Abstract
Background. In studies from high-income countries, human immunodeficiency virus type 1 (HIV-1)–infected persons have diminished responses to hepatitis B virus (HBV) vaccination, compared with HIV-1–uninfected persons, but data from other settings are limited.
Methods. We compared the immune response to HBV vaccination among HIV-1–infected and HIV-1–uninfected Kenyan adults and assessed the response of HIV-1–infected initial nonresponders to revaccination with a standard HBV vaccine series.
Results. Of 603 participants, 310 (51.4%) were HIV-1–infected, for whom the median CD4+ T-cell count was 557 cells/μL (interquartile range, 428–725 cells/μL); none were receiving antiretroviral therapy. Nonresponse to HBV vaccine was higher among HIV-1–infected participants, compared with HIV-1–uninfected participants (35.8% vs 14.3%; odds ratio, 3.33; P < .001). Of 102 HIV-1–infected initial nonresponders, 88 (86.3%) responded to revaccination, for an overall response, including to revaccination, of 94.9%. Among HIV-1–infected individuals, lower CD4+ T-cell counts and male sex were independent predictors of nonresponse to initial vaccination, and lower body mass index, higher plasma HIV-1 RNA levels, and longer time to revaccination predicted nonresponse to revaccination.
Conclusions. Kenyan adults had similar HBV vaccination responses as persons from high-income countries. Timely revaccination of HIV-1–infected nonresponders increased response to the vaccine to 95%.
Keywords: HBV vaccine, HIV-1, Africa
Africa, in addition to bearing the greatest human immunodeficiency virus type 1 (HIV-1) disease burden globally, has high endemicity for hepatitis B virus (HBV) infection [1]. Of the 360 million people infected with HBV worldwide, 50 million live in Africa [2]. Owing to shared routes of transmission and high prevalence of both infections, coinfection with HIV-1 and HBV is common [3], with a prevalence of 6%–20% in the region [3–5]. HIV-1/HBV coinfection is associated with significant morbidity, including cirrhosis and hepatocellular carcinoma. Preventing HBV infection, particularly in HIV-1–infected persons, is of public health importance.
HBV vaccination of people living with HIV-1 is a cornerstone of HBV prevention. HIV-1–infected persons, however, have diminished vaccine responses, including to HBV vaccine [6, 7]. In studies from the United States, >90% of healthy HIV-1–uninfected persons developed an immune response to HBV vaccination, compared with 20%–70% of HIV-1–infected persons [8]. Poor response to HBV vaccination in HIV-1–infected persons has been associated with high HIV-1 load [9] and low CD4+ T-cell count [6, 7]. Other predictors of nonresponse include older age, male sex, obesity, smoking, and alcohol use [8]. Several studies have demonstrated that the immune response to HBV vaccine among HIV-1–infected children and adults may be improved by using high vaccine doses, giving additional doses, or using immunomodulatory agents [10–16].
The World Health Organization (WHO) recommends that HIV-1–infected adults who do not respond to the primary vaccine series should be revaccinated with 3 doses of either the standard or double-strength vaccine series [1]. The US Advisory Committee on Immunization Practices recommends revaccination of HIV-1–infected nonresponders but adds that data are limited on the effectiveness of modified vaccination schedules or dosing [17].
While investigators have studied responses to HBV vaccination in HIV-1 infection in North American, European, and Asian populations, to our knowledge no study has explored the serological response to HBV vaccine among HIV-1–infected African adults. In addition, there are insufficient data to provide definite guidelines for revaccinating African HIV-1–infected persons who do not respond to an initial vaccine series. To address these knowledge gaps, we compared the immune response to HBV vaccination among HIV-1–infected and HIV-1–uninfected individuals and assessed factors associated with nonresponse. We also assessed the serological response to revaccination, using a repeat standard dose series, among HIV-1–infected individuals who did not develop an antibody response to the initial vaccine series.
METHODS
Study Design and Population
This prospective interventional study was conducted among HIV-1–infected and HIV-1–uninfected men and women attending a research clinic in Thika, Kenya. All participants were concurrently enrolled in the Partners PrEP Study, a phase III, multisite, randomized, double-blind, placebo-controlled trial of daily oral tenofovir-based preexposure prophylaxis (PrEP) for the prevention of HIV-1 acquisition. For that clinical trial, all participants were members of heterosexual HIV-1–serodiscordant couples (ie, couples in which one member was HIV-1 seropositive and the other seronegative). The trial design, eligibility criteria, and participant characteristics are described elsewhere [18]. In brief, at the time of enrollment, HIV-1–infected partners did not meet immunologic and clinical criteria for antiretroviral treatment (ART) initiation but were referred for ART during follow-up if they became eligible according to the national guidelines. HIV-1–uninfected partners were healthy, with normal renal, liver, and hematologic function at enrollment and were assigned to daily oral tenofovir treatment, emtricitabine/tenofovir treatment, or placebo. All participants were followed prospectively for a maximum of 36 months.
