Abstract
Understanding the interactions between varicella-zoster virus (VZV) and host cells can be addressed by using small molecule inhibitors of cellular enzymes. Roscovitine (Rosco) is a purine derivative that inhibits cyclin-dependent kinase 1 (cdk1), cdk2, cdk5, cdk7, and cdk9, which are key regulators of the cell cycle and transcription. Herpesviruses are known to interact with cell cycle proteins; thus, the antiviral effects of Rosco on VZV growth were evaluated. In a plaque reduction assay, 25 μM Rosco prevented VZV replication, and the antiviral effect was reversible for at least up to 24 h posttreatment. Rosco also reduced expression of the major transactivator, IE62, over 48 h. Confocal microscopy studies indicated that Rosco caused the immediate-early proteins ORF4 and IE62 to abnormally localize in infected cells and prevented cell-cell spread of VZV over 48 h. Rosco was found to inhibit VZV DNA synthesis as measured by real-time PCR, and this technique was used to estimate the 50% effective concentration (EC50) of 14 μM. This value was close to the EC50 estimate of 12 μM determined from plaque reduction assays. At 25 μM, Rosco was not cytotoxic over 48 h in a neutral red uptake assay, and proliferation was slowed as the cells accumulated in a G2-like state. These results demonstrate the importance of cdk's in VZV replication and suggest that cdk inhibitors could serve as useful VZV antivirals.
During primary infection, varicella-zoster virus (VZV), a human-restricted alphaherpesvirus, is carried within T cells to epithelial cells and neurons, resulting in the characteristic vesicular rash of varicella (chicken pox). Following recovery of the host, VZV establishes lifelong latency in sensory neurons. Reactivation from ganglia occurs in some 20% of the population, leading to resumed VZV replication in the skin, giving rise to the unilateral distribution of zoster (shingles). As such, the course of human infection requires VZV replication in a variety of host cell types that are dividing (basal keratinocytes), quiescent (memory T cells and dermal fibroblasts), and terminally differentiated (neurons) (1, 27). Although the molecular basis of VZV tissue tropism is not completely understood, the ability to grow in this wide host cell range relies upon expression of specific viral proteins that likely play important roles in infection. For example, when recombinant VZV mutant strains were created that did not express either of two viral kinases, open reading frame 47 (ORF47) or ORF66, there was no effect on viral replication in MeWo cells whatsoever. Yet the kinase ORF47 was essential in skin and T cells in the SCID-hu mouse model and in T cells grown in culture, whereas the viral kinase encoded by ORF66 was important for full infectivity in T cells (5, 12, 36).
The ability of VZV to replicate in noncycling cells is shared with herpes simplex viruses (HSV), which grow in similar cell types. HSV has acquired several viral genes that are critical for in vivo infection whose importance is cell type specific. These include several that alter nucleotide pool enzymes required for efficient viral DNA replication as well as transcriptional activators that play a cell division stage-dependent role in infection. For example, HSV VP16 and ICP0 are transcriptional activators that have key roles in nondividing cells but can be partially replaced by host cell functions in certain rapidly dividing cell types (9, 13). Furthermore, it has become apparent that HSV, and to some extent human cytomegalovirus (HCMV), require the activity of cell cycle-dependent factors for efficient viral replication (17, 21). During the cell cycle, division is tightly regulated by proteins known as cyclins and cyclin-dependent kinases (cdk's), which function together to control replication by mediating phosphorylation of key regulatory proteins such as retinoblastoma protein (Rb). HSV type 1 (HSV-1) and HCMV have been shown to require cdk activity for efficient replication in many cell types and inhibitors of cdk prevent infection (7, 45).
Roscovitine (Rosco) is a purine derivative that inhibits cdk1/cyclin B, cdk2/cyclin A or E, cdk5/p25, cdk7/cyclin H, and cdk9/cyclin T in in vitro kinase assays at concentrations below 1.0 μM (extracellular regulated kinases erk1 and erk2 and dual-specificity protein kinase Dyrk are inhibited at higher concentrations) (33, 43). Rosco inhibits cdk's by binding to the catalytic domain of the cdk molecule in place of ATP, which prevents transfer of a phosphate group to the substrate (33). Rosco and flavopiridol, another cdk inhibitor, prevented the replication of human immunodeficiency virus (HIV) type 1 by blocking Tat transactivation of the HIV type 1 promoter (10, 52). Flavopiridol inhibits a broader range of kinases than Rosco, including cdk4 and cdk6, and is a potent inducer of apoptosis in tumor cells (48). Extensive studies show that Rosco prevents HSV-1 at multiple steps: it suppressed viral but not cellular gene transcription (20, 47), inhibited viral DNA synthesis (46), and prevented reactivation from explanted neurons that harbored latent HSV-1 (44). Importantly, it was demonstrated that Rosco prevented viral replication by binding to cellular proteins and did not apparently act by inhibiting the known viral kinases (43).
We hypothesized that Rosco may affect VZV replication, since many of the cell types infected by VZV are similar to those infected by HSV-1. Here we show that Rosco prevented VZV replication at levels that were not cytotoxic, did not induce apoptosis in the host cells, and were lower than that needed to block HSV-1. VZV DNA replication and IE62 protein expression were inhibited and cell-cell spread was affected, indicating that Rosco inhibited cdk activity that was required either at multiple points in VZV replication or at an early stage that affected the entire cascade of VZV replication. These results support the concept that cellular functions that are essential for virus replication can provide useful targets for antiviral agents.
MATERIALS AND METHODS
Propagation of cells and virus.
Human foreskin fibroblasts (HFF) and MeWo cells (human melanoma cell line) were grown in minimal essential medium Eagle with Earle's salts and l-glutamine, supplemented with heat-inactivated fetal bovine serum (FBS), penicillin-streptomycin (5,000 IU/ml), amphotericin B (250 μg/ml), and nonessential amino acids. All media and supplements were purchased from Mediatech (Washington, D.C.). The recombinant parental Oka strain of VZV (38) was stored at −80°C and then grown on MeWo cells or HFF for up to 10 passages. Cell-free virus was prepared from infected MeWo cells by sonication in a bath sonicator in PSGC buffer (phosphate-buffered saline [PBS] with 5% sucrose, 0.1% sodium glutamate, 10% FBS) and was then diluted 1:5 in tissue culture medium before use. The cell-free virus, which could contain a small number of intact infected cells, was adsorbed to the cell monolayers for 2 h at 37°C, the inoculum was removed, the cells were washed one time with PBS, and then tissue culture medium containing the indicated drug was added (time zero). Tissue culture medium containing drugs or diluent alone (mock treatment) was refreshed daily.
Preparation of drugs.
Rosco was purchased from Calbiochem (San Diego, Calif.), and a 20 mM stock solution was prepared in dimethyl sulfoxide. Phosphonoacetic acid (PAA) was purchased from Sigma, diluted in PBS, neutralized with NaOH, and diluted to a final concentration of 100 μg/ml (714 mM) with tissue culture medium. Staurosporine, purchased from Sigma, was dissolved in dimethyl sulfoxide to make a stock solution of 100 ng/μl. All drugs were aliquoted and stored at −20°C until use. Final dilutions of drugs used in all experiments were prepared in 37°C tissue culture medium immediately before use.
