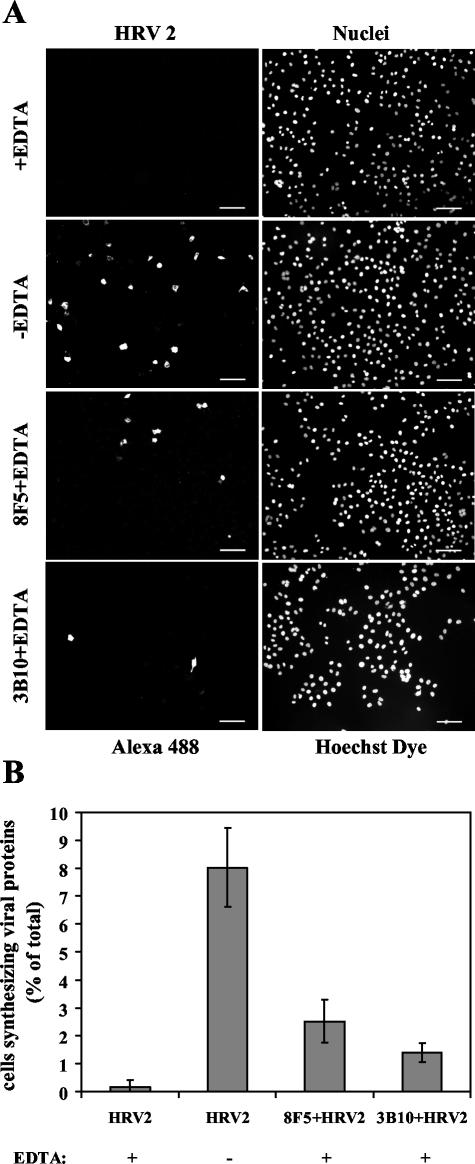
To confirm that EDTA inhibits infection of HeLa cells by HRV2, virus was incubated with HeLa-FcRII suspension cells at an MOI of 10 for 20 min at 34°C in MEM with or without 10 mM EDTA. Cells were extensively washed with EDTA-containing MEM and PBS, transferred into chamber slides, and further incubated in MEM supplemented with FCS, glutamine, and antibiotics for 18 h at 34°C. The cells were then processed for detection of de novo-synthesized viral proteins by indirect immunofluorescence microscopy by using MAb 8F5 (Fig. 7A). Essentially no viral proteins were synthesized when the cells were challenged with HRV2 in the absence of calcium ions, whereas roughly 9% of the cells produced virus antigen in the presence of Ca2+ (Fig. 7B). The rather low number of infected cells is most probably due to the short time of infection (20 min) and the repeated washing with EDTA-containing media before plating.
FIG. 7.
HeLa-FcRII cells are infected with antibody-complexed HRV2. (A) Cells were challenged with HRV2 (MOI, 10) in the presence or absence of 10 mM EDTA for 20 min at 34°C, washed, resuspended in infection medium, and further incubated for 18 h. Cells were fixed and stained for the presence of de novo-synthesized viral proteins by using MAb 8F5. Nuclei were stained with Hoechst dye. Images show 150 to 300 cells. Infection with HRV2-antibody complexes was carried out identically but in the presence of EDTA only. Bar, 100 μm. (B) Virus-synthesizing cells are given as percentage of the cells positive for viral antigen. One typical experiment out of three independent experiments is shown; results are presented as means ± standard deviations (error bars) of triplicates, with a total of about 1,500 to 2,000 cells examined.
Control experiments established that in internalized virus-antibody complexes the antibodies were under the limit of detection by fluorescence microscopy after 18 h due to lysosomal degradation. Therefore, staining with 8F5 and secondary antibody after 18 h was specific for newly synthesized virus. Under the conditions described above HRV2 complexed to 8F5 or 3B10 resulted in de novo viral synthesis in the presence of EDTA. HeLa-FcRII cells synthesizing viral proteins, as seen by immunofluorescence microscopy (Fig. 7A), were counted and related to the total number of cells (Fig. 7B). Viral proteins were seen in 1.4 and 2.5% of the cells upon challenge with 3B10- and 8F5-complexed HRV2, respectively. Taken together, complexation of the virus with virus-specific MAbs in conjunction with FcRII can mediate internalization and infection of HRV2.
