Abstract
Background. Organ dysfunction and tissue hypoxia in severe falciparum malaria result from an imbalance between oxygen delivery and demand. In severe malaria, microvascular obstruction from parasite sequestration decreases oxygen delivery. However, host microvascular function (defined as the capacity to increase oxygen delivery in response to ischemia) and oxygen consumption have not been assessed.
Methods. We used near-infrared resonance spectroscopy to measure thenar muscle microvascular function (StO2recov) and oxygen consumption (VO2) in 36 adults in Papua, Indonesia, with severe malaria, 33 with moderately severe malaria (MSM), 24 with severe sepsis, and 36 healthy controls.
Results. In the severe malaria group, the StO2recov of 2.7%/second was 16% and 22% lower than that in the MSM group (3.1%/second) and control group (3.5%/second), respectively (P < .001), and comparable to that in the severe sepsis group (2.5%/second). In the severe malaria group, StO2recov was inversely correlated with lactate level (r = −0.63; P < .001) and predicted death (area under the receiver operating characteristic curve, 0.71 [95% confidence interval {CI}, .51–.92]), with each percentage decrease associated with an increased odds of mortality (odds ratio, 2.49 [95% CI, 1.05–6.2]). Conversely, VO2 increased in the severe malaria group by 18%, compared with levels in the control and severe sepsis groups (P < .001), and was associated with parasite biomass (r = 0.49; P = .04).
Conclusions. Impaired microvascular function is associated with increased mortality among individuals with severe malaria, while oxygen consumption is increased. Tissue hypoxia may result not only from microvascular obstruction, but also from impaired ability of the microvasculature to match oxygen delivery to increased oxygen demand.
Keywords: severe malaria, Plasmodium falciparum, microvascular function, oxygen consumption, tissue hypoxia
Mortality from severe Plasmodium falciparum malaria has decreased with use of intravenous artesunate. However, case-fatality rates remain at 8% and 15% for African children and Asian adults, respectively [1, 2]. Adjunctive therapies to improve outcomes require delineation of the pathogenic processes causing severe malaria.
A major pathogenic mechanism in severe malaria is microvascular sequestration of parasitized erythrocytes, resulting in obstructed flow and tissue hypoxia [3]. In adults with severe malaria, we have used reactive-hyperemia peripheral arterial tonometry to show functional impairment of nitric oxide (NO)–dependent vascular reactivity in larger conductance vessels [4]. Other investigators have visualized rectal capillaries, using orthogonal polarizing spectroscopy, and demonstrated heterogeneous blood flow during severe malaria instead of complete blockage [5], with vessels having increased flow adjacent to obstructed vessels. This suggests that, in addition to obstruction, the microvascular function or capacity of patent capillaries to compensate for decreased oxygen delivery is impaired during severe malaria [6]. This is proposed to be a pathogenic mechanism in sepsis but has not been well characterized in malaria [7]. The methods above have provided valuable information about vascular pathology during severe malaria but have not characterized the effects on oxygen delivery.
Oxygen demand affects tissue hypoxia. Sepsis patients have decreased oxygen consumption proportional to disease severity, with a postulated mechanism being mitochondrial inhibition by NO [8]. This mechanism has been proposed in the past to contribute to severe disease in falciparum malaria [9]. However, systemic and organ-specific NO bioavailability is decreased in both African children and Asian adults with severe malaria [4, 10]. Oxygen consumption in malaria and the relation to disease severity has not been assessed, nor has this been compared between patients with sepsis and healthy controls.
Near-infrared resonance spectroscopy (NIRS) measures tissue oxygenation by comparing absorption of near-infrared light by oxyhemoglobin (O2Hb) and deoxyhemoglobin (HHb) [11] solely in microcirculatory vessels, the vessels most affected by malaria [11]. By inducing an ischemic stress, NIRS has been used to assess microvascular function and oxygen consumption in sepsis and other diseases [12–15].
We assessed skeletal muscle microvascular function and oxygen consumption using NIRS in Indonesian adults with malaria or sepsis and control subjects. We hypothesized that microvascular function would be impaired in malaria in proportion to disease severity and that oxygen consumption in severe malaria would be reduced similar to sepsis. We also examined the factors associated with microvascular function and oxygen consumption.
METHODS
The study was conducted at Mitra Masyarakat Hospital, Timika, Indonesia, an area with unstable malaria transmission [16], and was approved by ethics committees of the National Institute of Health Research and Development, Indonesia, and the Menzies School of Health Research, Australia. Written informed consent was obtained from patients or from relatives, if patients were comatose or too ill.
Patients ≥18 years of age with severe P. falciparum malaria, moderately severe P. falciparum malaria (MSM), or severe sepsis were enrolled. Severe malaria was defined as peripheral parasitemia with ≥1 modified World Health Organization (WHO) criterion of severity: a Glasgow coma score of <11, acute renal failure (defined as a creatinine level of >265 µmol/L after rehydration or as a urine output of <400 mL per 24 hours), hyperbilirubinemia (defined as a total bilirubin level of >50 µmol, with either a creatinine level of >130 µmol/L or a parasite count of >100 000 parasites/µL), blackwater fever, hypoglycemia (defined as a whole blood glucose level of <2.2 mmol/L), respiratory distress (defined as a respiratory rate of >32 breaths/minutes), acidosis (defined as a venous bicarbonate level of <15 mmol/L), shock (defined as a systolic blood pressure of <80 mm Hg after fluid resuscitation), and hyperparasitemia (defined as parasitized erythrocyte level of >10%) [4, 17]. Patients were excluded if hemoglobin levels were <60 g/L or if they received antimalarial treatment for >18 hours. MSM was defined as falciparum malaria with fever within the past 48 hours, a parasite count of >1000 parasites/µL, and the need for hospital admission because of the inability to tolerate oral therapy, but without WHO warning signs or severe criteria [4]. Severe sepsis was defined as clinical evidence of infection, ≥3 features of the systemic inflammatory response syndrome, and evidence of dysfunction of ≥1 organs, with or without septic shock, according to American College of Chest Physicians criteria [18], with no parasites detected by microscopy or rapid diagnostic testing. Healthy controls were nonrelated hospital visitors without fever in the past 48 hours and no parasitemia. All patients were evaluated by hospital physicians who were no involved in this study and were treated with antimalarials and antibiotics, using hospital protocols.