Infectious focus assay.
The yield of VZV-infected cells was determined by plaque assay following the protocol described in reference 35 with MeWo cell monolayers instead of Vero cells. After 5 days, the monolayers were fixed with 3.7% formalin for 15 min, washed twice with PBS, and then incubated with blocking buffer (10% FBS in PBS) for 30 min at room temperature. VZV plaques were detected by immunohistochemical staining with a human polyclonal immune serum as the primary antibody and alkaline phosphatase-conjugated anti-human immunoglobulin G for the secondary antibody (Jackson ImmunoResearch, West Grove, Pa.) followed by enzymatic deposition of Fast Red and Naphthol AS-MX phosphate (Sigma). The level of detection of the infectious focus assay was 3 PFU per specimen.
Flow cytometry analysis.
MeWo cells were seeded in each well of six-well plates and cultured with various concentrations of Rosco. After 24 or 48 h, the cells were removed with trypsin, washed with buffer (0.5% FBS and 0.1% sodium azide in PBS), resuspended in 70% ethanol, and fixed at −20°C for 1 h. Cells were washed twice and resuspended in 0.5 ml of buffer. RNase A (final concentration, 200 mg/ml) was added, and the mixture was incubated for 30 min at 4°C. Propidium iodide was added at a final concentration of 20 μg/ml, and the relative DNA content of the cells was analyzed with an LSRII flow cytometer and Modfit software (Becton Dickinson Information Systems, San Jose, Calif.).
NR cytotoxicity assay.
The Neutral Red (NR) cytotoxicity assay was performed according to previously described methods (3). NR was purchased from Sigma, diluted in tissue culture medium to 25 μg/ml, and incubated overnight at 37°C. Prior to use, NR solution was filtered (0.22-μm pore size) to remove fine dye crystals. MeWo cells (104) were seeded on 96-well plates and treated with 5.0 ng of staurosporine or 0 to 100 μM Rosco. After 24 or 48 h, the medium was removed from the MeWo cells, replaced with 0.25 ml of NR solution, and incubated for 2 h at 37°C. Cells were then rapidly washed and fixed in 0.25 ml of 0.5% formalin in Dulbecco's PBS with Ca2+ and Mg2+ (Mediatech). The NR dye that had been taken up by viable cells was extracted with 0.1 ml of desorb solution (1% glacial acetic acid, 50% ethanol). The plate was agitated on an orbital shaker for 25 min, and then the absorbance at 540 nm was recorded in a microtiter plate spectrophotometer.
Immunofluorescence confocal microscopy.
Subconfluent HFF were grown in chamber slides for 24 h and then inoculated with cell-free VZV for 2 h. The infected cells were washed with PBS, and tissue culture medium containing 25 μM Rosco or control medium was added. At 24 and 48 h postinfection, cells were fixed in acetone and washed three times in PBS. After blocking in 10% goat serum in PBS (blocking solution), cells were stained with monospecific mouse or rabbit antibodies to VZV proteins ORF4 and ORF62 (24) and secondary antibodies conjugated to rhodamine or fluorescein isothiocyanate (Caltech, Inc.). The slides were mounted and then processed with a Bio-Rad LaserSharp Plus imaging system (Bio-Rad, Hercules Calif.).
Immunoblotting.
Infected and uninfected MeWo cells were lysed and analyzed by Western blot analysis by following previously described methods (34). VZV IE62 protein was detected with rabbit polyclonal serum (generously provided by William Ruyechan, State University of New York, Buffalo). A mouse monoclonal antibody to human β-actin was added simultaneously to indicate the amount of protein in each lane (Sigma). The appropriate secondary antibodies conjugated to alkaline phosphatase (Jackson ImmunoResearch) were used to generate a chemiluminescent signal in the presence of the ImmuneStar reagent (Bio-Rad). The membranes were exposed to BioMax film (Kodak, Rochester, N.Y.) for exactly 30 min.
Real-time PCR.
Total cellular and viral DNA from VZV-infected MeWo cells was isolated with DNAzol reagent (Invitrogen, Carlsbad, Calif.). Real-time PCR was performed at the DNA Core Facility at the University of Rochester by using the probes and primers described by Hawrami and Breuer (18). All reactions (including standards) were performed in triplicate with an ABI 7900 HT TaqMan apparatus with the following cycle parameters: 1 cycle of 50°C (2 min) followed by 95°C (10 min) and 40 cycles of 95°C (15 s) followed by 60°C (1 min). Raw data were analyzed with Sequence Detection Software (ABI, Foster City, Calif.), and then the absolute viral genome copy number was calculated based on a standard curve obtained from serial dilutions ranging from 100 ng to 0.01 pg of a plasmid containing a 647-bp fragment of ORF38.
Apoptosis detection assays.
MeWo cells were grown in glass chamber slides, infected with VZV, treated with 25 μM Rosco for 24 h, fixed with 4% paraformaldehyde in PBS, and then stained with 0.1 μg of 4′,6′-diamidino-2-phenylindole (DAPI)/ml in PBS to visualize all nuclei (Sigma). Apoptotic cells were identified by using a TdT enzyme that transfers fluorescein to the ends of fragmented DNA (Roche Diagnostics Corp., Indianapolis, Ind.). VZV-infected cells were identified by using human polyclonal immune serum and a secondary antibody conjugated to tetramethyl rhodamine isothiocyanate (Jackson ImmunoResearch Laboratories, Inc.). The stained cells were examined by fluorescence microscopy, and digital photomicrographs were taken at a magnification of ×10. From these photographs, 300 DAPI-stained cell nuclei were counted, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling-positive and VZV-positive cells in the same field of view were counted, and the percentage of apoptotic cells was calculated.
RESULTS
Effects of Rosco on MeWo cells.
