Abstract
The absence of daily robust light-dark exposure patterns may contribute to sleep disturbances in persons with Alzheimer’s disease and related dementias (ADRD). Personal light-dark and activity-rest patterns were measured for healthy older adults and for persons with ADRD. Persons with ADRD experienced lower light levels, exhibited lower activity levels, and had greater levels of circadian disruption than healthy older adults during winter. Seasonal differences were observed for persons with ADRD; lower levels of light exposure and greater levels of circadian disruption were seen during the winter than during the summer, although activity levels did not differ for the two seasons.
Keywords: Alzheimer’s disease, circadian, circadian dysregulation, circadian light, phasor magnitude
INTRODUCTION
Persons with Alzheimer’s disease and related dementias (ADRD) are often difficult to manage because of sleep problems, nocturnal wandering, and daytime irritability. Irregular activity-rest patterns, possibly due to disruption of circadian rhythms, are among the most common reasons for nursing home placement.
Circadian rhythms are governed by the master clock in the suprachiasmatic nuclei (SCN), which has an intrinsic period slightly longer than 24 hours. The light-dark pattern incident on the retina entrains the SCN to the 24-hour day, coordinating and enabling biological rhythms to occur at the correct time of day and night for species survival. Without exposure to a regular, daily pattern of light and dark, circadian rhythms become irregular, compromising health and well-being [1].
Light treatment to promote circadian entrainment offers promise as a non-pharmacological method to regulate sleep and activity in persons with ADRD. Studies have shown that regular morning or evening light exposures, as well as all-day light exposures, can consolidate and improve nighttime sleep, increase daytime wakefulness, and reduce evening agitation [2–8].
Research has shown that the human circadian system is maximally sensitive to 460 nm (blue) light [9, 10]. Few photometric instruments are calibrated in terms of “circadian light” and can record light over extended periods of time. Consequently, there have been few field studies quantifying light-dark exposure patterns as they might affect circadian disruption.
The present paper provides personal light-dark exposure patterns and activity-rest patterns exhibited by non-institutionalized persons with ADRD and by healthy older adults. From those data, levels of circadian disruption exhibited by healthy adults and by persons with ADRD are compared. Levels of circadian disruption exhibited by persons with ADRD during the winter and during the summer are also evaluated.
MATERIALS AND METHODS
Subjects
Healthy older adults
Sixteen healthy older adults (13 females; mean ± standard deviation (SD) age = 73 ± 6 years) were recruited and completed the data collection for this study, which ran from September 2010 until February 2011. A research nurse evaluated physical health through interviews. The Mini-Mental Score Examination (MMSE) mean ± SD score was 29 ± 1.1 (range: 27–30). None of the subjects lived in nursing homes or were hospitalized during the study, and all subjects scored a zero (independent) on the Minimum Data Set Activities of Daily Living Scale [11] for the week prior to their participation in the study. All subjects completed a consent form approved by the Institutional Review Boards at all three institutions.
ADRD older adults
Twenty-one older adults (7 females) who were cognitively impaired (81 ± 6 years) participated in the study, which ran from May–August 2011 (summer) and November 2011 to March 2012 (winter). The mean ± SD score on the MMSE was 20.5 ± 2.9 points (range: 16–24). All participants had been previously diagnosed with dementia by a physician based on the DSM-IV and NINCDS-ADRDA criteria [12, 13].
Procedures
Participants continuously wore a Dimesimeter on a wrist for one week. They were asked to leave the device uncovered by clothing, and caregivers of ADRD subjects checked for compliance.
The Dimesimeter is a small disk (~2 cm diameter) that continuously records light (using red, green, and blue (RGB) sensors) and activity levels [14]. Upon downloading, the RGB values are converted into illuminance, circadian light (CLA), and circadian stimulus (CS) levels [15]. Briefly, illuminance is irradiance weighted by the photopic luminous efficiency function (V(λ)), an orthodox measure of the spectral sensitivity of the human fovea, peaking at 555 nm. CLA is irradiance weighted by the spectral sensitivity of the retinal phototransduction mechanisms stimulating the SCN, based on nocturnal melatonin suppression [15]. CS is a transformation of CLA into relative units from 0, the threshold for circadian system activation, to 1, response saturation.