A standardized history was documented, and a physical examination was performed. Venous blood was collected at enrollment and daily until discharge or death to measure biomarkers of severity, including lactate and plasma histidine rich protein 2, a measure of parasite biomass. Plasma was obtained within 20 minutes and stored at −70°C. Parasite counts were determined by thick and thin film microscopy. Hemoglobin level, biochemistry and acid-base parameters, and lactate level were measured with a bedside analyzer (i-STAT). NIRS measurements were performed daily until discharge or death. Plasma histidine rich protein 2 was measured using an enzyme-linked immunosorbent assay as previously described [4].
NIRS Analysis
NIRS noninvasively assesses microcirculatory oxygenation by measuring differential absorption of O2Hb and HHb [11]. According to Beer's law, this is confined to arterioles, capillaries, and venules of skeletal muscle with minimal interference from skin blood flow and myoglobin [11]. A clinical spectroscope (InSpectra 650, Hutchinson Technology, Hutchinson, MN) was used for the study. This uses a probe applied to the thenar eminence with a 15-mm gap between emitter and detector to measure optical attenuation at 680, 720, 760, and 800 nm: attenuation at 720 nm is sensitive for O2Hb, and attenuation at 760 nm is sensitive for O2Hb and HHb [19]. The attenuation is transformed into a wide-gap second derivative signal not affected by total tissue hemoglobin level, optical path length, and scattering [19]. Two measurements are displayed: tissue oxygen saturation (StO2; expressed as the ratio of the O2Hb signal to the sum of the O2Hb and HHb signals) and tissue hemoglobin index (THI; defined as the total thenar hemoglobin level, expressed as the sum of the relative tissue O2Hb and HHb signals).
To induce an ischemic stress, a vascular cuff was inflated to 200 mm Hg for 5 minutes and rapidly deflated. We assessed the following characteristics in all patients: (1) baseline StO2; (2) baseline THI; (3) StO2 at the end of occlusion (StO2low); (4) THI at end of occlusion (THIlow); (5) peak StO2 after release of occlusion (StO2peak); (6) difference in StO2peak and baseline StO2 (StO2diff); (6) peak THI after release of occlusion (THIhigh); (7) microvascular function or rate of skeletal muscle reoxygenation (StO2recov), defined as the rate of increase in StO2 in the first 14 seconds after release of occlusion, as previously described [12]; and (8) skeletal muscle tissue oxygen consumption (VO2), defined as the difference in tissue O2 content (THI × 1.39 × StO2) before and at the end of vascular occlusion, divided by the duration, as previously described [13]. Methemoglobin level was assessed concurrently with a CO-oximeter (Masimo Radical 7), as levels affect NIRS results [19, 20].
Statistical Methods
Statistical analysis was performed using Stata 11 software. Intergroup differences among malaria groups (MSM and severe malaria) and controls were compared by analysis of variance or the Kruskal-Wallis test, where appropriate. An a priori pairwise comparison, performed using the Sidak method, compared severe malaria and sepsis groups, severe malaria and MSM groups, and severe malaria and control groups. Pearson or Spearman correlation coefficients were determined depending on the normality of the distributions. Multivariable linear regression was used to adjust for confounding variables, with disease severity defined as the number of WHO severity criteria. Longitudinal associations were assessed by mixed effects modeling, using generalized estimating equations. Logistic regression was used to determine the association between binary outcomes, and goodness of fit was assessed by the Hosmer-Lemeshow goodness-of-fit test. A 2-sided P value of < .05 was considered statistically significant. Sample size calculation based on previous studies of endothelial function in severe malaria showed that 36 patients in each group would have 80% power to detect a 20% difference in microvascular function.
RESULTS
Patients
A total of 129 adults were enrolled: 36 had severe malaria, 33 had MSM, 20 had severe sepsis, 4 had septic shock, and 36 were controls. Among patients with severe malaria, 13 (36%) had 1 severity criterion, 8 (22%) had 2, and the remaining 15 (42%) had >2, with 2 severity criteria being the median. Among patients with severe sepsis, 13 (55%) had pneumonia, 7 (29%) had diarrheal disease, 3 (12%) had meningitis, and 1 (4%) had an undifferentiated febrile illness. There were 9 deaths (25% of patients) in the severe malaria group, 2 (8%) in the severe sepsis group, and 2 (50%) in the septic shock group. All patients with severe malaria received intravenous artesunate, with antibiotics coadministered in 19 (53%). All patients with MSM received intravenous artesunate initially and had treatment switched to oral artemisinin combination therapy once it could be tolerated. In patients with sepsis, 22 (92%) received ceftriaxone, with 9 also receiving gentamicin, and 2 received ampicillin and gentamicin. Baseline characteristics and laboratory results for all patients are shown in Table 1.
Table 1.