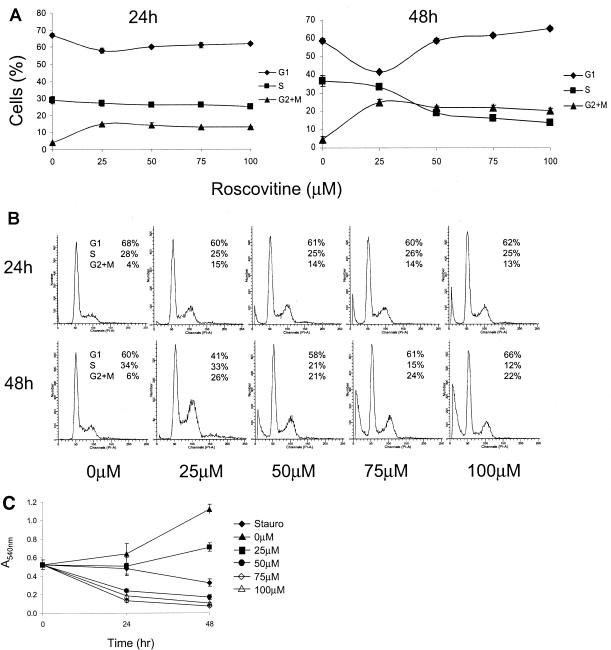
Tumor cell lines such as MeWo, a human melanoma cell line commonly used to cultivate VZV, have a disregulated cell cycle that leads to uncontrolled proliferation. The effects of Rosco, a cell cycle inhibitor, on MeWo cells were not known, although Rosco had inhibited the growth of 60 human tumor cell lines, including some melanomas (33). Cell cycle analysis of L1210 cells (mouse lymphocytic leukemia cells) showed that, at a concentration of 60 μM, Rosco caused arrest at the G1/S border and accumulation in G2 phase (33). Similar effects were also observed with MeWo cells treated with Rosco for 48 h. For cell cycle analysis, subconfluent MeWo cells were treated with 0 to 100 μM Rosco for 24 or 48 h, and then the percentage of cells in each phase of the cell cycle was determined by flow cytometry analysis of propidium iodide-stained cells (Fig. 1A). After 24 h of treatment, a slight accumulation in G2 phase was observed at all concentrations. By 48 h, more than 20% of the cells had accumulated in G2 phase in the presence of 25 μM and higher Rosco. At 50 to 100 μM, Rosco treatment caused a decrease in S, suggesting an arrest at the G1/S border. Representative histograms of the flow cytometry data (Fig. 1B) show that while 25 μM Rosco caused MeWo cells to accumulate in G2 after 48 h, reaching 26% of cells, there was little sign of cell death under the microscope or change in S phase (33% in treated cells compared to 34% in mock treated cells). In contrast, higher concentrations of Rosco resulted in the appearance of a cell population with sub-G1 DNA content, an indication of cell death and DNA fragmentation. This was also observed by microscopic evaluation where cells appeared rounded and nonadherent (data not shown).
FIG. 1.
Effects of Rosco on MeWo cells. (A) Flow cytometry analysis of propidium iodide-stained MeWo cells. The percentages of cells in the G1 (diamonds), S (squares), and G2 + M (triangles) phases are shown. Each point represents the average ± standard deviation of the results for six samples from two separate experiments. (B) Representative flow cytometry histograms of propidium iodide-stained MeWo cells. (C) NR uptake cytotoxicity assay. The effects of 0 to 100 μM Rosco on MeWo cells were compared to those of staurosporine (Stauro; diamonds).
To determine whether the MeWo cells treated with Rosco were either growing, alive but arrested, or dead, an NR dye uptake assay was performed. This assay depends on the ability of living cells to take up the dye and thus provides a colorimetric indicator of cell viability and relative cell number. MeWo cells were treated with 0 to 100 μM Rosco for 24 and 48 h, and NR was added during the last 2 h. The results of this assay agreed with the cell cycle analysis in that 25 μM Rosco slowed MeWo cell growth and the treatment was not cytotoxic (Fig. 1C). Higher concentrations of Rosco were as deleterious to MeWo cells as treatment with staurosporine, a known inducer of apoptosis. Mock-treated MeWo cells grew normally, more than doubling in number during the assay.
Rosco prevented VZV replication at concentrations lower than that needed to cause cell cycle arrest.
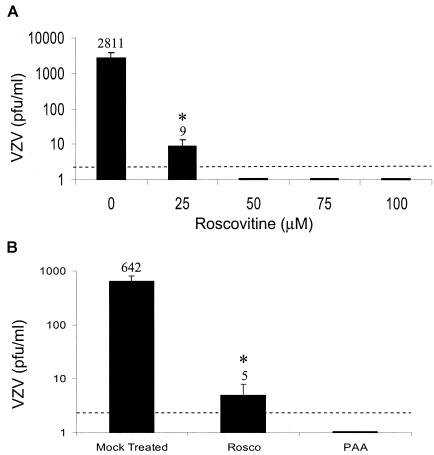
Previous studies demonstrated that similar concentrations of Rosco inhibited cell cycle progression and HSV-1 replication (45). Rosco prevented the replication of HSV-1 in HEL (human embryonic lung) cells at concentrations of 50 μM and higher and in Vero cells at 100 μM. Since MeWo cells were also arrested in the G1 phase in this concentration range, we tested whether Rosco caused a dose-dependent antiviral response to VZV (Fig. 2A). Subconfluent MeWo cells (∼105 cells) were inoculated with cell-free VZV (∼102 PFU) and then treated with Rosco for 24 h, allowing for 1 full round of virus replication. The amount of cell-cell spread, the exclusive route of VZV propagation in culture, was measured by titration of the treated, VZV-infected MeWo cells by infectious focus assay. VZV increased 10-fold in untreated MeWo cells, the normal rate of spread for this highly cell-associated virus, whereas an average of 9 PFU were detected in VZV-infected cells treated with 25 μM Rosco. In the presence of 50 to 100 μM Rosco, very little infectious VZV was recovered. An average of 1 to 2 plaques is shown in Fig. 2A, and these results represent four separate experiments where the yield ranged from undetectable to 3 PFU/ml, the limit of detection of this assay. In separate experiments, the antiviral effects of 25 μM Rosco were compared to those of PAA, a known inhibitor of the viral DNA polymerase (39). Rosco was nearly as effective as 100 μg of PAA/ml in preventing VZV replication (Fig. 2B). These experiments demonstrate that the cdk inhibitor Rosco prevented VZV replication at half the amount needed to prevent HSV-1 replication in HEL cells. Importantly, the antiviral effect of 25 μM Rosco occurred at a level that was not cytotoxic and did not halt cell growth, although the MeWo cells appeared to grow more slowly at this concentration.
FIG. 2.
Effects of Rosco on VZV replication. (A) The yield of VZV in MeWo cells treated with Rosco was determined by plaque assay. Each point represents the average ± standard deviation of the results from three experiments performed in duplicate. (B) Virus yield was determined in MeWo cells that were treated with 25 μM Rosco or 100 μg of PAA (714 mM)/ml or that were mock treated for 24 h. Each point represents an average ± standard deviation of the results from two experiments performed in duplicate. Asterisks indicate significant differences (P < 0.05) between Rosco-treated and mock-treated cultures as determined by Student's t test.
Antiviral effects of Rosco were reversible and did not induce apoptosis.