Procedures
As part of screening procedures for the Partners PrEP Study, all potential trial participants were assessed for HBV by use of HBV surface antigen (HBsAg; Murex Abbot Murex, Dartford, United Kingdom) and HBV surface antibody (HBsAb; Murex Abbot Murex) assays, and enrollees who were susceptible to HBV (ie, negative for HBsAg and negative for HBsAb) were offered vaccination. Participants who accepted vaccination received 3 doses of 20 μg of recombinant HBsAg (Euvax B [LG Life Sciences, Seoul, Korea], Revacc B [Bharat Biotech International, India], or Shanvac B [Shantha Biotech, India], depending on supplier availability) at 0, 1 to 3, and 6 months. For the present study, those who were susceptible to HBV infection, completed the vaccination series on schedule, and had an archived blood sample collected 6 months after completion of vaccine series were assessed. Immune response to HBV vaccine was measured by development of protective levels of HBsAb in serum, using the DiaSorin LIAISON anti-HBs II assay (DiaSorin, Saluggia, Italy). The testing was conducted at a research laboratory in Nairobi that participated in an external quality assurance program for testing related to HBV and HIV-1. Samples were tested in a batched fashion on archived frozen specimens collected at a scheduled clinical trial visit 6 months after the vaccine series was completed. Antibody titers were reported as dichotomous response/nonresponse, with titers <10 mIU/mL considered nonresponse.
HIV-1–infected participants who had a nonresponse to this initial vaccination were offered revaccination, using a 3-dose schedule at 0, 1, and 6 months of 20 μg of recombinant HBV surface antigen. Blood samples to assess antibody response were collected 4 weeks after the first and last dose. Participants who had developed protective antibodies at the time of the blood sample collection 4 weeks after the first dose did not receive the third revaccination dose.
Sociodemographic, behavioral, clinical, and laboratory data were collected in standard case report forms. Body mass index (BMI), calculated as the weight in kilograms divided by the height in meters squared, was categorized using the following WHO categories: underweight, <18.5; normal, 18.5–24.9; overweight, 25–29.9; and obese, ≥30.0 [19].
Among HIV-1–infected participants, plasma HIV-1 RNA concentrations were quantified on samples collected at the time of clinical trial enrollment in batch testing at the University of Washington, using the Abbott Real-Time HIV-1 RNA assay, with a limit of quantification of 80 copies/mL. CD4+ T-cell counts were assessed every 6 months, using the BD FACSCount (BD Biosciences).
The institutional review boards of the University of Washington and Kenyatta National Hospital approved the study. Participants provided written informed consent.
Statistical Analysis
The outcome variable was nonresponse to HBV vaccination, as measured by lack of development of protective levels of HBsAb. Binomial exact confidence intervals (CIs) were computed for the proportions of nonresponse.
We used logistic regression methods to assess the relationship between demographic, behavioral, and clinical characteristics and nonresponse to initial HBV vaccination and revaccination. Variables evaluated included age, sex, education, income, alcohol use, BMI, hormonal contraceptive use, WHO HIV-1 clinical stage, CD4+ T-cell count, HIV-1 plasma RNA concentration, and clinical trial randomization arm; for those undergoing revaccination, data on smoking, ART use, and time to revaccination were also collected and analyzed. Multivariate logistic regression models were fitted to determine independent predictors of nonresponsiveness to HBV vaccine. Overall nonresponse among the HIV-1–infected persons was analyzed by Kaplan-Meier methods.
Data were analyzed using Intercooled Stata, version 11.1.
RESULTS
The research site enrolled 495 HIV-1–serodiscordant couples into the Partners PrEP Study. Of HIV-1–infected enrollees, 122 (24.7%) were immune to HBV infection (HbsAb level, ≥10 IU/mL), 19 (3.8%) had chronic hepatitis B (HBsAg positive), and 354 (71.5%) were susceptible to HBV infection. Of the HIV-1–uninfected enrollees, 140 (28.3%) were immune to hepatitis B, 355 (71.7%) were susceptible, and none had chronic HBV infection, which was an exclusion criterion for the clinical trial. Of the 19 HIV-1–uninfected partners of HIV-1–infected participants who had chronic HBV infection, 12 (63.2%) were susceptible, and 7 (36.8%) were immune to HBV infection.
A total of 603 HBV-susceptible participants completed the vaccine regimen as scheduled and had a serum sample available for testing (Figure 1). Of these, 310 (51.4%) were HIV-1 infected, and 287 (47.6%) were male. The median age was 31 years (interquartile range [IQR], 26–38 years). For the HIV-1–infected participants, the median CD4+ T-cell count was 557 cells/μL (IQR, 428–725 cells/μL; range, 254–1833 cells/μL), and none had initiated ART at the beginning of the HBV vaccination series.