Baseline Demographic, Clinical, and Laboratory Values for Healthy Controls and Patients with Moderately Severe Malaria, Severe Malaria, or Severe Sepsis
| Variable | Healthy Controls (n = 36) | Moderately Severe Malaria (n = 33) | Severe Malaria (n = 36) | Severe Sepsis (n = 24) |
|---|---|---|---|---|
| Age, y, median (range) | 29 (19–54) | 30 (18–50) | 27 (18–55) | 27 (18–50) |
| Male sex, no. (%) | 27 (75) | 18 (55) | 29 (82) | 16 (67) |
| Fever duration before presentation, d, median (range) | NA | 3 (1–14) | 4 (1–14) | 3.5 (1–21) |
| Systolic blood pressure, mm Hg, mean (range)a | 118 (101–140) | 105 (95–136) | 106 (78–153) | 101 (61–140) |
| Diastolic blood pressure, mm Hg, mean (range)a | 70 (57–89) | 60 (47–90) | 64 (40–90) | 61 (31–87) |
| Pulse rate, beats/min, mean (range)a | 67 (52–108) | 81 (55–115) | 92 (61–120) | 109 (70–175) |
| Pulse oxygen saturation, % saturation, mean (range) | 99 (96–100) | 99 (94–100) | 98 (75–100) | 98 (86–100) |
| Temperature, °C, mean (range)a | 36.3 (35–37) | 36.8 (35.3–40.4) | 37.0 (35.1–39.6) | 37.9 (35–39.4) |
| White blood cell count, ×103 cells/µL, mean (range)a | 6.3 (2.8–9.2) | 4.2 (2–11.6) | 8.1 (2.7–31.8) | 15.5 (1.7–56.3) |
| Hemoglobin level, g/dL, mean (range)a | 12.9 (8.8–16.5) | 11.15 (6.8–15) | 11.0 (4.1–17) | 11.2 (6.8–14.6) |
| Platelet level, ×109 platelets/L, mean (range)a | 158 (112–247) | 48 (12–143) | 36 (11–188) | 103 (19–220) |
| Creatinine level, mmol/L, mean (range)a | NA | 96 (28–180) | 235.5 (66–1190) | 130 (57–626) |
| Lactate level, mmol/L, mean (range)a | NA | 1.06 (0.45–3.2) | 3.11 (0.54–20) | 2.67 (0.98–8.42) |
| Parasite density, parasites/µL, geometric mean (95% CI)a | NA | 9153 (6632–12 634) | 30 432 (8094–114 421) | NA |
Abbreviations: CI, confidence interval; NA, not available.
a P < .05, by analysis of variance, the Kruskal-Wallis test, or χ2 test, comparing the severe malaria, moderately severe malaria, severe sepsis, and healthy control groups.
StO2, THI, and Disease Severity
NIRS data are presented in Table 2, with the exception of 3 patients with uninterpretable results (2 with severe malaria and 1 with severe sepsis). There was no significant difference in StO2 on enrollment among patients with MSM, patients with severe malaria, and controls, but StO2 was 3.5% lower in patients with severe sepsis, compared with controls (P = .01). There was no difference in StO2low among groups or in StO2peak. Values for baseline THI, THIlow, and THIhigh did not significantly differ among patient groups. In the severe malaria and MSM groups, there was a decrease in THI over time; however, no change was seen in StO2 with clinical recovery in any group. There were no significant differences in methemoglobin percentages among groups for which CO-oximetry was performed.
Table 2.
Near Infrared Resonance Spectroscopy and CO-Oximetry Values for Healthy Controls and Patients with Moderately Severe Malaria, Severe Malaria, or Severe Sepsis
| Variable | Healthy Controls (n = 36) | Moderately Severe Malaria (n = 33) | Severe Malaria (n = 33) | Severe Sepsis (n = 23) |
|---|---|---|---|---|
| StO2 at baseline, %a | 88.7 (88–90) | 88.4 (87–90) | 88.8 (86–92) | 85.6 (83–88) |
| THI at baseline | 16.9 (16.1–17.7) | 17.0 (16.2–18.0) | 18.8 (17.6–20.0) | 16.9 (15.7–18.3) |
| StO2 at end of occlusion, % | 44.8 (41–48) | 42.0 (38–46) | 44.6 (42–50) | 43.5 (39–48) |
| THI at end of occlusion | 14.7 (13.9–15.6) | 13.6 (12.6–14.7) | 14.8 (13.9–15.8) | 14.0 (12.8–15.4) |
| StO2 recovery, %/seconda,b | 3.5 (3.3–3.7) | 3.1 (3.0–3.5) | 2.7 (2.4–3.0) | 2.5 (2.0–3.0) |
| StO2 peak after release, % | 97.0 (97–98) | 96.4 (96–97) | 95.1 (92–96) | 92.8 (90–96) |
| Absolute difference in tissue oxygen saturation, %a,c | 8.4 (7.5–9.2) | 7.9 (7.0–9.0) | 6.4 (5.5–6.9) | 7.3 (5.5–9.0) |
| Oxygen consumptiona | ||||
| Arbitrary units | 230 (208–251) | 255 (244–268) | 270 (252–298) | 232 (206–247) |
| Methemoglobin, % | 0.9 (.5–1.1) | 1.4 (.5–2.3) | 1.3 (1.1–1.6) | 1.3 (1.0–1.7) |
All values are mean (95% confidence interval).
Abbreviations: StO2, tissue oxygen saturation; THI, tissue hemoglobin index.
a P < .05, by analysis of variance or the Kruskal-Wallis test, comparing the severe malaria, moderately severe malaria, severe sepsis, and healthy control groups.
b Defined as the rate of increase in StO2 in the first 14 seconds after release of occlusion, as previously described [12].
c Defined as ([StO2 peak after release] – [StO2 at baseline]).