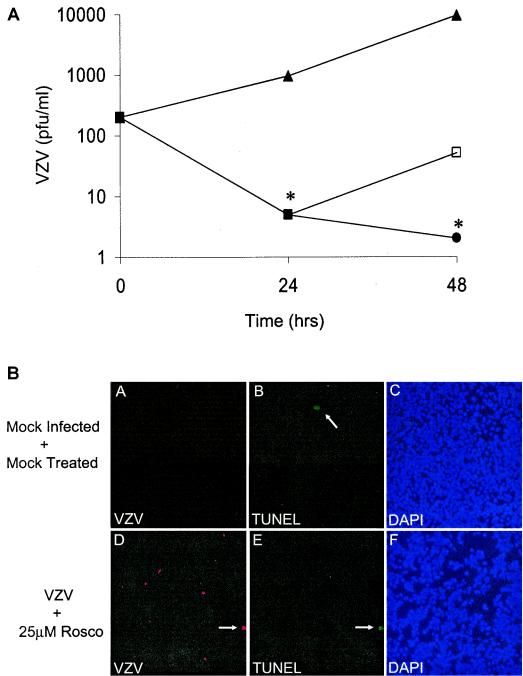
Toxicity and cell death are concerns when using antiviral agents that target cellular factors. Although 25 μM Rosco treatment did not appear overtly toxic to MeWo cells by flow cytometry and the NR uptake assay (Fig. 1), it was feasible that the number of treated cells remained fairly constant because dying cells were replaced by slowly dividing cells. To address this question, we studied the reversibility of Rosco on VZV replication. MeWo cells were infected with cell-free VZV and treated with 25 μM Rosco for 24 h. At this time, one group of infected cells was harvested to determine VZV yield, a second group was given fresh Rosco-containing medium, and the third group was given fresh medium containing no drugs. After a second 24-h incubation period, the yield of infectious VZV was determined for all groups. Indeed, the effects of Rosco were found to be reversible, since removal of Rosco allowed VZV replication with similar kinetics to the untreated cultures (Fig. 3A). Between 24 and 48 h after inoculation, VZV increased 9.9-fold with a doubling time of 7.2 h in untreated MeWo cells; when Rosco was removed, VZV increased 10.2-fold with a doubling time of 7.2 h. Again, Rosco significantly inhibited VZV replication compared to untreated cultures, and very little infectious VZV was recovered after a continuous 48 h of Rosco treatment.
FIG. 3.
Inhibition of VZV by Rosco is reversible. (A) MeWo cells were infected with VZV and treated with 25 μM Rosco for 24 h, and then tissue culture medium with (circles) or without (open squares) Rosco was added for 24 h. Mock-treated, infected cells were given medium lacking Rosco (triangles). Cells were harvested, and virus yield was determined by a standard plaque assay. Each point represents an average ± standard deviation of the results from three experiments performed in duplicate, and significance was evaluated by using Student's t test (*, P < 0.05). (B) Fluorescence micrographs of MeWo cells infected with VZV and treated with 25 μM Rosco for 24 h. Digital photos of the same field of view show VZV-infected cells (red) (A and D), apoptotic cells (green) (B and E), and cell nuclei (blue) (C and F). Magnification, ×10. TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling.
VZV yield after 24 h of Rosco treatment was lower than at time zero, which indicates that Rosco resulted in some loss of infectious virus that initially entered the MeWo cells. Several possibilities exist to explain why Rosco reduced infectious VZV yield after 24 h. First, Rosco could lengthen the eclipse phase, the time after infection when infectious virus cannot be recovered. Second, normal cell processes could degrade VZV components while Rosco inhibited virus replication. Third, entering VZV proteins could be toxic or induce cell death. And last, Rosco treatment could also render cells, whether infected or not, more susceptible to apoptosis. To address the latter possibility, two methods were employed to observe the effects of VZV infection and Rosco treatment on the levels of MeWo cell apoptosis: observation of condensed nuclei with DAPI stain and in situ terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling analysis. MeWo cells were grown on glass chamber slides, infected with cell-free VZV, and treated with Rosco for 24 h under the same conditions used for the antiviral tests. MeWo cells were also treated with a known inducer of apoptosis, camptothecin, to provide a positive control. Because few of the MeWo cells were infected with VZV, it was important to examine infected and apoptotic cells individually. Thus, infected cells were detected with polyclonal anti-VZV serum and a tetramethyl rhodamine isothiocyanate-conjugated fluorophore, and apoptotic cells were labeled with a TdT enzyme that transfers fluorescein to the ends of fragmented DNA. A total of 300 cells in each sample were examined visually, and the percentage of apoptotic cells was calculated. Camptothecin induced apoptosis in 25 to 30% of MeWo cells; however, in mock-treated and mock-infected control cells, less than 1% were apoptotic. Representative fluorescence micrographs showed the rare MeWo cell undergoing apoptosis in the negative control (Fig. 3B, panel B). Importantly, less than 1% of the cells treated with 25 μM Rosco, infected with VZV, or both were apoptotic (Fig. 3B, panels D and E). The images of DAPI-stained cells showed that similar numbers of cells were in each field of view (Fig. 3B, panels C and F). This indicated that Rosco treatment did not induce apoptosis in MeWo cells and that VZV infection did not sensitize the cells to the effects of Rosco.
Effects of Rosco on VZV cell-cell spread and immediate-early protein expression.
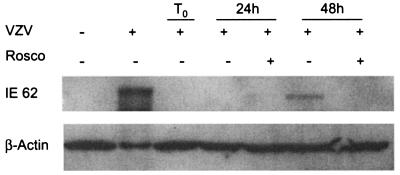
To determine the stage of VZV infection that was inhibited by Rosco, the expression of the major immediate-early transactivator protein, IE62, was studied by immunoblot analysis. IE62, homologue of HSV-1 ICP4, activates transcription of the immediate-early, early, and late gene classes, thereby initiating the entire VZV replication cascade (40). If Rosco interfered with the expression of IE62, then transcription of all gene classes would be impaired. Indeed, IE62 protein was not detected in VZV-infected MeWo cells treated with 25 μM Rosco for 48 h (Fig. 4). In untreated MeWo cells infected with VZV, IE62 protein was detectable after 48 h. Because the amount of input virus was low, no IE62 protein was detected at time zero or at 24 h in either sample. To avoid the problem of a low-titer inoculum that is standard for VZV, the expression of IE62 was next studied in individual cells.
FIG. 4.
Rosco reduces IE62 expression. VZV-infected MeWo cells were treated with 25 μM Rosco or mock treated for 24 and 48 h. Cell lysates were analyzed by immunoblotting with antibodies against IE62 and β-actin, which served as a loading control. Uninfected and untreated MeWo cells (first lane) and fully infected and untreated MeWo cells (second lane) served as negative and positive controls, respectively. +, present; −, absent.
