Abstract
Background
Anaplasma ovis infections can cause clinical symptoms in acute phase and lead to huge economic losses in flocks. The aim of the present study was to investigate the hematological and parasitological changes in experimental anaplasmosis in sheep with Iranian strain of A. ovis.
Method
Five male sheep without any blood parasite infection were selected. One hundred ml heparinized blood was collected from splenectomised sheep that showed 6% A. ovis parasitemia. Inoculums of 20 ml blood were administered intravenously to each test animal. Hematological, parasitological and clinical changes of experimental anaplasmosis were studied in 0-38 days post infection.
Result
Parasitemia was detected 3 days post infection and reached its maximum level on the day 12 of experiment in test animals. Then the parasitemia was declined, but the organism could be found persistently until the last day of study. The red cell counts, packed cell volume and hemoglobin concentration were decreased and mean corpuscular volume was increased significantly during the infection period. Reticulocytosis and basophilic stippling were also detected. No significant changes were observed in total and differential leukocyte count and animal body temperature.
Conclusion
Experimental A. ovis infection in sheep resulted in marked normocytic normochromic anemia at the beginning of the infection which became macrocytic normochromic by the development of the disease. There were negative correlations between parasitemia and RBC, PCV and Hb values, therefore hematological assessment can be considered as a practical diagnostic tool in ovine anaplasmosis.
Keywords: Anaplasma ovis, Sheep, Experimental, Anemia, Iran
Introduction
Anaplasma ovis is an intraerythrocytic rickettsial pathogen of sheep, goats and wild ruminants (1–5).The acute phase of the disease is characterized by severe anemia, fever, weight loss, abortion, lower milk production, pallor of mucous membrane, jaundice and often death (6, 7). Anaplasmosis is endemic in tropical and subtropical areas but is frequently reported in temperate regions of the world (7–9). Ovine anaplasmosis is mainly caused by A. ovis and A. marginale. Anaplasmosis is transmitted by ticks. Biting insects or inoculation of blood into susceptible animals can also transmit the disease (10, 11). Recovered animals become carriers. Splenectomy and intercurrent infections lower the resistance of the animals and render them more susceptible to the disease. Other stress factors such as malnutrition and pregnancy also increase the susceptibility of animals to anaplasmosis. During the acute stage of the disease, a diagnosis in the individual animal is usually made on the basis of clinical signs, the presence of the organism in blood smear and hematological evidence of infection (12).
Although some pathogenic aspects of anaplasmosis have been investigated before, but still this disease is a matter of importance for the researchers, thus the aim of this study was to evaluate the hematological, parasitological and clinical changes of experimental anaplasmosis in sheep with Iranian strain of A. ovis based on microscopic and molecular identification.
Material and Methods
Source of experimental animals
Twenty local breed sheep, about 5-6 month old were selected from a farm in Varamin, Tehran Province, Iran. The blood samples were collected in vacutainer tubes containing EDTA.
Microscopic examination
The collected blood samples were used to prepare thin blood smears for microscopic examination. Blood smears were fixed with methanol for five min, stained with Giemsa at a dilution of 5% in buffer solution for 30 min, and then examined for the presence of blood parasite such as Anaplasma, Theileria and Babesia spp. under oil immersion lens (100×). Blood smears were recorded as negative for all hemoparasites if no inclusion bodies were observed in a 200-oil-immersion field.
PCR analysis
DNA extraction was performed by using molecular biological system transfer kit (MBST Iran), based on the manufacturer's instructions. A PCR method was carried to detect Anaplasma spp. (A. ovis and A. marginale) based on the msp4 gene sequence and they were differentiated from each other by PCR-RFLP using HpaII enzyme similar to the method of Ahmadi-Hamedani and Khaki et al. (13).
Theileria and Babesia infection was ruled out by PCR technique using specific primers for 18SrRNA gene (14).
Selection of animals
Five male sheep were selected from above as recipient animals for the experimental study. All selected animals were tested negative for the infection of blood parasites microscopically which were confirmed by PCR method.
An A. ovis infected sheep with no other blood parasites based on blood smear and PCR examination, was selected as donor and splenectomised to induce high level of parasitemia.