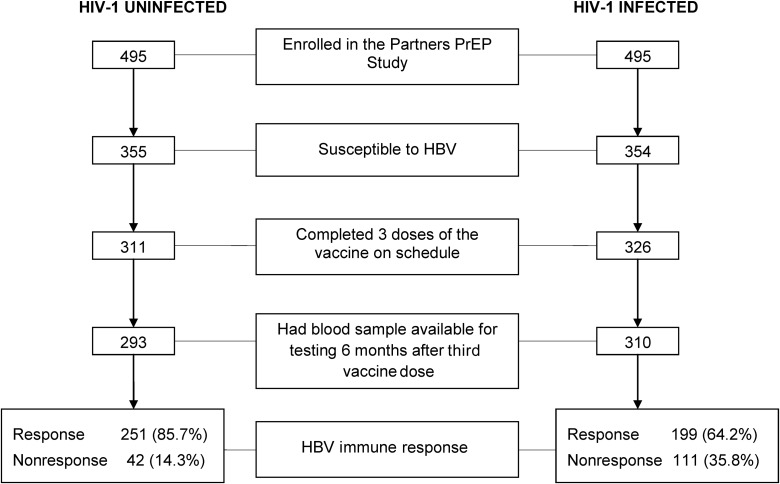
Figure 1.
Study participants and hepatitis B virus (HBV) vaccine immune response rates, by human immunodeficiency virus type 1 (HIV-1) status. Participants who were susceptible to HBV infection at enrollment into the Partners PrEP Study, completed the vaccination schedule, and had an archived blood sample collected 6 months after completion of the standard HBV vaccination series and their immune response rates, by HIV-1 status.
Response to HBV Vaccination
Six months after completing the standard HBV vaccination series, 111 (35.8%) of 310 HIV-1–infected participants did not have protective HBsAb titers, compared with 42 (14.3%) of 293 HIV-1–uninfected participants (odds ratio [OR] for nonresponse, 3.33; 95% CI, 2.23–4.98; P < .001), translating to a HBV vaccination response of 85.7% (95% CI, 81.1–89.5) among those who were HIV-1–uninfected and 64.2% (95% CI, 58.6–69.5) among those who were HIV-1–infected.
In multivariate analysis, among HIV-1–infected participants, sex and CD4+ T-cell counts were statistically significantly associated with nonresponse (Table 1). Men were more than twice as likely as women to be nonresponders (adjusted OR, 2.37; P = .02). Compared with participants who had a CD4+ T-cell count of >500 cells/μL at baseline, those with CD4+ T-cell counts of 350–500 cells/μL (adjusted OR, 1.98; P = .02) and 250–350 cells/µL (adjusted OR, 2.02; P = .06) were 2-fold more likely not to respond to the vaccine. Only 5 participants initiated ART during the vaccination period; of these, 3 did not respond to HBV vaccine. Among HIV-1–uninfected participants, there were no statistically significant predictors of nonresponse in univariate analysis.
Table 1.
Correlates of Nonresponse to Initial Hepatitis B Virus Vaccination, by Human Immunodeficiency Virus Type 1 (HIV-1) Status
| HIV-1 Uninfected (n = 293) |
HIV-1 Infected (n = 310) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Total, No. (%) | Nonresponders, No. (%) | OR (95% CI) | P | Total, No. (%) | Nonresponders, No. (%) | OR (95% CI) | P | aOR (95% CI) | P |
| Age, y | ||||||||||
| 18–24 | 30 (10.24) | 1 (3.33) | Ref | 67 (21.61) | 23 (34.33) | Ref | ||||
| 25–34 | 138 (47.10) | 24 (17.39) | 6.11 (.79–47.02) | .08 | 154 (49.68) | 51 (33.12) | 0.95 (.52–1.74) | .86 | 0.87 (.46–1.65) | .67 |
| 35–44 | 91 (31.06) | 11 (12.09) | 3.99 (.49–32.26) | .20 | 63 (20.32) | 22 (34.92) | 1.03 (.50–2.12) | .94 | 0.67 (.29–1.57) | .36 |
| >45 | 34 (11.60) | 6 (17.65) | 6.21 (.70–54.96) | .10 | 26 (8.39) | 15 (57.69) | 2.61 (1.03–6.59) | .04 | 1.42 (.48–4.22) | .53 |
| Sex | ||||||||||
| Female | 70 (23.89) | 9 (12.86) | Ref | 246 (79.35) | 78 (31.71) | Ref | ||||
| Male | 223 (76.11) | 33 (14.80) | 1.18 (.53–2.60) | .69 | 64 (20.65) | 33 (51.56) | 2.29 (1.31–4.01) | .004 | 2.37 (1.17–4.77) | .02 |
| Education, y | ||||||||||
| None | 5 (1.71) | 1 (20.00) | Ref | 5 (1.61) | 2 (40.00) | Ref | ||||
| 1–8 | 161 (54.95) | 16 (9.94) | 0.44 (.05–4.19) | .48 | 200 (64.52) | 71 (36.50) | 0.83 (.13–5.05) | .84 | ||
| 9–12 | 100 (34.13) | 20 (20.00) | 1.00 (.11–9.44) | 1.00 | 82 (26.45) | 28 (34.15) | 0.78 (.12–4.93) | .79 | ||
| >12 | 27 (9.22) | 5 (18.52) | 0.91 (.08–9.99) | .94 | 23 (7.43) | 10 (43.48) | 1.15 (.16–8.27) | .89 | ||
| Monthly income | ||||||||||