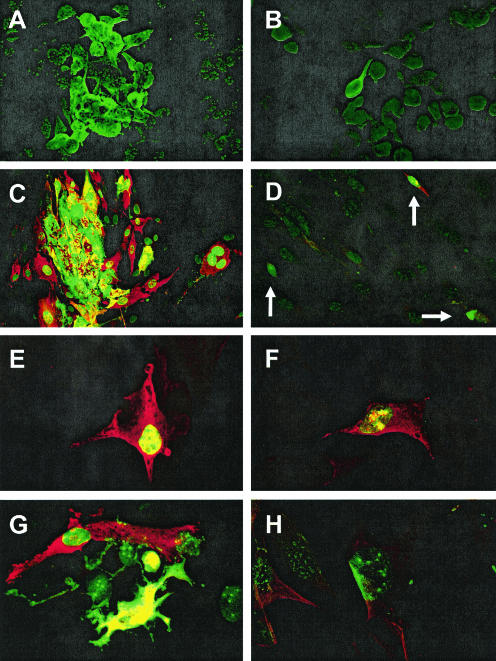
The effects of Rosco on VZV cell-cell spread and the intracellular location of viral proteins were examined by confocal microscopy. Using an antibody that recognizes the major immediate-early protein IE62, VZV was found only in single MeWo cells after treatment with Rosco for 24 h, whereas virus plaques grew to include more than 20 cells under normal conditions (Fig. 5A and B). To more closely examine the localization of VZV proteins, the confocal microscopy studies were repeated in HFF, which are less rounded than MeWo cells and have a more distinct separation of cytoplasm and nucleus. Antibodies that recognize IE62 and IE4, another immediate-early protein with a classically cytoplasmic localization pattern, were used to visualize the spread of VZV in HFF monolayers. After 48 h, IE62 was found in nuclei and IE4 was mainly cytoplasmic at the edges of expanding plaques, a pattern seen at early stages of infection. IE62 and IE4 partly colocalized in both the nuclei and cytoplasm in the center of the syncytia where late infection events were occurring (Fig. 5C). At higher magnification it was clearly visible that IE62 protein was abundant yet restricted to the nucleus, whereas IE4 was present both in the cytoplasm and the nucleus (Fig. 5E). In contrast, cultures treated with Rosco showed isolated cells that expressed either the early or late pattern of IE62 and IE4 localization (Fig. 5D). In many cases, IE62 expression was reduced and appeared as irregular patches that colocalized with IE4 in the nuclei (Fig. 5F). Small foci (Fig. 5G and H) in Rosco-treated HFF were very rare, and they consisted of one or two very bright cells surrounded by 5 to 6 cells in which IE62 was found in small puncta in the nucleus. This nuclear speckling pattern has not previously been seen at any stage of VZV infection and suggests that Rosco prevented normal expression and localization of IE62 protein in addition to blocking the spread of VZV.
FIG. 5.
Rosco prevents VZV spread and alters immeditate-early protein localization. VZV-infected MeWo cells were mock treated (A) or treated with 25 μM Rosco for 24 h (B) and then visualized with rabbit polyclonal serum specific for VZV protein IE62 and a fluorescein isothiocyanate conjugate to detect VZV plaques. HFF were infected with cell-free VZV (C) and mock treated or treated with 25 μM Rosco for 48 h (D to H), and the localization of VZV proteins IE4 (red) and IE62 (green; yellow indicates colocalization) was determined. (D to F) Individual VZV-infected cells (arrows in panel D) show the expected patterns of IE4 localization to the cytoplasm and in the nucleus of some cells and IE62 localization to the nucleus. (G and H) Rare foci showed IE62 in an unusual nuclear speckling pattern. Magnification, ×240 (A to D) and ×400 (E to H).
Rosco inhibited VZV DNA synthesis.
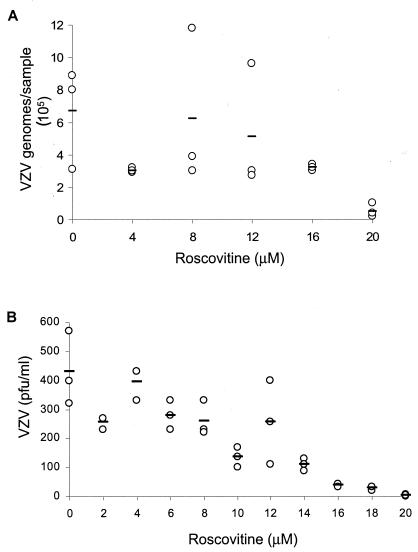
Rosco is known to block DNA synthesis of HSV-1 independently of immediate-early and early gene expression (46). We hypothesized that the restriction of VZV to single cells in the presence of Rosco was due to a block in viral DNA replication in a similar manner. To measure the effect of Rosco treatment on VZV DNA synthesis, we adapted the real-time PCR procedure developed by Hawrami and Breuer to quantitate VZV genomic DNA (18). Real-time PCR has been successfully used to detect VZV in clinical samples, to measure the genome copy number, and for typing isolates (18, 28, 31). Increasing amounts of Rosco, from 0 to 20 μM, resulted in a dose-dependent reduction in VZV genome copy number (Fig. 6A). In untreated cells, VZV replication generated more than 6 × 105 genomes per sample after 24 h, whereas infected cells treated with 20 μM Rosco contained approximately 5 × 104 copies. The increasing inhibition of VZV yield was also seen at low concentrations of Rosco. An infectious focus assay performed on VZV-infected MeWo cells treated with 0 to 20 μM Rosco for 24 h showed that virus yield began decreasing in the presence of 10 μM Rosco and approached zero with 20 μM Rosco (Fig. 6B). To estimate the 50% effective concentration (EC50), or the amount of Rosco that reduced VZV DNA synthesis and virus yield by half, linear regression analysis was performed on both sets of data. An EC50 of 14 μM was obtained by real-time PCR, and this agreed with the EC50 of 12 μM that was determined by plaque assay. This EC50 range applies to MeWo cells inoculated with a low multiplicity of infection of cell-free VZV; cell-associated virus, a high multiplicity of infection, or other cell types could produce different results. Importantly, real-time PCR provided a rapid and sensitive method to show that Rosco inhibited VZV DNA synthesis in a dose-dependent manner and at levels below that needed to block the cell cycle.
FIG. 6.
Effect of Rosco on VZV DNA synthesis. VZV-infected MeWo cells were treated with 0 to 20 μM Rosco for 24 h, and then total viral and cellular DNAs were extracted and analyzed by real-time PCR for VZV genome copy number (A) or titers were determined by standard plaque assay for infectious VZV yield (B). Each circle represents one sample; bars indicate the averages of the results from triplicate samples.
DISCUSSION
Taken together, the results of this study show that 25 μM Rosco prevents VZV replication, results in decreased immediate-early gene expression and abnormal localization, inhibits DNA replication, and does so without causing cytotoxicity or apoptosis in the host MeWo cells. We found that at least 50 μM Rosco was required to arrest MeWo cells in G1, which is similar to the 60 μM concentration used to arrest L1210 tumor cells and the 80 μM concentration used to arrest Vero cells. The cytotoxicity of Rosco depended on the cell type being studied. L1210 cells died rapidly in 120 μM Rosco, yet Vero cells tolerated doses of 120 and 200 μM (33, 45). In the results presented here, we showed that concentrations of Rosco above 50 μM caused cell death in MeWo cells. One group suggests that the antiviral effects of Rosco occur at concentrations that are also cytotoxic, since they observed cell death in stationary HFF treated with 33 μM Rosco at 2 days and beyond (16). This was not a concern in our study because the effects of Rosco on VZV replication were evident at 25 μM and lower, and the duration of experiments did not exceed 48 h.