The selected animals were purchased and transferred to Veterinary Research and Teaching Hospital, Faculty of Veterinary Medicine, University of Tehran. All animals were treated against internal and external parasites14 days prior to the study.
Experimental infection
The donor was subjected to blood collection for inoculation, three weeks after splenectomy while showing 6% A. ovis parasitemia. One hundred ml blood was collected in container with heparin anticoagulant from donor and an inoculum of 20 ml blood was administered intravenously to each test animal.
Clinical examination and Sampling
Two ml of blood sample was collected via the jugular vein into EDTA from each test animal prior inoculation (day 0) and weekly thereafter (days 3, 5, 8, 9, 12, 15, 18, 20, 23, 25, 27, 30, 32, 34, and 38 post inoculation) for hematological and parasitological analysis. Physical examination was performed and clinical signs including rectal temperature, anorexia, depression and color of the mucous membranes (e.g., conjunctival and oral) were recorded on each animal.
Microscopic examination
Blood smears were prepared and fixed with methanol for 5 min and stained with 5% Giemsa solution for 30 min and then examined for the presence of Anaplasma inclusion bodies under oil immersion lens (100×).
Parasitemia ratio was assessed by counting the number of infected red blood cells on examination of at least 200 microscopic fields. The number of infected cells was then expressed as a percentage.
Reticulocyte and basophilic stippling percentage was calculated by counting them per 1000 red cells in cresyl blue and Giemsa stained blood smears respectively.
Hematological assessment
Hematological parameters included, total erythrocyte count (RBC), packed cell volume (PCV), hemoglobin concentration (Hb), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), total white blood cells (WBC) and differential leukocyte count were estimated as described by Meyer and Harvey (2004) (15).
Statistical analysis
Analysis of variance (ANOVA) and Tukey tests were used for statistical differences between the groups. All values were expressed as mean ± standard error of mean (SEM) and P<0.05 was considered as statistically significant.
Result
Parasitological findings
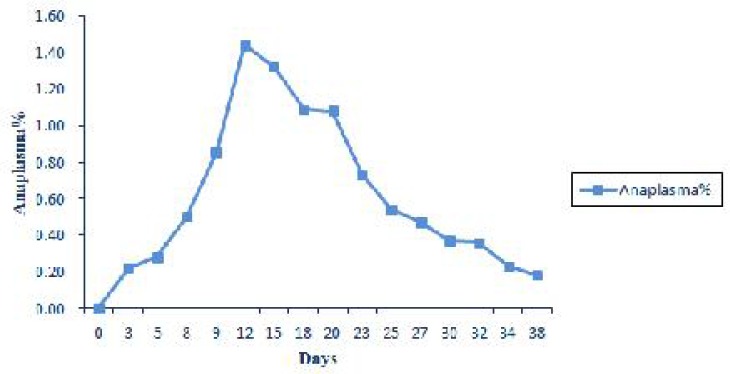
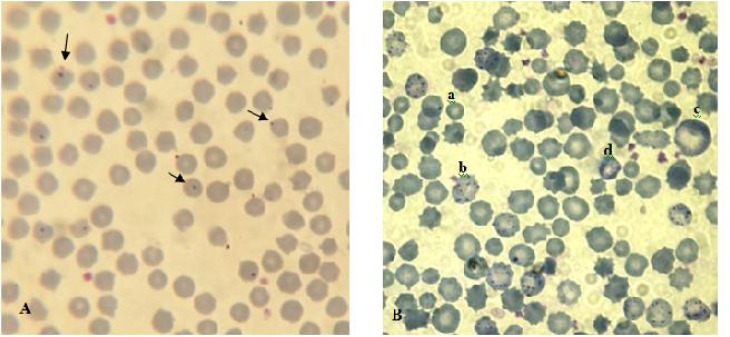
Microscopic examination of blood smears obtained from test animals revealed that the percentage of Anaplasma inclusion bodies increased significantly with the progress of infection and reached a peak of 1.44 ± 0.46% on the day 12 of experiment. Then, from this point to day 38, there was a gradual decline in parasitemia percentage to a minimum of 0.18 ± 0.03% (Fig. 1). In infected sheep erythrocytes 62.5-86% (Mean. 74.53%) of the inclusion bodies were located centrally or submarginally and the remaining 14-37.5% (Mean. 25.47%) were situated marginally (Fig. 2A).