Microvascular Function and Clinical Disease
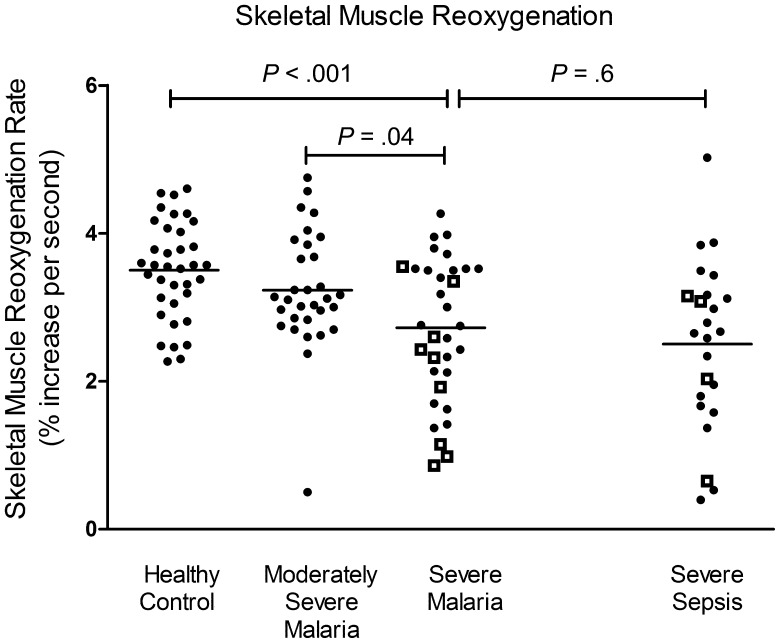
Among patients with severe malaria, the StO2recov was 16% and 22% lower than that for patients with MSM and controls (P < .001), decreased in proportion to disease severity, and was comparable to that for patients with severe sepsis (Table 2 and Figures 1–3). Post hoc pairwise comparison showed significant differences between the severe malaria and MSM groups and the severe malaria and control groups (Figures 1–3). There was no longitudinal change in StO2recov until death or discharge in all patients, regardless of severity or outcome. In the severe malaria group, the StO2diff was lower than that for patients in the MSM and control groups (P < .001), with no longitudinal change (Table 2). In severe malaria, there was a significant difference among the quartiles in age and parasite density (P = .04 and P = .03, respectively), with patients in the lowest quartile of microvascular reactivity being older (median age, 34 years) and having a higher median parasite density (247 180 parasites/µL). There were no other differences in clinical or laboratory variables among quartiles. In the severe sepsis group, there was a significant difference among the quartiles in white blood cell count and mean arterial blood pressure, with increased leukocytes (mean leukocyte count, 19.6 × 103 cells/µL) and lower mean arterial pressure (60 mm Hg) in the lowest microvascular reactivity quartile. Comparison of patients with severe sepsis and those with severe malaria in the lowest microvascular reactivity quartile revealed that the severe sepsis group had lower median blood pressure (60 mm Hg vs 77 mm Hg; P = .03) and higher mean leukocyte count (19.6 × 103 cells/µL vs 8.1 × 103 cells/µL; P = .03).
Figure 1.
Skeletal muscle reoxygenation rate (defined as the rate of increase in tissue oxygen saturation in the first 14 seconds after release of occlusion; P < .001, by analysis of variance) in healthy control, moderately severe malaria, severe malaria, and severe sepsis groups. Open squares represent fatal cases, and horizontal lines represent mean values for each group. Horizontal bars represent pairwise comparisons between groups.
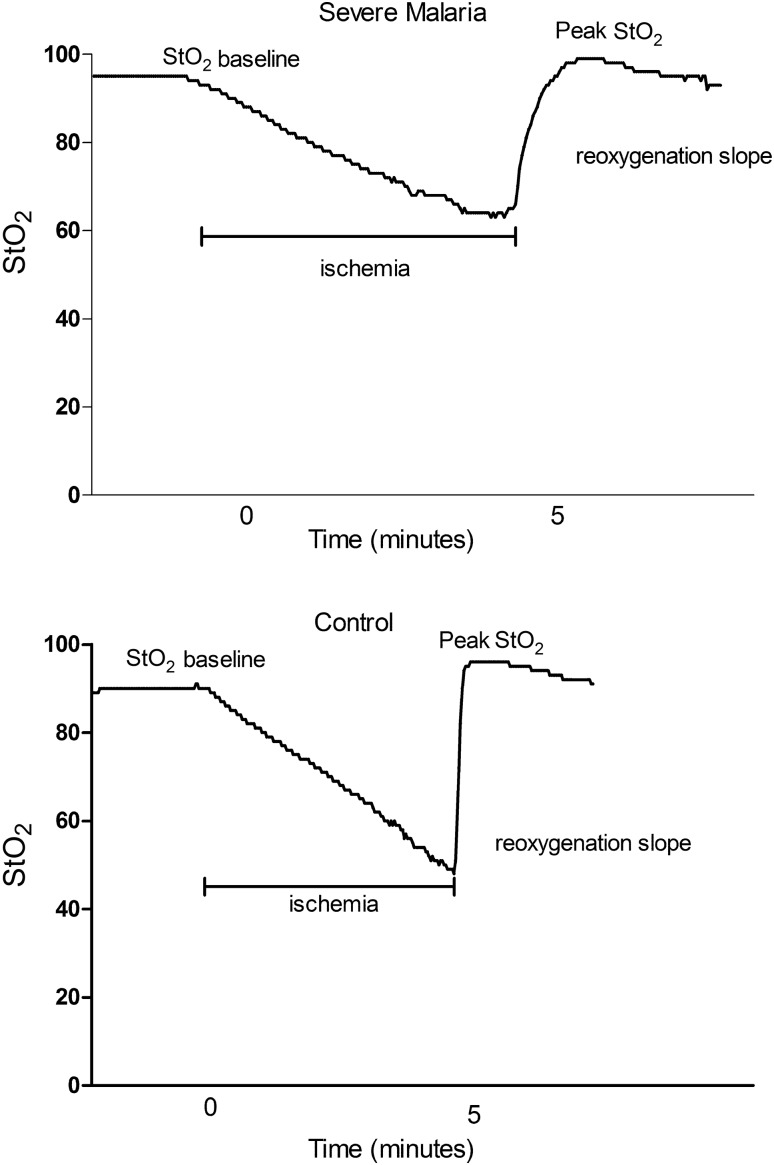
Figure 2.
Representative tissue oxygen saturation (StO2) curves from a patient with severe malaria (upper panel) and a healthy control (lower panel).