| None | 28 (0.10) | 6 (21.43) | Ref | 112 (36.13) | 43 (38.39) | Ref | ||||
| Any | 265 (0.90) | 36 (13.58) | 0.58 (.22–1.52) | .27 | 198 (63.87) | 68 (34.34) | 0.84 (.52–1.36) | .48 | ||
| Weekly alcoholic drinks | ||||||||||
| None | 249 (84.98) | 34 (13.65) | Ref | 290 (93.55) | 101 (34.83) | Ref | ||||
| Any | 44 (15.02) | 8 (18.18) | 1.41 (.60–3.28) | .43 | 20 (6.45) | 10 (50.00) | 1.87 (.75–4.65) | .18 | ||
| Hormonal contraceptive use (women only) | ||||||||||
| None | 22 (31.43) | 5 (22.73) | Ref | 83 (33.74) | 31 (37.35) | Ref | ||||
| Any | 48 (68.57) | 4 (8.33) | 0.31 (.07–1.29) | .11 | 163 (66.26) | 47 (28.83) | 0.68 (.39–1.19) | .18 | ||
| BMIa | ||||||||||
| <18.5 | 48 (16.38) | 8 (16.67) | Ref | 23 (7.42) | 9 (39.13) | Ref | … | |||
| 18.5–24.9 | 188 (64.16) | 23 (12.23) | 0.70 (.29–1.67) | .42 | 198 (63.87) | 75 (37.88) | 0.95 (.39–2.99) | .91 | … | |
| 25.0–29.9 | 44 (15.02) | 9 (20.45) | 1.29 (.45–3.69) | .64 | 69 (22.26) | 22 (31.88) | 0.73 (.27–1.94) | .53 | … | |
| ≥30.0 | 13 (4.44) | 2 (15.38) | 0.91 (.17–4.91) | .91 | 20 (6.45) | 5 (25.00) | 0.52 (.14–1.93) | .33 | … | |
| CD4+ T-cell count, cells/µL | ||||||||||
| >500 | N/A | N/A | N/A | N/A | 190 (61.29) | 55 (28.95) | Ref | |||
| 350–500 | N/A | N/A | N/A | N/A | 82 (26.45) | 38 (46.34) | 2.12 (1.24–3.62) | .01 | 1.98 (1.13–3.49) | .02 |
| <350 | N/A | N/A | N/A | N/A | 38 (12.26) | 18 (47.37) | 2.21 (1.09–4.50) | .03 | 2.02 (.96–4.27) | .06 |
| Plasma HIV-1 load, RNA copies/mL | ||||||||||
| <10 000 | N/A | N/A | N/A | N/A | 203 (65.70) | 63 (31.03) | Ref | |||
| 10 000–49 999 | N/A | N/A | N/A | N/A | 72 (23.30) | 31 (43.06) | 1.68 (.97–2.92) | .07 | 1.56 (.87–2.81) | .13 |
| ≥50 000 | N/A | N/A | N/A | N/A | 34 (11.00) | 17 (50.00) | 2.22 (1.07–4.63) | .03 | 1.81 (.84–3.91) | .13 |
| WHO HIV-1 clinical stage | ||||||||||
| 1 | N/A | N/A | N/A | N/A | 228 (73.55) | 83 (36.40) | Ref | … | ||
| 2 | N/A | N/A | N/A | N/A | 63 (20.32) | 19 (30.16) | 0.75 (.41–1.38) | .36 | … | |
| 3 | N/A | N/A | N/A | N/A | 19 (6.13) | 9 (47.37) | 1.57 (.61–4.03) | .35 | … | |
| Clinical trial randomization arm | ||||||||||
| PrEP | 196 (66.89) | 25 (12.56) | Ref | N/A | N/A | N/A | … | |||
| Placebo | 97 (33.11) | 17 (17.35) | 1.46 (.75–2.86) | .27 | N/A | N/A | N/A | … | ||
All factors were as measured at start of vaccination.
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; N/A, not applicable; OR, odds ratio; PrEP, preexposure prophylaxis; Ref, reference; WHO, World Health Organization.
a Body mass index (BMI) is calculated as the weight in kilograms divided by the height in meters squared.
Response to Revaccination Among HIV-1–Infected Individuals
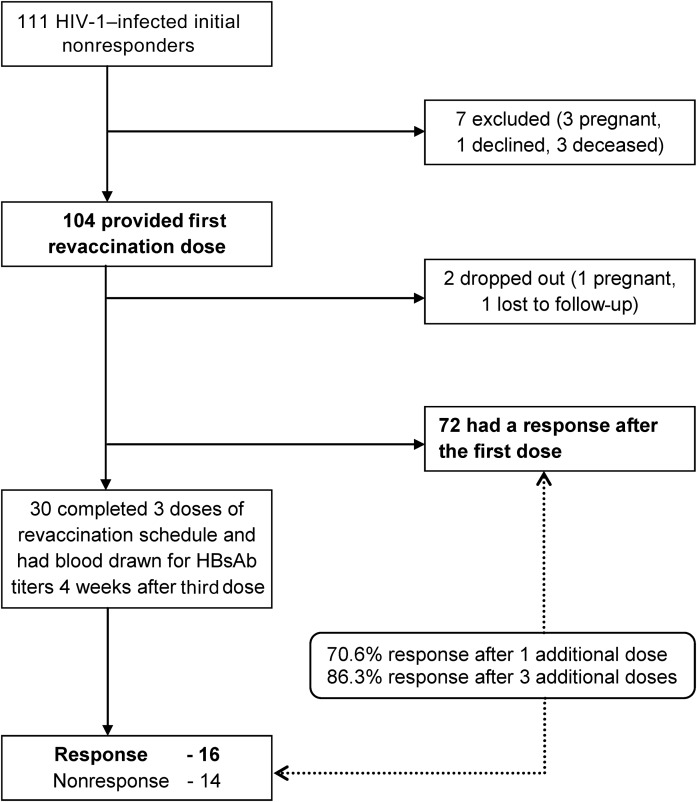
Of 111 HIV-1–infected subjects who did not respond to the initial vaccine series, revaccination was initiated for 104 and completed by 102 (Figure 2). The median age was 33 years (IQR, 28–40 years), and 33 (31.7%) were male. A minority reported use of alcohol (19 [18.3%]) or cigarettes (17 [16.3%]). The median CD4+ T-cell count at the time of initiation of the revaccination series was 459 cells/µL (IQR, 368–645 cells/µL), and 31 (29.8%) of the participants were using ART. The median time from the last dose of the initial vaccination series to the first dose of the revaccination series was 20.0 months (IQR, 12.1–24.2 months).