DISCUSSION
In contrast to findings for the major-group rhinovirus receptor ICAM-1 and the poliovirus receptor, which not only bind but also catalyze uncoating of their cognate viruses, our previous investigations suggested no such function for LDLRs (4, 12). We thus decided to systematically investigate whether the natural receptors of the minor-group rhinovirus HRV2 could be replaced by suitable surrogate receptors to mediate endocytosis into a compartment with a pH of ≤5.6, i.e., late endosomes that would allow for the conformational modification and uncoating.
Similar approaches using Fc receptors have been used previously for FMDV and poliovirus, other members of the picornavirus family. FMDV was shown to infect cells via virus-specific antibodies attaching to FcRIIB2 expressed in CHO cells (18). Although FMDV bound substantially to these cells in the absence of antibodies, infection occurred only when the virus was coated with antibodies. In subsequent studies the authors applied this system to propagate virus mutated in the receptor binding site (19). The same system appeared not suitable for poliovirus infection. However, it was later shown by Akio Nomoto's group that some selected MAbs, when bound to the high-affinity Fc-γ (FcRI) receptor, initiated infection of mouse cells whose poliovirus receptor homologue does not bind the virus (1). However, in the absence of the poliovirus receptor the efficiency of infection was very low. Nevertheless, this is remarkable since the poliovirus receptor was believed to be indispensable as it not only binds the virus but also catalyzes its disassembly. In another study it was shown that poliovirus 135S subviral particles were infectious upon internalization via FcRII (13). This underscores the fact that the receptor is dispensable once the conformational change has taken place.
The latter studies used cells devoid of the natural receptors; as a consequence they were not susceptible for infection. LDLRs are evolutionarily conserved, and HRV2 binds to cells from a number of species, e.g., mouse fibroblasts, but fails to replicate due to an intracellular block (39). Thus, the only available cell line, mouse M4 cells, which does not express any of the known minor-group HRV receptors could not be used for the present studies (27). Therefore, HeLa-H1 cells were employed in the presence of EDTA to exclude infection via the natural receptors. Furthermore, the MAbs are virus neutralizing (11, 35); they were employed at concentrations which, again, preclude infection of parental HeLa-H1 cells. With these precautions we clearly established that HeLa cells expressing the FcRIIB1 receptor could be infected with virus-antibody complexes.
Two isoforms of the FcRII receptor are known. These exhibit low affinity for IgG1 and IgG2a/b complexes. Whereas FcRIIB2 is internalized by the clathrin-mediated pathway, FcRIIB1 has a 47-amino-acid insertion in the cytoplasmic tail that actively prevents localization in clathrin-coated pits (21). Nevertheless, the latter isoform is internalized, albeit at a reduced level (22) and leads to phagocytic uptake of IgG-coated Toxoplasma gondii (16). Based on the findings that phagocytosis of small and large particles is dynamin I and clathrin independent (36), the B1 isoform can mediate clathrin-independent endocytosis and phagocytosis.