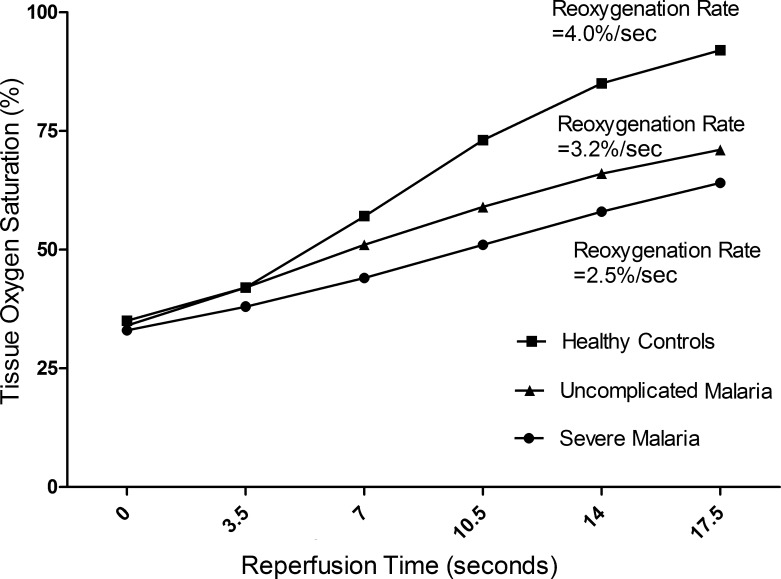
Figure 3.
Representative curves of skeletal muscle reoxygenation rate (defined as the rate of increase in tissue oxygen saturation in the first 14 seconds after release of occlusion) in patients with severe malaria, patients with moderately severe malaria, and healthy controls. The curves were chosen by having rates closest to the mean for the disease categories.
Microvascular Function and Biomarkers of Severity
There was a significant negative association between venous lactate level and StO2recov in all patients with malaria (r = −0.53; P < .001) and in patients with severe malaria (r = −0.63; P < .001), both at baseline and longitudinally, but not in the MSM group alone. In the severe malaria group, this association remained after adjustment for disease severity. There was no significant association between lactate level and StO2recov in the severe sepsis group (r = −0.27; P = .15).
Oxygen Consumption, Clinical Disease, and Biomarkers of Severity
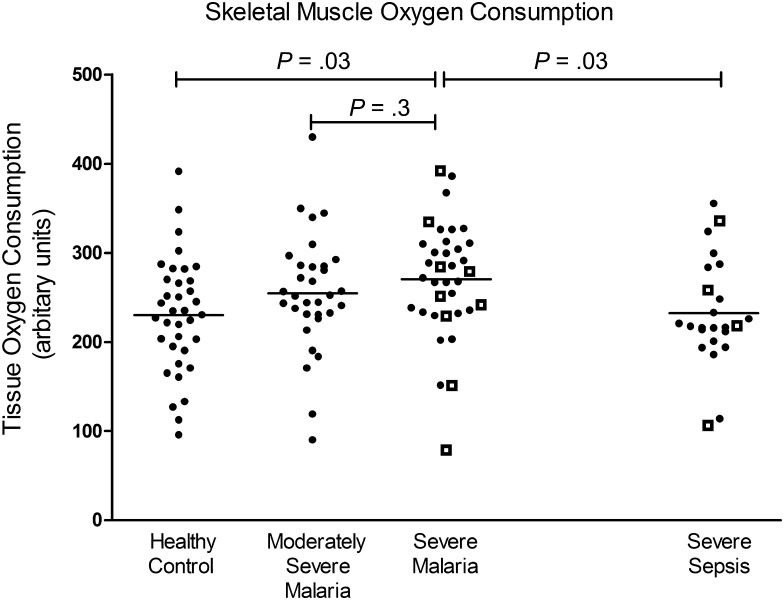
The mean VO2 among patients with severe malaria was 8% and 18% higher than that for the MSM and control groups, respectively (P < .001; Figure 4). Post hoc pairwise comparison showed a significant difference between the severe malaria and control groups but not between the severe malaria and MSM groups (Figure 4). Patients with severe malaria and those with MSM had higher VO2 than patients with severe sepsis (P < .001; Figure 4), with the VO2 in the severe malaria group 16% higher than that in the severe sepsis group (P = .03; Figure 4). In contrast, there was no difference between the severe sepsis and control groups. There was a significant negative association between lactate and VO2 among 35 patients with severe malaria (r = −0.37; P = .03) but not in the MSM or severe sepsis groups, both at baseline and longitudinally (Figure 4). This association in the severe malaria group remained significant on a multivariable model adjusted for microvascular function and disease severity. In 18 patients with severe malaria, VO2 and parasite biomass were significantly correlated (r = 0.49; P = .04). The rate of oxygen consumption decreased over time in all patients with severe malaria (P < .001) and in patients who survived severe malaria (P < .001), but this did not occur in patients who died of severe malaria. There was no association between StO2recov and VO2 in any of the disease groups and controls, suggesting that oxygen consumption was not supply dependent. There were no differences in age, sex, temperature, leukocyte count, blood pressure, hemoglobin level, and blood glucose level among the VO2 quartiles in either the severe malaria or severe sepsis groups.
Figure 4.
Skeletal muscle oxygen consumption in healthy controls, patients with moderately severe malaria, patients with severe malaria (P < .001, by analysis of variance), and patients with severe sepsis. Open squares represent fatal cases, and horizontal lines represent mean values for each group. Horizontal bars represent pairwise comparisons between disease groups.
Microvascular Function, Oxygen Consumption, and Mortality Area Under the Receiver Operating Characteristic Curve (ROC)
In the severe malaria group, microvascular function was 28% lower in patients who died, compared with patients who survived (P = .01). On univariate analysis, each percentage decrease in microvascular function was associated with an increased risk of death, with an odds ratio of 2.49 (95% CI, 1.05–6.2). The prognostic value of microvascular dysfunction in predicting death, as measured by the ROC, was 0.71 (95% CI, .51–.92). Microvascular function of <2.3%/second was 76% sensitive and 48% specific in predicting a fatal outcome. No significant difference in VO2 between survivors and nonsurvivors were seen in the severe malaria or severe sepsis groups.