Fig. 1.
Expression of changes in prasitemia during experimental period
Fig. 2.
Anaplasma ovis infected sheep blood smear. A: Anaplasma inclusion body. B: a. Anaplasma ovis inclusion body, b: Basophilic stippling, c: Macrocytic RBC,d: polychromatophilic RBC
Hematologic and clinical changes
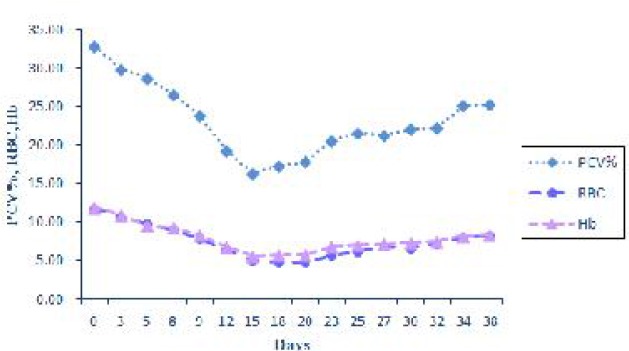
The data showed that the values of PCV, RBC and Hb decreased soon after the appearance of parasitaemia, reaching their lowest levels a few days after the peak of parasitaemia. After that there was a slight rise in the amount of these parameters until the last day of experiment (Fig. 3).
Fig. 3.
Expression of changes in PCV, RBC and Hb during experimental period
Reticulocytes count started to grow from 0.002 ± 0.002% on the day 8 to a high of 7.00 ± 3.76% on the day 23 post-inoculation followed by a downward trend to the end of study (Fig. 4).
Fig. 4.
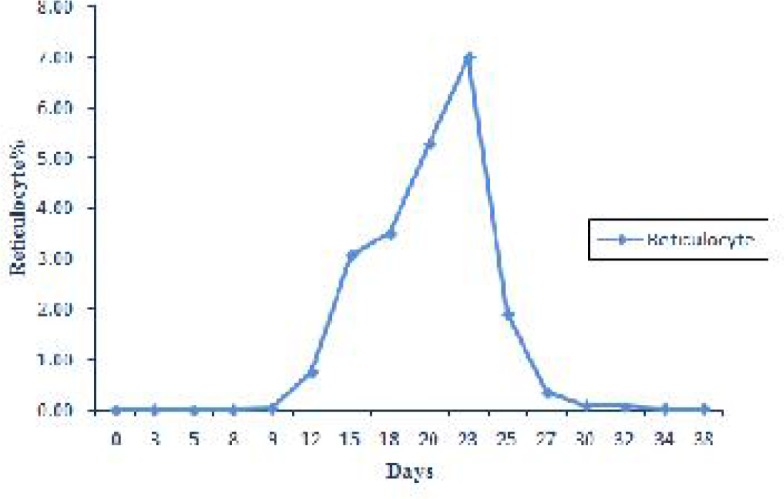
Expression of changes in reticulocyte % during experimental period
Basophilic stippling was detected microscopically 9 days post infection and reached a peak 2.00 ± 0.32 on the day 20 of experiment (Fig. 5 and 2B).
Fig. 5.