Figure 2.
Hepatitis B virus (HBV) vaccine immune response among human immunodeficiency virus type 1 (HIV-1)–infected initial nonresponders. Immune response to HBV standard dose revaccination after 1 additional dose or 3 additional doses among HIV-1–infected initial nonresponders.
Abbreviation: HBsAb, HBV surface antibody.
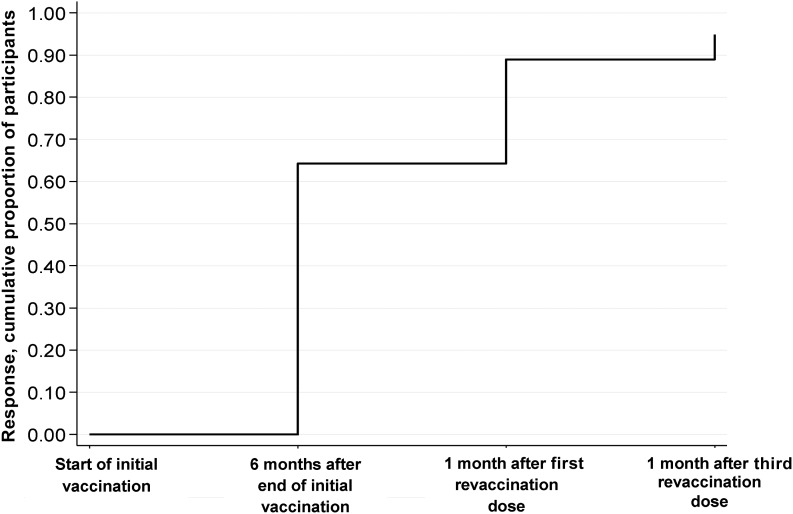
Following the first revaccination dose, 72 of 102 participants (70.6%) developed a positive antibody response. An additional 16 participants developed a positive antibody response after the third revaccination dose. Thus, of those who did not respond to the first vaccination series, 86.3% (88 of 102) responded during the repeat series. The cumulative response to initial and revaccination was thus 64.2% (95% CI, 58.6–69.5) after the initial series, 89.0% (95% CI, 85.1–92.2) after 1 revaccination dose, and 94.9% (95% CI, 91.8–97.0) after the complete 3-dose revaccination series (Figure 3).
Figure 3.
Cumulative proportion of response to additional doses of hepatitis B virus (HBV) vaccine among human immunodeficiency virus type 1 (HIV-1)–infected participants. Plot showing the cumulative proportion with immune response to additional doses of standard HBV vaccine among HIV-1–infected participants. Immune response was determined 6 months after completion of the initial vaccination series and 4 weeks after the first and third revaccination doses.
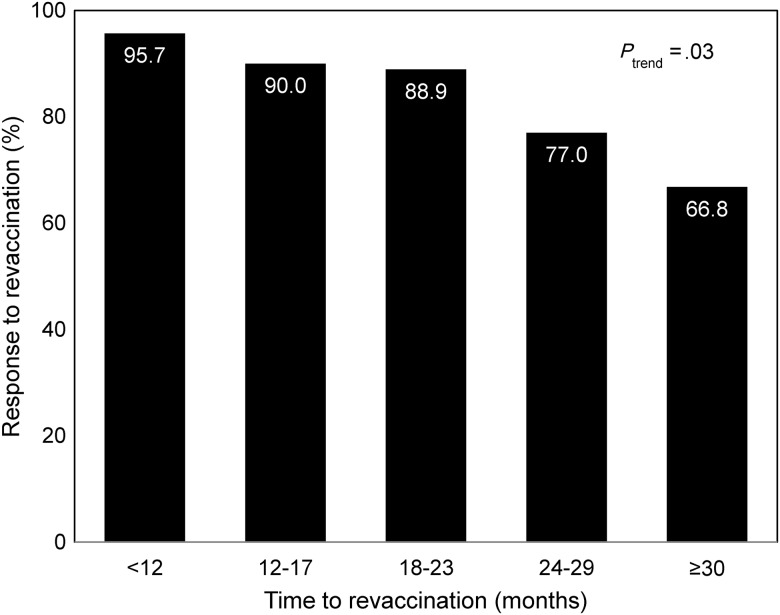
In multivariate analysis, 3 factors were associated with nonresponse to revaccination: BMI, plasma HIV-1 RNA load, and time from completion of initial vaccine series. Compared with participants with a normal BMI, those who were underweight were more likely not to respond (adjusted OR, 10.73; 95% CI, 1.01–113.88; P = .05). Participants with a baseline viral load of >50 000 copies/mL had a 6-fold greater odds of nonresponse, compared with those with a baseline viral load of <10 000 copies/mL (adjusted OR, 5.78; 95% CI, 1.17–28.62; P = .03). In addition, the odds of nonresponse increased with each additional month from the last dose of the initial vaccination series to the initiation of revaccination (adjusted OR, 1.19; 95% CI, 1.06–1.33; P = .004; Figure 4). Age, sex, education, contraceptive use, alcohol and cigarette use, CD4+ T-cell count, WHO stage, and ART use were not associated with response to revaccination. There was no association between the HBV vaccine type used and response to revaccination.