Data analysis
The Dimesimeter light and activity data were visually inspected to determine if subjects complied with the protocol. The continuous five-day period that resulted in the most complete data set was used in the analysis, and 94%, 96%, and 93% of the data in those five-day periods were used for the ADRD winter, ADRD summer, and healthy adults winter data sets respectively. Mean light and activity patterns were determined for each subject. From those data, two measures of circadian entrainment were calculated: relative activity (RA), based upon activity, and phasor magnitude, based upon both light and activity.
Circadian entrainment
RA is a “contrast” calculation based upon the most and least active periods in a day [6]; a higher value suggests less circadian disruption. A mean 24-hour activity-rest profile was determined for each subject from the hourly mean activity levels. The continuous 10-hour block with the highest mean activity level (M10) and the continuous 5-hour block with the lowest mean activity level (L5) were determined. The RA for each subject was calculated by the following ratio:
Phasor analysis is used to characterize the resonance between the 24-hour light-dark pattern and the 24-hour activity-rest pattern [16]. Similar to the activity data, the overall light level exposures were calculated by creating a mean 24-hour light-dark pattern from the hourly mean values for each subject. Since CS is a measure of the effectiveness of optical radiation on the retina for stimulating the human circadian system, the daily patterns of CS were used in the phasor analyses. Phasor angles and magnitudes were determined from the light and activity patterns. The higher the resonance, measured by the phasor magnitude, the greater the circadian entrainment. Phasor angles describe the phase relationship between the light-dark pattern and the activity-rest pattern. Entrained individuals typically have positive phasor angles of about 1 to 2 hours in the phasor diagram [17]. These “normal” people are usually active in bright light immediately after rising in the morning and continue to be active throughout the day and into the dim evening light. The continuous 10-hour block that had the highest mean CS level was also calculated to represent light exposures when the subject was typically awake (CS10).
Statistical analyses
Two-tailed, unpaired t-tests were used to compare outcome measures obtained for the three groups, healthy older adults during the winter, persons with ADRD during the winter and during the summer.
RESULTS
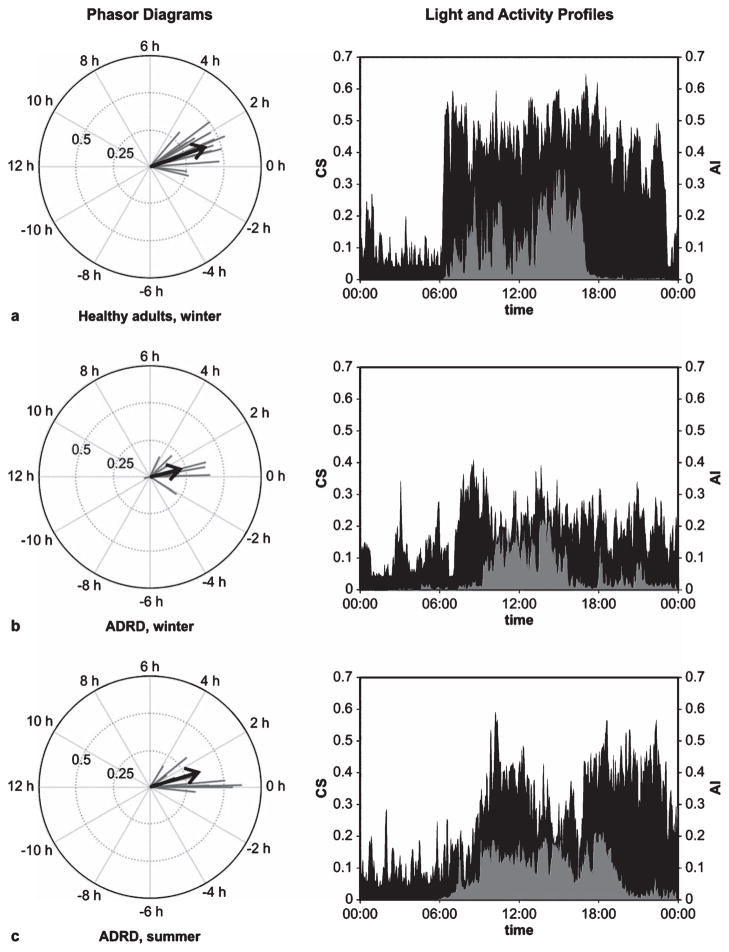
Table 1 provides the mean ± standard error of the mean (SEM) values for each of the outcome measures. The mean phasor magnitude for each group was determined from the arithmetic mean of the individual phasor magnitudes in that group; the mean phasor angles were based upon the vector sums of the individual phasor angles in the group. Figure 1 illustrates individual phasors, along with the group mean phasors together with 5-day light and activity profiles for a representative subject from each group.