DISCUSSION
Microvascular function, a measure of the capacity to increase flow and oxygen delivery in response to ischemia, is decreased in severe falciparum malaria and associated with an increased risk of death. In contrast, skeletal muscle oxygen consumption is increased in patients with severe malaria as compared to patients with MSM, control subjects, and patients with severe sepsis. These results suggest that tissue hypoxia in severe falciparum malaria could result not only from parasite sequestration and microcirculatory obstruction, but also from impaired ability of the microvasculature to match delivery to increased oxygen demand.
A decrease in baseline StO2 was observed in the severe sepsis group but not in the MSM or severe malaria groups. StO2 represents the aggregate of microvascular O2 hemoglobin saturations and may not reveal ischemic areas when blood flow is heterogeneous. However, the rate of StO2 increase after ischemia assesses microvascular function (ie, the capacity to increase oxygen delivery in response to hypoxia). This was reduced in falciparum malaria in proportion to disease severity and was associated with an increased risk of death, with both the rate of reoxygenation and the overall response being impaired. The impairment in the severe malaria group was comparable to that seen in the severe sepsis group, which was previously reported to be decreased [12]. Increased lactate level and metabolic acidosis due to decreased oxygen delivery are prognostic indicators of a fatal outcome in adult falciparum malaria [21]. Inverse associations between venous lactate level and microvascular function, cross-sectionally and longitudinally, suggest that failure of the microcirculation to respond adequately to ischemic stress may contribute to tissue hypoxia during severe malaria.
The hyperemic response during assessment of microvascular function measures the maximal ability of organs or tissues to increase blood flow on demand [6]. This results from increased delivery to patent capillaries and recruitment of additional capillaries, both of which are regulated by precapillary arterioles [6, 7]. Arteriolar dysfunction results in decreased functional capillary density and in the inability to compensate for obstructed capillaries [7]. Krogh's model states that, because of diffusion limitations, oxygen delivery by capillaries is limited to a fixed tissue volume [22], suggesting that decreased capillary density results in tissue hypoxia due to failure of adjacent capillaries to compensate for obstructed ones. Real-time visualization of hamster capillaries infected with Plasmodium berghei has demonstrated marked increase in the heterogeneity of blood flow velocity and distribution, with decreased functional capillary density a major lethal event [23]. This heterogeneous pattern has also been demonstrated in rectal capillaries of Asian adults with severe malaria, using orthogonal polarizing spectroscopy, with obstructed capillaries visualized adjacent to those with hyperdynamic flow [5].
A key mediator of arteriole function is NO bioavailability [24], which is impaired during severe malaria because of multiple factors, including hypoargininemia; increased concentrations of the endogenous inhibitor of NO synthase, asymmetric dimethylarginine; and quenching due to increased cell-free hemoglobin [4, 25, 26]. Decreased NO bioavailability in severe malaria is likely to play a key role in the impaired regulation of capillary density [4, 25, 26]; other possible causes include increased microvascular resistance through decreased erythrocyte deformability, which has been associated with a fatal outcome in falciparum malaria [27].
This study demonstrated increased skeletal muscle oxygen consumption in patients with malaria, compared with patients with severe sepsis and controls, that was proportional to disease severity. Our results are consistent with those of a study of Vietnamese adults in an intensive care unit, in whom systemic oxygen consumption was measured by thermodilution [28]: the study demonstrated increased consumption in a severe malaria group, compared with a septic shock group, but it did not include controls. In contrast, a study of Thai adults with cerebral malaria showed decreased cerebral oxygen consumption but did not measure overall consumption [29]. Increased host metabolism during severe disease could be a factor: in a murine model of P. berghei, severe disease was associated with increased oxygen consumption [30], which can result from alternative activation of macrophages [31]. This has been shown in human nematode and trypanosomal infections [32] but not in malaria. Our results do not support the hypothesis of impaired oxygen use due to host mitochondrial dysfunction; however, since we were unable to measure host and parasite parameters separately, we could not completely exclude this hypothesis. The results also do not demonstrate supply dependence of oxygen in malaria, in which decreased oxygen delivery would result in less oxygen utilization.
The association between oxygen consumption and parasite biomass could be related to a dose-response relationship in parasite induction of host metabolism, although we cannot exclude a contribution from parasite oxidative metabolism. In vitro, P. falciparum is microaerophilic, consuming minimal oxygen [33] and relying on anaerobic metabolism to produce energy. This suggests that the oxidative phosphorylation via the tricarboxylic acid cycle (TCA) seen in human mitochondria may be absent. However, in vitro studies have also shown measurable increases in oxygen use in parasitized erythrocytes, albeit at significantly less levels than that in other blood cells, such as leukocytes [34, 35]. Recent in vitro metabolomic studies of P. falciparum revealed an active TCA cycle involved in the metabolism of amino acids but not of carbohydrates [36]. African children with malaria had increased expression of P. falciparum genes involved with oxidative phosphorylation and respiration proportional to disease severity, suggesting the parasite TCA cycle could be active in vivo [37]. However, it is unclear whether the parasite biomass sequestered in the thenar muscles would have been large enough to induce a measurable difference in oxygen consumption, and parasite induction of host metabolism appears to be the most likely explanation.
Interestingly, an inverse association was noted between lactate level and oxygen consumption on univariate and multivariable analysis in patients with severe malaria. This suggests that, whereas an increase in oxygen use might theoretically lead to tissue hypoxia due to elevated oxygen demand, it is also associated with decreased lactate level. In vitro, host muscle and erythrocytes metabolize lactate when oxygen is limited but not absent [38], and both Plasmodium knowlesi and P. falciparum metabolize lactate at low oxygen tensions [39, 40]. NO is thought to impair mitochondrial function in patients with sepsis [8], and decreased NO bioavailability in patients with severe malaria [4] could lead to less inhibition and increased mitochondrial oxidation.