Figure 4.
Response to hepatitis B virus (HBV) revaccination among human immunodeficiency virus type 1 (HIV-1)–infected initial nonresponders, by time to revaccination. Bar chart depicting the percent response to HBV revaccination among HIV-1–infected persons with initial nonresponse to vaccination, by time to revaccination. The P value for trend is also shown.
DISCUSSION
In this study of HIV-1–infected and HIV-1–uninfected Kenyan adults, we found that standard HBV vaccination failed to result in protective immune responses for more than a third of those with HIV-1 infection, consistent with results from studies from the United States and other higher-income countries. Revaccination of HIV-1–infected initial nonresponders improved the overall response to 95%.
Lower CD4+ T-cell counts at the onset of vaccination and male sex were independent predictors of nonresponse to initial vaccination in HIV-1–infected individuals, whereas lower BMI, higher plasma HIV-1 RNA levels, and longer time to revaccination predicted nonresponse to revaccination. Higher plasma HIV-1 RNA levels were associated with nonresponse to initial HBV vaccination, but this association did not reach significance in multivariate analysis, possibly because of limited numbers in each HIV-1 RNA stratum. Low HBV vaccine immune response rates among men have been reported elsewhere, although the mechanism for this poor response is not clear [12, 13]. Malnutrition has been associated with impaired immune response to vaccines, including HBV vaccine among children and adults [20, 21], and some studies have found improved response rates with micronutrient supplementation [22, 23]. We did not observe an association between ART and response to vaccination or revaccination, although few participants initiated ART during the initial vaccination series. The role of ART in immune response to HBV vaccination is unclear. While some studies have demonstrated that participants receiving ART have better immune responses to vaccines [8, 24, 25], others have not [12, 13, 26].
Prior studies have suggested that modification of the standard HBV vaccine regimen by using higher HBV vaccine doses, increasing the number of HBV vaccine injections, or both significantly improves HBsAb seroconversion rates among HIV-1–infected adults [12–16, 25–28]. Our revaccination response rate was similar to that obtained in a French study of 20 HIV-1–infected participants, in which 55% of the subjects responded to an initial standard HBV vaccine dose and, of 9 initial nonresponders, 7 responded after 3 additional doses of standard HBV vaccine, translating to an overall response of 90% [16]. In a study of 65 subjects from Italy, the initial response rate to double-dose vaccine was 60% but increased to 89% after 2 additional double-dose injections [12]. Investigators in the United States reported a revaccination response rate of 59% among HIV-1–infected initial nonresponders revaccinated with 3–8 doses of standard HBV vaccine [25]. Another study of 144 participants conducted in the Netherlands observed that half of initial HIV-1 nonresponders responded to 3 monthly injections of double-dose HBV vaccine [13]. Thus, the poor response to HBV vaccine among HIV-1–infected adults can be overcome by providing additional doses of vaccine, and our results are consistent with this approach.
In this cohort of HIV-1–serodiscordant couples, >70% were susceptible to HBV at the time of study entry, and two-thirds of HIV-1–infected participants with chronic HBV/HIV-1 coinfection had partners who were HBV susceptible, underscoring the public health opportunity for HBV vaccination in this population. All HIV-1–infected persons in our study had relatively intact immune systems, with all having CD4+ T-cell counts of >250 cells/µL at the start of initial vaccination, which may in part explain the high rate of vaccine response; as reported in other studies [24, 29] provision of HBV vaccine to HIV-1–infected persons earlier in the course of HIV-1 infection, prior to development of advanced immunosuppression, is associated with a better HBV vaccine response. Among HIV-infected persons, postvaccination testing and revaccination of nonresponders with a full series is recommended [17]. While not definitive, our results suggest that revaccination should be initiated within 24 months of initial vaccination, for maximal results.