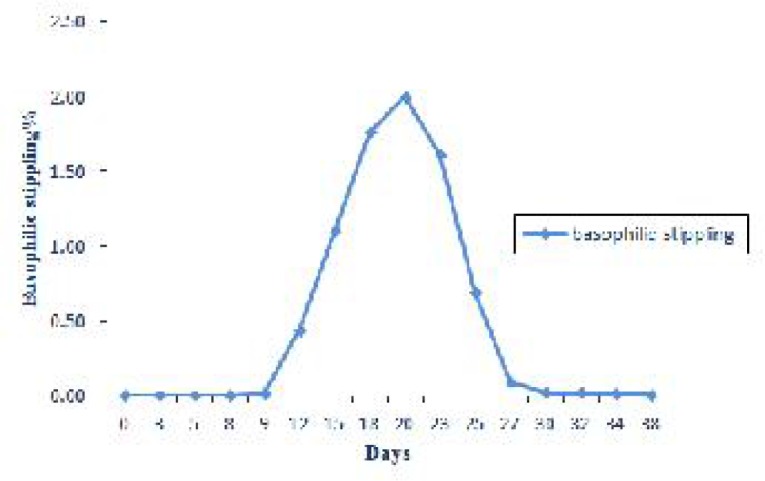
Expression of changes in basophilic stippling % during experimental period
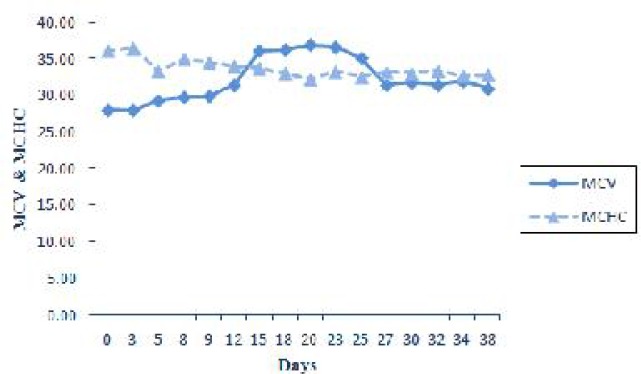
A significant increase in MCV was observed coincidentally with the rise of reticulocyte on the day 20 and 23 post infection. There was no significant difference in MCHC. This indicates the development of regenerative macrocytic normochromic anemia at this stage of study, but at the beginning of the experiment the anemia was nonregenerative by normocytic normochromic appearance (Fig. 6).
Fig. 6.
Expression of changes in MCV and MCHC during experimental period
Polychromasia was observed from the day 12 and increased gradually until day 20 which then returned to almost 0% by the end of experiment.
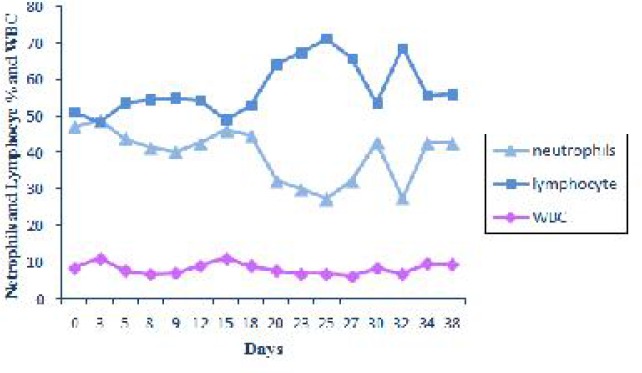
In comparison with the first day, neutrophils percentage decreased between 5-38 days post infection and it reached its minimum level on the day 25 post infection. The reverse pattern was observed in lymphocytes percentage. However these changes were not significant. There was also no significant difference in the mean of WBC count (Fig. 7).
Fig. 7.
Expression of changes in neurophils and lymphocytes % and WBC during experimental period
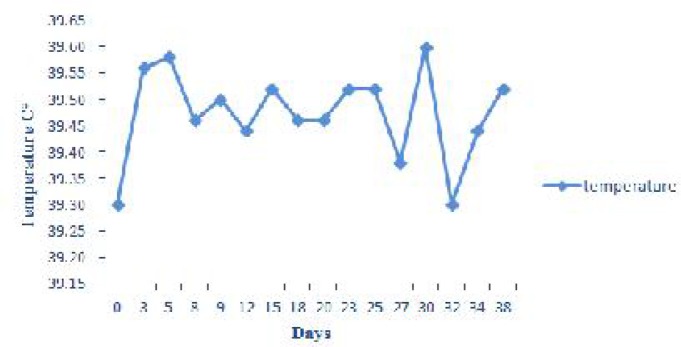
In our experiment during the days 3-38 post infection, body temperature over 39.5 was seen only in one or two sheep each day, thus no significant changes in mean body temperature were observed in statistical analysis (Fig. 8).