We initially expressed both isoforms in HeLa cells at similar levels (data not shown). However, at steady state FcRIIB1 was expressed at the plasma membrane only (e.g., Fig. 1), whereas FcRIIB2 was found at the plasma membrane as well as in endosomes (data not shown). Unexpectedly, FcRIIB1 was substantially more efficient in mediating binding and internalization of anti-HRV2 antibodies. Thus, we used this receptor for all further studies. Despite its low affinity for IgG complexes, we could demonstrate binding of the antibodies and antibody-induced internalization of FcRII (Fig. 4). Immunofluorescence microscopy revealed enhanced binding and internalization upon complexation of the antibodies with virus (data not shown). However, lack of suitable HRV2 antibodies allowed us to demonstrate neither colocalization of antibody, virus, and FcRII nor their cointernalization and colocalization in endocytic compartments. For this reason we intended to determine internalization of radiolabeled HRV2-antibody complexes but found that plasma membrane-bound complexes could not be removed even by treatment at pH 2. Therefore, internalization of virus-antibody complexes was demonstrated by lysosomal degradation of 35S-labeled HRV2 (Fig. 6). This again demonstrates that this minor-group HRV can be internalized by a clathrin-independent route under conditions where the clathrin-mediated pathway is blocked (2, 14, 33). During its natural entry route HRV2 dissociates from its receptors at a mildly low pH of about 6.0 in early endosomes (4) prior to uncoating (at a pH of ≤5.6) in late endosomes (25). Since antibody-FcRII complexes are not dissociated at low pH (pH > 2) and the antibodies employed recognize native virus as well as subviral particles, the virus most probably remains bound to the antibody-FcRII complex during its transit through late endosomes. Thus, RNA release must occur when the virus is still attached to FcRII via the antibody.
Although 8F5-HRV2 complexes were bound to HeLa-FcRII cells to a higher extent than 3B10-virus complexes, we did not see any gross differences between the two antibodies with respect to infection efficiency despite their different binding geometry. Whereas 8F5 binds bivalently over the twofold axis of the icosahedral symmetry of the viral capsid (10), 3B10 binds monovalently (11). Since both antibodies bind to the same epitope, we do not know whether a particular site is better suited for viral delivery.
In summary, we have demonstrated that LDLRs are dispensable for infection and can be replaced by FcRII receptors in conjunction with virus-neutralizing antibodies under conditions which inhibit binding of the virus to its natural receptors. Therefore, we believe that this system can be used for the characterization of HRV variants carrying mutations in their receptor binding site. Experiments along these lines are currently being pursued.
Acknowledgments
This work was funded by the Austrian Virology Foundation (D.B. and R.F.).
REFERENCES
- 1.Arita, M., H. Horie, and A. Nomoto. 1999. Interaction of poliovirus with its receptor affords a high level of infectivity to the virion in poliovirus infections mediated by the Fc receptor. J. Virol. 73:1066-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayer, N., D. Schober, M. Hüttinger, D. Blaas, and R. Fuchs. 2001. Inhibition of clathrin-dependent endocytosis has multiple effects on HRV2 cell entry. J. Biol. Chem. 276:3952-3962. [DOI] [PubMed] [Google Scholar]
- 3.Bayer, N., D. Schober, E. Prchla, R. F. Murphy, D. Blaas, and R. Fuchs. 1998. Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection. J. Virol. 72:9645-9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brabec, M., G. Baravalle, D. Blaas, and R. Fuchs. 2003. Conformational changes, plasma membrane penetration, and infection by human rhinovirus type 2: role of receptors and low pH. J. Virol. 77:5370-5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, M. S., J. Herz, and J. L. Goldstein. 1997. LDL receptor structure: calcium cages, acid baths and recycling receptors. Nature 388:629-630. [DOI] [PubMed] [Google Scholar]
- 6.Dirlam-Schatz, K. A., and A. D. Attie. 1998. Calcium induces a conformational change in the ligand binding domain of the low density lipoprotein receptor. J. Lipid Res. 39:402-411. [PubMed] [Google Scholar]
- 7.Ellinger, I., M. Schwab, A. Stefanescu, W. Hunziker, and R. Fuchs. 1999. IgG transport across trophoblast-derived BeWo cells: a model system to study IgG transport in the placenta. Eur. J. Immunol. 29:733-744. [DOI] [PubMed] [Google Scholar]