The results of apoptosis and cytotoxicity assays indicate that the antiviral effects of Rosco were not due to generalized cell death, nor were VZV-infected MeWo cells more susceptible to apoptosis in the presence of Rosco. Certain cell types, especially tumors and neuronal cells, undergo apoptosis in the presence of Rosco and other cell cycle inhibitors (50). Additionally, the combination of HSV-1 infection and Rosco treatment for more than 24 h was more toxic to Vero cells than the drug alone (45). This phenomenon was also reported for HIV-infected T cells and macrophages that selectively underwent apoptosis when treated with concentrations of Rosco that did not block the growth of uninfected cells (52). Although the MeWo cells in our studies did not appear apoptotic, it is possible that Rosco affected other unknown cellular functions. The MeWo cells remained permissive for VZV after Rosco treatment, since virus replication resumed after Rosco was removed.
The ability of Rosco to prevent VZV replication could be explained in two ways: either Rosco blocks multiple stages of VZV replication or Rosco inhibits a single step very early in infection that stops the progression of VZV replication. Evidence for the latter hypothesis was the reduction in IE62 protein expression in MeWo cells treated with Rosco for 48 h, although this does not eliminate the possibility that Rosco could also inhibit downstream events in the presence of VZV immediate-early and early proteins. In infected HFF treated with Rosco, it was evident that two immediate-early proteins, IE4 and IE62, had abnormal localization and were unable to initiate replication. IE62 must localize to the nucleus to transactivate VZV promoters and amplify its expression by auto induction (26). Since IE62 is the major transactivating protein, direct or indirect interference by Rosco would have profound downstream effects on VZV replication. If IE62 and the other immediate-early transactivators were not able to trigger the coordinated cascade of viral gene expression, then viral DNA synthesis could not occur. Indeed, we found that Rosco treatment prevented VZV DNA synthesis, as measured by real-time PCR. We do not yet know if Rosco inhibited DNA replication independently of immediate-early and early proteins. In the case of HSV-1, Rosco inhibited HSV-1 DNA synthesis even in the presence of immediate-early and early proteins (47). Further studies are in progress to determine whether Rosco inhibits the classes of VZV genes independently of one another.
The unusual speckling pattern of IE62 in a few VZV-infected HFF that were treated with Rosco is noteworthy. This punctate distribution of IE62 is entirely novel and may lead to new ideas about the timing and function of IE62 at early stages of infection. The IE62 puncta appeared in nuclei surrounding cells that expressed abundant IE62. It is plausible that these brightly staining cells were from the VZV inoculum because it was made by sonication and was not filtered or centrifuged before adsorption to HFF monolayers. Therefore, some infected cells could adhere to the chamber slide during the 48-h experiment. The intact infected cells in the inoculum could transfer IE62 protein and VZV genomes to adjacent cells by cell fusion or from virions, which contain large amounts of IE62 in the tegument (25). Under normal conditions, this leads to transactivation of VZV promoters, including ORF62, and amplification of IE62 expression. However, in the presence of Rosco, IE62 expression was inhibited and the small amount of IE62 protein in the cells was in the nucleus in a distribution reminiscent of ND10 domains. This is intriguing because HSV-1 DNA and immediate-early proteins (ICP0 and ICP4) are found in close proximity to ND10 domains at early stages of infection (6, 15, 32). IE62 is the homologue of HSV-1 ICP4, so it will be interesting to determine whether it also interacts with ND10 domain proteins such as PML. There is little information about the location of VZV DNA, immediate-early proteins, or mRNA transcription in the nucleus, so this should be a very fruitful avenue to pursue. In addition, these studies may benefit from using inhibitors like Rosco that block VZV gene expression at early stages.
Herpesviruses are known to interact with cell cycle regulatory components, such as cdk's and cyclins. HSV-1, HCMV, and Epstein-Barr virus block cellular DNA replication and halt the cell cycle at the G1/S transition in the host while at the same time modulating cyclin-cdk functions to create a nuclear environment that is optimized for viral DNA synthesis (reviewed in reference 17). It seems likely that VZV has similar effects on the cell cycle, and we are currently investigating this issue. The primary targets of Rosco in nonneuronal cells are cdk2-cyclinE/A, cdk1-cyclinB, cdk7-cyclin H, and cdk9/cyclin T (33, 52), yet it is possible that other unknown cellular or viral kinases may be inhibited by the concentrations of Rosco used in this study. VZV encodes two kinases, ORF47 and ORF66, which could potentially be inhibited by Rosco. ORF47 kinase phosphorylates IE62 and ORF9 proteins (5, 22, 23, 37), but ORF47 kinase activity is not inhibited by Rosco in vitro (T. K. Kenyon, personal communication). On the other hand, cdk1 phosphorylates gI, an essential VZV glycoprotein in vivo (34), and this interaction is specifically inhibited by Rosco in vitro (53). ORF66 protein kinase phosphorylates IE62, but this is likely not the only target, as ORF66 is not essential for growth in cultured cells (19, 26). It will be interesting to test whether in vitro ORF66 kinase activity is inhibited by Rosco. The question as to whether Rosco targets virus proteins was recently addressed, and it was determined that no HSV-1 proteins from infected cell extracts bound to immobilized purvalanol, a purine-derived cdk inhibitor related to Rosco (43). Because VZV kinases could be substrates for cdk inhibitors, experiments are planned to determine whether any VZV proteins bind to immobilized Rosco and purvalanol.
Rosco falls into the group of purine-derived pharmacological cdk inhibitors that have potential clinical applications. Originally designed to block proliferation of cancer cells, cdk inhibitors such as flavopiridol are well tolerated in humans (48, 51). Rosco was effective in a rodent cancer model (41), has been studied in a rodent model of herpetic keratitis (2), and has recently been given to humans in phase 1 trials with no signs of toxicity (14). Whether purine-derived pharmacological cdk inhibitors can be used as antivirals remains an unanswered question that should be pursued because there is a great need for improved treatments for VZV infections.
Zoster is an extremely painful condition often associated with serious complications such as nerve damage, vision impairment, and postherpetic neuralgia, and the incidence is highest in elderly people and those who are immunosuppressed (29). Varicella and zoster are treated with acyclovir and its derivatives, but pain and other sequelae often persist despite therapy (42, 49). In addition, there are reports of acyclovir-resistant VZV arising during long-term treatment for chronic zoster in immune-deficient patients (4, 8, 30). Resistance to antiviral agents that target cellular genes is unlikely to arise by mutations in the virus genome, so cdk inhibitors such as Rosco would have that advantage if they were developed as new treatments for VZV infections. This has been our experience and that of others, since HSV-1 and VZV mutants resistant to Rosco could not be isolated under conditions where resistance to acyclovir easily arose (45; J. F. Moffat, unpublished data).
Cellular proteins that are essential for virus replication are novel antiviral targets (reviewed in reference 11). This area of drug development has not been deeply mined because of concerns over cytotoxicity and harmful side effects. While not disregarding toxicity, there are compelling reasons to pursue antiviral applications for cdk inhibitors. Compounds that block cdk's required for several viruses would be broadly effective, and cdk inhibitors have additive effects with acyclovir and could be combined with the current nucleoside analogs (43). Before cdk inhibitors can be applied against herpesvirus infections, more studies must be done to identify the cellular kinases that are involved in virus replication. To that end, experiments addressing the role of cdk's in VZV replication are currently under investigation.