Fig. 8.
Expression of changes in temperature during experimental period
The mucus membranes were anemic on the day 12-23 post infection without any evidence of clinical icterus. During these days infected animals showed varying degrees of anorexia and listlessness.
Discussion
Studies of experimental ovine anaplasmosis are very scarce in Iran and little information had been provided. This preliminary study was done for the first time in Iran. Our experiment indicated that parasitemia increased significantly along with the progress of infection and displayed its highest level 12 days post infection. The parasitemia reached to maximum level one or two weeks after first parasites detectable in the blood smear of infected animals (12). The parasitaemia then diminished slowly, but the organism could constantly be found until observations were stopped 38 days post infection. Decline parasite numbers in nonsplenectomised sheep, may be due to removal the infected erythrocyte from the circulation by reticuloendothelial system (16).
A few days after the peak of parasitemia, progressive anemia was appeared, in which the values of Hb, RBC and PCV gradually decreased from 3 days post inoculation and reached approximately their lowest levels on the day 15 post infection. In our experiment there was no evidence of intravascular hemolysis, therefore anemia occurred as a result of phagocytosis of infected erythrocytes to removal parasites during the period of rising parasitemia. Hemoglobinuria was an unusual clinical sign of anaplasmosis, because anemia results from extravascular opsonization and phagocytosis of parasitized erythrocytes by reticuloendothelial cells (17). However the severity of anemia may be as a result of immune-mediated destruction of non parasitized erythrocytes in addition to that of parasitized (18).
We observed negative correlations (P<0.01) among parasitemia and RBC count (r=−0.494), Hb (r=−0.496) and PCV (r=−0.570). The anemia increased as the severity of parasitemia increased, as Nazifi et al. (19) noted previously in cattle anaplasmosis.
In our experiment in infected sheep erythrocytes 62.5-86% (Mean. 74.53%) of the inclusion bodies were located centrally or submarginally and the remaining 14-37.5% (Mean. 25.47%) were situated marginally. Splitter et al. (12) revealed that within erythrocytes 60-70% of A. ovis inclusion bodies were located marginally and 30-40% submarginally or centrally. In the case of A. ovis, bacterial inclusions were found 35–40% of the time in the central or submarginal part of the erythrocyte of the host, and the remaining 60–65% of the time in the marginal part (20).
Hematological values including packed cell volume, hemoglobin concentration and erythrocyte count in animals infected with A. ovis initiated to increase gradually on the day 18-23 post infection until the last day of experiment.
The results of the current experiment indicated that at the early stage of the study, during the days 3-18 post infection, reticulocytes and basophilic stippling were not appeared or presented at very low numbers and there were no changes in MCV and MCHC. Therefore at this stage of the disease anemia was nonregenerative with normocytic normochromic appearance. Thereafter by development of infection reticulocytes gradually increased and reached its maximal level on the day 23 post infection. On the other hand basophilic stippling was detected 9 days post infection and increased significantly up to day 20 of experiment. A significant increased in MCV coincided with highest reticulocytosis. These findings indicated evidence of macrocytic normochromic anemia in the middle stage of experiment. Reticulocytosis, polychromasia and basophilic stippling in blood smears suggested a regenerative anemia in infected animal (21). Naqid et al. (16) reported a macrocytic normochromic anemia in goats naturally infected with A. ovis. Anemia of camel anaplasmosis was macrocytic normocromic (22). No significant changes in MCV and MCHC in the goat naturally infected with A.ovis were observed (13).
The hematological findings showed a significant decrease in RBC, Hb, PCV, and MCHC in cattle infected with A. marginale (23). No significant changes in MCV value were noted so that, anemia was found to be normocytic hypochromic.
Our experiment showed that there were no significant difference in the means of WBC counts, lymphocytes, neutrophils, eosinophils, and monocytes. Total leukocyte count usually remained unchanged in experimental anaplasmosis (12).