Our study had limitations. Ideally, HBV antibody testing is done within 4 weeks of completion of vaccination; however, we analyzed samples from 6 months after completion of vaccination, because the sampling was done as part of the HIV-1 prevention clinical trial. It is possible that some participants had waning antibodies and thus were misclassified as nonresponders, and our relatively low antibody response to vaccination among HIV-1–uninfected participants (85.7%) may thus be in part due to waning antibodies. In addition, we did not measure antibodies against hepatitis B core antigen (anti-HBc), although persons with isolated core antibody positivity (ie, those who were positive for anti-HBc, negative for HBsAg, and negative for HBsAb) may have had a past infection with waning HBsAb and may have been misclassified as susceptible to HBV. The HBsAb assay used in our study did not quantify antibody titers, and thus we cannot describe differences in titers. Finally, the ultimate measure of success in vaccination would be to follow participants and determine the incidence of HBV infection in the vaccinated participants, but this would be prohibitively expensive and time-consuming, and the development of HBsAb titers has been clearly demonstrated in multiple populations to be a strong surrogate marker for protection from HBV infection [17].
To the best of our knowledge, this is the first study to explore the immune response to HBV vaccination and revaccination among HIV-1–infected adults in Africa, where the HIV-1 prevalence is highest. Our findings add to the body of research on HBV vaccine immune responses and may help guide policy on the best practices for revaccinating HIV-1–infected persons who do not respond to the standard HBV vaccination schedule.
In summary, as has been seen in high-income settings, HIV-1–infected adults in Kenya had suboptimal response to standard HBV vaccine, and HIV-1–uninfected adults had high response. HIV-1–infected initial nonresponders achieved a 95% cumulative response with revaccination with a standard vaccine series. Determination of the HBsAb response after vaccination of HIV-1–infected adults and timely revaccination of nonresponders with 3 additional HBV vaccine doses can significantly increase the development of protective antibody titers.
Notes
Acknowledgments. We thank the HIV-1–serodiscordant couples enrolled at the Partners PrEP Study in Thika, Kenya, for their participation; and the study staff in Thika and the Clinical Trials Laboratory in Nairobi, for their contribution to this work.
Financial support. This work was supported by the Fogarty International Center, US National Institutes of Health (grant D43 TW000007), and by the Bill and Melinda Gates Foundation (grant 47674).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Essential prevention and care interventions for adults and adolescents living with HIV in resource-limited settings. Geneva: 2008. WHO. [Google Scholar]
- 2.Kiire CF. The epidemiology and prophylaxis of hepatitis B in sub-Saharan Africa: a view from tropical and subtropical Africa. Gut. 1996;38(Suppl 2):S5–12. doi: 10.1136/gut.38.suppl_2.s5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barth RE, Huijgen Q, Taljaard J, Hoepelman AI. Hepatitis B/C and HIV in sub-Saharan Africa: an association between highly prevalent infectious diseases. A systematic review and meta-analysis. Int J Infect Dis. 2010;14:e1024–31. doi: 10.1016/j.ijid.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Harania RS, Karuru J, Nelson M, Stebbing J. HIV, hepatitis B and hepatitis C coinfection in Kenya. AIDS. 2008;22:1221–2. doi: 10.1097/QAD.0b013e32830162a8. [DOI] [PubMed] [Google Scholar]
- 5.Bwogi J, Braka F, Makumbi I, et al. Hepatitis B infection is highly endemic in Uganda: findings from a national serosurvey. Afr Health Sci. 2009;9:98–108. [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HN, Harrington RD, Crane HM, Dhanireddy S, Dellit TH, Spach DH. Hepatitis B vaccination in HIV-infected adults: current evidence, recommendations and practical considerations. Int J STD AIDS. 2009;20:595–600. doi: 10.1258/ijsa.2009.009126. [DOI] [PubMed] [Google Scholar]
- 7.van den Berg R, van Hoogstraten I, van Agtmael M. Nonresponsiveness to hepatitis B vaccination in HIV seropositive patients; possible causes and solutions. AIDS Rev. 2009;11:157–64. [PubMed] [Google Scholar]
- 8.Kim HN, Harrington RD, Van Rompaey SE, Kitahata MM. Independent clinical predictors of impaired response to hepatitis B vaccination in HIV-infected persons. Int J STD AIDS. 2008;19:600–4. doi: 10.1258/ijsa.2007.007197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Overton ET, Sungkanuparph S, Powderly WG, Seyfried W, Groger RK, Aberg JA. Undetectable plasma HIV RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clin Infect Dis. 2005;41:1045–8. doi: 10.1086/433180. [DOI] [PubMed] [Google Scholar]