- 8.Fass, D., S. Blacklow, P. S. Kim, and J. M. Berger. 1997. Molecular basis of familial hypercholesterolaemia from structure of LDL receptor module. Nature 388:691-693. [DOI] [PubMed] [Google Scholar]
- 9.Gessner, J. E., H. Heiken, A. Tamm, and R. E. Schmidt. 1998. The IgG Fc receptor family. Ann. Hematol. 76:231-248. [DOI] [PubMed] [Google Scholar]
- 10.Hewat, E. A., and D. Blaas. 1996. Structure of a neutralizing antibody bound bivalently to human rhinovirus 2. EMBO J. 15:1515-1523. [PMC free article] [PubMed] [Google Scholar]
- 11.Hewat, E. A., T. C. Marlovits, and D. Blaas. 1998. Structure of a neutralizing antibody bound monovalently to human rhinovirus 2. J. Virol. 72:4396-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofer, F., M. Gruenberger, H. Kowalski, H. Machat, M. Huettinger, E. Kuechler, and D. Blass. 1994. Members of the low density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proc. Natl. Acad. Sci. USA 91:1839-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang, Y., J. M. Hogle, and M. Chow. 2000. Is the 135S poliovirus particle an intermediate during cell entry? J. Virol. 74:8757-8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huber, M., M. Brabec, N. Bayer, D. Blaas, and R. Fuchs. 2001. Elevated endosomal pH in HeLa cells overexpressing mutant dynamin can affect infection by pH-sensitive viruses. Traffic 2:727-736. [DOI] [PubMed] [Google Scholar]
- 15.Hunziker, W., P. Male, and I. Mellman. 1990. Differential microtubule requirements for transcytosis in MDCK cells. EMBO J. 9:3515-3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joiner, K. A., S. A. Fuhrman, H. M. Miettinen, L. H. Kasper, and I. Mellman. 1990. Toxoplasma gondii: fusion competence of parasitophorous vacuoles in Fc receptor-transfected fibroblasts. Science 249:641-646. [DOI] [PubMed] [Google Scholar]
- 17.Lonberg-Holm, K., and N. M. Whiteley. 1976. Physical and metabolic requirements for early interaction of poliovirus and human rhinovirus with HeLa cells. J. Virol. 19:857-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mason, P. W., B. Baxt, F. Brown, J. Harber, A. Murdin, and E. Wimmer. 1993. Antibody-complexed foot-and-mouth disease virus, but not poliovirus, can infect normally insusceptible cells via the Fc receptor. Virology 192:568-577. [DOI] [PubMed] [Google Scholar]
- 19.Mason, P. W., E. Rieder, and B. Baxt. 1994. RGD sequence of foot-and-mouth disease virus is essential for infecting cells via the natural receptor but can be bypassed by an antibody-dependent enhancement pathway. Proc. Natl. Acad. Sci. USA 91:1932-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mellman, I., T. Koch, G. Healey, W. Hunziker, V. Lewis, H. Plutner, H. Miettinen, D. Vaux, K. Moore, and S. Stuart. 1988. Structure and function of Fc receptors on macrophages and lymphocytes. J. Cell Sci. Suppl. 9:45-65. [DOI] [PubMed] [Google Scholar]
- 21.Miettinen, H. M., K. Matter, W. Hunziker, J. K. Rose, and I. Mellman. 1992. Fc receptor endocytosis is controlled by a cytoplasmic domain determinant that actively prevents coated pit localization. J. Cell Biol. 116:875-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miettinen, H. M., J. K. Rose, and I. Mellman. 1989. Fc receptor isoforms exhibit distinct abilities for coated pit localization as a result of cytoplasmic domain heterogeneity. Cell 58:317-327. [DOI] [PubMed] [Google Scholar]
- 23.Neubauer, C., L. Frasel, E. Kuechler, and D. Blaas. 1987. Mechanism of entry of human rhinovirus 2 into HeLa cells. Virology 158:255-258. [DOI] [PubMed] [Google Scholar]
- 24.Nykjaer, A., and T. E. Willnow. 2002. The low-density lipoprotein receptor gene family: a cellular Swiss army knife? Trends Cell Biol. 12:273-280. [DOI] [PubMed] [Google Scholar]
- 25.Prchla, E., E. Kuechler, D. Blaas, and R. Fuchs. 1994. Uncoating of human rhinovirus serotype 2 from late endosomes. J. Virol. 68:3713-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravetch, J. V. 1994. Fc receptors: rubor redux. Cell 78:553-560. [DOI] [PubMed] [Google Scholar]
- 27.Reithmayer, M., A. Reischl, L. Snyers, and D. Blaas. 2002. Species-specific receptor recognition by a minor-group human rhinovirus (HRV): HRV serotype 1A distinguishes between the murine and the human low-density lipoprotein receptor. J. Virol. 76:6957-6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossmann, M. G. 2002. Picornavirus structure overview, p. 27-50. In B. L. Semler and E. Wimmer (ed.), Molecular biology of picornaviruses. ASM Press, Washington, D.C.