The means of WBC counts, lymphocytes, eosinophils, and monocytes in the infected goat were lower than in the uninfected, but neutrophils counts were higher in the infected animals than in the uninfected animals (13). These differences were not statistically significant. In the study of camel anaplasmosis, (22), a significant rise in total leukocyte count was observed due to increased lymphocyte and decreased nutrophil counts. All the Sahiwal and crossbred cattle infected with A.maginale showed significant decreased in WBCs count (24). As molecular evaluation of Theileria and Babesia spp. was not performed in these studies, the noted changes in WBC total and differential counts might be related to Babesia or Theileria.
Changes in mean body temperature were not statistically significant, but some of infected animals demonstrated fever during several days post infection. These findings may be related to individual characteristic and defense system of each animal. In experimental anaplasmosis body temperature were usually unchanged, although it increased as high as 41°C in several animals at the peak of parasitaemia (12). Without any evidence of clinical icterus, the mucus membranes were anemic during the days 12-23 post infection. It may be as a result of decrease in PCV, RBC and Hb.
Conclusion
Anaplasma ovis can cause marked anemia that was normocytic normochromic at the beginning of the infection. By the development of the disease and appearance of reticulocytosis, basophilic stippling and polychromasia MCV increased and anemia became macrocytic normochromic. We observed negative correlation between parasitemia and RBC, PCV and Hb values. No significant changes were noticed in the mean of total and differential WBC count. Although some of infected animals showed fever during several days post infection but in most of them body temperature was unchanged. Therefore no significant changes in body temperature were observed. Palness of mucus membranes and anorexia were exhibited during the anemic phase of infection.
Acknowledgement
The authors declare that there is no conflict of interest.
References
- 1.Kuttler KL. Anaplasma infections in wild and domestic Ruminants: a review. J Wildl Dis. 1984;20:12–20. doi: 10.7589/0090-3558-20.1.12. [DOI] [PubMed] [Google Scholar]
- 2.Zaugg JL, Goff WL, Foreyt W, Hunter DL. Susceptibility of elk (Cervus elaphus) to experimental infection with Anaplasma marginale and A. ovis . J Wildl Dis. 1996;32:62–66. doi: 10.7589/0090-3558-32.1.62. [DOI] [PubMed] [Google Scholar]
- 3.Radostitis OM, Gay CC, Blood DC, Hinchcliff KW. Veterinary Medicine. 9th ed. London, New York, Philadelphia: WB Saunders Co; 2000. pp. 1261–1265. [Google Scholar]
- 4.Yabsley MJ, Davidson WR, Stallknecht DE, Varela AS, Swift PK, Devos JC, Jr, Dubay SA. Evidence of tick-borne organisms in mule deer (Odocoileus hemionus) from the western United States. Vector Borne Zoonotic Dis. 2005;5:351–362. doi: 10.1089/vbz.2005.5.351. [DOI] [PubMed] [Google Scholar]
- 5.de la Fuente J, Atkinson MW, Naranjo V, Fernandez de Mera IG, Mangold AJ, Keating KA, Kocan KM. Sequence analysis of the msp4 gene of Anaplasma ovis strains. Vet Microbiol. 2007;119:375–381. doi: 10.1016/j.vetmic.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Splitter EJ, Twiehaus MJ, Castro ER. Anaplasmosis in sheep in the United States. J Am Vet Medl Assoc. 1955;127:244–245. [PubMed] [Google Scholar]
- 7.Kocan KM, de la Fuente J, Guglielmone AA, Melendez RD. Antigens and alternatives for control of Anaplasma marginale infection in cattle. Clin Microbiol Rev. 2003;16:698–712. doi: 10.1128/CMR.16.4.698-712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Fuente J, Torina A, Caracappa S, Tumino G, Furlá R, Almazán C, Kocan KM. Serologic and molecular characterization of Anaplasma species infection in farm animals and ticks from Sicily. Vet Parasitol. 2005b;133:357–362. doi: 10.1016/j.vetpar.2005.05.063. [DOI] [PubMed] [Google Scholar]