- 10.Bloom A, Jackson K, Kiviat A, Zheng H, Sax P, Gandhi R. Repeat hepatitis B vaccination may lead to seroprotection in HIV-infected patients who do not respond to an initial series. J Acquir Immune Defic Syndr. 2009;50:110–3. doi: 10.1097/QAI.0b013e318183acc0. [DOI] [PubMed] [Google Scholar]
- 11.Cornejo-Juarez P, Volkow-Fernandez P, Escobedo-Lopez K, Vilar-Compte D, Ruiz-Palacios G, Soto-Ramirez LE. Randomized controlled trial of hepatitis B virus vaccine in HIV-1-infected patients comparing two different doses. AIDS Res Ther. 2006;3:9. doi: 10.1186/1742-6405-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruciani M, Mengoli C, Serpelloni G, et al. Serologic response to hepatitis B vaccine with high dose and increasing number of injections in HIV infected adult patients. Vaccine. 2009;27:17–22. doi: 10.1016/j.vaccine.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 13.de Vries-Sluijs TE, Hansen BE, van Doornum GJ, et al. A prospective open study of the efficacy of high-dose recombinant hepatitis B rechallenge vaccination in HIV-infected patients. J Infect Dis. 2008;197:292–4. doi: 10.1086/524690. [DOI] [PubMed] [Google Scholar]
- 14.Fonseca MO, Pang LW, de Paula Cavalheiro N, Barone AA, Heloisa Lopes M. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine. 2005;23:2902–8. doi: 10.1016/j.vaccine.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 15.Potsch DV, Oliveira ML, Ginuino C, et al. High rates of serological response to a modified hepatitis B vaccination schedule in HIV-infected adults subjects. Vaccine. 2010;28:1447–50. doi: 10.1016/j.vaccine.2009.11.066. [DOI] [PubMed] [Google Scholar]
- 16.Rey D, Krantz V, Partisani M, et al. Increasing the number of hepatitis B vaccine injections augments anti-HBs response rate in HIV-infected patients. Effects on HIV-1 viral load. Vaccine. 2000;18:1161–5. doi: 10.1016/s0264-410x(99)00389-8. [DOI] [PubMed] [Google Scholar]
- 17.Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep. 2006;55:1–33. ; quiz CE1–4. [PubMed] [Google Scholar]
- 18.Mujugira A, Baeten JM, Donnell D, et al. Characteristics of HIV-1 serodiscordant couples enrolled in a clinical trial of antiretroviral pre-exposure prophylaxis for HIV-1 prevention. PLoS One. 2011;6:e25828. doi: 10.1371/journal.pone.0025828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. BMI classification. Geneva: 2012. WHO http://apps.who.int/bmi/index.jsp?introPage=intro_3.html. Accessed 16 June 2012. [Google Scholar]
- 20.Caidi H, Bennis IF, Mouan N, El Aouad R. [Evaluation of the response to vaccination against poliomyelitis and measles in malnourished children in Morocco] East Mediterr Health J. 2004;10:474–81. [PubMed] [Google Scholar]
- 21.Fabrizi F, Dixit V, Martin P, Jadoul M, Messa P. Meta-analysis: the impact of nutritional status on the immune response to hepatitis B virus vaccine in chronic kidney disease. Dig Dis Sci. 2012;57:1366–72. doi: 10.1007/s10620-011-1987-1. [DOI] [PubMed] [Google Scholar]
- 22.Miyagawa K, Hayashi Y, Kurihara S, Maeda A. Co-administration of l-cystine and l-theanine enhances efficacy of influenza vaccination in elderly persons: nutritional status-dependent immunogenicity. Geriatr Gerontol Int. 2008;8:243–50. doi: 10.1111/j.1447-0594.2008.00483.x. [DOI] [PubMed] [Google Scholar]
- 23.Langkamp-Henken B, Wood SM, Herlinger-Garcia KA, et al. Nutritional formula improved immune profiles of seniors living in nursing homes. J Am Geriatr Soc. 2006;54:1861–70. doi: 10.1111/j.1532-5415.2006.00982.x. [DOI] [PubMed] [Google Scholar]
- 24.Landrum ML, Huppler Hullsiek K, Ganesan A, et al. Hepatitis B vaccine responses in a large U.S. military cohort of HIV-infected individuals: another benefit of HAART in those with preserved CD4 count. Vaccine. 2009;27:4731–8. doi: 10.1016/j.vaccine.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Psevdos G, Kim JH, Groce V, Sharp V. Efficacy of double-dose hepatitis B rescue vaccination in HIV-infected patients. AIDS Patient Care STDS. 2010;24:403–7. doi: 10.1089/apc.2009.0340. [DOI] [PubMed] [Google Scholar]
- 26.Pettit NN, DePestel DD, Malani PN, Riddell Jt. Factors associated with seroconversion after standard dose hepatitis B vaccination and high-dose revaccination among HIV-infected patients. HIV Clin Trials. 2010;11:332–9. doi: 10.1310/hct1105-332. [DOI] [PubMed] [Google Scholar]
- 27.Cardell K, Akerlind B, Sallberg M, Fryden A. Excellent response rate to a double dose of the combined hepatitis A and B vaccine in previous nonresponders to hepatitis B vaccine. J Infect Dis. 2008;198:299–304. doi: 10.1086/589722. [DOI] [PubMed] [Google Scholar]
- 28.Launay O, van der Vliet D, Rosenberg AR, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA. 2011;305:1432–40. doi: 10.1001/jama.2011.351. [DOI] [PubMed] [Google Scholar]
- 29.Pasricha N, Datta U, Chawla Y, et al. Immune responses in patients with HIV infection after vaccination with recombinant Hepatitis B virus vaccine. BMC Infect Dis. 2006;6:65. doi: 10.1186/1471-2334-6-65. [DOI] [PMC free article] [PubMed] [Google Scholar]