Our studies had several limitations. Lack of microbiological facilities prevented us from excluding bacterial infections in patients with malaria and from defining the bacterial etiology in severe sepsis. Assessment of microvascular function and oxygen consumption in thenar muscles may not reflect the situation in other organs. Hemozoin, a product of hemoglobin metabolism by P. falciparum, could affect NIRS results, although the optimal wavelength for absorption is 650 nm, which is below the spectra used by NIRS [19, 41]. In addition, it cannot explain the abnormal results in patients with sepsis, in whom hemozoin is absent. To begin to significantly affect NIRS results, tissue hemozoin concentrations would have to be >10% of the tissue hemoglobin level of 10 000 µg/mL, which approximates to a parasitemia of well over 50% [42]. Parasitemia in our study did not reach such levels, and analysis of muscle biopsy specimens from Thai adults with severe noncerebral or cerebral malaria showed that a median of 5% and 14% of erythrocytes, respectively, were parasitized [43].
In conclusion, microvascular function was impaired in patients with severe malaria and was associated with increased mortality, and skeletal muscle oxygen consumption was unexpectedly increased. Tissue hypoxia and organ failure may result not only from mechanical microvascular obstruction due to parasite sequestration, but also from a functional impairment of the microvasculature to match oxygen delivery to increased oxygen demand. Further studies are needed to fully characterize the factors causing both impaired microvascular function and increased oxygen consumption.
Notes
Acknowledgments. We thank Retno Gitawati, Indri Rooslamiati, Sri Muliati, and Erens Meokbum, for their support; Yohanes Kalvein Mira Mangngi, for nursing assistance; Ferryanto Chalfein, Prayoga, Kim Piera, Tonia Woodberry, and Gabriela Minigo, for technical and logistical assistance; Mitra Masyarakat Hospital staff, for clinical support; and Mauritz Okeseray, Paulus Sugiarto, Jeanne Rini Poespoprodjo, and Lembaga Pengembangan Masyarakat Amungme Kamoro, for support and assistance.
Financial support. The study was funded by the Australian National Health and Medical Research Council (NHMRC ICRG ID 283321; grants 605807 and 496600; and fellowships to N. M. A. and T. W. Y.) and the Wellcome Trust (ICRG GR071614MA and fellowship to R. N. P.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Potential conflicts of interest.: All authors: No potential conflicts of interest.
References
- 1.SEAQUAMAT Group. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366:717–25. doi: 10.1016/S0140-6736(05)67176-0. [DOI] [PubMed] [Google Scholar]
- 2.Dondorp AM, Fanello CI, Hendriksen IC, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet. 2010;376:1647–57. doi: 10.1016/S0140-6736(10)61924-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dondorp AM, Pongponratn E, White NJ. Reduced microcirculatory flow in severe falciparum malaria: pathophysiology and electron-microscopic pathology. Acta Trop. 2004;89:309–17. doi: 10.1016/j.actatropica.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Yeo TW, Lampah DA, Gitawati R, et al. Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med. 2007;204:2693–704. doi: 10.1084/jem.20070819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dondorp AM, Ince C, Charunwatthana P, et al. Direct in vivo assessment of microcirculatory dysfunction in severe falciparum malaria. J Infect Dis. 2008;197:79–84. doi: 10.1086/523762. [DOI] [PubMed] [Google Scholar]
- 6.Vallet B. Vascular reactivity and tissue oxygenation. Intensive Care Med. 1998;24:3–11. doi: 10.1007/s001340050507. [DOI] [PubMed] [Google Scholar]
- 7.Ellis CG, Jagger J, Sharpe M. The microcirculation as a functional system. Crit Care. 2005;9(Suppl 4):S3–8. doi: 10.1186/cc3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brealey D, Brand M, Hargreaves I, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–23. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 9.Clark IA, Alleva LM, Mills AC, Cowden WB. Pathogenesis of malaria and clinically similar conditions. Clin Microbiol Rev. 2004;17:509–39. doi: 10.1128/CMR.17.3.509-539.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anstey NM, Weinberg JB, Hassanali MY, et al. Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J Exp Med. 1996;184:557–67. doi: 10.1084/jem.184.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mancini DM, Bolinger L, Li H, Kendrick K, Chance B, Wilson JR. Validation of near-infrared spectroscopy in humans. J Appl Physiol. 1994;77:2740–7. doi: 10.1152/jappl.1994.77.6.2740. [DOI] [PubMed] [Google Scholar]
- 12.Creteur J, Carollo T, Soldati G, Buchele G, De Backer D, Vincent JL. The prognostic value of muscle StO2 in septic patients. Intensive Care Med. 2007;33:1549–56. doi: 10.1007/s00134-007-0739-3. [DOI] [PubMed] [Google Scholar]
- 13.Doerschug KC, Delsing AS, Schmidt GA, Haynes WG. Impairments in microvascular reactivity are related to organ failure in human sepsis. Am J Physiol Heart Circ Physiol. 2007;293:H1065–71. doi: 10.1152/ajpheart.01237.2006. [DOI] [PubMed] [Google Scholar]
- 14.Skarda DE, Mulier KE, Myers DE, Taylor JH, Beilman GJ. Dynamic near-infrared spectroscopy measurements in patients with severe sepsis. Shock. 2007;27:348–53. doi: 10.1097/01.shk.0000239779.25775.e4. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro NI, Arnold R, Sherwin R, et al. The association of near-infrared spectroscopy-derived tissue oxygenation measurements with sepsis syndromes, organ dysfunction and mortality in emergency department patients with sepsis. Crit Care. 2011;15:R223. doi: 10.1186/cc10463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karyana M, Burdarm L, Yeung S, et al. Malaria morbidity in Papua Indonesia, an area with multidrug resistant Plasmodium vivax and Plasmodium falciparum. Malar J. 2008;7:148. doi: 10.1186/1475-2875-7-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94(Suppl. 1):S1–90. [PubMed] [Google Scholar]