- 9.Torina A, Alongi A, Naranjo V, Scimeca S, Nicosia S, Di Marco V, Caracappa S, Kocan KM, de la Fuente J. Characterization of Anaplasma infections in Sicily, Italy. Ann NY Acad. Sci. 2008;1149:90–93. doi: 10.1196/annals.1428.065. [DOI] [PubMed] [Google Scholar]
- 10.Aguirre DH, Gaido AB, Vinabal AE, de Echaide ST, Guglielmone AA. Transmission of Anaplasma marginale with adult Boophilus microplus ticks fed as nymphs on calves with different levels of rickettsaemia. Parasite. 1994;1:405–407. doi: 10.1051/parasite/1994014405. [DOI] [PubMed] [Google Scholar]
- 11.de Echaide ST, Knowles DP, Mcguire TC, Palmer GH, Suarez CE, Mcelwain TF. Detection of cattle naturally infected with Anaplasma marginale in a region of endemicity by nested PCR and a competitive enzyme linked immunosorbent assay using recombinant major surface protein 5. J Clin Microbiol. 1998;36:777–782. doi: 10.1128/jcm.36.3.777-782.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Splitter EJ, Anthony HD, Twiehaus MJ. Anaplasma ovis in the United States: experimental studies with sheep and goats. Am J Vet Res. 1956;17:487–491. [PubMed] [Google Scholar]
- 13.Ahmadi-Hamedani M, Khaki Z, Rahbari S, Kazemi B, Bandehpour M. Molecular identification of anaplasmosis in goats using a new PCR-RFLP method. Iran J Vet Res. 2009;10:367–372. [Google Scholar]
- 14.Shayan P, Ebrahimzadeh E, Tageldin MH, Amininia N, Eckert B. Molecular study of malignant theileriosis at Barka region in the sultanate Oman. Iranian J Parasitol. 2011;1(6):66–72. [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer DJ, Harvey JW. Veterinary laboratory medicine. 3rd ed. London: WB. Saunders Co; 2004. pp. 17–24, 63-65, 163. [Google Scholar]
- 16.Naqid IA, Zangana IZ. Hematological and serological (cELIZA) studies of caprine anaplasmosis in Duhok governorate of Kurdistan region of Iraq. J Duhok Univ. 2010;13(1):153–161. [Google Scholar]
- 17.Allen PC, Kuttler KL, Amerault BS. Clinical chemistry of anaplasmosis: blood chemical changes in infected mature cows. Am J Vet Res. 1981;42:322–325. [PubMed] [Google Scholar]
- 18.Stoltsz WH. Ovine and Caprine anaplasmosis. In: Coetzer JAW, Tustin RC, editors. Infectious disease of livestock. 2nd ed. Vol. 1. Oxford university press; 2004. pp. 617–624. [Google Scholar]
- 19.Nazifi S, Razavi SM, Mansourian M, Nikahval B, Moghaddam M. Studies on correlations among parasitaemia and some hemolytic indices in two tropical diseases (theileriosis and anaplasmosis) in Fars province of Iran. Trop Anim Health Prod. 2008;40:47–53. doi: 10.1007/s11250-007-9052-y. [DOI] [PubMed] [Google Scholar]
- 20.Shompole S, Waghela SD, Rurangirwa FR, McGuire T. Cloned DNA probes identify Anaplasma ovis in goats and reveal a high prevalence of infection. J Clin Microbiol. 1989;27:2730–2735. doi: 10.1128/jcm.27.12.2730-2735.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Douglas JW, Wardrop KJ. Schalm's veterinary hematology. 6th ed. WB; 2010. pp. 153–155. [Google Scholar]
- 22.Alsaad KM. Clinical, hematological and biochemical studies of anaplasmosis in Arabian one-humped camels (Camelus dromedaries) J Anim and Vet Adv. 2009;8(9):1794–97. [Google Scholar]
- 23.Arslan SH, Shukur AA. Clinical and hematological studies on theileriosis and anaplasmosis in local breed cattle in Mousl region. Iraq J Vet Sci. 1994;7(2):93–100. [Google Scholar]
- 24.Atif FA, Khan MS, Iqbal HJ, Roheen T. Prevalence of tick-born disease in Punjab (Pakistan) and hematological profile on Anaplasma marginale infection in indigenous and crossbred cattle. Pakistan J Sci. 2012;4(1):11–15. [Google Scholar]