- 29.Rudenko, G., L. Henry, K. Henderson, K. Ichtchenko, M. S. Brown, J. L. Goldstein, and J. Deisenhofer. 2002. Structure of the LDL receptor extracellular domain at endosomal pH. Science 298:2353-2358. [DOI] [PubMed] [Google Scholar]
- 30.Savolainen, C., S. Blomqvist, M. N. Mulders, and T. Hovi. 2002. Genetic clustering of all 102 human rhinovirus prototype strains: serotype 87 is close to human enterovirus 70. J. Gen. Virol. 83:333-340. [DOI] [PubMed] [Google Scholar]
- 31.Schober, D., P. Kronenberger, E. Prchla, D. Blaas, and R. Fuchs. 1998. Major and minor receptor group human rhinoviruses penetrate from endosomes by different mechanisms. J. Virol. 72:1354-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skern, T., C. Neubauer, L. Frasel, P. Grundler, W. Sommergruber, M. Zorn, E. Kuechler, and D. Blaas. 1987. A neutralizing epitope on human rhinovirus type 2 includes amino acid residues between 153 and 164 of virus capsid protein VP2. J. Gen. Virol. 68:315-323. [DOI] [PubMed] [Google Scholar]
- 33.Snyers, L., H. Zwickl, and D. Blaas. 2003. Human rhinovirus type 2 is internalized by clathrin-mediated endocytosis. J. Virol. 77:5360-5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomassini, E., T. Graham, C. DeWitt, D. Lineberger, J. Rodkey, and R. Colonno. 1989. cDNA cloning reveals that the major group rhinovirus receptor on HeLa cells is intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. USA 86:4907-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tormo, J., E. Stadler, T. Skern, H. Auer, O. Kanzler, C. Betzel, D. Blaas, and I. Fita. 1992. Three-dimensional structure of the Fab fragment of a neutralizing antibody to human rhinovirus serotype 2. Protein Sci. 1:1154-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tse, S. M., W. Furuya, E. Gold, A. D. Schreiber, K. Sandvig, R. D. Inman, and S. Grinstein. 2003. Differential role of actin, clathrin, and dynamin in Fc gamma receptor-mediated endocytosis and phagocytosis. J. Biol. Chem. 278:3331-3338. [DOI] [PubMed] [Google Scholar]
- 37.Uncapher, C. R., C. M. Dewitt, and R. J. Colonno. 1991. The major and minor group receptor families contain all but one human rhinovirus serotype. Virology 180:814-817. [DOI] [PubMed] [Google Scholar]
- 38.Unkeless, J. C. 1979. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J. Exp. Med. 150:580-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yin, F. H., and N. B. Lomax. 1986. Establishment of a mouse model for human rhinovirus infection. J. Gen. Virol. 67:2335-2340. [DOI] [PubMed] [Google Scholar]