Table 1.
Mean ± standard error of the mean (SEM) values of each of the measures described in the data analysis section
| Group | Phasor magnitude*+ | Phasor angle | RA* | M10* | L5 | CS10*+ |
|---|---|---|---|---|---|---|
| Healthy adults | 0.40 ± 0.02 | 1.31 ± 0.26 | 0.68 ± 0.02 | 0.47 ± 0.01 | 0.09 ± 0.01 | 0.15 ± 0.02 |
| ADRD, winter | 0.22 ± 0.04 | 0.98 ± 0.57 | 0.50 ± 0.05 | 0.34 ± 0.02 | 0.11 ± 0.01 | 0.09 ± 0.02 |
| ADRD, summer | 0.35 ± 0.05 | 1.13 ± 0.44 | 0.51 ± 0.06 | 0.36 ± 0.03 | 0.12 ± 0.02 | 0.17 ± 0.03 |
A significant difference (p < 0.05) between the Healthy adults, Winter and the ADRD, Winter groups;
A significant difference (p < 0.05) between the ADRD, Summer and ADRD, Winter groups.
Fig. 1.
Phasor diagrams with light and activity profiles. Individual phasors (black lines) are shown with the average phasors (arrows) for each of the three groups, (a) healthy adult subjects studied during winter, (b) a group of subjects with ADRD studied during winter, and (c) another group of ADRD subjects studied during summer. Representative 5-day average light (CS, gray) and activity (AI, black) profiles are shown for a selected individual from each group (a–c). The representative individuals are those having the median phasor magnitude within each group.
The t-tests revealed that during the winter, those with ADRD exhibited more circadian disruption than healthy adults as reflected by their significantly shorter phasor magnitudes and lower RA values. Those with ADRD studied in the winter also had significantly shorter phasor magnitudes than those studied in the summer. ADRD adults were less active during waking hours than healthy adults, as measured by M10. ADRD adults studied in the winter were exposed to less light than healthy adults in the winter and than ADRD adults in the summer, as measured by CS10 (Fig. 1).
DISCUSSION
We used light-dark and activity-rest patterns to assess circadian disruption. Our results are consistent with previous studies that have reported activity levels and sample light measurements using a photopic illuminance meter in older adults [18–20]. The present results are, however, the first field study to examine the synchrony between the calibrated light stimulus pattern and the activity response pattern to assess circadian disruption. Measurements obtained with the Dimesimeter revealed that those with ADRD experienced more circadian disruption than healthy older adults. For comparison, schoolteachers and day-shift nurses, who typically exhibit little circadian disruption, had mean phasor magnitudes of 0.53 and 0.46, respectively, while healthy adults had a mean phasor magnitude of 0.40 [17, 21]. Rotating-shift nurses who worked one night a week had a mean phasor magnitude of 0.30, whereas those who worked two nights and three nights a week (and therefore were more disrupted) had a mean phasor magnitude of 0.12 and 0.05, respectively [17]. RA and phasor magnitudes, used here as measures of circadian disruption, were significantly correlated (r2 = 0.32; p < 0.0001). Although MMSE scores did not correlate well with lower phasor magnitudes (r2 < 0.01), there was a significant correlation between CS10 values and phasor magnitudes in the ADRD groups (r2 = 0.7; p < 0.0001), suggesting that higher amounts of circadian light exposures during the day reduce circadian disruption.
Irregular activity-rest patterns are common in those with ADRD. Our data suggest that more deliberate light interventions, particularly during the winter, could reduce circadian disruption and thereby reduce sleep disturbances in older persons, particularly those with ADRD.
Acknowledgments
The National Institute on Aging (R01 AG034157) funded the study. Barbara Plitnick, Andrew Bierman, Dennis Guyon, and Ines Martinovic of the LRC and Ashritha Epur of CWRU are acknowledged for their assistance.
Footnotes
Authors’ disclosures available online (http://www.jalz.com/disclosures/view.php?id=1310).