Acknowledgments
This work was supported by the Hendricks Research Fund, SUNY Upstate Medical University, and Public Health Service grants R01 AI052168 (J.F.M.) and R01 EY07397 (P.R.K.).
REFERENCES
- 1.Arvin, A. M. 1996. Varicella-zoster virus, p. 2547-2585. In B. N. Fields, D. N. Knipe, and P. M. Howley (ed.), Virology, 2nd ed. Lippincott-Raven, Philadelphia, Pa.
- 2.Avunduk, A. M., E. D. Varnell, and H. E. Kaufman. 2003. The effect of roscovitine on herpetic keratitis. Exp. Eye Res. 76:679-683. [DOI] [PubMed] [Google Scholar]
- 3.Babich, H., A. Sedletcaia, and B. Kenigsberg. 2002. In vitro cytotoxicity of protocatechuic acid to cultured human cells from oral tissue: involvement in oxidative stress. Pharmacol. Toxicol. 91:245-253. [DOI] [PubMed] [Google Scholar]
- 4.Bernhard, P., and N. Obel. 1995. Chronic ulcerating acyclovir-resistant varicella zoster lesions in an AIDS patient. Scand. J. Infect. Dis. 27:623-625. [DOI] [PubMed] [Google Scholar]
- 5.Besser, J., M. H. Sommer, L. Zerboni, C. P. Bagowski, H. Ito, J. Moffat, C. C. Ku, and A. M. Arvin. 2003. Differentiation of varicella-zoster virus ORF47 protein kinase and IE62 protein binding domains and their contributions to replication in human skin xenografts in the SCID-hu mouse. J. Virol. 77:5964-5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boutell, C., A. Orr, and R. D. Everett. 2003. PML residue lysine 160 is required for the degradation of PML induced by herpes simplex virus type 1 regulatory protein ICP0. J. Virol. 77:8686-8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bresnahan, W. A., I. Boldogh, P. Chi, E. A. Thompson, and T. Albrecht. 1997. Inhibition of cellular Cdk2 activity blocks human cytomegalovirus replication. Virology 231:239-247. [DOI] [PubMed] [Google Scholar]
- 8.Breton, G., A. M. Fillet, C. Katlama, F. Bricaire, and E. Caumes. 1998. Acyclovir-resistant herpes zoster in human immunodeficiency virus-infected patients: results of foscarnet therapy. Clin. Infect. Dis. 27:1525-1527. [DOI] [PubMed] [Google Scholar]
- 9.Cai, W., and P. A. Schaffer. 1991. A cellular function can enhance gene expression and plating efficiency of a mutant defective in the gene for ICP0, a transactivating protein of herpes simplex virus type 1. J. Virol. 65:4078-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chao, S. H., K. Fujinaga, J. E. Marion, R. Taube, E. A. Sausville, A. M. Senderowicz, B. M. Peterlin, and D. H. Price. 2000. Flavopiridol inhibits P-TEFb and blocks HIV-1 replication. J. Biol. Chem. 275:28345-28348. [DOI] [PubMed] [Google Scholar]
- 11.Coen, D. M., and P. A. Schaffer. 2003. Antiherpesvirus drugs: a promising spectrum of new drugs and drug targets. Nat. Rev. Drug Discov. 2:278-288. [DOI] [PubMed] [Google Scholar]
- 12.Cohrs, R. J., D. H. Gilden, P. R. Kinchington, E. Grinfeld, and P. G. Kennedy. 2003. Varicella-zoster virus gene 66 transcription and translation in latently infected human ganglia. J. Virol. 77:6660-6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daksis, J. I., and C. M. Preston. 1992. Herpes simplex virus immediate early gene expression in the absence of transinduction by Vmw65 varies during the cell cycle. Virology 189:196-202. [DOI] [PubMed] [Google Scholar]
- 14.Dancey, J., and E. A. Sausville. 2003. Issues and progress with protein kinase inhibitors for cancer treatment. Nat. Rev. Drug Discov. 2:296-313. [DOI] [PubMed] [Google Scholar]
- 15.Everett, R. D., G. Sourvinos, and A. Orr. 2003. Recruitment of herpes simplex virus type 1 transcriptional regulatory protein ICP4 into foci juxtaposed to ND10 in live, infected cells. J. Virol. 77:3680-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evers, D., J. Breitenbach, K. Borysko, L. Townsend, and J. Drach. 2002. Inhibition of cyclin-dependent kinase 1 by purines and pyrrolo[2, 3-d]pyrimidines does not correlate with antiviral activity. Antimicrob. Agents Chemother. 46:2470-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flemington, E. K. 2001. Herpesvirus lytic replication and the cell cycle: arresting new developments. J. Virol. 75:4475-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawrami, K., and J. Breuer. 1999. Development of a fluorogenic polymerase chain reaction assay (TaqMan) for the detection and quantitation of varicella zoster virus. J. Virol. Methods 79:33-40. [DOI] [PubMed] [Google Scholar]
- 19.Heineman, T. C., K. Seidel, and J. I. Cohen. 1996. The varicella-zoster virus ORF66 protein induces kinase activity and is dispensable for viral replication. J. Virol. 70:7312-7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan, R., L. Schang, and P. A. Schaffer. 1999. Transactivation of herpes simplex virus type 1 immediate-early gene expression by virion-associated factors is blocked by an inhibitor of cyclin-dependent protein kinases. J. Virol. 73:8843-8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalejta, R. F., and T. Shenk. 2002. Manipulation of the cell cycle by human cytomegalovirus. Front. Biosci. 7:d295-d306. [DOI] [PubMed] [Google Scholar]
- 22.Kenyon, T. K., J. I. Cohen, and C. Grose. 2002. Phosphorylation by the varicella-zoster virus ORF47 protein serine kinase determines whether endocytosed viral gE traffics to the trans-Golgi network or recycles to the cell membrane. J. Virol. 76:10980-10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenyon, T. K., J. Lynch, J. Hay, W. Ruyechan, and C. Grose. 2001. Varicella-zoster virus ORF47 protein serine kinase: characterization of a cloned, biologically active phosphotransferase and two viral substrates, ORF62 and ORF63. J. Virol. 75:8854-8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinchington, P., D. Bookey, and S. Turse. 1995. The transcriptional regulatory proteins encoded by varicella-zoster virus open reading frames (ORFs) 4 and 63, but not ORF 61, are associated with purified virus particles. J. Virol. 69:4274-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinchington, P., J. Hougland, A. Arvin, W. Ruyechan, and J. Hay. 1992. The varicella-zoster virus immediate-early protein IE62 is a major component of virus particles. J. Virol. 66:359-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinchington, P. R., K. Fite, A. Seman, and S. E. Turse. 2001. Virion association of IE62, the varicella-zoster virus (VZV) major transcriptional regulatory protein, requires expression of the VZV open reading frame 66 protein kinase. J. Virol. 75:9106-9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ku, C. C., J. A. Padilla, C. Grose, E. C. Butcher, and A. M. Arvin. 2002. Tropism of varicella-zoster virus for human tonsillar CD4(+) T lymphocytes that express activation, memory, and skin homing markers. J. Virol. 76:11425-11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaGuardia, J. J., R. J. Cohrs, and D. H. Gilden. 1999. Prevalence of varicella-zoster virus DNA in dissociated human trigeminal ganglion neurons and nonneuronal cells. J. Virol. 73:8571-11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liesegang, T. J. 1999. Varicella zoster viral disease. Mayo Clin. Proc. 74:983-998. [DOI] [PubMed] [Google Scholar]