- 18.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM consensus conference committee. American college of chest physicians/society of critical care medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 19.Myers DE, Anderson LD, Seifert RP, et al. Noninvasive method for measuring local hemoglobin oxygen saturation in tissue using wide gap second derivative near-infrared spectroscopy. J Biomed Opt. 2005;10:034017. doi: 10.1117/1.1925250. [DOI] [PubMed] [Google Scholar]
- 20.Myers D, McGraw M, George M, Mulier K, Beilman G. Tissue hemoglobin index: a non-invasive optical measure of total tissue hemoglobin. Crit Care. 2009;13(Suppl 5):S2. doi: 10.1186/cc8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day NP, Phu NH, Mai NT, et al. The pathophysiologic and prognostic significance of acidosis in severe adult malaria. Crit Care Med. 2000;28:1833–40. doi: 10.1097/00003246-200006000-00025. [DOI] [PubMed] [Google Scholar]
- 22.Krogh A. The number and the distribution of capillaries in muscle with the calculation of the oxygen pressure necessary for supplying tissue. J Physiol (Lond) 1919;52:409–515. doi: 10.1113/jphysiol.1919.sp001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martini J, Gramaglia I, Intaglietta M, van der Heyde HC. Impairment of functional capillary density but not oxygen delivery in the hamster window chamber during severe experimental malaria. Am J Pathol. 2007;170:505–17. doi: 10.2353/ajpath.2007.060433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaughn MW, Kuo L, Liao JC. Effective diffusion distance of nitric oxide in the microcirculation. Am J Physiol. 1998;274:H1705–14. doi: 10.1152/ajpheart.1998.274.5.H1705. [DOI] [PubMed] [Google Scholar]
- 25.Yeo TW, Lampah DA, Tjitra E, et al. Increased asymmetric dimethylarginine in severe falciparum malaria: association with impaired nitric oxide bioavailability and fatal outcome. PLoS Pathog. 2010;6:e1000868. doi: 10.1371/journal.ppat.1000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeo TW, Lampah DA, Tjitra E, et al. Relationship of cell-free hemoglobin to impaired endothelial nitric oxide bioavailability and perfusion in severe falciparum malaria. J Infect Dis. 2009;200:1522–9. doi: 10.1086/644641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dondorp AM, Angus BJ, Chotivanich K, et al. Red blood cell deformability as a predictor of anemia in severe falciparum malaria. Am J Trop Med Hyg. 1999;60:733–7. doi: 10.4269/ajtmh.1999.60.733. [DOI] [PubMed] [Google Scholar]
- 28.Day NP, Phu NH, Bethell DP, et al. The effects of dopamine and adrenaline infusions on acid-base balance and systemic haemodynamics in severe infection. Lancet. 1996;348:219–23. doi: 10.1016/s0140-6736(96)09096-4. [DOI] [PubMed] [Google Scholar]
- 29.Warrell DA, White NJ, Veall N, et al. Cerebral anaerobic glycolysis and reduced cerebral oxygen transport in human cerebral malaria. Lancet. 1988;2:534–8. doi: 10.1016/s0140-6736(88)92658-x. [DOI] [PubMed] [Google Scholar]
- 30.Cooper AL, Dascombe MJ, Rothwell NJ, Vale MJ. Effects of malaria on O2 consumption and brown adipose tissue activity in mice. J Appl Physiol. 1989;67:1020–3. doi: 10.1152/jappl.1989.67.3.1020. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen KD, Qiu Y, Cui X, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–8. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Ann Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 33.Scheibel LW, Ashton SH, Trager W. Plasmodium falciparum: microaerophilic requirements in human red blood cells. Exp Parasitol. 1979;47:410–8. doi: 10.1016/0014-4894(79)90094-8. [DOI] [PubMed] [Google Scholar]
- 34.Murphy AD, Doeller JE, Hearn B, Lang-Unnasch N. Plasmodium falciparum: cyanide-resistant oxygen consumption. Exp Parasitol. 1997;87:112–20. doi: 10.1006/expr.1997.4194. [DOI] [PubMed] [Google Scholar]
- 35.Ribaut C, Reybier K, Reynes O, et al. Electrochemical impedance spectroscopy to study physiological changes affecting the red blood cell after invasion by malaria parasites. Biosens Bioelectron. 2009;24:2721–5. doi: 10.1016/j.bios.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 36.Olszewski KL, Mather MW, Morrisey JM, et al. Branched tricarboxylic acid metabolism in Plasmodium falciparum. Nature. 2010;466:774–8. doi: 10.1038/nature09301. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Daily JP, Scanfeld D, Pochet N, et al. Distinct physiological states of Plasmodium falciparum in malaria-infected patients. Nature. 2007;450:1091–5. doi: 10.1038/nature06311. [DOI] [PubMed] [Google Scholar]
- 38.Gladden LB. Lactate metabolism: a new paradigm for the third millennium. J Physiol. 2004;558:5–30. doi: 10.1113/jphysiol.2003.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moulder JW. The metabolism of malarial parasites. Annu Rev Microbiol. 1948;2(1 vol.):101–20. doi: 10.1146/annurev.mi.02.100148.000533. [DOI] [PubMed] [Google Scholar]
- 40.Makler MT, Ries JM, Williams JA, et al. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am J Trop Med Hyg. 1993;48:739–41. doi: 10.4269/ajtmh.1993.48.739. [DOI] [PubMed] [Google Scholar]
- 41.Serebrennikova YM, Patel J, Garcia-Rubio LH. Interpretation of the ultraviolet-visible spectra of malaria parasite Plasmodium falciparum. Appl Opt. 2010;49:180–8. doi: 10.1364/AO.49.000180. [DOI] [PubMed] [Google Scholar]
- 42.Newman DM, Heptinstall J, Matelon RJ, et al. A magneto-optic route toward the in vivo diagnosis of malaria: preliminary results and preclinical trial data. Biophys J. 2008;95:994–1000. doi: 10.1529/biophysj.107.128140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis TM, Pongponratan E, Supanaranond W, et al. Skeletal muscle involvement in falciparum malaria: biochemical and ultrastructural study. Clin Infect Dis. 1999;29:831–5. doi: 10.1086/520444. [DOI] [PubMed] [Google Scholar]