References
- 1.Carvalho-Bos SS, Riemersma-van der Lek RF, Waterhouse J, Reilly T, Van Someren EJW. Strong association of the rest-activity rhythm with well-being in demented elderly women. Am J Geriatr Psychiatry. 2007;15:92–100. doi: 10.1097/01.JGP.0000236584.03432.dc. [DOI] [PubMed] [Google Scholar]
- 2.Dowling GA, Hubbard EM, Mastick J, Luxenberg JS, Burr RL, Van Someren EJ. Effect of morning bright light treatment for rest-activity disruption in institutionalized patients with severe Alzheimer’s disease. Int Psychogeriatr. 2005;17:221–236. doi: 10.1017/S1041610205001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamadera H, Ito T, Suzuki H, Asayama K, Ito R, Endo S. Effects of bright light on cognitive and sleep-wake (circadian) rhythm disturbances in Alzheimer-type dementia. Psychiatry Clin Neurosci. 2000;54:352–353. doi: 10.1046/j.1440-1819.2000.00711.x. [DOI] [PubMed] [Google Scholar]
- 4.Lyketsos CG, Lindell Veiel L, Baker A, Steele C. A randomized, controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long-term care. Int J Geriatr Psychiatry. 1999;14:520–525. [PubMed] [Google Scholar]
- 5.Fetveit A, Skjerve A, Bjorvatn B. Bright light treatment improves sleep in institutionalized elderly–an open trial. Int J Geriatr Psychiatry. 2003;18:520–526. doi: 10.1002/gps.852. [DOI] [PubMed] [Google Scholar]
- 6.Van Someren E, Kessler A, Mirmirann M, Swaab D. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biol Psychiatry. 1997;41:955–963. doi: 10.1016/S0006-3223(97)89928-3. [DOI] [PubMed] [Google Scholar]
- 7.Ancoli-Israel S, Gehrman P, Martin JL, Shochat T, Marler M, Corey-Bloom J, Levi L. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s disease patients. Behav Sleep Med. 2003;1:22–36. doi: 10.1207/S15402010BSM0101_4. [DOI] [PubMed] [Google Scholar]
- 8.Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: A randomized controlled trial. JAMA. 2008;299:2642–2655. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- 9.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris JN, Fries BE, Morris SA. Scaling ADLs Within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54:M546–M553. doi: 10.1093/gerona/54.11.m546. [DOI] [PubMed] [Google Scholar]
- 12.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. 4. American Psychiatric Association; Arlington, VA: 2000. [Google Scholar]
- 13.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease. Neurology. 1984;34:939. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 14.Lighting Research Center RPI. Dimesimeter -Light and Activity Measurement System Description and Calibration. Rensselaer Polytechnic Institute; 2011. [Accessed October 19, 2011]. http://www.lrc.rpi.edu/programs/lightHealth/pdf/DimesimeterDoc.pdf, September 2, 2011. [Google Scholar]
- 15.Rea MS, Figueiro MG, Bierman A, Bullough JD. Circadian light. J Circadian Rhythms. 2010;8:2. doi: 10.1186/1740-3391-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rea M, Bierman A, Figueiro M, Bullough J. A new approach to understanding the impact of circadian disruption on human health. J Circadian Rhythms. 2008;6:7. doi: 10.1186/1740-3391-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller D, Figueiro M, Bierman A, Schernhammer E, Rea M. Ecological measurements of light exposure, activity and circadian disruption. Lighting Res Technol. 2010;42:271–284. doi: 10.1177/1477153510367977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ancoli-Israel S, Klauber M, Jones D, Kripke DF, Martin J, Mason W, Pat-Horenczyk R, Fell R. Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing-home patients. Sleep. 1997;20:18–23. [PubMed] [Google Scholar]
- 19.Campbell S, Kripke D, Gillin J, Hrubovcak J. Exposure to light in healthy elderly subjects and Alzheimer’s patients. Physiol Behav. 1988;42:141–144. doi: 10.1016/0031-9384(88)90289-2. [DOI] [PubMed] [Google Scholar]
- 20.Shochat T, Martin J, Marler M, Ancoli-Israel S. Illumination levels in nursing home patients: Effects on sleep and activity rhythms. J Sleep Res. 2000;9:373–379. doi: 10.1046/j.1365-2869.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- 21.Rea MS, Brons JA, Figueiro MG. Measurements of light at night (LAN) for a sample of female school teachers. Chronobiol Int. 2011;28:673–680. doi: 10.3109/07420528.2011.602198. [DOI] [PMC free article] [PubMed] [Google Scholar]