- 30.Linnemann, C. C., Jr., K. K. Biron, W. G. Hoppenjans, and A. M. Solinger. 1990. Emergence of acyclovir-resistant varicella zoster virus in an AIDS patient on prolonged acyclovir therapy. AIDS 4:577-579. [DOI] [PubMed] [Google Scholar]
- 31.Loparev, V. N., K. McCaustland, B. P. Holloway, P. R. Krause, M. Takayama, and D. S. Schmid. 2000. Rapid genotyping of varicella-zoster virus vaccine and wild-type strains with fluorophore-labeled hybridization probes. J. Clin. Microbiol. 38:4315-4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maul, G. G., A. M. Ishov, and R. D. Everett. 1996. Nuclear domain 10 as preexisting potential replication start sites of herpes simplex virus type-1. Virology 217:67-75. [DOI] [PubMed] [Google Scholar]
- 33.Meijer, L., A. Borgne, O. Mulner, J. P. Chong, J. J. Blow, N. Inagaki, M. Inagaki, J. G. Delcros, and J. P. Moulinoux. 1997. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 243:527-536. [DOI] [PubMed] [Google Scholar]
- 34.Moffat, J., H. Ito, M. Sommer, S. Taylor, and A. M. Arvin. 2002. Glycoprotein I of varicella-zoster virus is required for viral replication in skin and T cells. J. Virol. 76:8468-8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moffat, J. F., L. Zerboni, P. R. Kinchington, C. Grose, H. Kaneshima, and A. M. Arvin. 1998. Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J. Virol. 72:965-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moffat, J. F., L. Zerboni, M. H. Sommer, T. C. Heineman, J. I. Cohen, H. Kaneshima, and A. M. Arvin. 1998. The ORF47 and ORF66 putative protein kinases of varicella-zoster virus determine tropism for human T cells and skin in the SCID-hu mouse. Proc. Natl. Acad. Sci. USA 95:11969-11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng, T. I., L. Keenan, P. R. Kinchington, and C. Grose. 1994. Phosphorylation of varicella-zoster virus open reading frame (ORF) 62 regulatory product by viral ORF 47-associated protein kinase. J. Virol. 68:1350-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niizuma, T., L. Zerboni, M. H. Sommer, H. Ito, S. Hinchliffe, and A. M. Arvin. 2003. Construction of varicella-zoster virus recombinants from parent Oka cosmids and demonstration that ORF65 protein is dispensable for infection of human skin and T cells in the SCID-hu mouse model. J. Virol. 77:6062-6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oberg, B. 1989. Antiviral effects of phosphonoformate (PFA, foscarnet sodium). Pharmacol. Ther. 40:213-285. [DOI] [PubMed] [Google Scholar]
- 40.Perera, L. P., J. D. Mosca, M. Sadeghi-Zadeh, W. T. Ruyechan, and J. Hay. 1992. The varicella-zoster virus immediate early protein, IE62, can positively regulate its cognate promoter. Virology 191:346-354. [DOI] [PubMed] [Google Scholar]
- 41.Pippin, J. W., Q. Qu, L. Meijer, and S. J. Shankland. 1997. Direct in vivo inhibition of the nuclear cell cycle cascade in experimental mesangial proliferative glomerulonephritis with Roscovitine, a novel cyclin-dependent kinase antagonist. J. Clin. Investig. 100:2512-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rowbotham, M. C., and K. L. Petersen. 2001. Zoster-associated pain and neural dysfunction. Pain 93:1-5. [DOI] [PubMed] [Google Scholar]
- 43.Schang, L., A. Bantly, M. Knockaert, F. Shaheen, L. Meijer, M. Malim, N. Gray, and P. Schaffer. 2002. Pharmacological cyclin-dependent kinase inhibitors inhibit replication of wild-type and drug-resistant strains of herpes simplex virus and human immunodeficiency virus type 1 by targeting cellular, not viral, proteins. J. Virol. 76:7874-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schang, L. M., A. Bantly, and P. A. Schaffer. 2002. Explant-induced reactivation of herpes simplex virus occurs in neurons expressing nuclear cdk2 and cdk4. J. Virol. 76:7724-7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schang, L. M., J. Phillips, and P. A. Schaffer. 1998. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J. Virol. 72:5626-5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schang, L. M., A. Rosenberg, and P. A. Schaffer. 2000. Roscovitine, a specific inhibitor of cellular cyclin-dependent kinases, inhibits herpes simplex virus DNA synthesis in the presence of viral early proteins. J. Virol. 74:2107-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schang, L. M., A. Rosenberg, and P. A. Schaffer. 1999. Transcription of herpes simplex virus immediate-early and early genes is inhibited by roscovitine, an inhibitor specific for cellular cyclin-dependent kinases. J. Virol. 73:2161-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Senderowicz, A. M. 1999. Flavopiridol: the first cyclin-dependent kinase inhibitor in human clinical trials. Investig. New Drugs 17:313-320. [DOI] [PubMed] [Google Scholar]
- 49.Snoeck, R., G. Andrei, and E. De Clercq. 1999. Current pharmacological approaches to the therapy of varicella zoster virus infections: a guide to treatment. Drugs 57:187-206. [DOI] [PubMed] [Google Scholar]
- 50.Somerville, L., and J. Cory. 2000. Enhanced roscovitine-induced apoptosis is mediated by a caspase-3-like activity in deoxyadenosine-resistant mouse leukemia L1210 cells. Anticancer Res. 20:3347-3355. [PubMed] [Google Scholar]
- 51.Stadler, W. M., N. J. Vogelzang, R. Amato, J. Sosman, D. Taber, D. Liebowitz, and E. E. Vokes. 2000. Flavopiridol, a novel cyclin-dependent kinase inhibitor, in metastatic renal cancer: a University of Chicago Phase II Consortium study. J. Clin. Oncol. 18:371-375. [DOI] [PubMed] [Google Scholar]
- 52.Wang, D., C. de la Fuente, L. Deng, L. Wang, I. Zilberman, C. Eadie, M. Healey, D. Stein, T. Denny, L. E. Harrison, L. Meijer, and F. Kashanchi. 2001. Inhibition of human immunodeficiency virus type 1 transcription by chemical cyclin-dependent kinase inhibitors. J. Virol. 75:7266-7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ye, M., K. M. Duus, J. Peng, D. H. Price, and C. Grose. 1999. Varicella-zoster virus Fc receptor component gI is phosphorylated on its endodomain by a cyclin-dependent kinase. J. Virol. 73:1320-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]